Abstract
Impulse oscillometry (IOS) is a variant of forced oscillation technique, described by Dubois over 50 years ago, which permits passive measurement of lung mechanics. In this method, sound waves are superimposed on normal tidal breathing, and the disturbances in flow and pressure caused by the external waves are used to calculate parameters describing the resistance to airflow and reactive parameters that mostly relate to efficient storage and return of energy by the lung. It requires minimal patient cooperation and can be done easily in subjects who are unable to perform spirometry. Importantly, IOS can differentiate small airway obstruction from large airway obstruction and is more sensitive than spirometry for peripheral airway disease. It has been used to study various respiratory disorders, especially asthma and is suitable for measuring bronchodilatory response as well as bronchoprovocation testing. IOS parameters seem to be able to pick up early changes in lung functon such that they are superior to spirometry in predicting loss of control in asthmatic patients and possibly in identifying early airway disease in smokers. Such comparisons, especially for chronic obstructive pulmonary disease, are made difficult by widespread use of spirometric parameters as the diagnostic gold standard. Here, we discuss the principles and technique of IOS and review its application in obstructive airway diseases.
KEY WORDS: Impulse oscillometry, lung function, obstructive airway disease, small airway obstruction
INTRODUCTION
Mechanical properties of the lung are important determinants as well as indicators of lung function and thus help in the diagnosis and monitoring of several lung disorders. Of these, the most common are obstructive airway diseases (OAD) that afflict almost 10% of the world population and numbers may be even higher in India due to indoor and outdoor pollution.[1,2,3] Asthma and chronic obstructive pulmonary disease (COPD) are responsible for the bulk of OAD burden. Spirometry, the most commonly performed lung function test in clinical practice, is considered to be the gold standard diagnostic test for OAD. However, the forceful expiratory and inspiratory maneuvers of spirometry require patient cooperation and physical capacity that is usually lacking in young children below 4 years age, elderly, and those with physical and cognitive limitations. Other than technical difficulties, there are also some fundamental limitations. While COPD is already considered to be predominantly a small airway disease, with almost 80-90% of terminal bronchioles being lost by the time emphysema is clearly visible on CT, there is increasing recognition that small airway disease is also an important aspect of asthma.[4,5] Physiological principles of spirometry dictate that maximal expiratory flow (MEF) is governed by resistance of the airway segment upstream of the choke point (CP).[6] The CP starts centrally and moves peripherally, during expiration. FEV1 is theoretically a poor measure of peripheral/small airway disease since, during the initial blow, the chokepoint is central and MEF is mostly unaffected by peripheral airway resistance. This forms the basis of using mid or late expiratory flows (MEF 25-75) as an index of peripheral/small airway disease. However, this too is shown to be inadequate. For example, many cleanup workers and firefighters who were exposed to toxic fumes during the 9/11 World Trade Center attack and its aftermath developed persistent respiratory symptoms suggestive of airway disease but had absolutely normal spirometry including normal MEF 25-75. Importantly, the presence of small airway disease was firmly established using IOS in this group.[7]
IOS is a noninvasive method, which uses sound waves to measure respiratory mechanics. It is based on the principle of forced oscillation technique (FOT), first described by Dubois et al. in 1956.[8] Two main advantages of the IOS/FOT are: (a) Performing the test is relatively easy since it is a passive method that requires minimal cooperation and (b) it measures resistance and reactance at different frequencies in lung offering important information about regional inhomogeneity and lung periphery.[9,10] In IOS, the only requirement is for the subject to be relaxed and breathing normally while sound waves are being superimposed on the breathing. This does not require any effort from the subject and hence is feasible to do in many situations like in children, very elderly people, in subjects who are on ventilators, who underwent surgery or when spirometry related bronchospasm is a concern. The second advantage is that IOS can detect subtle changes in the small airway function even in the setting of normal spirometry, as illustrated above, thus providing valuable information for early diagnosis and monitoring of airway diseases. In this review, we describe the principles of IOS and discuss the progress in adopting this relatively new methodology in OAD.
IMPULSE OSCILLOMETRY METHODOLOGY
Principle
In FOT, the sound waves, generated with the help of a loudspeaker are transmitted into the lungs of the subject. These sound waves, which are essentially pressure waves, cause changes in the pressure and this change in pressure drives changes in airflow. By measuring the magnitude of change in the pressure and flow, one can determine the mechanical properties of the lung. Waves of lower frequencies travel deep into lungs till alveoli and are reflected back while those of higher frequencies are reflected from the larger airways. Thus, the parameters calculated at different frequencies give measures of different regions in the lungs. The main difference is that in FOT, the sound waves of different frequencies were transmitted sequentially, whereas in IOS, an impulse, which can be mathematically decomposed into different frequencies, is transmitted. This helps in reducing the time of test and also provides a high signal to noise resolution.
Technique
An impulse consisting of a mixture of sound waves of different frequencies is generated by the loud speaker at the mouth. As this wave passes into the lungs, it causes changes in pressure as well as in the flow of air. The frequencies of the waves delivered in IOS ranges from 5 to 30 Hz. While frequencies higher than 30 Hz can cause discomfort to the patient, the parameters measured at <5 Hz are influenced by breath dynamics. A pressure transducer and a pnuemochromatograph are present at the mouthpiece, to measure the pressure and flow, respectively [Figure 1]. During testing, the Subject should be in sitting position with the head in neutral or slightly extended position with a nose clip. A technician (or the subject) should firmly support the cheeks of the subject during measurement.
Figure 1.
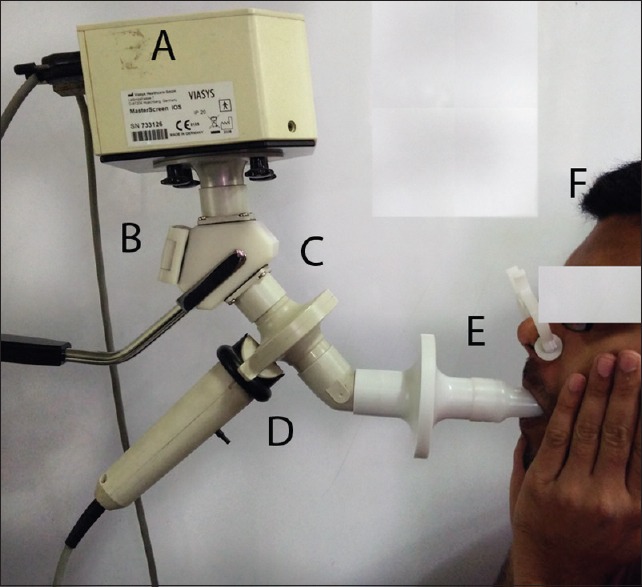
Impulse oscillometry system showing loud speaker (A), screen flap (B), Y-adapter (C), pnuemochomatograph (D), mouth piece (E) and subject wearing nose clip and supporting cheeks with the hands (F)
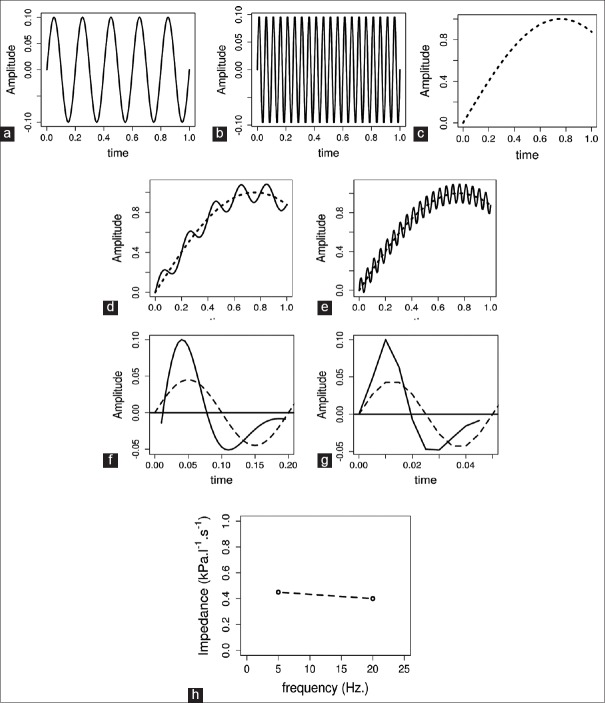
For elucidation of the mathematical aspect of this technique, let us consider a simplistic simulated scenario in which, sound waves at two frequencies 5 Hz and 20 Hz were passed into the lung sequentially. The pure sinusoidal waves at these frequencies are shown in Figure 2a and b. Flow recordings before the superposition of the waves show normal airflow during tidal breathing [Figure 2c]. This shows the normal flow of air in and out of lungs at a typical breathing frequency (16-20/min). When the sound waves are overlapped on the tidal breathing, they result in a change in the flow and now flow recording shows a complex signal consisting of both respiratory and sound wave induced components, i.e. one can observe the basic respiratory flow pattern at the breathing frequency as well as disturbances in the flow because of the superimposed waves [Figure 2d and e]. These are separated using baseline approximation technique. In short, straight line segment is inserted between the start and end points of the flow recordings due to each wave and consider this as the baseline. The recordings after the baseline correction show the separation of the respiratory component and that due to external waves [Figure 2f and g]. The same procedures apply to the recordings of pressure, and now, we have the recordings of flow and pressure with respect to time. These recordings in time scale have to be converted into the frequency scale to further calculate the parameters of our interest. Fast Fourier transform, a mathematical technique is used to convert this time scale to frequency scale. Respiratory input impedance (Zrs) is calculated as the ratio of the resulting pressure and flow changes due to the external pressure waves.
Figure 2.
Elucidation of Impulse oscillometry methodology. Sine waves at 5 Hz (a) and at 20 Hz (b). Flow recording of normal tidal breathing (c). Flow recording when tidal breathing is superimposed by 5 Hz waves (d) and 20 Hz waves (e). (f-g) show simulated change in flow (solid line) when a pressure wave (dashed line) with frequency 5 Hz (f) or 20 Hz (g) is applied
However, IOS differs slightly from the above example (FOT), where the 5 and 20 Hz pressure waves are shown separately for simplicity. In IOS, rather than sending the pressure waves of different frequencies sequentially, an impulse that mathematically consists of all frequencies from 5 Hz to 30 Hz is sent into the lungs. The disadvantage is that the impulse used in the IOS can be a little forceful to the subject when compared to the gentler plain sinusoidal waves of FOT and may even change the lung mechanics slightly. In addition, if one desires to track within-breath changes of Zrs, the discontinuous nature of IOS can reduce temporal resolution. However, the advantages overcome these limitations for most pulmonologists. First, with IOS we can calculate the impedance at every frequency from 5 to 30, whereas, in FOT, we can calculate only at the frequencies of sine waves we use. Second, this results in improved signal to noise ratio and makes it a better tool for detecting regional abnormalities that have small effects on lung mechanics. Last but not least, this also decreases the duration of the test. Together, this leads to increased efficiency for diagnostic applications in a PFT laboratory.
IMPULSE OSCILLOMETRY PARAMETERS AND THEIR INTERPRETATION
Impedance
Respiratory impedance is the sum of all forces which oppose the generated impulse. Impedance measured at any frequency is the ratio of the difference in pressure and changes in the flow at that frequency. Depending on the region where the pressure is measured, the impedance varies. For example, pressure difference at the mouth and in the alveoli gives impedance of the airways and the difference at the mouth, and pleural pressures give a total impedance of the lung. In IOS, the pressure measured at the mouth is compared to atmospheric pressure, which is the pressure outside the chest wall. This defined as respiratory system Zrs and includes the in-phase (real) component which is the resistive component (Rrs) and an out-of-phase (imaginary) component which is a reactive component (Xrs). Simply put, Rrs can be viewed as the energy dissipation whereas Xrs as energy storage. Since IOS measures input impedance, abnormalities of chest wall and skeletal muscles will also be reflected in the measurement.
Resistance
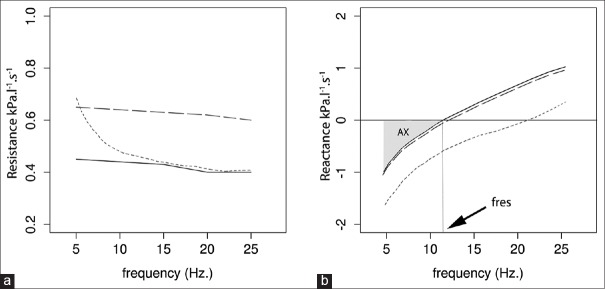
The resistance derived from impedance includes the resistance due to central airways, peripheral airways, lung tissue, and chest wall, although the latter two are usually negligible. Almost, 80% of the resistance is contributed by central airways and only 20% by small airways (<2 mm in diameter) in adults.[11,12] This is mainly because of the high total cross-sectional area of small airways. However, in children the contribution of small airways is higher than in adults. Resistance values are considered normal within the range of 150% of predicted values. Resistance is independent of the frequency in healthy subjects. In central airway obstruction, the resistance at all frequency increases, while, in small airway obstruction, the resistance at lower frequencies increases but is unchanged at higher frequencies that do not reach the small airways [Figure 3a]. This frequency dependency of resistance might be normal in children, but indicates small airway obstruction in adults.
Figure 3.
Measurements of resistance (a). and reactance (b). on a frequency scale. Bold, long-dash and short-dash lines represent measurements in normal, central airway obstruction and peripheral airway obstruction respectively
Reactance
Reactance includes two components, the inertia of the air column to move (inertance) and the capacitance of the lung. Capacitance can be interpreted as a property which reflects elasticity of the lung. The capacitance component of the reactance is defined to be negative in sign and inertance is defined as positive. Unlike resistance, reactance is frequency dependent. Since, the elastic properties of lungs majorly reside at the periphery, at low frequencies, the capacitance component dominate, and total lung reactance is negative, whereas, at higher frequencies, the inertia of the air column in larger airways dominates making the total reactance positive [Figure 3b]. Importantly and perhaps counterintuitively, here elastance or capacitance refers to energy return properties of the lung, similar to electric circuits, not stiffness during inflation – the more intuitive definition for clinicians. Therefore, in either fibrosis or emphysema or small airway disease, the reactance at lower frequencies would change in the same direction, i.e. become even more negative. Hence, the direction of change of reactance does not differentiate between obstructive or restrictive diseases.
Resonant frequency
Resonant frequency (fres) is defined as the frequency at which the inertial properties of airway and the capacitance of lung periphery are equal [Figure 3b], i.e., the frequency at which total reactance is zero. We cannot attribute fres to a specific mechanical property of lungs, but it can be used to separate low frequencies where capacitance component dominates from high frequencies where the inertial component takes over. The normal value of fres in adults is 7-12 Hz. In children, it is higher and increases with decreasing age. In lung diseases, both obstructive and restrictive, fres is increased above normal. This is because of reactance becoming more negative at low frequencies in each of these diseases, as discussed above.
Area of reactance
Area of reactance (AX) includes the area under the reactance curve from lowest frequency to the fres. It is the area between X-axis below zero and reactance curve in the graph [Figure 3b]. It includes the total area dominated by the capacitance and reflects the elastic properties of the lung. As seen with reactance and fres, this also increases in any disease of lung periphery. AX is a single measurement that summarizes the above parameters and is also shown to be correlated with resistance at lower frequencies.
Coherence
Coherence is another important parameter and is used to determine the validity and quality of the test results. It reflects the reproducibility the impedance measurements. It is a value between 0 and 1 and, ideally, should be >0.8 at 5 Hz and >0.9 at 20 Hz for the measurement to be considered valid. However, it is important to note that these values are for adults, and there are no standard values reported in children. Coherence can be decreased because of improper technique, irregular breathing, glottis closure, and swallowing.
Reference values
Normal values for adult and pediatric population are essential for easy interpretation of the test. However, studies that aim to determine predictive equations for IOS parameters were few worldwide, and there were no studies done on the Indian population. There is an urgent need for conducting such studies in many areas around the globe, including India.
Among those studies, many were done in children. All those studies found standing height as the single most important parameter which helps in predicting resistance. Age is also shown to have strong correlation with resistance and reactance values. The resistance and Frequency of resonance decrease with increasing height and age, whereas, reactance increases. The relationship between reactance and height is linear while between resistance and height is mostly exponential as suggested these studies. Table 1 shows a comparison of the predictive equations published in different populations.
Table 1.
Predictive equations of R5 and X5 in different populations
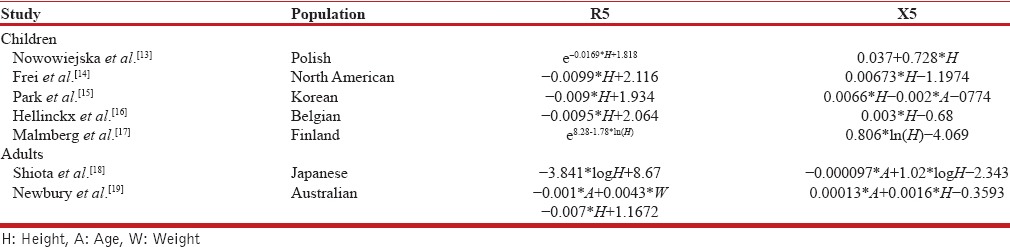
The studies done to determine reference values in adults are very few, and they have also identified height and age as most important covariates. Shiota et al., have shown the predictive equations revealed significant differences in smokers and nonsmokers, but not between men and women in the Japanese population.[18] Newbury et al., determined predictive equations in the Australian population and both studies have concluded age and height as significant predictors.[19] The equations determined by these studies are compared in Table 1. More studies have to be conducted in different regions of the world to validate existing findings and to provide robust global references of the parameters.
IMPULSE OSCILLOMETRY IN OBSTRUCTIVE AIRWAY DISEASES
IOS has been used to study a number of disease states, including asthma, COPD, and interstitial lung diseases. Most of the studies have been done in asthmatic children and are aimed at monitoring the progress of disease and elucidating the effect of bronchodilators.
Diagnosis of asthma includes a demonstration of reversible obstruction and bronchial hyperresponsiveness. IOS has been demonstrated to be sensitive and accurate on both counts. Decrease in R5 by 30-35% is considered as a positive bronchodilator response.[20,21] Other parameters such as R10 and AX have also been shown to change in response to the bronchodialators. Asthmatic patients have increased R5, fres, and AX while the X5 is more negative. In bronchoprovocation testing, a similar response, i.e., an increase in R5, fres, and AX and decrease in the X5 is observed. A 20% decrease in FEV1 is shown to be equivalent to 50% decrease in X5, which has been shown to be a more sensitive parameter for identifying bronchial hyperreactivity.[22] Schulze et al. showed that even at lower doses of methacholine, there were significant changes in IOS parameters, reflecting the sensitivity of the procedure. This allows bronchoprovocative tests to be performed with smaller doses of bronchoconstricting agents.[23]
IOS has also been shown to be effective in monitoring asthma. Shi et al. compared baseline spirometric and IOS parameters between asthmatic children subsequently presenting with exacerbations and children with controlled asthma. They found that there was no difference in baseline spirometric values except small differences in FEV1/forced vital capacity, but IOS parameters, R5, frequency dependence of resistance (R5 – R20), and AX were significantly different between the two groups. Receiving operator characteristics (ROC) showed baseline R5 – R20 and AX were effective in predicting asthma control at subsequent visit to the clinic with area under the curves being 0.91 and 0.90, respectively.[24] Similarly, Gonem et al. showed that entropy of impedence measured over time can differentiate the frequent exacerbators from less frequent exacerbators among asthmatic patients.[25]
In COPD patients, Gong et al. have shown that the reactive parameters were correlated with lung function more than resistance. They have also proposed that changes in X5 over time might be used for monitoring the disease.[26] Frantz et al. reported that inspite of normal spirometry, the subjects with symptoms of COPD have higher pulmonary resistance and lower pulmonary reactance. Their finding indicates that IOS is a more sensitive technique in detecting the subtle changes in the lung function.[27] Even though, IOS is very useful in the diagnosis, there were limited studies in COPD to prove use of the technique for monitoring the disease progress. The fact that spirometry is a diagnostic standard for COPD complicates matters. Evaluation of COPD Longitudinally for better definition of Predictive Surrogate Endpoints had baseline IOS done in 2054 subjects with COPD (GOLD criteria), 233 nonsmoking controls, and 322 smoking controls.[28] COPD was associated with increased R5, R5 – R20, and reactance. However, 5-10% of smokers with normal spirometry had “abnormal” IOS. These subjects tended to have lower (but normal) FEV1, were older and were heavier smokers; possibly reflecting real disease undiagnosed by spirometry. IOS parameters overlapped between the groups and thus IOS based diagnosis will be different from spirometry. This has been confirmed by others who report that healthy smokers often have abnormal IOS compared to nonsmokers.[29]
CONCLUSION
IOS is a very useful tool in measuring the mechanical properties of lung, which helps in diagnosis and monitoring the progress of disease. It is very easy to perform in children and elderly subjects. Repeat variability for all IOS parameters is higher than FEV1 (~10% vs. ~5%), but is acceptable in clinical practice. Notably, IOS parameters may be less variable than other commonly measured spirometry parameters such as FEF50 (~20%). IOS may be more sensitive than spirometry in detecting airway abnormalities. It is also a better tool to predict asthma control and exacerbations. Since the diagnosis of COPD is based on spirometric definitions, it is difficult to compare IOS. However, since IOS is better for detecting small airway disease in asthma and postenvironmental exposure[7] even where spirometry is normal, the presumption is that IOS should be more sensitive in identifying early COPD. The main limitation is the lack of reference values and extensive evaluation over different disease conditions. However, these are not limitations of the technique itself and can be overcome once the technique is adopted widely and more thorough studies are conducted. In India, another limitation could be its cost and lack of portability. One need to have a dedicated laboratory to perform the test and it is not possible to use in the field studies. However, given the value, it adds to the clinical diagnosis and monitoring the prognosis, IOS has the potential to be a part of routine pulmonary examination. Further technological advances that reduce the form factor, making it inexpensive and portable, as well as novel analytical methods for ease of diagnosis are on the horizon. Such developments would make IOS a viable alternative to spirometry in routine clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138:3–6. doi: 10.1378/chest.10-0645. [DOI] [PubMed] [Google Scholar]
- 2.Kodgule R, Salvi S. Exposure to biomass smoke as a cause for airway disease in women and children. Curr Opin Allergy Clin Immunol. 2012;12:82–90. doi: 10.1097/ACI.0b013e32834ecb65. [DOI] [PubMed] [Google Scholar]
- 3.Murtagh E, Heaney L, Gingles J, Shepherd R, Kee F, Patterson C, et al. Prevalence of obstructive lung disease in a general population sample: The NICECOPD study. Eur J Epidemiol. 2005;20:443–53. doi: 10.1007/s10654-005-1248-8. [DOI] [PubMed] [Google Scholar]
- 4.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 5.Contoli M, Bousquet J, Fabbri LM, Magnussen H, Rabe KF, Siafakas NM, et al. The small airways and distal lung compartment in asthma and COPD: A time for reappraisal. Allergy. 2010;65:141–51. doi: 10.1111/j.1398-9995.2009.02242.x. [DOI] [PubMed] [Google Scholar]
- 6.Thien F. Measuring and imaging small airways dysfunction in asthma. Asia Pac Allergy. 2013;3:224–30. doi: 10.5415/apallergy.2013.3.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oppenheimer BW, Goldring RM, Herberg ME, Hofer IS, Reyfman PA, Liautaud S, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest. 2007;132:1275–82. doi: 10.1378/chest.07-0913. [DOI] [PubMed] [Google Scholar]
- 8.Dubois AB, Botelho SY, Comroe JH., Jr A new method for measuring airway resistance in man using a body plethysmograph: Values in normal subjects and in patients with respiratory disease. J Clin Invest. 1956;35:327–35. doi: 10.1172/JCI103282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith HJ, Reinhold P, Goldman MD. Forced oscillation technique and impulse oscillometry. European Respiratory Monograph. Ch. 5. In: Gosselink R, Stam H, editors. Lung Function Testing. UK: European Respiratory Society Publications; 2005. pp. 72–105. [Google Scholar]
- 10.Bickel S, Popler J, Lesnick B, Eid N. Impulse oscillometry: Interpretation and practical applications. Chest. 2014;146:841–7. doi: 10.1378/chest.13-1875. [DOI] [PubMed] [Google Scholar]
- 11.Hogg JC, Williams J, Richardson JB, Macklem PT, Thurlbeck WM. Age as a factor in the distribution of lower-airway conductance and in the pathologic anatomy of obstructive lung disease. N Engl J Med. 1970;282:1283–7. doi: 10.1056/NEJM197006042822302. [DOI] [PubMed] [Google Scholar]
- 12.Mead J. The lung's “quiet zone”. N Engl J Med. 1970;282:1318–9. doi: 10.1056/NEJM197006042822311. [DOI] [PubMed] [Google Scholar]
- 13.Nowowiejska B, Tomalak W, Radlinski J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3-18 years. Pediatr Pulmonol. 2008;43:1193–7. doi: 10.1002/ppul.20926. [DOI] [PubMed] [Google Scholar]
- 14.Frei J, Jutla J, Kramer G, Hatzakis GE, Ducharme FM, Davis GM. Impulse oscillometry: Reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest. 2005;128:1266–73. doi: 10.1378/chest.128.3.1266. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Yoon JW, Shin YH, Jee HM, Wee YS, Chang SJ, et al. Reference values for respiratory system impedance using impulse oscillometry in healthy preschool children. Korean J Pediatr. 2011;54:64–8. doi: 10.3345/kjp.2011.54.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellinckx J, De Boeck K, Bande-Knops J, van der Poel M, Demedts M. Bronchodilator response in 3-6.5 years old healthy and stable asthmatic children. Eur Respir J. 1998;12:438–43. doi: 10.1183/09031936.98.12020438. [DOI] [PubMed] [Google Scholar]
- 17.Malmberg LP, Pelkonen A, Poussa T, Pohianpalo A, Haahtela T, Turpeinen M. Determinants of respiratory system input impedance and bronchodilator response in healthy Finnish preschool children. Clin Physiol Funct Imaging. 2002;22:64–71. [PubMed] [Google Scholar]
- 18.Shiota S, Katoh M, Fujii M, Aoki S, Matsuoka R, Fukuchi Y. Predictive equations and the reliability of the impulse oscillatory system in Japanese adult subjects. Respirology. 2005;10:310–5. doi: 10.1111/j.1440-1843.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 19.Newbury W, Crockett A, Newbury J. A pilot study to evaluate Australian predictive equations for the impulse oscillometry system. Respirology. 2008;13:1070–5. doi: 10.1111/j.1440-1843.2008.01375.x. [DOI] [PubMed] [Google Scholar]
- 20.Song TW, Kim KW, Kim ES, Park JW, Sohn MH, Kim KE. Utility of impulse oscillometry in young children with asthma. Pediatr Allergy Immunol. 2008;19:763–8. doi: 10.1111/j.1399-3038.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 21.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112:317–22. doi: 10.1067/mai.2003.1627. [DOI] [PubMed] [Google Scholar]
- 22.Bailly C, Crenesse D, Albertini M. Evaluation of impulse oscillometry during bronchial challenge testing in children. Pediatr Pulmonol. 2011;46:1209–14. doi: 10.1002/ppul.21492. [DOI] [PubMed] [Google Scholar]
- 23.Schulze J, Smith HJ, Fuchs J, Herrmann E, Dressler M, Rose MA, et al. Methacholine challenge in young children as evaluated by spirometry and impulse oscillometry. Respir Med. 2012;106:627–34. doi: 10.1016/j.rmed.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Aledia AS, Galant SP, George SC. Peripheral airway impairment measured by oscillometry predicts loss of asthma control in children. J Allergy Clin Immunol. 2013;131:718–23. doi: 10.1016/j.jaci.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 25.Gonem S, Umar I, Burke D, Desai D, Corkill S, Owers-Bradley J, et al. Airway impedance entropy and exacerbations in severe asthma. Eur Respir J. 2012;40:1156–63. doi: 10.1183/09031936.00228611. [DOI] [PubMed] [Google Scholar]
- 26.Gong SG, Yang WL, Zheng W, Liu JM. Evaluation of respiratory impedance in patients with chronic obstructive pulmonary disease by an impulse oscillation system. Mol Med Rep. 2014;10:2694–700. doi: 10.3892/mmr.2014.2528. [DOI] [PubMed] [Google Scholar]
- 27.Frantz S, Nihlén U, Dencker M, Engström G, Löfdahl CG, Wollmer P. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respir Med. 2012;106:1116–23. doi: 10.1016/j.rmed.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Crim C, Celli B, Edwards LD, Wouters E, Coxson HO, Tal-Singer R, et al. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med. 2011;105:1069–78. doi: 10.1016/j.rmed.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Shinke H, Yamamoto M, Hazeki N, Kotani Y, Kobayashi K, Nishimura Y. Visualized changes in respiratory resistance and reactance along a time axis in smokers: A cross-sectional study. Respir Investig. 2013;51:166–74. doi: 10.1016/j.resinv.2013.02.006. [DOI] [PubMed] [Google Scholar]