Abstract
Tubulointerstitial fibrosis mediates the development of end-stage renal disease from renal injuries of all etiologies and is considered an important predictor of renal survival. Transforming growth factor-β (TGF-β) is one of the most important growth factors which promotes tubulointerstitial fibrosis, but the mechanisms whereby this occurs are not well-defined. This is because TGF-β has pleiotropic effects that depend upon the target cell type. This review discusses how TGF-β signaling in each of the relevant cell types (e.g. tubular epithelium, fibroblasts) may contribute to tubulointerstitial fibrosis progression and suggests ways in which future research can improve our understanding of TGF-β-mediated tubulointerstitial fibrosis.
Keywords: TGF-beta, tubulointerstitial fibrosis, extracellular matrix, chronic kidney disease
Renal fibrosis, characterized by the accumulation of extracellular matrix (ECM), is the final common pathway whereby all renal injuries lead to end-stage renal disease. Although fibrosis in the kidney can occur as either tubulointerstitial fibrosis or glomerulosclerosis, injury to both the tubulointerstitial and glomerular compartments ultimately results in tubulointerstitial fibrosis. Exactly how glomerular injury causes tubulointerstitial fibrosis is poorly understood, but it is well established that the best predictor of renal survival from kidney disease of any etiology is tubulointerstitial fibrosis. Fibrosis in the renal tubulointerstitium, like fibrosis in all organs, results from increased ECM synthesis, reduced degradation, or a combination of both. Dysregulation of this equilibrium can occur during tissue repair due to persistent inflammation, increased reactive oxygen species (ROS) production, changes in protease levels as well as altered cell-ECM interactions. These cellular events are regulated at the molecular level by numerous proteins including growth factors. One of the most important growth factors that plays a role in renal fibrosis is transforming growth factor-β (TGF-β).
TGF-β has 3 mammalian isoforms (TGF-β1, -β2, and -β3) that are a part of the TGF-β superfamily which also includes the bone morphogenic proteins (BMPs) and activins. TGF-β is secreted in a latent form due to non-covalent binding to the latency-associated peptide (LAP) and anchored to the ECM by latent TGF-β binding proteins (LTBPs) (Figure 1).1 To become active, TGF-β is released from the LAP by numerous mechanisms, including proteolytic cleavage (e.g. MMPs, plasmin), ROS, heat, or through conformational change as induced by thrombospondin and certain integrins.2 Once activated, TGF-β ligands bind to the TGF-β type II receptor (TβRII) which phosphorylates the TGF-β type I receptor leading to activation of multiple intracellular signaling pathways.3, 4 Canonical TGF-β signaling is mediated by the Smads, but other downstream proteins (e.g. MAPK, PI3K, TAK) can modulate Smad signaling and exert Smad-independent effects.5, 6 These complex signaling pathways allow TGF-β to regulate multiple cellular events involved in tissue development, immune function, wound repair, and cancer biology.
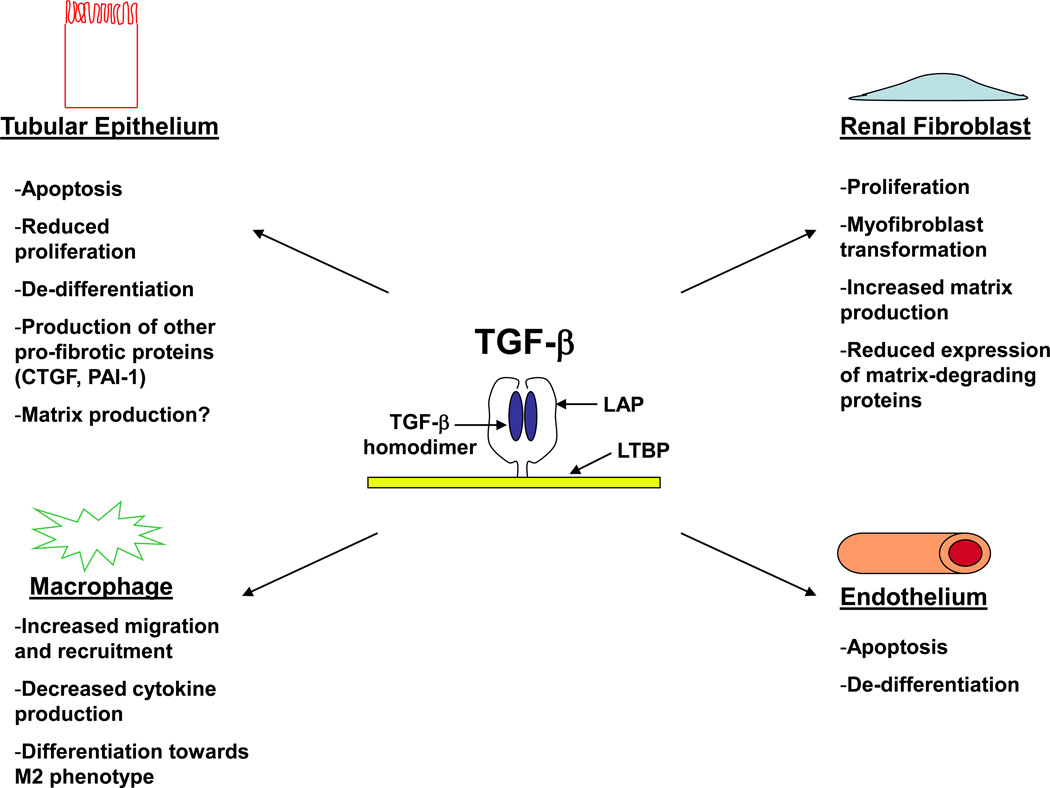
Figure 1.
TGF-β’s effects vary depending upon target cell type. TGF-β ligands are secreted as mature homodimers non-covalently bound to the LAP. This latent complex is anchored to the ECM by LTBP, and the mature TGF-β homodimer must be separated from the LAP to become active and bind to the TGF-β type II receptor. TGF-β has pleiotropic effects that vary depending upon the target cell type. Shown above are the different cellular responses to TGF-β by various cell types involved in tubulointerstitial fibrosis (tubular epithelium, renal fibroblast, macrophage, and endothelium). Many of these cellular responses were determined by in vitro studies, and validation of these effects in vivo has yet to be confirmed.
Abbreviations: TGF-β, Transforming growth factor-β; LAP, latency associated peptide; ECM, extracellular matrix; LTBP, latent TGF-β binding protein
A large body of evidence suggests that TGF-β plays a critical role in the pathogenesis of tubulointerstitial fibrosis. The best direct evidence for these effects is that over-expression of TGF-β1 by renal tubular epithelial cells resulted in tubulointerstitial fibrosis in the absence of any injury,7 and conversely, a blocking antibody to TGF-β ameliorated interstitial matrix accumulation after unilateral ureteral obstruction (UUO), a model of severe tubulointerstitial fibrosis.8 Despite this compelling evidence that TGF-β promotes tubulointerstitial fibrosis, the mechanism whereby it does so remains unclear. This is partly due to the complexity of tubulointerstitial fibrosis progression which involves many different cell types: interstitial fibroblasts produce matrix components, inflammatory cells generate pro-fibrotic growth factors, and tubular epithelial cells are often the initial target of injury (Figure 2). The lack of a clearly defined mechanism whereby TGF-β promotes fibrosis is also due to the fact that TGF-β receptors are ubiquitously expressed and TGF-β has pleiotropic effects which are cell type specific and vary in different forms of injury. In addition to these factors, the role of TGF-β in renal injury has been primarily studied by modulating TGF-β activity through systemic approaches. This strategy only reveals the aggregate response of the kidney to TGF-β and does not elucidate the mechanism whereby TGF-β increases tubulointerstitial fibrosis. In this review, we will outline what is known about the specific effects of TGF-β signaling on the response to injury by each cell type involved in tubulointerstitial fibrosis (Figure 1) in order to define some of the important questions that future research should address.
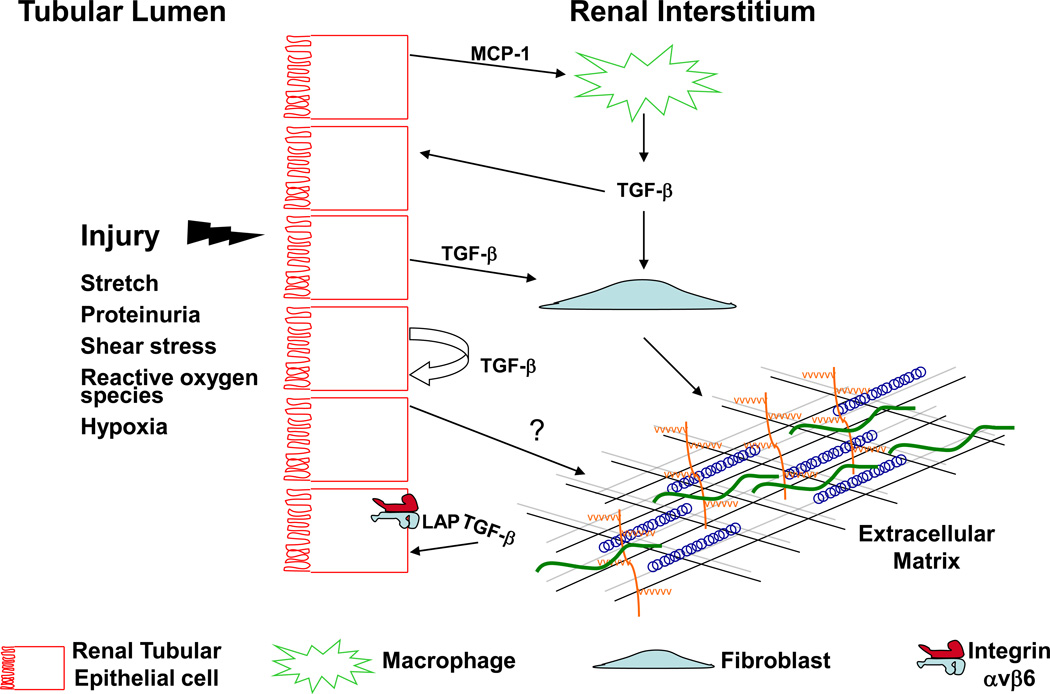
Figure 2.
Proposed cellular interactions that lead to TGF-β-mediated tubulointerstitial fibrosis. Various forms of injury (e.g. mechanical stretch, hypoxia) target the renal tubular epithelium leading to production of inflammatory cytokines such as MCP-1 which recruits macrophages. The infiltrating macrophages are potent sources of TGF-β which can signal on neighboring epithelial cells or renal fibroblasts. TGF-β from either macrophages or injured tubular epithelium stimulates fibroblasts to produce matrix components such as collagen I and fibronectin. The increased TGF-β production by injured epithelium can signal in an autocrine fashion leading to further TGF-β production, de-differentiation, and possibly increased collagen IV production. Tubular injury may also increase integrin αvβ6 expression and activation of latent TGF-β.
Abbreviations: MCP-1, monocyte chemoattractant protein-1
TGF-β signaling and renal fibroblasts
The fibroblast, considered the primary cell responsible for matrix synthesis, is thought to be the main mediator of TGF-β’s pro-fibrotic effects. This perception is based on several in vitro studies showing increased fibroblast proliferation and matrix production in response to TGF-β. Specifically, TGF-β induces a morphologic transformation of human subcutaneous fibroblasts into myofibroblasts, potent matrix-producing cells, by increasing expression of α-smooth muscle actin (α-SMA).9 TGF-β also stimulates fibroblasts’ transcription of collagen I and fibronectin, important components of tubulointerstitial fibrosis.10, 11 In addition, TGF-β signaling in fibroblasts promotes matrix accumulation by suppressing its degradation through down-regulation of certain MMPs and increased expression of tissue inhibitor of MMP (TIMP).12, 13
There are several reasons why validation of these in vitro findings with in vivo studies is critical. First, not all fibroblasts respond equally to TGF-β. Thus, studies on dermal fibroblasts may not necessarily predict cellular behavior of renal fibroblasts. Furthermore, although many early in vitro studies were performed on renal fibroblasts (NKF-49F cell line), heterogeneic responses to TGF-β by subclones of NKF-49F cells have been reported.14 Perhaps this is because NKF-49F cells are a mixture of cortical and medullary fibroblasts which have morphologic and perhaps functional differences. Second, the response of cells to TGF-β is determined by the dose; for example, TGF-β can stimulate matrix production in renal cortical fibroblasts at one dose (0.2ng/mL) but suppress collagen secretion at higher amounts (1ng/mL).15 Whether the concentration of active TGF-β in the injured renal tubulointerstitium results in fibroblasts’ suppression or stimulation of ECM is unclear and an important reason why in vivo studies are necessary. Finally, TGF-β’s actions are not just cell-specific but also dependent upon the surrounding microenvironment and can be modulated by other growth factors and cytokines.16 As the cell culture milieu is quite different from that of the injured kidney, the fibroblast’s response to TGF-β in vitro may poorly predict its actions after injury in vivo.
The intracellular signaling pathways whereby TGF-β mediates its profibrotic effects on renal fibroblasts are also not well delineated. Both p21-activated kinase-2 (PAK2) and c-Abl kinases have been shown to mediate TGF-β’s proliferative effects on renal fibroblasts but do not have this effect on other renal cell types such as mesangial cells.17 The signaling pathways whereby TGF-β stimulates matrix production have been investigated in mesangial cells,18–20 but have not yet been defined in renal fibroblasts. This is an important area of study because one could potentially identify fibroblast-specific TGF-β signaling pathways that could be safer and more specific pharmaceutical targets.
TGF-β signaling and renal tubular epithelial cells
The renal tubular epithelium is often the cellular target of injury, and there is also increasing literature supporting its role as an effector of tubulointerstitial fibrosis. Increased expression of TGF-β and its receptors have been demonstrated in the damaged epithelium in multiple models of glomerular and renal tubular injury.21–23 These include: 1) the UUO injury model, the classic rodent model of renal tubulointerstitial fibrosis in which the collecting duct epithelium is directly injured by the increased intraluminal pressure from obstruction; 2) diabetic models of injury resulting in proteinuria that targets the proximal tubule epithelium as an important extraglomerular site of injury; 3) models of acute kidney injury using toxins or ischemia/reperfusion which target the proximal tubule due to its high metabolic requirements and unique vascular supply. In these settings of injured renal tubules, epithelial TGF-β signaling is thought to promote tubulointerstitial fibrosis by affecting tubular cell integrity and increasing ECM production by both tubular epithelial cells and neighboring fibroblasts.
TGF-β signaling can impair renal tubule cell integrity leading to tubular atrophy, a hallmark of tubulointerstitial fibrosis, by inducing either epithelial apoptosis or autophagy. TGF-β, a strong promoter of epithelial apoptosis, has been shown to induce renal tubule cell apoptosis in vitro.24 Furthermore, the protective effects of a TGF-β blocking antibody (1D11) on the response to UUO were partially attributed to reduced tubular apoptosis in vivo.8 Recently, TGF-β was shown to cause tubular destruction as over-expression of TGF-β1 in renal tubules in vivo led to autophagy and tubular degeneration.7 One of the problems with this elegant model is that the levels of TGF-β1 in these mice were very high (>200ng/mL), which is likely much higher than those produced by most chronic disease states.
In tubulointerstitial fibrosis, there is concurrent tubular atrophy which raises the question: does epithelial apoptosis cause increased matrix accumulation? In the pulmonary literature, the increased apoptosis induced in the lungs of TGF-β1 over-expressing mice was mediated by the early growth response gene-1 (Egr-1), and inhibiting Egr-1 not only rescued the mice from TGF-β-dependent apoptosis but also reduced fibrosis, establishing a link between the two.25 A similar connection between TGF-β-dependent apoptosis and fibrosis has not yet been proven in the kidney. A putative mechanism whereby apoptosis leads to fibrosis is through the phagocytosis of apoptotic cells by macrophages.26 This stimulates the production of TGF-β which may induce matrix production by surrounding fibroblasts. The tubular atrophy induced by epithelial TGF-β signaling contributes to the injury and impaired renal function observed in tubulointerstitial fibrosis even if a causal relationship with ECM production has not yet been established.
TGF-β facilitates the de-differentiation of injured renal epithelial cells into more mesenchymal-like cells, and it is possible that this process results in increased ECM synthesis. During this phenotypic change, tubular cells lose epithelial markers such as E.cadherin and ZO-1, express mesenchymal-like proteins such as α-SMA and vimentin, and undergo cytoskeletal alterations resulting in dissolution of tight junctions and adaptation of a more migratory structure.27 Numerous mechanisms are responsible for this change in phenotype. TGF-β mediated the loss of E.cadherin in renal epithelial cells in vitro through induction of integrin-linked kinase (ILK) in a Smad-dependent fashion.28 TGF-β also induced the expression of α-SMA in renal epithelial cells through pathways involving β-catenin as well as the GTPase Rho.29, 30 TGF-β can induce all of these morphologic changes in renal tubular epithelial cells as well as increase the expression of collagen I, IV, and fibronectin in vitro, suggesting that the alteration in phenotype may cause the increased matrix production.31, 32 Alternatively, it is possible that these are two independent effects induced by TGF-β and there is no causal relationship. TGF-β may also stimulate the de-differentiation of injured epithelial cells in vivo as Smad3 null epithelial cells had better preservation of epithelial markers after UUO.33 Whether these de-differentiated tubular epithelial cells in vivo actually migrate through the tubular basement membrane and populate the interstitium as matrix-producing fibroblasts is controversial.34, 35 Even if these mesenchymal-like epithelial cells never become bona fide interstitial fibroblasts, they still may contribute to peritubular fibrosis. Further research is necessary to determine how much autocrine ECM production occurs in response to renal tubular TGF-β signaling in vivo.
TGF-β signaling in injured tubular epithelium may also promote tubulointerstitial fibrosis by increasing ECM production of neighboring fibroblasts. TGF-β autoinduces its own production in a feedforward process that depends on the coordinated actions of Smad3, ERK, and p38 in renal proximal tubules.36, 37 Therefore, injury to tubules results in increased epithelial synthesis of TGF-β which can signal on nearby fibroblasts. Such paracrine signaling likely accounts for the proliferating fibroblasts and interstitial matrix accumulation observed in mice over-expressing TGF-β1 in renal tubules.7
Epithelial TGF-β signaling can also increase the expression of other pro-fibrotic growth factors such as connective tissue growth factor (CTGF), which has been shown to stimulate matrix production by increasing transcription of collagen I in both renal proximal tubules and fibroblasts in vitro. Plasminogen activator inhibitor-1 (PAI-1) is another protein downstream of TGF-β with clear pro-fibrotic actions in the kidney.38, 39 Also, in non-renal cells, TGF-β signaling has been shown to modify the actions of epidermal growth factor receptor ligands, hepatocyte growth factor, and insulin-like growth factor.16, 40, 41 Future research is required to define how TGF-β signaling alters these growth factors’ actions in the injured renal tubulointerstitium.
Renal tubular epithelium also plays an important role in TGF-β activation. Abundant TGF-β is sequestered in its LAP-bound latent form anchored to the matrix, so activation is a critical rate-limiting step in controlling TGF-β signaling.1 The αv integrins are important in vivo activators of TGF-β.42 In particular, integrin αvβ6 has been shown to mediate TGF-β-dependent tubulointerstitial fibrosis following UUO injury.43 The expression of integrin αvβ6 is restricted to epithelial cells where it binds the LAP of TGF-β1 and –β3 which are tethered to the ECM. In order for integrin αvβ6 to induce the conformational change necessary to activate TGF-β, there must be an intact actin cytoskeleton as well as integrin activation, presumably causing traction on the matrix via binding to TGF-β/LAP.44, 45 Furthermore, latent TGF-β must be anchored to the ECM through one of the LTBPs for integrin αvβ6-mediated activation to occur.46 As such, integrin αvβ6’s interaction with latent TGF-β that is anchored to the ECM may be one way for epithelial cells to transmit mechanical stress and/or cytoskeletal changes to the surrounding matrix.47 The cellular stress from UUO-induced stretch imposed on collecting duct epithelial cells may activate integrin αvβ6 leading to TGF-β activation. This mechanical strain from increased intraluminal pressure may not be limited to obstructive nephropathy. Reduction in nephron number, as occurs in the 5/6 nephrectomy model or chronic kidney disease, has been shown to increase single nephron glomerular filtration rate (GFR) and, consequently, induce fluid shear stress to the tubules.48 This intratubular shear stress can activate TGF-β independent of proteinuria or hypertension.49 Therefore, changes in intratubular fluid pressure caused by declining renal function may promote TGF-β-mediated tubulointerstitial fibrosis through effects on tubular epithelium.50
As discussed above, injured renal tubular epithelium has been shown to contribute to TGF-β-dependent tubulointerstitial fibrosis by inducing apoptosis/autophagy, increasing TGF-β and other growth factor production and through augmented TGF-β activation. Paradoxically, when our group injured mice lacking TGF-β signaling in the collecting duct tubular epithelium, the conditional knockout mice sustained greater fibrosis and injury.51 These results suggest that, though excessive TGF-β signaling is deleterious, some TGF-β signaling is necessary for maintenance of the normal epithelial structure. Consistent with this, reduced epithelial TGF-β activity in lung epithelia resulted in emphysematous changes,52 and dominant negative TβRII expression in the exocrine pancreas perturbed epithelial homeostasis.53
TGF-β signaling and inflammatory cells
Inflammation plays an important role in the pathophysiology of tubulointerstitial fibrosis.54 Most renal injuries have some component of an inflammatory infiltrate composed of T cells, macrophages, and neutrophils with varying distribution based upon the nature of injury. In chronic renal injury, the macrophage is the predominant immune cell and though it exerts beneficial effects by phagocytosis of cellular debris and apoptotic cells, macrophages are also associated with worsened tubulointerstitial injury.55 Macrophages’ detrimental actions may be mediated, in part, by augmenting TGF-β activity. Macrophages directly increase TGF-β activity by both producing large amounts of TGF-β as well as generating ROS which activate latent TGF-β. Also, macrophages produce inflammatory cytokines such as IL-6 which alters TGF-β receptor trafficking leading to heightened signaling.56
Not only do inflammatory cells modulate TGF-β activity, but TGF-β affects immune responses as evidenced by the early death of TGF-β1 null mice from overwhelming inflammation.57 Although this finding implies that TGF-β has an immunosuppressive effect, the precise effect of TGF-β on immune responses is complicated and likely depends upon the cellular milieu (Table 1). TGF-β’s effects on monocytes/macrophages vary depending upon cellular differentiation: this growth factor stimulates monocyte migration and adhesion but suppresses inflammatory cytokine production by tissue macrophages (Figure 3).58 The effect of TGF-β signaling on inflammation may also vary depending upon the cell type where signaling is altered: TGF-β signaling in macrophages may decrease the production of proinflammatory growth factors and cytokines whereas TGF-β signaling within injured epithelium likely heightens the inflammatory response.59, 60 For example, Smad3 deficient mice have attenuated macrophage infiltration following injury by UUO,33 suggesting that TGF-β may promote inflammation in injured kidneys. One possible explanation for this increased macrophage infiltration is that TGF-β can stimulate monocyte chemoattractant protein-1 (MCP-1) expression in renal proximal tubules.61 Thus, it is likely that TGF-β signaling in injured tubular epithelium results in increased macrophage infiltration which, if prolonged, can lead to synthesis of growth factors such as TGF-β that stimulates neighboring fibroblasts to produce matrix components (Figure 2,3).
Table 1.
TGF-β has been described as a pro-inflammatory and anti-inflammatory growth factor, and its specific effect depends upon the target cell and microenvironment. This table shows seemingly contradictory effects of TGF-β in modulating renal inflammation. TGF-β can have immunosuppressive effects on immune cells but pro-inflammatory effects on renal parenchyma.73 This is consistent with the study by Cao et al where only macrophages ex vivo were treated with TGF-β.74 Also, the global deletion of TβRII produced multi-organ inflammation, likely as a result of altered signaling in inflammatory cells. The conflicting reports of the other studies listed may be due to how their models differentially affected the activity of Smad7, a known modulator of inflammation and NF-κB.75, 76 Overexpressing latent TGF-β1 as well as deleting Smad4 both appeared to have dramatic effects on Smad7 activity which may explain the inflammatory effects.74, 77
| TGF-β as pro-inflammatory | TGF-β as anti-inflammatory | ||
|---|---|---|---|
| TGF-β1 increased inflammatory cytokine (MCP-1, IL-8) production by proximal tubule cells in vitro |
Qi W, et al. Am J Physiol Renal Physiol 290: F703-F709, 2006. |
TGF-β1-treated macrophages attenuated inflammation following adriamycin nephrosis |
Cao Q et al. J Am Soc Nephrol 21:933- 942, 2010. |
| Smad3−/− mice had attenuated macrophage infiltration after UUO |
Sato M. et al. J Clin Invest 12:1486- 1494, 2003. |
Overexpression of latent TGF-β1 prevented inflammation in UUO model |
Wang W et al. J Am Soc Nephrol 16:1371–1383,2005. |
| Smad3−/− mice had less IL-6 after acute kidney injury |
Nath et al. Am J Physiol Renal Physiol 301 :F436–442, 2011. |
Deletion of Smad4 in renal tubules enhanced renal inflammation after UUO |
Meng X, et al. Kidney Int Nov 2011. |
| ALK5 inhibitor targeted to proximal tubule reduced inflammation after UUO |
Prakash J et al. Pharm Res 10:2427- 39, 2008. |
Deletion of TβRII caused increased inflammation in kidney and other organs |
Leveen P, et al. Blood 100 (2) 560–8, 2002. |
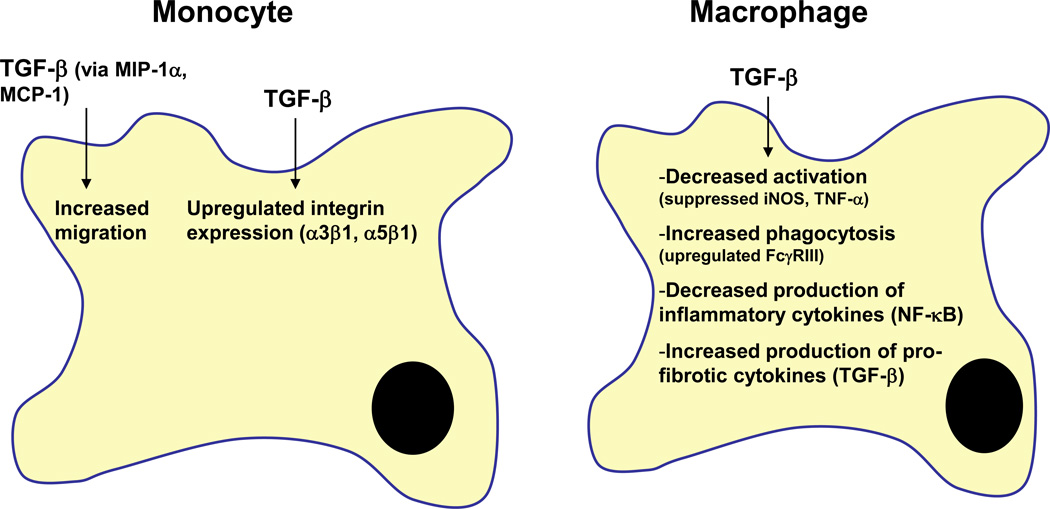
Figure 3.
TGF-β’s effects on monocytes/macrophages. TGF-β promotes monocyte migration by increasing chemoattractant cytokines such as MIP-1α and MCP-1. Cell/cell and cell/matrix interactions of monocytes are also enhanced by TGF-β through upregulated expression of integrins. In contrast to these stimulatory effects on monocytes, TGF-β suppresses activation of macrophages as evidenced by decreased iNOS and TNF-α expression. TGF-β signaling reduces macrophage production of inflammatory cytokines such as IFN-γ but increases the synthesis of pro-fibrotic factors like TGF-β.
Abbreviations: MIP-1α, macrophage inflammatory protein-1α; MCP-1, monocyte chemoattractant protein-1; iNOS, inducible nitric oxide synthase; TNF-α, tumor necrosis factor-a; IFN-γ, interferon-γ
TGF-β and the microvasculature
Capillary degeneration is integrally linked to tubulointerstitial fibrosis, and endothelial cell dropout may play a causal role in the progression of fibrosis.62 Loss of postglomerular capillaries creates a hypoxic environment which further stimulates fibrosis.63 Exactly how TGF-β signaling in the endothelium promotes fibrosis in vivo is unclear. Endothelial injury can stimulate TGF-β production as shown in animal models of increased salt intake and salt-sensitive hypertension in which TGF-β production was upregulated by endothelial cells of renal arterioles.64, 65 Conversely, TGF-β may have adverse effects on the renal microvasculature by promoting both endothelial apoptosis and de-differentiation leading to increased matrix production.66, 67 TGF-β may also modulate angiogenesis, a pivotal event in the restoration of healthy renal vasculature after injury. TGF-β signaling on endothelial cells in vitro has been shown to have a net anti-angiogenic effect.68 However, TGF-β’s in vivo effect on angiogenesis is complicated as this growth factor induces renal proximal tubular expression of both VEGF-A and thrombospondin-1 which have pro-angiogenic and anti-angiogenic effects, respectively, in vivo69–71 These findings suggests that TGF-β signaling on injured epithelium may affect the adjacent vasculature and that divergent effects on angiogenesis may occur. Interestingly, a neutralizing TGF-β antibody protected against the vascular loss that occurred 5 wks after ischemia-reperfusion, implying that TGF-β may mediate chronic microvascular changes that result from acute kidney injury.72 Whether these changes were due to TGF-β-mediated anti-angiogenesis or due to other effects on the endothelium (e.g. apoptosis) is unclear. To better define the role of TGF-β in the endothelial response to injury, future studies should examine endothelial-specific TGF-β signaling in vivo as well as analyze the TGF-β-mediated crosstalk between injured tubular epithelium and the surrounding vasculature.
Conclusions
TGF-β clearly plays a key role in the progression of tubulointerstitial fibrosis, but the mechanisms whereby this occurs remain undefined. Understanding how TGF-β mediates tubulointerstitial fibrosis is challenging because multiple cell types are involved in fibrosis and the responses to TGF-β vary dependent upon cell type and cellular milieu. Fortunately, the advent of Cre/lox technology allows for selective inhibition of TGF-β signaling in specific compartments of the kidney. This tool will improve our understanding of cell-autonomous TGF-β signaling, providing important information regarding the effects of inhibiting TGF-β activity in targeted cell types in vivo. Another important direction for future studies is elucidating cell-specific downstream pathways of TGF-β. These pathways likely mediate the divergent effects of TGF-β, and defining how these pathways differ in epithelial cells and fibroblasts may identify pharmacologic targets with safer profiles than TGF-β. Also, a better understanding of how inhibiting TGF-β signaling alters the activity of other growth factors following renal injury is crucial and could have important therapeutic implications. As the population with chronic kidney disease continues to expand, a safe and effective way to modulate TGF-β-dependent tubulointerstitial fibrosis would make a huge medical and economic impact.
Acknowledgments
Supported in part by a Veterans Affairs Career Development Award (L.G.), the Vanderbilt Physician Scientist Development Award (L.G.), NIH grants DK065123 (R.Z.); DK075594 (R.Z.); DK65123 (R.Z.); DK083187 (R.Z.); AHA established investigator award (R.Z.); a Merit Award from the Department of Veterans Affairs (R.Z.), and the George O’Brien Center Grant (R.Z.).
References
- 1.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280(9):7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol. 2008;40(6–7):1068–1078. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita H, ten Dijke P, Franzen P, Miyazono K, Heldin CH. Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-beta. J Biol Chem. 1994;269(31):20172–20178. [PubMed] [Google Scholar]
- 5.Brown KA, Pietenpol JA, Moses HL. A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling. J Cell Biochem. 2007;101(1):9–33. doi: 10.1002/jcb.21255. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19(1):128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, et al. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 177(2):632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, et al. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000;58(6):2301–2313. doi: 10.1046/j.1523-1755.2000.00414.x. [DOI] [PubMed] [Google Scholar]
- 9.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignotz RA, Endo T, Massague J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987;262(14):6443–6446. [PubMed] [Google Scholar]
- 11.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, et al. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leco KJ, Khokha R, Pavloff N, Hawkes SP, Edwards DR. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem. 1994;269(12):9352–9360. [PubMed] [Google Scholar]
- 14.Tang N, Cunningham K, Enger MD. TGF beta elicits opposite responses in clonal subpopulations of NRK-49F cells. Exp Cell Res. 1991;196(1):13–19. doi: 10.1016/0014-4827(91)90450-9. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez RJ, Sun MJ, Haverty TP, Iozzo RV, Myers JC, Neilson EG. Biosynthetic and proliferative characteristics of tubulointerstitial fibroblasts probed with paracrine cytokines. Kidney Int. 1992;41(1):14–23. doi: 10.1038/ki.1992.3. [DOI] [PubMed] [Google Scholar]
- 16.Uttamsingh S, Bao X, Nguyen KT, Bhanot M, Gong J, Chan JL, et al. Synergistic effect between EGF and TGF-beta1 in inducing oncogenic properties of intestinal epithelial cells. Oncogene. 2008;27(18):2626–2634. doi: 10.1038/sj.onc.1210915. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005;19(1):1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- 18.Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem. 2004;279(4):2632–2639. doi: 10.1074/jbc.M310412200. [DOI] [PubMed] [Google Scholar]
- 19.Runyan CE, Schnaper HW, Poncelet AC. Smad3 and PKCdelta mediate TGF-beta1-induced collagen I expression in human mesangial cells. Am J Physiol Renal Physiol. 2003;285(3):F413–F422. doi: 10.1152/ajprenal.00082.2003. [DOI] [PubMed] [Google Scholar]
- 20.Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-beta1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int. 1999;56(5):1710–1720. doi: 10.1046/j.1523-1755.1999.00733.x. [DOI] [PubMed] [Google Scholar]
- 21.Basile DP, Rovak JM, Martin DR, Hammerman MR. Increased transforming growth factor-beta 1 expression in regenerating rat renal tubules following ischemic injury. Am J Physiol. 1996;270(3 Pt 2):F500–F509. doi: 10.1152/ajprenal.1996.270.3.F500. [DOI] [PubMed] [Google Scholar]
- 22.Sutaria PM, Ohebshalom M, McCaffrey TA, Vaughan ED, Jr, Felsen D. Transforming growth factor-beta receptor types I and II are expressed in renal tubules and are increased after chronic unilateral ureteral obstruction. Life Sci. 1998;62(21):1965–1972. doi: 10.1016/s0024-3205(98)00166-0. [DOI] [PubMed] [Google Scholar]
- 23.Goumenos DS, Tsakas S, El Nahas AM, Alexandri S, Oldroyd S, Kalliakmani P, et al. Transforming growth factor-beta(1) in the kidney and urine of patients with glomerular disease and proteinuria. Nephrol Dial Transplant. 2002;17(12):2145–2152. doi: 10.1093/ndt/17.12.2145. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikawa M, Hishikawa K, Idei M, Fujita T. Trichostatin a prevents TGF-beta1-induced apoptosis by inhibiting ERK activation in human renal tubular epithelial cells. Eur J Pharmacol. 642(1–3):28–36. doi: 10.1016/j.ejphar.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 25.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200(3):377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13(10):2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112(4):503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, et al. Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am J Pathol. 2004;165(6):1955–1967. doi: 10.1016/s0002-9440(10)63247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, et al. Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol. 2003;284(5):F911–F924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159(4):1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61(5):1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 33.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112(10):1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 21(8):1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 35.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 121(2):468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988;263(16):7741–7746. [PubMed] [Google Scholar]
- 37.Zhang M, Fraser D, Phillips A. ERK, p38, and Smad signaling pathways differentially regulate transforming growth factor-beta1 autoinduction in proximal tubular epithelial cells. Am J Pathol. 2006;169(4):1282–1293. doi: 10.2353/ajpath.2006.050921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krag S, Danielsen CC, Carmeliet P, Nyengaard J, Wogensen L. Plasminogen activator inhibitor-1 gene deficiency attenuates TGF-beta1-induced kidney disease. Kidney Int. 2005;68(6):2651–2666. doi: 10.1111/j.1523-1755.2005.00737.x. [DOI] [PubMed] [Google Scholar]
- 39.Oda T, Jung YO, Kim HS, Cai X, Lopez-Guisa JM, Ikeda Y, et al. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int. 2001;60(2):587–596. doi: 10.1046/j.1523-1755.2001.030002587.x. [DOI] [PubMed] [Google Scholar]
- 40.Naim R, Naumann A, Barnes J, Sauter A, Hormann K, Merkel D, et al. Transforming growth factor-beta1-antisense modulates the expression of hepatocyte growth factor/scatter factor in keloid fibroblast cell culture. Aesthetic Plast Surg. 2008;32(2):346–352. doi: 10.1007/s00266-007-9078-6. [DOI] [PubMed] [Google Scholar]
- 41.Gardner S, Alzhanov D, Knollman P, Kuninger D, Rotwein P. TGF-beta inhibits muscle differentiation by blocking autocrine signaling pathways initiated by IGF-II. Mol Endocrinol. 25(1):128–137. doi: 10.1210/me.2010-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176(6):787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, et al. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(−/−) mice. Am J Pathol. 2003;163(4):1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, et al. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest. 2006;116(6):1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 46.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165(5):723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keski-Oja J, Koli K, von Melchner H. TGF-beta activation by traction? Trends Cell Biol. 2004;14(12):657–659. doi: 10.1016/j.tcb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Essig M, Terzi F, Burtin M, Friedlander G. Mechanical strains induced by tubular flow affect the phenotype of proximal tubular cells. Am J Physiol Renal Physiol. 2001;281(4):F751–F762. doi: 10.1152/ajprenal.2001.281.4.F751. [DOI] [PubMed] [Google Scholar]
- 49.Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-beta in rats. Am J Physiol. 1998;274(4 Pt 2):F635–F641. doi: 10.1152/ajprenal.1998.274.4.F635. [DOI] [PubMed] [Google Scholar]
- 50.Rohatgi R, Flores D. Intratubular hydrodynamic forces influence tubulointerstitial fibrosis in the kidney. Curr Opin Nephrol Hypertens. 19(1):65–71. doi: 10.1097/MNH.0b013e32833327f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gewin L, Bulus N, Mernaugh G, Moeckel G, Harris RC, Moses HL, et al. TGF-beta receptor deletion in the renal collecting system exacerbates fibrosis. J Am Soc Nephrol. 21(8):1334–1343. doi: 10.1681/ASN.2010020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, et al. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422(6928):169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 53.Bottinger EP, Jakubczak JL, Roberts IS, Mumy M, Hemmati P, Bagnall K, et al. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997;16(10):2621–2633. doi: 10.1093/emboj/16.10.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferenbach D, Kluth DC, Hughes J. Inflammatory cells in renal injury and repair. Semin Nephrol. 2007;27(3):250–259. doi: 10.1016/j.semnephrol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Sean Eardley K, Cockwell P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005;68(2):437–455. doi: 10.1111/j.1523-1755.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang XL, Topley N, Ito T, Phillips A. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J Biol Chem. 2005;280(13):12239–12245. doi: 10.1074/jbc.M413284200. [DOI] [PubMed] [Google Scholar]
- 57.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 59.Kitamura M, Suto T, Yokoo T, Shimizu F, Fine LG. Transforming growth factor-beta 1 is the predominant paracrine inhibitor of macrophage cytokine synthesis produced by glomerular mesangial cells. J Immunol. 1996;156(8):2964–2971. [PubMed] [Google Scholar]
- 60.Alexandrow MG, Moses HL. Transforming growth factor beta and cell cycle regulation. Cancer Res. 1995;55(7):1452–1457. [PubMed] [Google Scholar]
- 61.Qi W, Chen X, Polhill TS, Sumual S, Twigg S, Gilbert RE, et al. TGF-beta1 induces IL-8 and MCP-1 through a connective tissue growth factor-independent pathway. Am J Physiol Renal Physiol. 2006;290(3):F703–F709. doi: 10.1152/ajprenal.00254.2005. [DOI] [PubMed] [Google Scholar]
- 62.Bohle A, Mackensen-Haen S, Wehrmann M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res. 1996;19(3–4):191–195. doi: 10.1159/000174072. [DOI] [PubMed] [Google Scholar]
- 63.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, et al. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295(4):F1023–F1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanders PW. Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol. 2009;297(2):F237–F243. doi: 10.1152/ajprenal.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jing L, Zhang JZ, Zhao L, Wang YL, Guo FY. High-expression of transforming growth factor beta1 and phosphorylation of extracellular signal-regulated protein kinase in vascular smooth muscle cells from aorta and renal arterioles of spontaneous hypertension rats. Clin Exp Hypertens. 2007;29(2):107–117. doi: 10.1080/10641960701195447. [DOI] [PubMed] [Google Scholar]
- 66.Rouschop KM, Claessen N, Pals ST, Weening JJ, Florquin S. CD44 disruption prevents degeneration of the capillary network in obstructive nephropathy via reduction of TGF-beta1-induced apoptosis. J Am Soc Nephrol. 2006;17(3):746–753. doi: 10.1681/ASN.2005080808. [DOI] [PubMed] [Google Scholar]
- 67.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19(12):2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pepper MS, Belin D, Montesano R, Orci L, Vassalli JD. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990;111(2):743–755. doi: 10.1083/jcb.111.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakagawa T, Li JH, Garcia G, Mu W, Piek E, Bottinger EP, et al. TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int. 2004;66(2):605–613. doi: 10.1111/j.1523-1755.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 70.Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, et al. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis. 2001;37(3):601–611. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- 71.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12(7):1448–1457. doi: 10.1681/ASN.V1271448. [DOI] [PubMed] [Google Scholar]
- 72.Spurgeon KR, Donohoe DL, Basile DP. Transforming growth factor-beta in acute renal failure: receptor expression, effects on proliferation, cellularity, and vascularization after recovery from injury. Am J Physiol Renal Physiol. 2005;288(3):F568–F577. doi: 10.1152/ajprenal.00330.2004. [DOI] [PubMed] [Google Scholar]
- 73.Saxena V, Lienesch DW, Zhou M, Bommireddy R, Azhar M, Doetschman T, et al. Dual roles of immunoregulatory cytokine TGF-beta in the pathogenesis of autoimmunity-mediated organ damage. J Immunol. 2008;180(3):1903–1912. doi: 10.4049/jimmunol.180.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao Q, Wang Y, Zheng D, Sun Y, Lee VW, Zheng G, et al. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 21(6):933–942. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ng YY, Hou CC, Wang W, Huang XR, Lan HY. Blockade of NFkappaB activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney Int Suppl. 2005;(94):S83–S91. doi: 10.1111/j.1523-1755.2005.09421.x. [DOI] [PubMed] [Google Scholar]
- 76.Chen HY, Huang XR, Wang W, Li JH, Heuchel RL, Chung AC, et al. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes. 60(2):590–601. doi: 10.2337/db10-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang W, Huang XR, Li AG, Liu F, Li JH, Truong LD, et al. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol. 2005;16(5):1371–1383. doi: 10.1681/ASN.2004121070. [DOI] [PubMed] [Google Scholar]