Abstract
Adherence to antiretroviral therapy (ART) is crucial for thwarting HIV disease progression and reducing secondary HIV transmission, yet youth living with HIV (YLH) struggle with adherence. The highest rates of new HIV infections in the United States occur in young African American men. A sample of 387 HIV-positive young African American males on ART was selected from a cross-sectional assessment of (YLH) receiving medical care within the Adolescent Trials Network for HIV/AIDS Interventions (ATN) from 2010 to 2012 (12–24 years old, median 22.00, SD 2.08). Participants completed self-reported adherence, demographic, health, and psychosocial measures. Sixty-two percent self-reported 100% ART adherence. Optimal data analysis identified frequency of cannabis use during the past 3 months as the strongest independent predictor of adherence, yielding moderate effect strength sensitivity (ESS) = 27.1, p < 0.001. Among participants with infrequent cannabis use, 72% reported full adherence; in contrast, only 45% of participants who used cannabis frequently reported full adherence. Classification tree analysis (CTA) was utilized to improve classification accuracy and to identify the pathways of ART adherence and nonadherence. The CTA model evidenced a 38% improvement above chance for correctly classifying participants as ART adherent or nonadherent. Participants most likely to be adherent were those with low psychological distress and minimal alcohol use (82% were adherent). Participants least likely to be adherent were those with higher psychological distress and engaged in weekly cannabis use (69% were nonadherent). Findings suggest multiple profiles of ART adherence for young African American males living with HIV and argue for targeted psychosocial interventions.
Introduction
In the United States, the HIV/AIDS epidemic has disproportionally affected racial and ethnic minority groups, adolescents and young adults, and men who have sex with men (MSM) at higher rates than other demographic groups.1–6 Young people, ages 13–24, are disproportionately impacted by HIV with a little under a quarter of new HIV infections occurring among those less than 24 years of age, although this age bracket comprises just 15.4% of the general population.7,8 In addition, African Americans accounted for 56% of young people (ages 13–24 years) diagnosed with having HIV in 2014; a decrease from 65% in 2009, but relatively unchanged from the 55% in 2004.2,8 Although the Centers for Disease Control and Prevention (CDC) has created programs to increase HIV awareness for youth, the rate of HIV infection in the United States for African Americans under the age of 24 years continues to disproportionally negatively affect this population.7
Since 1996, antiretroviral therapy (ART), the combination of three or more antiretroviral medications (ARVs), has been the standard of care for HIV/AIDS.9 ART has substantially increased the survival rate and quality of life among individuals living with HIV/AIDS9,10 and is the only method for effectively treating HIV/AIDS among adolescents and young adults.11 ART is also an important tool for HIV prevention efforts because individuals on ART can significantly decrease the likelihood of HIV transmission to their sexual partners when they are virally suppressed.12–14
Historically, for ART to be most effective in preventing HIV virologic failure, ART adherence of 95% or greater is strongly recommended.1,15–17 However, the necessary adherence rate to best prevent virologic failure varies based on class of medication and frequency of dosage.18 For example, unboosted protease inhibitors (PIs) require an adherence level of 95%, but boosted PIs require an adherence level of just 80%, and non-nucleoside reverse transcriptase inhibitors require adherence levels even below boosted PIs.19 In 2015, Gordon et al. evaluated three current first-line ART regimen types among 1915 participants on ART, with the purpose of establishing the necessary adherence threshold to maintain virologic suppression for current ARVs. An 80–90% medication progression ratio to each of the three regimens was found to be associated with a virologic failure rate of just 3.5%, significantly below the 20% accepted virologic failure rate used to define adherence threshold requirements in older studies.20 Despite the notable improved potency of newer first-line ARVs compared with ARVs of the 2000s, the goal for ART adherence remains 100% given the complexity of some ART regimens, the risk of developing resistance to ARVs, the effect of inflammatory processes, and increased mortality with suboptimal adherence.20,21
Adherence to ART regimens for adolescents and young adults living with HIV (YLH) is often less than optimal. A 2014 systematic review and meta-analysis of adherence to ART among YLH (ages of 12–24) across the globe identified that overall only 62.3% were adherent to their therapy regimens.22 Of note, this review included studies that assessed adherence rates by self-report, pharmacological measurement (e.g., pill count), or viral load suppression. Adherence was found to be the lowest among YLH from North America, with only 53% being adherent.22
To date, several studies have investigated the predictors of ART adherence among YLH, yet few have focused specifically on understanding predictors of ART adherence among young African American males. Reisner et al.'s (2009) systematic review of all published studies of ART adherence among YLH between the ages of 13 and 24 years revealed five broad areas as being associated with ART adherence,23 specifically (1) demographic factors; (2) psychosocial factors, (3) disease factors (i.e., HIV viral load, CD4+ T-cell count); (4) treatment factors, and (5) physician factors.
Substance use and psychological distress have frequently been found to be associated with ART nonadherence,24–27 while some support has been found for housing instability,28 later HIV disease stage,29 and more complicated medication regimen factors30 as being associated with ART nonadherence. Factors linked with ART adherence for YLH include cognitive thought processes such as positive beliefs about the benefit of ART,31 self-efficacy,32 and motivational readiness,33 as well as social support.34 In addition, recent pilot studies with YLH have evaluated novel interventions such as cell phone support35 as well as utilizing motivational interviewing techniques and financial incentives to increase ART adherence.36
To date, such studies have shown promise for improving ART adherence among YLH. However, limitations of the aforementioned studies include small sample sizes as well as significant variability among samples (e.g., behavioral vs. perinatal acquisition; disparate sociodemographics and adherence methodology), which notably limits our understanding of predictors and pathways of ART adherence outcomes for young African American males living with HIV.
Given that young African American males, and within this demographic group MSM,37 face the greatest risk of acquiring HIV and that a limited number of studies examining predictors of ART adherence specific to this population exist, the current study had the following three aims: (1) identify the strongest predictor(s) of ART adherence among a multisite sample of young African American males living with HIV; (2) illustrate the pathways and profiles of ART adherence for this demographic group; and (3) identify possible targets of interventions for future intervention and prevention programs.
Methods
Participants
Participants (total n = 2216) were originally recruited from participating sites of the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) in 2010–2011 for a network-wide assessment of current health status and behavioral risk factors among YLH. To be eligible to participate, individuals had to be living with HIV, be between the ages of 12 and 24 years, understand written and verbal English, and received care (minimum of one visit) at one of the 20 Adolescent Medicine Trial Unit study sites. Participants were excluded from the study if they exhibited signs that they were unable to complete study measures, such as acute psychosis or intoxication. A subsample, which included all African American males who acquired HIV through behavioral mechanisms (91.2% through sexual contact) and who were currently taking antiretroviral medications (n = 387; age range, 12–24 years;, M = 21.3 years SD = 2.1 years), was selected for the current study.
The vast majority of participants did not identify as heterosexual (88.1%). Of the original 2216 participants, 899 were excluded from the current study because they were not taking HIV medications, 514 were excluded because they acquired HIV perinatally, 209 were excluded because they did not identify as male, and another 207 were excluded because they did not identify as black or African American. See Table 1 for number of study participants from each participating site of the ATN.
Table 1.
The Number of Study Participants from Each ATN Site 2010–2011
| ATN site | N |
|---|---|
| 1. University of South Florida | 3 |
| 2. Children's Hospital of Los Angeles | 11 |
| 3. Children's National Medical Center | 22 |
| 4. Children's Hospital of Philadelphia | 30 |
| 5. Stroger Hospital of Cook County and the CORE Center | 60 |
| 6. University Pediatric Center at University of Puerto Rico | 0 |
| 7. Montefiore Medical Center | 24 |
| 8. Mount Sinai Medical Center | 12 |
| 9. University of California at San Francisco | 13 |
| 10. Tulane Medical Center | 17 |
| 11. University of Maryland | 18 |
| 12. University of Miami School of Medicine | 11 |
| 13. Children's Diagnostic and Treatment Center | 5 |
| 14. St. Jude Children's Research Hospital | 43 |
| 15. Lurie Children's Hospital | 24 |
| 16. Baylor College of Medicine | 27 |
| 17. Wayne State University | 40 |
| 18. Johns Hopkins University | 19 |
| 19. The Fenway Institute | 3 |
| 20. University of Colorado at Denver | 5 |
| Total, N | 387 |
Procedures
Study staff enrolled potential participants after obtaining informed consent from the youth or parental/guardian consent and assent for youth who were minors. Once enrolled in the study, participants completed an assessment battery in a private room or space through an Audio Computer-Assisted Self-Interview (ACASI) within 2 weeks of enrollment. After completing the ACASI, participants were debriefed with a face-to-face interview with a study staff member. All ACASI administrations occurred between 2010 and 2011 and were completed in a single visit. Institutional Review Board approval was obtained at all study sites.
Measures
General demographics
Age, marital status, school status, completed education, employment status, total monthly income, living situation, housing stability, access to technology, sexual orientation, age diagnosed with having HIV, and HIV disclosure status were determined through the ACASI and were included as predictors of ART adherence.
Mental health
Brief symptom inventory
Mental health functioning was assessed with the Brief Symptom Inventory (BSI),38 a 53-item self-report measure that consists of three global indices (global severity, positive symptom distress, and positive symptom total) as well as nine primary symptom subscales (somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism). The questionnaire asks participants to rate on a 5-point Likert scale (from Not at all to Extremely) how much a problem has caused distress over the past 7 days. The BSI has been successfully used with ethnic minority YLH,33 and in the current study, the nine BSI subscales and overall global severity index scale evidenced appropriate levels of internal consistency as Cronbach's alpha ranged from 0.74 to 0.98.
Mental healthcare
Mental healthcare utilization over the prior 12 months was assessed through self-report. Specifically, participants reported on their desire/need for a mental health professional, actual mental health treatment received, number of mental health treatment sessions received, and if they experienced suicidal ideation over the past 12 months.
Substance use
The alcohol, smoking, and substance involvement screening test
Substance use during the past 3 months was assessed with the alcohol, smoking, and substance involvement screening test (ASSIST)39, a 10-item questionnaire that assesses the use of drugs (such as tobacco, alcohol, cannabis, cocaine, amphetamines, inhalants, sedatives, hallucinogens, opioids, and other substances) as well as the frequency of such usage with a 5-point Likert scale (Never to Daily). For the current study, each question assessing the frequency of usage (during the past 3 months) for a specific substance (e.g., cocaine) was entered as an independent predictor. Individual substances were only entered in the model if at least 5% of the sample endorsed some frequency of use. The ASSIST has demonstrated strong convergent, construct, predictive, and discriminative validity and is able to discriminate between low, moderate, and high-risk substance use.39
CRAFFT
The CRAFFT40 is a six-item self-report measure that assesses consequences for illicit drug/alcohol use as well as abuse/dependence. A score of two or higher on the CRAFFT in previous research has been found to be an optimal cutoff score for identifying any drug/alcohol problem or a substance disorder/dependence among an adolescent clinical population (n = 538) where 75% of participants were of a racial or ethnic minority group status.40 In the current study, a total CRAFFT score was created by collapsing the scores across all six items and demonstrated strong internal consistency (α = 0.72).
Social support
Social support questions
Six questions were included to assess the level of perceived social support in key areas related to medication adherence (i.e., keeping medical appointments, taking HIV medication, telling your partner about your HIV status, using condoms, avoiding drug use, and avoiding alcohol use). Participants rated their perceived level of social support with a 5-point Likert scale (from Strongly Disagree to Strongly Agree). For the current study, a total social support scale was created by collapsing the scores across all six questions. Cronbach's alpha = 0.80, indicating an appropriate level of internal consistency among items.
Self-efficacy
Self-efficacy for healthcare
Six questions (three related to medical appointments; three related to medication routine) were administered related to the participants' confidence for keeping future medical appointments and their medication routine. Participants were asked to rate the aforementioned questions with a 5-point Likert scale (from Very sure I can to Very sure I cannot). Lower scores indicated a stronger perceived ability to manage their health needs. Previous research with YLH has found the self-efficacy for medication routine questions to have robust reliability.33 For the current study, a total self-efficacy healthcare scale was created by collapsing the scores across all six questions. In addition, a self-efficacy for keeping medical appointments scale and a self-efficacy for keeping medication routine scale were created as well. Cronbach's alpha for the above said scales was 0.80, 0.69, and 0.81, respectively.
Motivation
Rollnick's readiness ruler
Participants were asked to rate on a scale from 1 (Not ready) to 10 (Completely ready) their motivation to adhere to their prescribed ARVs and make at least four medical appointments per year.41 Previous research with YLH has recommended this tool to assess readiness to change.33 In the current study, a total healthcare motivation item was created by collapsing participants' responses on the above said questions, yielding an adequate level of reliability (α = 0.64). These two questions of healthcare motivation were entered as predictors of ART adherence as well as the total healthcare motivation composite item.
Healthcare provider relationship
Healthcare provider relationship questions
Five questions were included in the overall adherence assessment that focused on the perceived relationship/interaction of the patient with his healthcare provider (e.g., I feel understood by my healthcare provider). Participants were asked to assess their perceived relationship/interaction with their healthcare provider with a 5-point Likert scale (from Strongly Disagree to Strongly Agree). Higher scores indicated a stronger perceived relationship with their healthcare provider. For the current study, a total healthcare provider relationship scale was created by collapsing the scores across all five questions, which demonstrated an appropriate level of internal reliability (α = 0.85).
Adherence
Adherence assessment
Participants were asked to report the number of missed doses over the last weekend and over the last seven days. A dose was defined as all pills required to be taken at a specific time. The adherence questionnaire was developed based on modification of previous Pediatric AIDS Clinical Trials Group (PACTG) and ATN studies supported by findings from Simoni et al.'s42 review of the ART adherence literature and has been used in prior studies with YLH.34,43 Nineteen follow-up questions assessing the barriers and facilitators of adherence originally developed for the Reaching for Excellence in Adolescent Care and Health (REACH) study44 were modified.
Self-report of medication adherence is the most commonly used method for capturing ART adherence among YLH and although there are limitations to this methodology, it has been found to be a valid method to assess ART adherence among YLH.42,45 For the purpose of the current study, only adherence questions related to medication factors (e.g., pill burden and number of daily doses), facilitators (medication reminders), and barriers (recent stressors) of adherence were included as predictors of adherence. In addition, only questions that had adequate response variability were included.
Data analysis
Missing data
Observations with missing data were not imputed as missing data were a relatively low occurrence.
Optimal data analysis
Optimal data analysis (ODA) is a statistical procedure that maximizes classification accuracy of a class variable (i.e., adherence vs. nonadherence).46,47 ODA has several advantages over other predictive statistical analyses that have been used in the adherence literature with YLH. For example, ODA is a nonparametric analysis and therefore does not have to meet the assumptions of parametric tests and uses an exact permutation probability producing an always valid Type-1 error rate.47 Other statistical techniques commonly used (e.g., multiple regression and multivariate analysis of variance) attempt to maximize a variance ratio, which presumes that the attributes selected are significant predictors for every member of a sample, have the same direction of influence for every member of a sample, and have the same coefficient value for all members of a sample, whereas ODA identifies the optimal cut-point of an attribute for maximizing the classification accuracy of the class variable.47
In comparison with statistical techniques that attempt to maximize a likelihood function (e.g., logistic regression), which have been used appropriately by other ART adherence researchers (e.g., Rabound et al.43), ODA has been found to provide greater accuracy of predicting class outcomes.48 In the current study, UniODA was conducted for 72 theoretically relevant variables (Table 2) using software for Windows, thus determining which attributes are independent significant predictors for ART adherence outcomes.
Table 2.
Predictors of ART Adherence Outcomes Included in Optimal Data Analysis
| Construct item/scale/subscales | Number of predictors | Predictors |
|---|---|---|
| Demographic | 15 | Age |
| Marital status | ||
| School status | ||
| Completed education | ||
| Employment status | ||
| Total monthly income | ||
| Living situation | ||
| Housing stability | ||
| Anticipate moving | ||
| Sexual orientation | ||
| Age diagnosed with having HIV | ||
| HIV disclosure status | ||
| Cell phone access | ||
| Computer with internet access | ||
| E-mail access | ||
| Mental health functioning | 12 | BSI scales |
| Global severity | ||
| Positive symptom distress | ||
| Positive symptoms total | ||
| Somatization | ||
| Obsessive-compulsive | ||
| Interpersonal sensitivity | ||
| Depression | ||
| Anxiety | ||
| Hostility | ||
| Phobic anxiety | ||
| Paranoid ideation | ||
| Psychoticism | ||
| Mental healthcare utilization (over the prior 12 months) | 4 | Perceived need for mental health |
| Mental health treatment received | ||
| Number of times counseling sought | ||
| Suicidal ideation | ||
| Substance use and severity | 7 | ASSIST (past 3 months freq) |
| Tobacco use | ||
| Alcohol use | ||
| Cannabis use | ||
| Cocaine use | ||
| Amphetamine use | ||
| Sedatives use | ||
| CRAFFT total score | ||
| Social support | 1 | Total social support for healthy living |
| Self-efficacy | 3 | Self-efficacy for medical appointments |
| Self-efficacy for medication routines | ||
| Total self-efficacy for medical care | ||
| Motivation | 3 | Motivation to take meds as prescribed |
| Motivation to keep medical appointments | ||
| Total motivation for medical care | ||
| Healthcare provider relationship | 1 | Total healthcare provider relationship score |
| Adherence assessment | ||
| Medication factors | 3 | Number of daily dosages |
| Daily pill burden | ||
| Difficulty taking pill | ||
| Facilitators to adherence | 10 | Did something to help remember |
| Labels | ||
| Calendar | ||
| Pill boxes | ||
| Beepers | ||
| Timers | ||
| Programmable wrist watches | ||
| Buddy system | ||
| Take pills when a certain event occurs | ||
| Other | ||
| Barrier to adherence | 13 | Experienced difficulty in past 7 days |
| No access at drugstore | ||
| Prescription elapsed | ||
| Sickness | ||
| Forgetfulness | ||
| Schedule interference | ||
| Needed break (did not feel like taking) | ||
| Living situation change | ||
| Worried about others finding out | ||
| Other illness | ||
| Family/friends do not help remind | ||
| Reminds me of HIV+ status | ||
| Other reason | ||
| Total number of predictors | 72 | |
ART, antiretroviral therapy; ASSIST, alcohol, smoking, and substance involvement screening test; BSI, brief symptom inventory.
Classification tree analysis
In addition, to conducting UniODA, classification tree analysis (CTA) was performed. CTA is an iterative ODA procedure that allows for the creation of a nonlinear multiattribute tree model, which hierarchically maximizes the mean percent accuracy classification of a binary outcome. In the current study, an enumerated CTA model was constructed using automated CTA software for Windows. Enumerated CTA selects the constellation of predictors, which will provide the greatest effect strength sensitivity (ESS) for maximizing the classification accuracy of the class variable. CTA allows for the development of incredibly granular models, which may result in near perfect classification accuracy. However, these models often have endpoints with small numbers of observations from the larger sample, which can limit the potential generalizability of such CTA models.49 Thus, in the current study, the CTA program was instructed to maintain a minimum of 10% of the overall sample in each endpoint, which has been identified as a statistically appropriate method to identify a replicable model.50
Results
Descriptive analyses
The dependent variable was 100% adherence to ART over the past 7 consecutive days, as measured by self-report. Participants who reported not missing a single dose were coded as ART adherent and all other participants were coded as ART nonadherent. In the current sample, 62.3% of participants reported 100% adherence over the past seven days. Means, standard deviations, and/or frequency analyses for all study variables were conducted and can be referenced in Table 3 (demographic, mental health utilization, and adherence characteristics) and Table 4 (BSI, substance use, and healthcare characteristics).
Table 3.
Demographic, Mental Health Utilization, and Adherence Characteristics for YAAM Living with HIV 12–24 Years of Age
| na(%) | |
|---|---|
| Demographics and HIV characteristics | |
| Age (years) | |
| Mean | 21.32 |
| Standard deviation | 2.06 |
| Median | 22.00 |
| Minimum | 12 |
| Maximum | 24 |
| Current marital status | |
| Single | 337 (87.1) |
| Living with a steady partner | 39 (10.1) |
| Married | 3 (0.8) |
| Separated | 1 (0.3) |
| Divorced | 0 (0) |
| Widowed | 0 (0) |
| Other | 7 (1.8) |
| Are you in school these days? | |
| No | 124 (32.0) |
| Yes | 166 (42.9) |
| No, I have graduated | 73 (18.9) |
| Yes, but I am on summer/winter/spring break now | 24 (6.2) |
| Highest level of education or grade completed | |
| Eighth grade or less | 6 (1.6) |
| More than eighth grade did not complete high school | 65 (16.8) |
| High school graduate | 127 (32.8) |
| GED | 22 (5.7) |
| Some college or technical education or higher | 134 (34.6) |
| Technical school graduate | 11 (2.8) |
| College graduate | 18 (4.7) |
| Some graduate school | 4 (1.0) |
| Are you currently employed? | |
| Yes | 169 (43.7) |
| No | 218 (56.3) |
| How much money did you make altogether during the past 30 days? | |
| <$500 | 98 (25.3) |
| $51–$249 | 69 (17.8) |
| $250–$499 | 53 (13.7) |
| $500–$999 | 65 (16.8) |
| $1000–$2999 | 61 (15.8) |
| $3000–$4999 | 4 (1) |
| $5000 or more | 1 (0.3) |
| Rather not answer | 16 (4.1) |
| Do not know | 50 (5.2) |
| Where are you currently living or staying most of the time? | |
| Your own home or apartment | 110 (28.4) |
| At a parent's house or apartment | 173 (44.7) |
| At another family member's house or apartment | 52 (13.4) |
| At a nonfamily member's house or apartment | 22 (5.7) |
| Foster home or group home | 1 (0.3) |
| In a rooming, boarding, halfway house, or a shelter | 18 (4.7) |
| On the street(s) | 3 (0.8) |
| Some other places not mentioned | 8 (2.1) |
| In the last year, how many times have you moved? | |
| Mean | 1.70 |
| Standard deviation | 3.44 |
| Median | 1.00 |
| Minimum | 0.00 |
| Maximum | 35.00 |
| How do you identify your sexual orientation? | |
| Straight | 46 (11.9) |
| Gay | 262 (67.7) |
| Queer | 1 (0.3) |
| Bisexual | 64 (16.5) |
| Questioning | 8 (2.1) |
| Other | 4 (1) |
| Refused to answer | 1 (0.3) |
| Do not know | 1 (0.3) |
| Do you have access to a cell phone? | |
| Yes | 360 (93.0) |
| No | 27 (7.0) |
| Do you have access to e-mail? | |
| Yes | 270 (69.8) |
| No | 117 (30.2) |
| Do you have access to a computer with internet? | |
| Yes | 231 (59.7) |
| No | 156 (40.3) |
| How old were you when you found out you were HIV positive? | |
| Mean | 18.74 |
| Standard deviation | 2.19 |
| Median | 19.0 |
| Minimum | 5.0 |
| Maximum | 24.0 |
| Have you disclosed your HIV status to anyone? | |
| Yes | 336 (86.8) |
| No | 51 (13.2) |
| Mental healthcare utilization | |
| In the past 12 months, did you want or need help with personal or family problems from a mental health professional such as a social worker, psychiatrist, psychologist, or counselor? | |
| Yes | 127 (32.8) |
| No | 260 (67.2) |
| In the past 12 months, have you seen a psychiatrist, psychologist, marriage and family therapist, or social worker about the way you were feeling or behaving (only if answer from the above is Yes) | |
| N | 127 |
| Yes | 93 (73.2) |
| No | 34 (26.8) |
| In the past 12 months, how many times have you sought counseling? | |
| Mean | |
| Standard deviation | 1.87 |
| Median | 4.73 |
| Minimum | 0.00 |
| Maximum | 0.00 |
| In the past 12 months, did you ever seriously consider attempting suicide? | 33.00 |
| Yes | 51 (13.2) |
| No | 336 (86.8) |
| Adherence characteristics | |
| Number of daily dosages | |
| Mean | 1.11 |
| Standard deviation | 0.38 |
| Median | 1.00 |
| Minimum | 1.00 |
| Maximum | 4.00 |
| Number of daily pills | |
| Mean | 2.34 |
| Standard deviation | 1.53 |
| Median | 2.00 |
| Minimum | 0.00 |
| Maximum | 8.00 |
| Pill that is hard to take | |
| N | 366 |
| Yes | 25 (6.5) |
| No | 361 (93.3) |
| Barriers to adherence | |
| Did an event occur in the last 7 days that made it more difficult to take your medicine? | |
| Yes | 54 (14) |
| No | 333 (86) |
| No access at drug store | |
| N | 168 |
| Yes | 20 (11.9) |
| No | 148 (88.1) |
| Prescription elapsed | |
| N | 166 |
| Yes | 42 (10.9) |
| No | 122 (72.6) |
| Sickness | |
| N | 168 |
| Yes | 22 (13.1) |
| No | 146 (86.9) |
| Forgot | |
| N | 168 |
| Yes | 120 (71.4) |
| No | 48 (28.6) |
| Schedule interference | |
| N | 168 |
| Yes | 25 (14.9) |
| No | 143 (85.1) |
| Did not feel like taking (needed break) | |
| N | 168 |
| Yes | 30 (17.9) |
| No | 138 (82.1) |
| Living situation changed | |
| N | 168 |
| Yes | 21 (12.5) |
| No | 147 (87.5) |
| Worried about others finding out | |
| N | 168 |
| Yes | 29 (17.3) |
| No | 138 (82.7) |
| Other illness | |
| N | 168 |
| Yes | 22 (13.1) |
| No | 146 (86.9) |
| Lack of family support | |
| N | 168 |
| Yes | 18 (10.7) |
| No | 150 (89.3) |
| Reminds me of HIV+ status | |
| N | 168 |
| Yes | 35 (20.8) |
| No | 133 (79.2) |
| Other reason | |
| N | 168 |
| Yes | 34 (20.2) |
| No | 134 (79.8) |
| Facilitators of adherence | |
| Use of labels | |
| N | 167 |
| Yes | 48 (28.6) |
| No | 119 (70.8) |
| Use of calendar | |
| N | 167 |
| Yes | 60 (35.7) |
| No | 107 (63.7) |
| Use of pill boxes | |
| N | 168 |
| Yes | 79 (47.0) |
| No | 89 (53.0) |
| Use of beepers | |
| N | 167 |
| Yes | 37 (22.0) |
| No | 130 (77.4) |
| Use of timers | |
| N | 168 |
| Yes | 62 (36.9) |
| No | 106 (63.1) |
| Use of programmable wrist watches | |
| N | 168 |
| Yes | 20 (11.9) |
| No | 148 (88.1) |
| Use of buddy system | |
| N | 168 |
| Yes | 58 (34.5) |
| No | 110 (65.5) |
| Take pill when a certain thing happens | |
| N | 168 |
| Yes | 80 (47.6) |
| No | 88 (52.4) |
| Other | |
| N | 168 |
| Yes | 54 (32.1) |
| No | 114 (67.9) |
| Doses missed (past 7 days) | |
| N | 387 |
| 0 | 241 (62.3) |
| 1 | 61 (15.8) |
| 2 | 42 (10.9) |
| 3 | 15 (3.9) |
| 4+ | 28 (7.2) |
n = 387 unless otherwise noted.
GED, general equivalency diploma.
Table 4.
Brief Symptom Inventory Subscales and Global Indices, Substance Use, and Healthcare-Related Characteristics—Descriptive (n = 387)
| M (SD) | A | |
|---|---|---|
| BSI dimensions | ||
| Somatization (7 items) | 0.70 (0.78) | 0.87 |
| Obsessive-compulsive (6 items) | 1.04 (0.97) | 0.87 |
| Interpersonal sensitivity (4 items) | 0.95 (1.03) | 0.85 |
| Depression (6 items) | 0.95 (0.97) | 0.88 |
| Anxiety (6 items) | 0.70 (0.85) | 0.87 |
| Hostility (5 items) | 0.99 (0.97) | 0.85 |
| Phobic anxiety (5 items) | 0.54 (0.82) | 0.85 |
| Paranoid ideation (5 items) | 1.19 (1.02) | 0.82 |
| Psychoticism (5 items) | 0.86 (0.88) | 0.74 |
| Global indices | ||
| Global severity index (53 items) | 0.89 (0.80) | 0.98 |
| Positive symptom distress index | 1.72 (0.69) | |
| Positive symptom total (53 items) | 23.52 (14.43) | |
| CRAFFT | ||
| CRAFFT total score (6 items) n = 386 | 2.32 (1.78) | 0.72 |
| Car ride intoxicated? | Yes (64.6%) | |
| Substance use to relax/fit in? | Yes (46.0%) | |
| Substance use alone? | Yes (49.7%) | |
| Forgetfulness while using substance? | Yes (27.9%) | |
| Family concern about substance use? | Yes (27.5%) | |
| Trouble while using substance? | Yes (16.1%) | |
| CRAFFT score >2 (indicated abuse/dependence) | 227 (58.8%) | |
| ASSISST | ||
| Current frequency of tobacco use | 1.53 (1.76) | |
| Current frequency of alcohol use | 1.51 (1.11) | |
| Current frequency of cannabis use | 1.44 (1.64) | |
| Current frequency of cocaine use | 0.12 (0.50) | |
| Current frequency of amphetamine use | 0.14 (0.48) | |
| Current frequency of sedative use | 0.14 (0.63) | |
| Motivation readiness for healthcare | ||
| Total motivation (2 items) | 19.12 (2.36) | 0.64 |
| Motivation for keeping medical appointments | 9.57 (1.33) | |
| Motivation to take HIV medications | 9.55 (1.41) | |
| Self-efficacy for medical care | ||
| Total self-efficacy for medical care (6 items) | 7.65 (2.32) | 0.80 |
| Self-efficacy for taking medication (3 items) | 2.84 (0.97) | 0.80 |
| Self-efficacy for keeping medical appointments (3 items) | 3.04 (1.12) | 0.70 |
| Social support for healthy living | ||
| Total social support for healthy living (6 items) | 25.75 (4.59) | 0.80 |
| Healthcare provider relationship | ||
| Total healthcare provider relationship (5 items) | 4.70 (0.59) | 0.85 |
UniODA analysis
In total, 28 of 72 attributes were significantly associated with ART adherence outcomes (Table 5). Consistent with previous literature, statistically significant attributes were in the predicted direction. Four of the 28 significant attributes evidenced moderate ESS (25–50%) for classifying ART adherence outcomes, while the remaining 24 statistically significant attributes exhibited relatively weak ESS (<25%) for classifying adherence outcomes.
Table 5.
Univariate Associations of Antiretroviral Therapy Adherence Among African American Males (Ages 12–24) Living with HIV
| Optimal discriminant analysis | Training analysis | ||||
|---|---|---|---|---|---|
| Attribute | UniODA model | N | % Adherent | p | ESSa |
| Moderate effect strength predictors | |||||
| Frequency of cannabis useb | If monthly or more, predict nonadherence | 144 | 45.1 | <0.00 | 27.1 |
| If once or twice, predict adherence | 243 | 72.4 | |||
| Frequency of alcohol use | If monthly or more, predict nonadherence | 163 | 47.2 | <0.00 | 27.0 |
| If once or twice, predict adherence | 224 | 73.2 | |||
| CRAFFT total score | If score suggestive of substance abuse, predict nonadherence | 227 | 52.0 | <0.00 | 25.5 |
| If score does not suggest substance abuse, predict adherence | 159 | 76.7 | |||
| Frequency of tobacco use | If monthly or more, predict nonadherence | 146 | 47.3 | <0.00 | 24.1 |
| If once or twice, predict adherence | 241 | 71.4 | |||
| Relatively weak predictors | |||||
| BSI positive symptom distress index | If two or more, predict nonadherence | 225 | 53.3 | <0.00 | 22.1 |
| If one or less, predict adherence | 162 | 74.7 | |||
| BSI global severity index | If a little bit or more, predict nonadherence | 216 | 53.7 | <0.00 | 20.3 |
| If not at all, predict adherence | 171 | 73.1 | |||
| BSI positive symptom total | If 24 or more, predict nonadherence | 181 | 52.5 | <0.00 | 19.5 |
| If 23 or less, predict adherence | 206 | 70.9 | |||
| Did an event occur that made it more difficult to take medicine? (past 7 days) | If yes, predict nonadherence | 54 | 31.5 | <0.00 | 18.3 |
| If no, predict adherence | 333 | 67.3 | |||
| BSI obsessive-compulsive subscale mean | If a little bit or more, predict nonadherence | 223 | 55.2 | <0.00 | 17.9 |
| If not at all, predict adherence | 163 | 72.4 | |||
| BSI hostility subscale | If a little bit or more, predict nonadherence | 278 | 56.5 | <0.00 | 17.7 |
| If not at all, predict adherence | 109 | 77.1 | |||
| Other strategy, which facilitates adherence | If no, predict nonadherence | 114 | 52.6 | <0.02 | 17.7 |
| If yes, predict adherence | 54 | 72.2 | |||
| BSI depression subscale | If a little bit or more, predict nonadherence | 202 | 54.5 | <0.00 | 17.4 |
| If not at all, predict adherence | 185 | 70.8 | |||
| BSI psychoticism subscale | If a little bit or more, predict nonadherence | 253 | 56.1 | <0.00 | 17.1 |
| If not at all, predict adherence | 134 | 73.9 | |||
| Total self-efficacy for medical care (mean) | If pretty sure I can or less, predict nonadherence | 140 | 51.4 | <0.00 | 16.7 |
| If very sure I can, predict adherence | 247 | 68.4 | |||
| Current living situation | If living at own place, boarding/halfway house, or homeless, predict nonadherence | 132 | 50.8 | <0.01 | 16.7 |
| If living at parents' home, at family members' or nonfamily members' home, foster home, school dorm, or other place not mentioned, predict adherence | 255 | 68.2 | |||
| No. of total daily pills | If two or more, predict nonadherence | 204 | 55.9 | <0.01 | 14.3 |
| If one or less, predict adherence | 183 | 69.4 | |||
| Total self-efficacy for keeping medical appointments | If pretty sure I can or less, predict nonadherence | 163 | 54.6 | <0.01 | 13.8 |
| If very sure I can, predict adherence | 224 | 67.9 | |||
| Total self-efficacy for taking medications mean | If pretty sure I can or less, predict nonadherence | 113 | 51.3 | <0.01 | 13.6 |
| If very sure I can, predict adherence | 274 | 66.8 | |||
| BSI anxiety subscale | If a little bit or more, predict nonadherence | 230 | 57.0 | <0.03 | 13.5 |
| If not at all, predict adherence | 157 | 70.1 | |||
| BSI interpersonal sensitivity subscale | If a little bit or more, predict nonadherence | 159 | 54.7 | <0.03 | 13.2 |
| If not at all, predict adherence | 228 | 67.5 | |||
| Total motivation readiness for healthcare mean | If unsure, predict nonadherence | 80 | 47.5 | <0.00 | 13.0 |
| if ready and able, predict adherence | 307 | 66.1 | |||
| Readiness to take prescription medication | If unsure, predict nonadherence | 55 | 41.8 | <0.00 | 12.4 |
| If ready and able, predict adherence | 332 | 65.7 | |||
| Age | If 22 years of age or older, predict nonadherence | 196 | 56.6 | <0.04 | 12.2 |
| If 21 years of age or less, predict adherence | 191 | 68.1 | |||
| Is there a pill that is hard for you to take? | If yes, predict nonadherence | 25 | 28.0 | <0.00 | 9.5 |
| If no, predict adherence | 361 | 64.8 | |||
| Frequency of amphetamine use | If once or twice, predict nonadherence | 37 | 40.5 | <0.00 | 8.8 |
| If never, predict adherence | 350 | 64.6 | |||
| Readiness to go to medical appointments | If unsure, predict nonadherence | 54 | 48.2 | <0.03 | 8.4 |
| If ready and able, predict adherence | 333 | 64.6 | |||
| Frequency of cocaine use | If once or twice, predict nonadherence | 29 | 41.4 | <0.02 | 6.7 |
| If never, predict adherence | 358 | 64.0 | |||
| No. of daily doses prescribed | If two or more, predict nonadherence | 33 | 45.5 | <0.04 | 6.1 |
| If one or less, predict adherence | 354 | 63.8 | |||
ESS, effect strength sensitivity, which is a standardized measure of effect strength.
Frequency of all substance use variables is during the past 3 months.
ESS, effect strength sensitivity.
Effect strength
All four attributes that exhibited moderate ESS for ART adherence outcomes were variables of participant substance use or abuse and met experiment-wise type one error rate criteria (Table 5). The attribute with the highest ESS for classifying ART adherence outcomes was frequency of cannabis use during the past 3 months, ESS = 27.1, p < 0.001. Participants who reported at least monthly use of cannabis during this time period were predicted to be nonadherent to ART (54.9% were actually nonadherent), while those participants who reported cannabis use of less than twice during this time period were predicted to be adherent of their ART regimen (72.4% were actually adherent). In addition, 24 attributes exhibited a significant, but relatively weak, ESS for ART adherence outcomes (Table 5).
Classification tree analysis
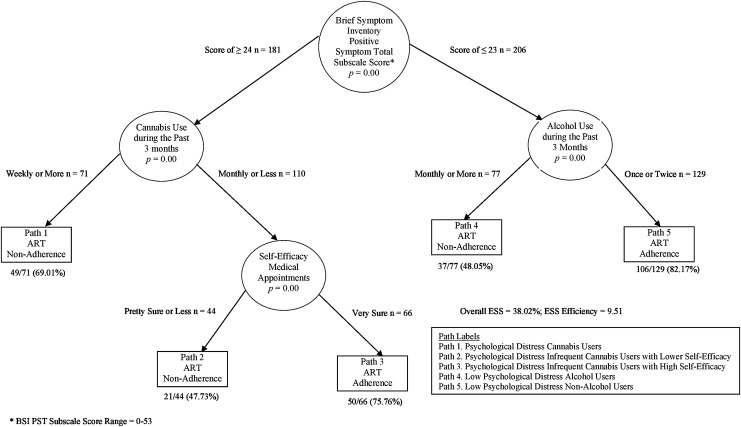
The enumerated CTA yielded an overall model (Fig. 1) that delivered moderate ESS (38.0%) for classifying participants as either being ART adherent or nonadherent. The model provided a 38% improvement above chance for classifying members of the sample as either being ART adherent or nonadherent. The CTA model yielded an overall accuracy in classification (PAC) mean of 68% (see Table 6 for complete CTA model performance statistics).
FIG. 1.
Classification tree analysis model for classifying ART adherence and nonadherence n = 387. ART, antiretroviral therapy.
Table 6.
ART Adherence Classification Tree Model Performance Summary
| Performance index | Performance parameter |
|---|---|
| Sensitivity | 156/241 (64.7%) |
| Specificity | 107/146 (73.3%) |
| Positive predictive value | 156/195 (80.0%) |
| Negative predictive value | 107/192 (55.7%) |
| Overall accuracy (PAC) | 263/387 (68.0%) |
| Effect strength sensitivity | 38.0% (moderate strength) |
PAC, percentage accurately classified, the percentage of the total sample that is correctly classified by the model.
Profiles of ART adherence behavior
The CTA model (Fig. 1) identified five distinct profiles of ART adherence outcomes. Two profiles identified the pathways predicting ART adherence and the remaining three profiles identified pathways predicting ART nonadherence. Profiles of ART adherence are discussed first (in order of greatest classification accuracy) and then profiles of ART nonadherence (in order of highest classification accuracy obtained). Once more, each of the five profiles of ART adherence behavior is outlined below in the order of highest classification accuracy obtained.
Path 5—Low psychological distress nonalcohol users (profile of adherence)
Path 5 represented the pathway with the greatest likelihood of engaging in ART adherent behavior (82% of participants [106/129] in this path were ART adherent). These individuals reported low levels of total psychological symptoms and were engaged in minimal alcohol use during the past 3 months (Table 7). Therefore, in the current sample of young African American males living with HIV, the greatest likelihood for ART adherence was among those who reported both minimal psychological distress and near absence of alcohol use.
Table 7.
Descriptive Information of Classification Tree Analysis Model Pathways (n = 387)
| Variables, M (SD) | ||||
|---|---|---|---|---|
| Pathways | BSIPST | Cannabis use | Alcohol use | Self-efficacya |
| Path 1. Psychological distress cannabis users | 35.70 (8.23) | 3.72 (0.45) | 2.00 (1.06) | 1.35 (0.44) |
| Path 2. Psychological distress infrequent cannabis users with lower self-efficacy | 37.68 (8.60) | 0.66 (0.78) | 1.66 (1.08) | 2.04 (0.41) |
| Path 3. Psychological distress infrequent cannabis users with high self-efficacy | 36.76 (8.37) | 0.33 (0.59) | 1.18 (1.05) | 1.10 (0.15) |
| Path 4. Low psychological distress alcohol users | 13.56 (8.36) | 1.56 (1.56) | 2.68 (0.60) | 1.24 (1.49) |
| Path 5. Low psychological distress nonalcohol users | 11.16 (6.80) | 0.96 (1.49) | 0.67 (0.47) | 1.23 (0.40) |
Lower scores = higher levels of self-efficacy.
BSIPST, brief symptom inventory positive symptom total subscale score.
Path 3—Psychological distress infrequent cannabis users with high self-efficacy (profile of adherence)
Participants who exhibited greater levels of psychological distress were initially predicted to be ART nonadherent. However, as elucidated in Fig. 1, there was a significant three-way interaction among the attributes of total number of psychological symptoms, frequency of cannabis use, and self-efficacy. Specifically, participants who initially were predicted to be ART nonadherent based on their total number of psychological symptoms reported, but who refrained from frequent cannabis use, and who had high levels of self-efficacy for keeping medical appointments were predicted to be ART adherent (76% of participants [50/66] were in fact adherent). Such findings highlight the potential protective role that self-efficacy for engaging in medical care may have with regard to ART adherence behaviors among young African American males living with HIV.
Path 1—Psychological distress cannabis users (profile of nonadherence)
Overall, the CTA model evidenced greater difficulty expounding the profiles of ART nonadherence with greater accuracy than chance. However, the model did identify one robust profile of ART nonadherence behavior. Specifically, participants who reported a greater number of psychological distress symptoms and who used cannabis weekly or more during the past 3 months were predicted to be ART nonadherent (69% were accurately classified [49/71]). Path 1 underscores the deleterious influence that comorbid psychological distress and frequent cannabis use for young African American males living with HIV can have with regard to ART adherent behavior. In the CTA model, no other constellation of attributes was found to be a more accurate pathway for correctly classifying participants as being ART nonadherent.
Path 4—Low psychological distress alcohol users (profile of nonadherence)
In isolation, the profile of Low Psychological Distress Alcohol Users did not perform better than chance for classifying participants as ART nonadherent (48% [37/77] of participants correctly predicted to be ART nonadherent). However, in the context of the overall CTA model, this pathway identifies the moderating effect of alcohol use for participants with lower levels of psychological symptoms and ART adherence behavior. Increased alcohol use represented a notable reduction of ART adherence, even among those with low psychological distress.
Path 2—Psychological distress infrequent cannabis users with lower self-efficacy (profile of nonadherence)
Participants of Path 2 reported high levels of psychological symptoms, infrequent cannabis use (monthly use or less), and lower levels of self-efficacy for keeping future medical appointments. In the CTA model, these participants were predicted to be ART nonadherent. However, only 48% (21/44) of these participants were indeed ART nonadherent. Although Path 2 did not evidence a high degree of classification accuracy, it demonstrated that lower levels of self-efficacy are associated with reduced ART adherence among participants with greater psychological distress. In the CTA model, participants in Path 2 only differed from participants in Path 3 in their level of self-efficacy to keep future medical appointments (Table 7). Yet, there is a considerable decrease in the proportion of participants who were ART adherent, going from 76% (in Path 3) to only 52% (in Path 2); a reduction of nearly 24%. Thus, in the context of the overall CTA model, higher levels of self-efficacy for keeping future medical appointments were associated with an increase in ART adherence, in particular, for participants with higher levels of psychological distress.
Discussion
The development and improvement of ART have afforded individuals living with HIV/AIDS an opportunity to live significantly longer and healthier lives than before treatment was available. Adherence to ART is strongly associated with reduced secondary transmission of HIV, reduced likelihood of virus mutations, increased immunological functioning, slowed disease progression, and increased life expectancy.9,10,51 That being said, the benefit of ART is directly contingent upon an individual's ability to adhere to the regimen. Given the high adherence rate requirement (of at least 80% for new ARV regimens and >95% for regimens with unboosted PIs), this poses a significant challenge for the many youth and young adults living with HIV who struggle greatly with adherence to ART.
Research examining factors associated with ART adherence among YLH has found that psychological distress (e.g., depression, anxiety, suicidal ideation24,26,27,32), cognitive belief systems (e.g., decisional balance, self-efficacy, motivation31,33), and substance use26,27,29 are the most consistent attributes significantly associated with adherence behaviors. The current study builds upon this literature by identifying substance use attributes (e.g., frequency of current cannabis, alcohol, and tobacco use) as well as the likelihood of having a substance abuse issue as being the strongest independent predictors of ART adherence outcomes among a large multisite sample of young African American males living with acquired HIV. The fact that substance use is associated with ART adherence outcomes is not remarkable on its own as this has been well documented in the adherence literature.26,27,29,52 However, what is noteworthy is that substance use variables were the strongest independent predictors of ART adherence outcomes when competing with 72 theoretically relevant variables.
To enhance the understanding of how factors associated with adherence interact with one and other and to identify pathways of adherence, a multiattribute tree model was created utilizing CTA. The CTA model evidenced a 38% improvement above chance for accurately classifying participants as being ART adherent, or nonadherent, and identified five pathways of adherence outcomes. The attributes of psychological distress, frequency of alcohol and cannabis use (during the past 3 months), and self-efficacy (for keeping medical appointments) were found to be the most pertinent attributes for predicting ART adherence outcomes. When examining the five pathways of the CTA model (Fig. 1), several important points should be highlighted. (1) Clear pathways of adherence and nonadherence exist for young African American males living with HIV. Specifically, the combination of low levels of psychological distress symptoms and minimal alcohol use is most predictive of ART adherence. In contrast, the grouping of higher levels of psychological distress symptoms and weekly cannabis use is most predictive of ART nonadherence. (2) Higher levels of alcohol use represented a notable risk factor for being ART nonadherent, even for those who reported low-levels of psychological distress. (3) Self-efficacy was identified as a protective factor for participants who reported higher levels of psychological distress, but only in the context of monthly or less cannabis use. (4) The CTA model performed better at identifying accurate pathways of adherence versus nonadherence, suggesting that the factors or mechanisms associated with nonadherence are more idiosyncratic or other important attributes were not included in the current model (e.g., neurocognitive functioning, discrimination, ethnic identity).
One of the larger studies investigating predictors of adherence among minority youth living with HIV found cognitive thought processes (e.g., motivational readiness, self-efficacy, and decisional balance) as being significantly associated with adherence.33 Interestingly, substance use and psychological distress were not related to adherence (limited variance of these two factors may have influenced these findings).
Although, the results of the current study seem to differ from MacDonnell's findings, the goals of the studies were different. This study's aim was to maximize classification accuracy of ART adherence outcomes and not to elucidate the mechanisms that lead individuals toward ART adherence. In the context of this study's findings and the previous literature, young African American males living with HIV absent of the negative effects of psychological distress and substance use may develop cognitive processes (e.g., increased self-efficacy, motivation) that foster ART adherent behaviors. Conversely, individuals who have higher levels of psychological distress and are engaging in substance use may be less likely to develop the cognitive schemas that promote ART adherent behaviors.
Clinical implications
YLH experience psychological distress at greater levels than their noninfected peers.53,54 Unfortunately, young African American males are less likely than Caucasian youth to receive mental healthcare as a result of economic, social, cultural, and demographic barriers, even within treatment centers (e.g., ATN sites) that provide comprehensive medical and social services.55 This is alarming given that the current study found the constellation of psychological distress and substance use variables to be most predictive of ART adherence outcomes. Early and ongoing assessment of psychological distress and substance use should be a part of routine HIV care for young African American males living with HIV. In particular, administering the BSI and the ASSIST as part of HIV care can identify those at high and low risk for adherence to ART.
In the current study, patients who exhibited higher levels of psychological distress (scores of 24 or higher on the BSI Positive Symptom Total Scale) and were engaging in weekly or more cannabis use were most likely to be ART nonadherent. Young African American males living with HIV who are identified as having mental health and substance use issues will require greater levels of intervention resources than the current standard of care to aid in the adherence to ART.
The current study also identified that increased levels of self-efficacy for medical care engagement can act as a protective factor and foster higher levels of ART adherence behaviors among those at additional risk (e.g., higher levels of psychological distress). African Americans have an extensive history of mistreatment by healthcare systems and as a result have higher levels of medical care mistrust than other racial groups.56
Primary care providers working with young African American males living with HIV should be sensitive of how the abovesaid cultural factors influence self-efficacy for medical care engagement and physician–patient relationships. Medical providers need to be trained in the cultural and developmental needs of young African American males and be comfortable discussing such issues in a nonjudgmental manner.57 In addition, working to create more youth-friendly treatment spaces (both the physical and social environments) will likely increase self-efficacy for medical care engagement. See Tanner et al.'s article for an in-depth review of important attributes for establishing youth-friendly clinics for YLH.57
Integrated multidisciplinary health teams, which practice within a developmentally and culturally sensitive manner, are likely to be best prepared to address the factors influencing ART adherence behaviors for young African American males living with HIV. It is plausible that mental healthcare and issues of substance use may be overlooked or minimized in treatment settings dedicated to the treatment of HIV given the potential severity of the disease. However, this study demonstrates the notable role of both mental health and substance use for young African American males living with HIV with respect to ART adherence behaviors. Treatment of HIV cannot solely focus on the medical aspects of the disease, but requires a great emphasis on psychosocial functioning as well, as such factors greatly impact patients' ability to tolerate and effectively participate in ART. Thus, the assessment and treatment of psychosocial factors should not only occur during the intake of new patients but should also be an integral part of routine and continued HIV care.
Intervention/prevention programs
As MacDonell et al.33 have previously articulated, successful intervention and prevention programs must target the large range of factors that influence ART adherence behaviors. These include cultural, developmental, societal-level, psychosocial, and individual-level factors. Harper58 provides an excellent discussion of the need of HIV intervention/prevention programming for young MSM to be culturally grounded by including elements of both societal-level and individual-level factors. Thus, multifaceted behavioral interventions presented within developmentally and culturally sensitive frameworks are likely to be the most successful for increasing ART adherence behaviors for YLH as well as reducing the rate of new HIV infections in communities at greater risk for the transmission of the virus.
Individuals living with HIV who also use drugs are at particular risk for suboptimal ART adherence.59 The deleterious effects of alcohol related to poor disease outcome and ART nonadherence among both adolescent and adults living with HIV are well documented.52,60 In fact, lower levels of alcohol use among minority youth living with HIV have been associated with an increase in HIV medical care appointment adherence.61 Programs aimed at reducing cannabis use among YLH62 have highlighted the difficulty of this task as cannabis is reportedly often used as a mechanism to reduce the negative side effects of ARVs (e.g., nausea, reduced appetite).
As medical cannabis has become legal in many states, some youth living with HIV are being prescribed cannabis to treat medication side effects. However, these youth are using cannabis at much higher rates than prescribed62 and as the current study highlights, frequent cannabis use is most strongly predictive of ART nonadherence among young African American males living with HIV. Thus, there is a robust need for the development of efficacious substance use reduction programs for this population that can be embedded within interdisciplinary care teams and delivered in a culturally and developmentally sensitive framework. Fortunately, recent research has begun to evaluate interventions such as motivational interviewing63,64 and cognitive-behavioral therapy65,66 to reduce the deleterious effects that substance use and psychological distress can have on adherence for YLH.
Future research
Replication of the current statistical methodology with a longitudinal research design would provide greater credence for a prediction model of ART adherence outcomes. Prominent findings in the current study underscore the importance of psychological functioning, recreational substance use, and self-efficacy for accurately classifying patients' ART adherence outcomes. However, these findings do not expound upon the underlying mechanisms that explain how such attributes influence ART adherence behaviors. It is likely that mental health and substance use variables significantly influence cognitive frameworks. Thus, future research is therefore encouraged to clarify the interactive relationship of mental health, substance use, and cognitive processes with respect to ART adherence behaviors.
The present study also presents the opportunity for primary care providers to increase their accuracy with respect to who might struggle with ART adherence by understanding patients' mental health, substance use, and self-efficacy profiles. However, future research focused on evaluating the specific psychosocial measures or tools that can be used most effectively in primary care settings to predict ART adherence behaviors is necessary. Specifically, the development of a standardized psychosocial assessment battery that provides a risk profile for ART nonadherence would have great clinical utility.
Limitations
Several limitations in this study should be noted. First and foremost, the use of a cross-sectional dataset does not allow for a causal prediction model to be substantiated. Second, in the current study, a number of constructs of interest were limited by ceiling effects (i.e., physician–patient relationship variables) or not being as broad of a measurement (i.e., social support) as desired. In addition, some constructs of interest to the current study, such as neurocognitive functioning, stigma and discrimination, ethnic identity, and decisional balance, were not available. Third, the dependent variable of complete adherence was based on participant self-report over a seven-day period. Although self-report has been established as a reliable and valid measure of ART adherence,42,45 it is susceptible to response bias and a multimethod assessment approach of adherence (i.e., self-report with pill monitoring devices) or a direct measure of viral load would be ideal.
Given that a seven-day recall period was used in the present study, ART adherence was defined as taking 100% of all doses prescribed, which is ideal, but slightly above and beyond what is necessary for optimal viral load suppression (i.e., needing to take 95% of doses prescribed for unboosted PIs and 80% for boosted PIs).12–14,19 Last, although the age range of participants in the current sample included individuals between the ages of 12 and 24 years, the vast majority of participants were between the ages of 18 and 24 years of age. Thus, generalizability for young African American males living with HIV under the age of 18 is somewhat limited.
In sum, this study provides a strong empirical basis for including psychosocial factors as focal points of HIV treatment and interventions. An emphasis by treatment providers and interventionists for the overall mental health functioning across psychological and emotional domains is likely critical for improving ART adherence outcomes among young African American males living with HIV. In addition, given the high comorbidity of HIV and recreational substance use (i.e., tobacco, alcohol, and cannabis) and the negative impact of such use for ART adherence outcomes, treatment settings are implored to incorporate substance use reduction and treatment intervention as part of routine patient care.
The medical needs of young African American males living with HIV are undoubtedly to be best met within integrated primary care treatment teams, consisting of multidisciplinary professionals focused on both the medical and psychosocial needs of this population. Scholars have voiced the need for multifaceted, interdisciplinary, and integrated health services for individuals living with HIV with a focus on adherence, substance use, and mental health.67
Evidence-based treatments for the aforementioned issues already exist and behavioral interventions with a strong focus on cognitive-behavioral principles, motivational interviewing, psychoeducation, and problem solving techniques have already been shown to be effective among individuals living with HIV—including those with comorbid substance use and/or mental health needs.66,68,69 Fortunately, intervention researchers have started to demonstrate the feasibility of incorporating motivational interviewing and cognitive-behavioral interventions into integrated HIV youth treatment settings.36,63–66 However, much work is still needed in the development of such interventions to best serve the psychosocial and medical needs of young African American males living with HIV.
Acknowledgments
The study was scientifically reviewed by the ATN Behavioral Leadership Group. Network, scientific, and logistical support was provided by the ATN Coordinating Center (C. Wilson and C. Partlow) at The University of Alabama at Birmingham. Network operations and data management support was provided by the ATN Data and Operations Center at Westat, Inc., (J. Korelitz and B. Driver). The authors acknowledge the contribution of the investigators and staff at the sites that participated in this study. The following ATN sites participated in this study: University of South Florida, Tampa (Emmanuel, Lujan-Zilbermann, Julian), Children's Hospital of Los Angeles (Belzer, Flores, Tucker), Children's National Medical Center (D' Angelo, Hagler, Trexler), Children' s Hospital of Philadelphia (Douglas, Tanney, DiBenedetto), John H. Stroger Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center (Martinez, Bojan, Jackson), University of Puerto Rico (Febo, Ayala-Flores, Fuentes-Gomez), Montefiore Medical Center (Futterman, Enriquez-Bruce, Campos), Mount Sinai Medical Center (Steever, Geiger), University of California, San Francisco (Moscicki, Auerswald, Irish), Tulane University Health Sciences Center (Abdalian, Kozina, Baker), University of Maryland (Peralta, Gorle), University of Miami School of Medicine (Friedman, Maturo, Major-Wilson), Children' s Diagnostic and Treatment Center (Puga, Leonard, Inman), St. Jude's Children's Research Hospital (Flynn, Dillard), Children's Memorial Hospital (Garofalo, Brennan, Flanagan), Baylor College of Medicine (Paul, Calles, Cooper), Wayne State University (Secord, Cromer, Green-Jones), Johns Hopkins University School of Medicine (Agwu, Anderson, Park), The Fenway Institute, Boston (Mayer, George, Dormitzer), and University of Colorado Denver (Reirden, Hahn, Witte). The investigators are grateful to the members of the local Youth Community Advisory Boards for their insight and counsel and are particularly indebted to the youth who participated in this study.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Forstein M, Cournos F, Douaihy A, et al. Practice Guideline for the Treatment of Patients with HIV/AIDS. Washington, DC: American Psychiatric Association, 2000 [Google Scholar]
- 2.HIV among African-Americans. 2011. CDC HIV/AIDS Fact Sheet. Centers for Disease Control and Prevention Web Site. Available at: www.cdc.gov/hiv/topics/aa/PDF/aa.pdf (Last accessed July7, 2014)
- 3.Hall HI, Byers RH, Ling Q, Espinoza L. Racial/ethnic and age disparities in HIV prevalence and disease among men who have sex with men in the United States. Am J Public Health 2007;97:1066–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurencin CT, Christensen DM, Taylor ED. HIV/AIDS and the African-American community: A state of emergency. J Natl Med Assoc 2008;100:35–43 [DOI] [PubMed] [Google Scholar]
- 5.Palacio H, Kahn JG, Richards TA, Morin SF. Effect of race and/or ethnicity in use of antiretrovirals and prophylaxis for opportunistic infection: A review of the literature. Public Health Rep 2002;117:233–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton MY, Jones RL, Wolitski RJ, Cleveland JC, Dean HD, Fenton KA. A review of the Centers for Disease Control and Prevention's response to the HIV/AIDS crisis among blacks in the United States, 1981–2009. Am J Public Health 2009;99:351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estimated HIV incidence among adults and adolescents in the United States, 2007–2010. Centers for Disease Control and Prevention Web Site. Available at: www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf (Last accessed July25, 2015)
- 8.HIV Surveillance Report, 2014. 2015. Centers for Disease Control and Prevention. Available at: www.cdc.gov/hiv/library/reports/surveillance/ (Last accessed April26, 2016)
- 9.The Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet 2009;372:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palella FJ, Delaney KM, Moorman AC, et al. The HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998;338:853–860 [DOI] [PubMed] [Google Scholar]
- 11.Panel on antiretroviral guidelines for adults and adolescents. 2012. Updated 2014. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services. Available at: www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf (Last accessed July7, 2014)
- 12.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 2010;5:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porco TC, Martin JN, Page-Shafer KA, et al. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS 2004;18:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MS, McCauley M, Gamble TR. HIV treatment as prevention and HPTN 052. Curr Opin HIV AIDS 2012;2:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies G, Koeing LJ, Stratford D, et al. Overview and implementation of an intervention to prevent adherence failure among HIV-infected adults initiating antiretroviral therapy: Lessons learned from project HAART. AIDS Care 2006;18:895–903 [DOI] [PubMed] [Google Scholar]
- 16.Paterson DL, Swindells S, Mohr J. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21–30 [DOI] [PubMed] [Google Scholar]
- 17.Wutoh AK, Elekwachi O, Clarke-Tasker V, Daftary M, Powell NJ, Campusano G. Assessment and predictors of antiretroviral adherence in older HIV-infected patients. J Acquir Immune Defic Syndr 2003;33:106–114 [DOI] [PubMed] [Google Scholar]
- 18.Maggiolo F, Airoldi M, Kleinloog HD, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI-or-PI treated patients. HIV Clin Trials 2007;8:282–292 [DOI] [PubMed] [Google Scholar]
- 19.Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother 2015;45:372–379 [DOI] [PubMed] [Google Scholar]
- 20.Gordon LL, Gharibian D, Chong K, Chun H. Comparison of HIV virologic failure rates between patients with variable adherence to three antiretroviral regimen types. AIDS Patient Care STDS 2015;29:384–388 [DOI] [PubMed] [Google Scholar]
- 21.Kahana S, Rohan J, Allison S, Frazier TW, Drotar D. A meta-analysis of adherence to antiretroviral therapy and virologic responses in HIV-infected children, adolescents, and young adults. AIDS Behavior 2013;1:41–60 [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: Systematic review and meta-analysis. AIDS 2014;28:1945–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, Safren SA. A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Top HIV Medicine 2009;17:14–25 [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy DA, Durako SJ, Moscicki A, et al. No change in health risk behaviors over time among HIV infected adolescents in care: Role of psychological distress. J Adolesc Health 2001;29:57–63 [DOI] [PubMed] [Google Scholar]
- 25.Bouris A, Voisin D, Pilloton M, et al. Project nGage: Network supported HIV care engagement for younger black men who have sex with men and transgender persons. J AIDS Clin Re 2013;4:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comulada WS, Swenderman DT, Rotheram-Borus M, Mattes KM, Weiss RE. Use of HAART among young people living with HIV. Am J Health Behav 2003;27:389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosek SG, Harper GW, Domanico R. Predictors of medication adherence among HIV infected youth. Psychol Health Med 2005;10:166–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez J, Bell D, Camacho R, et al. Adherence to antiviral drug regimens in HIV-infected adolescent patients engaged in care in a comprehensive adolescent and young adult clinic. J Natl Med Assoc 2000;2:55–61 [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy DA, Belzer M, Durako SJ, et al. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med 2005;159:764–770 [DOI] [PubMed] [Google Scholar]
- 30.Murphy DA, Sarr M, Durako SJ, et al. Barriers to HAART adherence among Human Immunodeficiency Virus-infected adolescents. Arch Pediatr Adolesc Med 2003;157:249–255 [DOI] [PubMed] [Google Scholar]
- 31.Belzer ME, Fuchs DN, Luftman GS, Tucker DJ. Antiretroviral adherence issues among HIV-positive adolescents and young adults. J Adolesc Health 1999;25:316–319 [DOI] [PubMed] [Google Scholar]
- 32.Naar-King S, Templin T, Wright K, et al. Psychosocial factors and medication adherence in HIV-positive youth. AIDS Patient Care STDS 2006;1:44–47 [DOI] [PubMed] [Google Scholar]
- 33.MacDonell KE, Naar-King S, Murphy DA, Parsons JT, Harper GW. Predictors of medication adherence in high risk youth of color living with HIV. J Pediatr Psychol 2010;35:593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams PL, Storm D, Montepiedra G, et al. Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics 2006;118:1745–1757 [DOI] [PubMed] [Google Scholar]
- 35.Belzer ME, MacDonell KK, Clark LF, et al. Acceptability and feasibility of a cell phone support intervention for youth living with HIV with nonadherence to antiretroviral therapy. AIDS Patient Care STDS 2015;29:338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster C, McDonald S, Frize G, et al. “Payment by results”—Financial incentives and motivational interviewing, adherence interventions in young adults with perinatally acquired HIV-1 infection: A pilot program. AIDS Patient Care STDS 2014;28:1–5 [DOI] [PubMed] [Google Scholar]
- 37.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006–2009. PLoS One 2011;6:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Derogatis L, Spencer M. The Brief Symptoms Inventory (BSI): Administration, Scoring, and Procedures Manual. Baltimore, MD: Clinical Psychometric Research, Inc., 1982 [Google Scholar]
- 39.WHO ASSIST Working Group. The Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST): Guidelines for Use in Primary Care (Draft version 1.1 for field testing). Geneva: World Health Organization, 2002 [Google Scholar]
- 40.Knight JR, Sherritt L, Shrier L, Harris S, Chang G. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med 2002;156:607–614 [DOI] [PubMed] [Google Scholar]
- 41.Stott NC, Rollnick S, Rees MR, Pill RM. Innovation in clinical method: Diabetes care and negotiating skills. Fam Pract 1995;12:413–418 [DOI] [PubMed] [Google Scholar]
- 42.Simoni JM, Kurth AE, Pearson CR, et al. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav 2006;10:227–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabound J, Li M, Walmsley S, et al. Once daily dosing improves adherence to antiretroviral therapy. AIDS Behav 2011;15:1397–1409 [DOI] [PubMed] [Google Scholar]
- 44.Murphy DA, Wilson CM, Durako SJ, et al. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA. AIDS Care 2001;13:27–40 [DOI] [PubMed] [Google Scholar]
- 45.Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response. A meta-analysis. J Acquir Immune Defic Syndr 2005;38:445–448 [DOI] [PubMed] [Google Scholar]
- 46.Suzuki H, Bryant FB, Edwards JD. Tracing prospective profiles of juvenile delinquency and non-delinquency: An optimal classification tree analysis. Optimal Data Anal 2010;1:125–143 [Google Scholar]
- 47.Yarnold PR, Soltysik RC. Using the ODA software 29–55. Optimal Data Analysis: A Guidebook with Software for Windows; Washington, DC, 2005 [Google Scholar]
- 48.Yarnold PR. UniODA vs. logistic regression and fisher's linear discriminant analysis: Modeling 10-year population change. Optimal Data Anal 2015;4:139–145 [Google Scholar]
- 49.Yarnold PR, Bryant FB, Smith JH. Manual vs. automated CTA: Predicting freshman attrition. Optimal Data Anal 2013;2:48–53 [Google Scholar]
- 50.Yarnold PR. Selecting the minimum denominator in manual and enumerated CTA. Optimal Data Anal 2015;4:14–20 [Google Scholar]
- 51.Flynn PM, Rudy BJ, Douglas SD, et al. Virologic and immunologic outcomes after 24 weeks in HIV type 1-infected adolescents receiving highly active antiretroviral therapy. J Infect Dis 2004;190:271–279 [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez A, Barinas J, O'Cleirigh C. Substance use: Impact on adherence and HIV medical treatment. Curr HIV/AIDS Rep 2011;8:223–234 [DOI] [PubMed] [Google Scholar]
- 53.Brown LK, Lourie KJ, Pao M. Children and adolescents living with HIV and AIDS: A review. J Child Psychol Psychiatry 2000;41:81–96 [PubMed] [Google Scholar]
- 54.Pao M, Lyon M, D'Angelo LJ, et al. Psychiatric diagnoses in adolescents seropositive for the human immunodeficiency virus. Arch Pediatr Adolesc Med 2000;154:240–244 [DOI] [PubMed] [Google Scholar]
- 55.Whiteley LB, Brown LK, Swenson R, Kapogiannis BG, Harper GW. Disparities in mental health care among HIV-infected youth. J Int Assoc Provid AIDS Care 2014;13:29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandon D, Issac L, LaVeist T. The legacy of Tuskegee and trust in medical care: Is Tuskegee responsible for race differences in mistrust of medical care? J Natl Med Assoc 2005;97:951–956 [PMC free article] [PubMed] [Google Scholar]
- 57.Tanner AE, Philbin MM, Duval A, et al. “Youth friendly” clinics: Considerations for linking and engaging HIV-infected adolescents into care. AIDS Care 2014;26:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harper GW. Sex isn't that simple: Culture and context in HIV prevention interventions for gay and bisexual male adolescents. Am Psychol 2007;62:803–819 [DOI] [PubMed] [Google Scholar]
- 59.Binford MC, Kahana SY, Altice FL. A systematic review of antiretroviral adherence interventions for HIV-infected people who use drugs. Curr HIV/AIDS Rep 2012;4:287–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azar MM, Springer SA, Meyer JP, et al. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 2010;112:178–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Outlaw A, Naar-King S, Green-Jones M, et al. Predictors of optimal HIV appointment adherence in minority youth: A prospective study. J Pediatr Psychol 2010;35:1011–1015 [DOI] [PubMed] [Google Scholar]
- 62.Esposito-Smythers C, Brown LK, Wolff J, et al. Substance abuse treatment for HIV infected young people: An open pilot trial. J Subst Abuse Treat 2014;46:244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy DA, Chen X, Naar-King S, Parsons JT. Alcohol and marijuana use outcomes in the healthy choices motivational interviewing intervention for HIV-positive youth. AIDS Patient Care STDS 2012;26:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naar-King S, Parsons JT, Murphy D, Kolmodin K, Harris DR. A multisite randomized trial of a motivational intervention targeting multiple risks in youth living with HIV: Initial effects on motivation, self-efficacy, and depression. J Adolesc Health 2010;46:422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kennard BD, Brown LT, Hawkins L, et al. Development and implementation of health and wellness CBT for individuals with depression and HIV. Cogn Behav Pract 2014;237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Safren S, O'Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol 2009;28:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet 2010;376:367–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daughters SB, Magidson JF, Schuster RM, Safren SA. ACT healthy: A combined cognitive-behavioral depression and medication adherence treatment for HIV-infected substance users. Cogn Behav Pract 2010;17:309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parsons JT, Rosof E, Mustanski B. Patient related factors predicting HIV medication adherence among men and women with alcohol problems. J Health Psychol 2007;12:357–370 [DOI] [PMC free article] [PubMed] [Google Scholar]