Abstract
Objective
To identify factors associated with racial differences in level of cognitive function in old age.
Methods
Older Black (n=5,950) and White (n=3,469) residents of a geographically defined urban community were randomly split into exploratory and confirmatory subgroups. A global measure of cognition was derived from 4 brief performance tests and potential correlates of cognition (candidates) were selected from demographic, health-related, and experiential measures. In the exploratory subgroup using a stepwise search algorithm, we examined the cognitive difference by race and then allowed candidate measures and race by candidate measure interactions to enter the model.
Results
The cognitive score in the exploratory subgroup (mean = 0.257, SD = 0.714) was a mean of 0.403-unit lower in Black persons than White persons (SE = 0.021, p<0.001), and race accounted for 7% of cognitive variability. After the candidate selection process, 16 measures were retained including 12 candidate measures and the 2-way interactions of race with education, age, reading/cognitive activity, and neuroticism. In this model, which accounted for 45% of the variability in global cognition, race was no longer associated with global cognition (coefficient = 0.012, SE = 0.110, p = 0.912). Findings were replicated in the confirmatory subgroup.
Conclusion
These cross-sectional analyses suggest that consideration of demographic, health-related, and experiential factors greatly attenuates racial differences in late-life level of cognition.
Keywords: cognitive function, racial differences, population study, neuroticism, cognitive activity
Introduction
Prior research suggests that Black persons are more likely to develop dementia than White persons (Chin, Negash, & Hamilton, 2011). However, longitudinal studies of cognitive function do not suggest strong racial differences in rates of cognitive decline, with some studies reporting no racial differences (Atkinson et al., 2005; Masel & Deek, 2009; Castora-Binkley, Peronto, Edwards, & Small, 2013; Mariske et al., 2013) and other studies reporting slightly slower decline in Black (Sloan & Wang, 2005; Alley, Suther, & Crimmins, 2007; Karlamangla et al., 2009; Early et al., 2013; Wilson, Capuano, Sytsma, Bennett, & Barnes, 2015) or White (Lyketsos, Chen, & Anthony, 1999; Sachs-Ericsson, & Blazer, 2005; Sawyer, Sachs-Ericsson, Preacher, & Blazer, 2009; Wolinsky et al., 2011) persons. The apparent absence of strong racial differences in cognitive decline suggests that the association of race with risk of dementia is primarily due to racial differences in late-life level of cognitive function.
The aim of the present study was to identify factors that may be contributing to racial differences in level of cognitive function in old age. Analyses are based on a biracial population of more than 9,000 older persons. They completed 4 brief cognitive tests from which a measure of global cognition was derived and candidate cognitive correlates were selected from demographic, health-related, and experiential measures. The population was randomly divided into exploratory and confirmatory subgroups. In a stepwise search algorithm in the exploratory subgroup, we assessed the racial differences in cognition with no covariates in the model and tracked the association as candidate measures and race by candidate measure interactions were allowed to enter the analysis. The final model was replicated in the confirmatory subgroup.
Methods
Participants
Analyses are based on persons from the Chicago Health and Aging Project (Bienias, Beckett, Bennett, Wilson, & Evans, 2003), a study of risk factors for dementia and other chronic conditions of old age. Four adjacent neighborhoods on the South side of Chicago were censused, and all residents aged 65 years or older were invited to participate in an in-home interview that included assessment of potential risk factors for dementia and administration of brief performance tests of cognitive function. Race was assessed by self report using the 1990 U.S. Census questions. Those with missing candidate (n=1,321) or cognitive (n=62) measures were excluded from analyses. Compared to the 9,419 individuals included in analyses, the 1,383 excluded individuals were older (77.8 vs 72.7, t[1,630.8] = 21.0, p<,001), had fewer years of education (11.4 vs 12.4, t[1,624.3]=8.0, p<0.001), and were less likely to be male (30.4% vs 39.7%, χ2 [1]= 43.4, p< 0.001). The proportion of Black persons in the excluded (62.3%) and included (63.2%) subgroups did not differ (χ2 [1]= 0.4, p= 0.511). Of the 9,419 eligible individuals, 5,950 (63.2%) were Black persons and 3,469 were White persons. Compared with the White persons, the Black persons were younger and had fewer years of education and lower scores at baseline on the composite measure of global cognition (Table 1).
Table 1.
Demographic Characteristics and Cognitive Function Scores of 9,419 Older Adults from a Biracial Sample
| Black Persons (n=5,950) |
White Persons (n=3,469) |
|
|---|---|---|
| Age (years) | 71.3(5.7; 61.2–102.8) | 75.1 (7.4; 64.4–101.2) |
| Education (years) | 11.5 (3.4; 0–30) | 13.8 (3.3; 0–30) |
| Cognitive function score | 0.107 (0.716; −3.020–1.541) | 0.501 (0.631; −3.020–1.730) |
| Women, n, % | 3594, 60% | 2077, 60% |
Note. Data are presented as M (SD; range) unless otherwise indicated.
Assessment of Cognitive Function
Cognition was assessed with four brief performance tests as part of the initial in-home interview. A brief story (East Boston story) was read to the participant and immediate and delayed recall of 12 story ideas provided measures of episodic memory (Albert et al., 1991; Wilson et al., 2002). The oral version of the Symbol Digit Modalities Test (Smith, 1982) provided a measure of perceptual speed. Global cognition was assessed with the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975). In a previous analysis, these four measures loaded on a single factor that accounted for more than 70% of their covariation (Wilson et al., 1999). Therefore, raw scores on each measure were converted to z scores, using the population-based estimates of the mean and SD, and the z scores were averaged to yield a composite global cognitive score. Support for the validity of this measure comes from prior research showing that it captures variability in trajectories of cognitive aging (Wilson, Mendes de leon, Bennett, Bienias, & Evans, 2004; Wilson et al., 2012) and predicts important outcomes such as dementia (Rajan, Wilson, Weuve, Barnes, & Evans, 2015) and death (Wilson, Barnes, Mendes de Leon, & Evans, 2009) in both Black and White persons.
Candidate Measures
As part of the in-home interview, information was collected on a range of factors with a possible relation to cognition. For the purposes of these analyses, we identified 20 possible correlates of cognitive function which had the potential to impact racial differences in cognitive function. These included 4 demographic measures: age, education, sex, and Southern U.S. origin. Preliminary analyses using a measure of socioeconomic status based on education, occupation, and income were similar to analyses using education alone. Because education was less likely to be missing than socioeconomic status, we included education rather than socioeconomic status in analyses. There were 10 health-related measures. Body mass index was expressed as weight (in kilograms) divided by height (in meters squared). Diabetes was based on self-report of a previous diagnosis or use of insulin or oral hypoglycemics (Arvanitakis, Bennett, Wilson, & Barnes, 2010). Hypertension, stroke, and cancer were based on self-report. In addition, blood pressure was measured using the Hypertension Detection and Follow-up Program protocol (Hypertension Detection and Follow-up Program Cooperative Group, 1977) with 2 readings taken with the participant seated and the right arm at heart level. The mean of each reading provided measures of systolic and diastolic blood pressure (Wilson, Boyle, et al., 2014). Lower extremity physical function was based on 3 physicial performance tests: time to complete an 8 foot walk, time to do 5 chair stands, and duration of a full tandem stand (up to 10 seconds) (Guralnik et al., 1994). Scores on each task were converted to z scores using the population mean and standard deviation and the z scores were averaged to yield a composite measure of lower limb function, as previously described (Wilson, Rajan, et al., 2014). Functional impairment was assessed with 3 measures used in the Established Populations for Epidemiologic Studies of the Elderly program: the 6-item Katz scale of basic self-care activities such as bathing and dressing (Katz & Akpom, 1976); the 3-item Rosow-Breslau measure of mobility (Rosow & Breslau, 1966); and the 5-item Nagi measure of global motor functional competence (Nagi, 1976). In addition, there were 7 measures of experiential factors. These included a 10-item version (Kohout, Berkman, Evans, & Cornoni-Huntley, 1993) of the Center for Epidemiological Studies Depression scale (Radloff, 1977). The personality traits of neuroticism, denoting a tendency to respond to stress with negative emotions, and extraversion, a disposition to be energetic and sociable, were assessed with 4-item short forms of these traits derived from standard 12-item scales of each trait (Costa & McCrae, 1992). The 4-item scales are highly correlated with their 12-item counterparts (Wilson, Krueger, et al. 2005). Each scale has been associated with mortality (Wilson, Krueger, et al., 2005) and the neuroticism measure has been associated with risk of incident dementia (Wilson, Barnes, Bennett, et al, 2005) and rate of cognitive decline (Wilson, Bennett, et al., 2005). Frequency of reading and other cognitively stimulating activities was rated from 1 (once a year or less) to 5 (daily or nearly every day). Scores for each activity (e.g., reading a book, visiting a museum) were averaged to yield a composite measure (Wilson et al., 1999; Wilson, Mendes de Leon, et al., 2002; Wilson, Bennett, et al., 2002). Persons were also asked about participation in 5 physical activities (e.g., walking for exercise, bicycling) during the past 2 weeks. Hours per week in any physicial activitiy was used in analyses (Wilson, Mendes de Leon, et al., 2002; Wilson, Bennett, et al., 2002). A previously established (Barnes, Mendes de Leon, Wilson, Bienias, & Evans, 2004) measure of social engagement was based on religious service attendance, participation in activities outside the home, and part-time or full-time employment. Social network size was the number of friends and relatives who had contact with the participant at least once per month, as previously described (Barnes et al., 2004).
Statistical Analysis
Descriptive analyses were performed using means and standard deviations for continuous measures and percentages for categorical measures. To cross-validate the findings, the entire cohort of participants was split into exploratory and confirmatory subgroups. Participants were assigned to either one of the two subgroups based on a Bernoulli random sampling scheme, where each participant had an equal probability of being selected into the exploratory or confirmatory subgroup. The selection process was performed under a sampling without replacement scheme. Based on this random selection process, 4,703 persons were assigned to the exploratory subgroup and 4,716 to the confirmatory subgroup. To assess the effectiveness of this randomization scheme, we used two-sample independent t-test and chi-square test statistics to compare the exploratory and confirmatory subgroups. For the candidate measure selection process, we used a stepwise search algorithm with candidate measures centered. The p-value threshold for either entering or staying in the model was set at 0.05. An indicator for Black race was forced into the primary model, and allowed to stay in the model whether or not it met the p-value threshold of 0.05. This approach allowed characterization of racial differences in cognitive function level and identification of measures that could potentially explain the racial differences. We examined the variance inflation factor in the final model and found collinearity among the model terms to be low. After the stepwise selection process, we examined the association of the selected measures in the confirmatory subgroup for purposes of cross-validation. We used a least absolute shrinkage and selection operator (lasso) to examine the robustness of our selection model (Tibshirani, 2006). We found that the two-way interactions of black race with age, education, neuroticism, and cognitive activity were also selected in the lasso technique giving us confidence in our findings. However, several of the main effects selected as part of our stepwise search algorithm, including the black main effect, were not selected in the lasso technique. All analyses were performed using SAS software (SAS Institute Inc., 2011).
Results
Subgroup Formation
We randomly divided the total study population into exploratory and confirmatory subgroups. The proportion of Black persons in the exploratory (64%) and confirmatory (62%) subgroups was similar (p = 0.12) and the two subgroups had similar levels of global cognitive function at baseline (exploratory mean = 0.257, SD = 0.714; confirmatory mean = 0.248, SD = 0.709; p = 0.54). As shown in Table 2, the subgroups were similar on a wide range of candidate demographic, health-related, and experiential measures.
Table 2.
Candidate Measures in Exploratory and Confirmatory Subgroups of Older Population-Based Sample
| Candidate variables | Exploratory subgroup | Confirmatory subgroup |
|---|---|---|
| Age (years) | 72.6 (6.5) | 72.7 (6.7) |
| Education (years) | 12.3 (3.5) | 12.4 (3.5) |
| Women, n, % | 2811, 60% | 2860, 61% |
| Southern U.S. Origin, n, % | 2164, 51% | 2068, 49% |
| Cancer, n, % | 865, 18% | 862, 18% |
| Stroke, n, % | 381, 8% | 422, 9% |
| Diabetes, n, % | 339, 7% | 339, 7% |
| Hypertension, n, % | 2450, 52% | 2391, 51% |
| Reading/cognitive activity | 3.2 (0.67) | 3.2 (0.67) |
| Physical activity | 3.1 (5.1) | 3.1 (5.0) |
| Physical function | 10.3 (3.7) | 10.3 (3.7) |
| ADL limitations | 0.22 (0.81) | 0.21 (0.82) |
| Rosow-Breslau limitations | 0.56 (0.92) | 0.57 (0.92) |
| Nagi limitations | 0.93 (1.3) | 0.94 (1.3) |
| Body mass index (kg/m2) | 27.9 (6.0) | 27.9 (6.0) |
| Systolic blood pressure | 137.8 (19.2) | 138.0 (19.5) |
| Diastolic blood pressure | 77.8 (11.1) | 77.9 (11.2) |
| Social network | 7.5 (6.3) | 7.5 (6.2) |
| Social engagement | 2.4 (1.7) | 2.4 (1.7) |
| Depressive symptoms | 1.5 (1.9) | 1.5 (1.9) |
| Neuroticism | 5.4 (2.3) | 5.4 (2.2) |
| Extraversion | 8.5 (2.1) | 8.5 (2.2) |
Note. Data are presented as M (SD) unless otherwise indicated.
Exploratory Subgroup
At baseline, scores on the composite measure of global cognition ranged from −3.012 to 1.730 and were approximately normally distributed (mean = 0.257, SD = 0.714, skewness = 1.11). To quantify racial differences in global cognition in the exploratory population, the initial model regressed the global cognitive score on an indicator for Black race. This initial step is shown in the first row of Table 3, where the global cognitive score was a mean of 0.403-unit lower in Black persons than White persons and race accounted for approximately 7% of the variability in global cognition. The candidate measures were selected using a forward stepwise search algorithm, with race forced as the primary variable and included in all subsequent iterations. The candidate measure or interaction with the strongest partial correlation with residual variation in the global cognitive score (and a p-value less than 0.05) entered the equation next. As shown by the second row of Table 3, in the presence of Black race, age was the second measure to enter the selection model. The addition of age to the model increased the total amount of cognitive variation explained to nearly 25%, and because Black participants were younger than White participants (Table 1), it increased the mean Black-White difference to 0.571-unit. The selection process continued to add covariates from a pool of candidate demographic, health-related, and experiential measures, as well as the 2-way interactions of race with each candidate measure; the order of entry for new terms was determined by the strength of their association with variability in global cognition not explained by previously entered covariates. As shown in Table 3, the final model included 16 measures in addition to race, of which 12 were main effects and 4 were terms for the interaction of race with a candidate measure. This race-centered model explained nearly 45% of the variation in global cognition in the exploratory subgroup.
Table 3.
Association of Candidate Measures with Level of Global Cognition in the Exploratory Population Subgroup and Estimated Black-White Difference in Global Cognition after Each Candidate Measure was Added to the Model
| Stepwise selection | Black-White difference | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Candidate variable | Coefficient | SE | p | Step | Partial R2 | Model R2 | Coefficient | SE | p |
| Black race | −0.403 | 0.021 | <0.001 | 0 | 0.0735 | 0.0735 | −0.403 | 0.021 | <0.001 |
| Age (per year) | −0.047 | 0.001 | <0.001 | 1 | 0.1759 | 0.2494 | −0.571 | 0.019 | <0.001 |
| Reading/cognitive activity | 0.360 | 0.014 | <0.001 | 2 | 0.0983 | 0.3477 | −0.406 | 0.019 | <0.001 |
| Race × education | 0.061 | 0.003 | <0.001 | 3 | 0.0438 | 0.3915 | −0.396 | 0.019 | <0.001 |
| Physical function | 0.032 | 0.002 | <0.001 | 4 | 0.0215 | 0.4129 | −0.358 | 0.018 | <0.001 |
| Body mass index | 0.011 | 0.001 | <0.001 | 5 | 0.0086 | 0.4215 | −0.367 | 0.018 | <0.001 |
| Education (per year) | 0.027 | 0.004 | <0.001 | 6 | 0.0053 | 0.4268 | −0.317 | 0.020 | <0.001 |
| Neuroticism | −0.022 | 0.004 | <0.001 | 7 | 0.0045 | 0.4313 | −0.318 | 0.020 | <0.001 |
| Male gender | −0.097 | 0.017 | <0.001 | 8 | 0.0041 | 0.4354 | −0.321 | 0.020 | <0.001 |
| ADL limitations | −0.062 | 0.011 | <0.001 | 9 | 0.0035 | 0.4389 | −0.328 | 0.020 | <0.001 |
| Depressive symptoms | 0.026 | 0.005 | <0.001 | 10 | 0.0036 | 0.4425 | −0.336 | 0.020 | <0.001 |
| Stroke | −0.098 | 0.029 | <0.001 | 11 | 0.0013 | 0.4438 | −0.336 | 0.020 | <0.001 |
| Race × age | −0.008 | 0.003 | <0.001 | 12 | 0.0013 | 0.4451 | −0.305 | 0.022 | <0.001 |
| Cancer | 0.049 | 0.020 | 0.015 | 13 | 0.0007 | 0.4458 | −0.301 | 0.022 | <0.001 |
| Social engagement | 0.012 | 0.005 | 0.028 | 14 | 0.0006 | 0.4464 | −0.304 | 0.022 | <0.001 |
| Race × read/cog. activity | −0.062 | 0.028 | 0.029 | 15 | 0.0006 | 0.4470 | −0.098 | 0.096 | 0.307 |
| Race × neuroticism | −0.015 | 0.007 | 0.038 | 16 | 0.0005 | 0.4475 | 0.012 | 0.110 | 0.912 |
Note. Estimated from a series of stepwise linear regression analyses.
As candidate measures were added to the model, the coefficient for the estimated main effect of Black race fluctuated and eventually became non-significant (right-hand columns of Table 3).
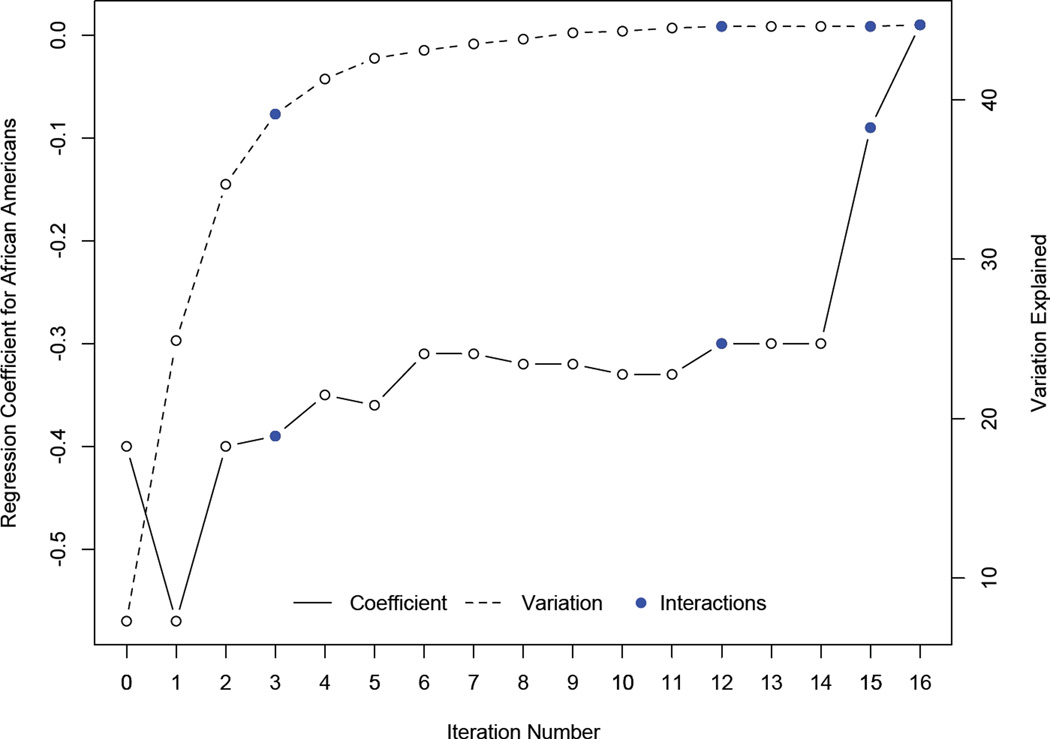
Figure 1, which is also based on these analyses, tracks the shift in the size of the coefficient for the Black-White cognitive difference (solid line in figure, seventh column in Table 3) as candidate measures and interactions entered the model and the amount of explained cognitive variability increased (dashed line in figure, sixth column in Table 3). The figure shows that the amount of explained cognitive variability increased relatively little after the fifth iteration. The factors contributing heavily to diminished Black-White cognitive differences were reading/cognitive activity (step 2; 0.165 reduction in coefficient) and its interaction with race (step 15; 0.206 reduction), the interaction of race and neuroticism (step 16; 0.110 reduction), the main effect of education (step 6; 0.050 reduction) and its interaction with race (step 3; 0.010 reduction), and the interaction of race with age (step 12; 0.031 reduction).
Figure 1.
Mean Black-White difference in global cognition (solid line in relation to left vertical axis) and percent of explained variation in global cognition (dashed line in relation to right vertical axis) after each iteration in a series of stepwise linear regression models in the exploratory population subgroup.
Use of tobacco (Ohara et al., 2015) and alcohol (Stampfer, Kang, Chen, Cherry, & Grodstein, 2005) has been associated with cognitive health in old age. However, adding these variables to the final model did not influence racial differences in cognition.
Because reading/cognitive activitiy and education are correlated but behaved differently with race, we repeated the final model with terms for the 2-way interaction of education and reading/cognitive activity and the 3-way interaction of race, education, and reading/cognitive activity. There was a significant 2-way interaction between education and reading/cognitive activity such that the association of either measure with cogntion was decreased at higher levels of the other measure. However, we did not find any modification of this 2-way interaction by race.
Confirmatory Subgroup
To assess the reproducibility of the candidate associations observed in the exploratory population subgroup, we constructed a model in the exploratory subgroup with terms for race and the 16 previously selected candidate measures and then repeated this model in the confirmatory population subgroup (Table 4). Results in the confirmatory subgroup were similar to those obtained in the exploratory subgroup, with racial differences in global cognition primarily attributed to the interaction of race with age, education, reading/cognitive activity, and neuroticism.
Table 4.
Association of Candidate Measures with Level of Global Cognition in the Exploratory and Confirmatory Population Subgroups
| Exploratory subgroup | Confirmatory subgroup | |||||
|---|---|---|---|---|---|---|
| Candidate measure | Coefficient | SE | p | Coefficient | SE | p |
| Black race | 0.012 | 0.109 | 0.91 | −0.036 | 0.090 | 0.62 |
| Age | −0.020 | 0.002 | <0.001 | −0.023 | 0.002 | <0.001 |
| Reading/cognitive activity | 0.228 | 0.023 | <0.001 | 0.178 | 0.023 | <0.001 |
| Race × education | 0.030 | 0.005 | <0.001 | 0.032 | 0.005 | <0.001 |
| Physical function | 0.029 | 0.002 | <0.001 | 0.027 | 0.002 | <0.001 |
| Body mass index | 0.010 | 0.001 | <0.001 | 0.011 | 0.001 | <0.001 |
| Education | 0.028 | 0.004 | <0.001 | 0.022 | 0.001 | <0.001 |
| Neuroticism | −0.021 | 0.006 | <0.001 | −0.030 | 0.003 | <0.001 |
| Male gender | −0.089 | 0.017 | <0.001 | −0.126 | 0.017 | <0.001 |
| ADL limitations | −0.058 | 0.011 | <0.001 | −0.053 | 0.011 | <0.001 |
| Depressive symptoms | 0.026 | 0.005 | <0.001 | 0.012 | 0.005 | 0.010 |
| Stroke | −0.100 | 0.029 | <0.001 | −0.061 | 0.027 | 0.030 |
| Race × age | −0.009 | 0.002 | <0.001 | −0.010 | 0.003 | 0.002 |
| Cancer | 0.048 | 0.020 | 0.002 | 0.069 | 0.020 | <0.001 |
| Social engagement | 0.012 | 0.005 | 0.021 | 0.011 | 0.005 | 0.035 |
| Race × read/cog. activity | −0.071 | 0.028 | 0.013 | −0.066 | 0.027 | 0.008 |
| Race × neuroticism | −0.016 | 0.007 | 0.037 | −0.014 | 0.007 | 0.042 |
Note. Estimated from linear regression models.
Discussion
In a biracial urban population of more than 9,000 older pesons, we considered factors that might explain cross-sectional Black-White differences in level of cognitive function. Adjustment for selected demographic, health-related, and experiential measures accounted for some of the racial differences in cognition but did not eliminate them. The racial gap was mostly explained by 4 candidate measures. In particular, racial differences were larger at higher levels of reading/cognitive activity and neuroticism. Black persons were also younger than White persons and had lower levels of education and reading/cognitive activity as well as a higher level of neuroticism. Racial differences due to other factors were minimal. Overall, the results suggest that racial differences in cognitive function in old age mainly reflect racial differences in life experiences and health prior to old age.
Prior research on racial disparities in late-life cognition has focused on factors related to education (e.g., years of schooling, quality of education) and literacy (e.g., language performance tests, self report) (Sisco et al., 2013; Carvalho et al., 2014). Consistent with that research, we found that adjusting for years of schooling and a self-report measure of frequency of reading and other cognitive activities substantially reduced the Black-White gap in cognitive function but did not eliminate it.
A novel finding was that a substantial proportion of the racial disparity in cognition was explained by an interaction between race and the neuroticism trait which indicates a tendency to react to stress with negative emotions. The association of higher neuroticism with lower level of cognition was stronger in Black persons than White persons. There are important differences in the life circumstances and social conditions of Black and White persons, and the increased stress associated with being a member of a historically marginalized minority group is well established (Mays, Cochran, & Barnes, 2007). The “weathering” hypothesis about racial disparities in health refers to the cumulative adverse health impact that older Black persons experience due to persistent social stressors including material hardship, structural and interpersonal discrimination, and multiple caregiving demands (Geronimus, Hicken, Keene, & Bound, 2006). Black persons who are especially reactive to stress, as indicated by a high level of the neuroticism trait, coupled with cumulative exposure to and high-effort coping with stressors, would be at a particular disadvantage, consistent with the observed interaction of race with neuroticism in the present analyses.
We also found an interaction between race and self reported frequency of reading and related cognitive activities such that the association of higher levels of reading/cognitive activity with higher cognitive function was weaker in Black persons than White persons. The theory of cumulative disadvantage describes socially stratified changes over time in access to resources and exposure to risks (Ferraro, Shippee, & Shafer, 2009). If disadvantage is cumulative, Black persons are likely to be especially disadvantaged in old age. As a result, they would be expected to have less access to resources (e.g., cognitively stimulating activities) and eventually to derive less benefit from exposure to resources. Our finding of a weaker link between reading/cognitive activity and cognitive function in Black persons compared to White persons is consistent with this hypothesis though the mechanisms linking disadvantage with reading/cognitive activity are uncertain.
Although education and reading/cognitive activity are related, their associations with cognitive function were differentially modified by race. Thus, education had a stronger association with cognition in Black persons relative to White persons whereas reading/cognitive activity had a weaker association with cognition in Black persons relative to White persons. The finding for education is consistent with a number of prior studies (Cagney & Lauderdale, 2002; Luo & Waite, 2005). The basis of this dissociation is not known, but it may be related to the fact that formal education occurs in early life when the cumulative level of disadvantage is hypothesized to be lower than in late life when reading/cognitive activity was assessed. From this perspective, the factors believed to enhance cognitive reserve, such as education (Stern et al., 1994) and reading/cognitive activity (Wilson et al., 2013), are likely to be similar in Black and White persons but their impact on reserve depends on when during the life span the experiences occur and the extent to which the cumulative burden of stressful life experiences limit exposure to potentially healthy activities or the ability to derive benefit from such exposures. Another consideration is that healthy cognitive development depends not only on years of formal schooling but also on the complex interaction of family characteristics, school environment, and early life socioeconomic status. Given that selection pressures for Black persons to achieve educational gains were often greater than for White persons, due to the effects of institutional and individual racial discrimination, it is possible that formal schooling might play a relatively stronger role in the cognitive development of Blacks than Whites.
There was an interaction between race and age such that age had a stronger (negative) correlation with cognitive function in Black persons than White persons. Late-life level of cognition reflects early life cognitive level minus late-life cognitive decline. Because there appears to be no (Atkinson et al., 2005; Masel & Deek, 2009; Castora-Binkley et al., 2013; Mariske et al., 2013) or minimal (Lyketsos et al., 1999; Sloan & Wang, 2005; Sachs-Ericsson & Blazer, 2005; Alley et al., 2007; Sawyer et al., 2009; Karlamangala et al., 2009; Wolinsky et al., 2011; Early et al., 2013; Wilson et al., 2015) racial difference in late-life rate of cognitive decline, the observed interaction of race and age suggests a cohort effect. That is, there was a stronger tendency among Black persons than White persons for those born earlier to be less cognitively healthy prior to old age than individuals born more recently. This interpretation is consistent with other evidence suggesting that the Black-White gap in cognitive health may be narrowing (Dickens & Flynn, 2006; Murray, 2006).
This study has three notable strengths. It was conducted in a large geographically defined biracial population, and we measured a wide range of demographic, health, and experiential factors that have been shown in previous research to be associated with cognition. Also, the population was randomly divided in half and analytic results in one half were replicated in the second half, suggesting that the results are reliable.
Several study limitations should be noted. Because much of the racial disparity in cognitive function appears to emerge in early life (Peoples & Kagan; 1995; Burchinal et al., 2011), the factors associated with this disparity in late life may differ from the factors associated with the disparity in early life. In addition, the correlational results do not allow us to conclude that these candidate measures caused the observed racial disparities in cognitive function. It is also possible that a different set of candidate measures, including measures of mental health, or different criteria for selecting candidate measures might lead to a different set of measures accounting for racial differences in cognition. However, we used candidate measures previously shown to be associated with cognition and sensitivity to selection criteria was examined using both forward stepwise and stepwise search algorithms. Our findings for two-way interactions of black race with education, age, neuroticism, and reading/cognitive activity were also confirmed using lasso regression. Because dementia was only ascertained in a subset of the population (Bienias et al., 2003), we could not determine the extent to which persons with dementia affected results. The tests used in this study may measure cognition with less error in White persons than Black persons, but it seems unlikely that test bias is responsible for a substantial proportion of the observed Black-White cognitive score difference given the success of candidate measures in accounting for the difference in both population subgroups. Finally, analyses are based on a composite measure of global cognition and it is possible results would differ with measures of specific cognitive domains.
Acknowledgments
This research was supported by National Institute on Aging grants R01AG11101, R01AG22018, and P30AG10161. The funding organization had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
The authors thank the residents of Morgan Park, Washington Heights, and Beverly who participated in the Chicago Health and Aging Project; Ms. Ann Marie Lane for community development and oversight of project coordination; Ms. Michelle Bos, Ms. Holly Hadden, Mr. Flavio LaMorticella, and Ms. Jennifer Tarpey for study coordination; and the staff of the Rush Institute for Healthy Aging.
Contributor Information
Robert S. Wilson, Rush Alzheimer’s Disease Center, Department of Neurological Sciences, Department of Behavioral Sciences, Rush University Medical Center, Chicago, IL.
Kumar B. Rajan, Rush Institute for Healthy Aging, Department of Internal Medicine, Rush University Medical Center, Chicago, IL.
Lisa L. Barnes, Rush Alzheimer’s Disease Center, Department of Neurological Sciences, Department of Behavioral Sciences, Rush University Medical Center, Chicago, IL.
Jennifer Weuve, Rush Institute for Healthy Aging, Department of Internal Medicine, Rush University Medical Center, Chicago, IL.
Denis A. Evans, Rush Institute for Healthy Aging, Department of Internal Medicine, Rush University Medical Center, Chicago, IL
REFERENCES
- Albert M, Scherr P, Taylor J, Evans DA, Funkenstein H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. International Journal of Neuroscience. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- Alley D, Suthers K, Crimmins E. Education and cognitive decline in older Americans: results from the AHEAD sample. Research on Aging. 2007;29:73–94. doi: 10.1177/0164027506294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Bennett DA, Wilson RS, Barnes LL. Diabetes and cognitive systems in older black and white persons. Alzheimer Disease and Associated Disorders. 2010;24:37–42. doi: 10.1097/WAD.0b013e3181a6bed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson HH, Cesari M, Kritchevsky SB, Penninx BW, Fried LP, Guralnik JM, Williamson JD. Predictors of combined cognitive and physical decline. Journal of the American Geriatrics Society. 2005;53:1197–1202. doi: 10.1111/j.1532-5415.2005.53362.x. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63:2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project. Journal of Alzheimer’s Disease. 2003;5:349–355. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- Burchinal M, McCartney K, Steinberg L, Crosnoe R, Friedman SL, McLoyd V, Pianta R the NICHD Early Child Care Research Network. Examining the Black-White achievement gap among low-income children using the NICHD study of early child care and youth development. Child Development. 2011;82:1404–1420. doi: 10.1111/j.1467-8624.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- Cagney KA, Lauderdale DS. Education, wealth, and cognitive function in later life. Journals of Gerontology, Series B. Psychological Sciences and Social Sciences. 2002;57:P163–P172. doi: 10.1093/geronb/57.2.p163. [DOI] [PubMed] [Google Scholar]
- Carvalho JO, Tommet D, Crane PK, Thomas ML, Claxton A, Habeck C, Manly JJ, Romero HR. Deconstructing racial differences: the effects of quality of education and cerebrovascular factors. Journals of Gerontology, Series B. Psychological Sciences and Social Sciences. 2015;70:545–556. doi: 10.1093/geronb/gbu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castora-Binkley M, Peronto CL, Edwards JD, Small BJ. A longitudinal analysis of the influence of race on cognitive performance. Journals of Gerontology, Series B. Psychological Sciences and Social Sciences. 2015;70:512–518. doi: 10.1093/geronb/gbt112. [DOI] [PubMed] [Google Scholar]
- Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Disease and Associated Disorders. 2011;25:187–195. doi: 10.1097/WAD.0b013e318211c6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Neo Personality Inventory-Revised. Lutz, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Dickens WT, Flynn JR. Black Americans reduce the racial IQ gap: evidence form standardization samples. Psychological Science. 2006;17:913–920. doi: 10.1111/j.1467-9280.2006.01802.x. [DOI] [PubMed] [Google Scholar]
- Early DR, Widaman KF, Harvey D, Beckett L, Park LQ, Farias ST, Reed BR, DeCarli C, Mungas D. Demographic predictors of cognitive decline in ethnically diverse older persons. Psychology and Aging. 2013;28:633–645. doi: 10.1037/a0031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro KF, Shippee TP, Shafer MH. Cumulative inequality theory for research on aging and the life course. In: Benstom VL, Gans D, Putney NM, Silverstein M, editors. Handbook of theories of aging. 2nd. New York, NY: Springer; 2009. pp. 413–435. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Seeman TE, Tinetti ME, Nevitt MC, Berkman LF. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful aging. Aging (Milano) 1994;6:410–419. doi: 10.1007/BF03324272. [DOI] [PubMed] [Google Scholar]
- Hypertension Detection and Follow-up Program Group. Race, education, and prevalence of hypertension. American Journal of Epidemiology. 1977;106:351–361. [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. American Journal of Epidemiology. 2009;170:331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S, Akpom CA. A measure of primary sociobiological functions. International Journal of Health Services. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. Journal of Aging and Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Luo Y, Waite L. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. Journals of Gerontology, Series B. Psychological Sciences and Social Sciences. 2005;60:S93–S101. doi: 10.1093/geronb/60.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Chen LS, Anthony JC. Cognitive decline in adulthood: an 11.5-year follow-up of the Baltimore Epidemiologic Catchment Area Study. American Journal of Psychiatry. 1999;156:58–65. doi: 10.1176/ajp.156.1.58. [DOI] [PubMed] [Google Scholar]
- Marsiske M, Dzierzewski JM, Thomas KR, Kasten L, Jones RN, Johnson KE, Willis SL, Whitfield KE, Ball KK, Rebok GW. Race-related disparities in 5-year cognitive level and change in untrained Active participants. Journal of Aging and Health. 2013;85:1035–1275. doi: 10.1177/0898264313497794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masel MC, Peek MK. Ethnic differences in cognitive function over time. Annals of Epidemiology. 2009;19:778–783. doi: 10.1016/j.annepidem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annual Review of Psychology. 2007;58:201–225. doi: 10.1146/annurev.psych.57.102904.190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. Changes over time in the black-white difference on mental tests: evidence from the children of the 1979 cohort of the National Longitudinal Survey of Youth. Intelligence. 2006;34:527–540. [Google Scholar]
- Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Memory Fund Quarterly -Health & Society. 1976;54:439–467. [PubMed] [Google Scholar]
- Ohara T, Ninomiya T, Hata J, Ozawa M, Yoshida D, Mukai N, Kiyohara Y. Midlife and late-life smoking and risk of dementia in the community: the Hisayama study. Journal of the American Geriatrics Society. 2015;63:2332–2339. doi: 10.1111/jgs.13794. [DOI] [PubMed] [Google Scholar]
- Peoples CE, Fagan JF, Drotar D. The influence of race on 3-year old children’s performance on the Stanford-Binet: Fourth Edition. Intelligence. 1995;21:69–82. [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rajan KB, Wilson RS, Weuve J, Barnes LL, Evans DA. Cognitive impairment 18 years before clinical diagnosis of Alzheimer’s disease dementia. Neurology. 2015;85:898–904. doi: 10.1212/WNL.0000000000001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosow I, Breslau N. A Gutman health scale for the aged. Journal of Gerontology. 1966;21:556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- Sachs-Ericsson N, Blazer DG. Racial differences in cognitive decline in a sample of community dwelling older adults. American Journal of Geriatric Psychiatry. 2005;13:968–975. doi: 10.1176/appi.ajgp.13.11.968. [DOI] [PubMed] [Google Scholar]
- Sawyer K, Sachs-Ericsson N, Preacher KJ, Blazer DG. Racial differences in the influence of the APOE epsilon 4 allele on cognitive decline in a sample of community dwelling older adults. Gerontology. 2008;55:32–40. doi: 10.1159/000137666. [DOI] [PubMed] [Google Scholar]
- Sisco S, Gross AL, Shih RA, Sachs BC, Glymour MM, Bangen KJ, Benitez A, Skinner J, Schneider BC, Manly JJ. The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. Journals of Gerontology, Series B. Psychological Sciences and Social Sciences. 2015;70:557–567. doi: 10.1093/geronb/gbt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan FA, Wang J. Disparities among older adults in measures of cognitive function by race or ethnicity. Journals of Gerontology, Series B. Psychological Sciences and Social Sciences. 2005;60:242–250. doi: 10.1093/geronb/60.5.p242. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test manual-revised. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. New England Journal of Medicine. 2005;352:245–253. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. Journal of the American Medical Association. 1994;271:1004–1010. [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. Journal of the Royal Statistical Society, Series B (Methodological) 1996;58:267–288. [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Mendes de Leon CF, Evans DA. Cognition and survival in a biracial urban population of old people. Intelligence. 2009;37:545–550. [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Beckett LA, Morris MC, Gilley DW, Bienias JL, Scherr PA, Evans DA. Cognitive activity in older persons from a geographically defined population. Journals of Gerontology, Series B. Psychological Sciences and Social Sciences. 1999;54:155–160. doi: 10.1093/geronb/54b.3.p155. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, Mendes de Leon CF, Morris MC, Schneider JA, Evans DA. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Mendes de Leon CF, Bienias JL, Morris MC, Evans DA. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology. 2005;30:11–17. doi: 10.1016/j.psyneuen.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Levine SR, Yu L, Hoganson GM, Buchman AS, Schnieder JA, Bennett DA. Harm avoidance and cerebral infaraction. Neuropsychology. 2014;28:305–311. doi: 10.1037/neu0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81:314–321. doi: 10.1212/WNL.0b013e31829c5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Capuano AW, Sytsma J, Bennett DA, Barnes LL. Cognitive aging in older Black and White persons. Psychology and Aging. 2015;30:279–285. doi: 10.1037/pag0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, Dong X, Leurgans SE, Evans DA. Cognitive decline after hospitalization in a community population of older persons. Neurology. 2012;78:950–956. doi: 10.1212/WNL.0b013e31824d5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Gu L, Bienias JL, Mendes de Leon CF, Evans DA. Neuroticism, extraversion, and mortality in a defined population of older persons. Psychosomatic Medicine. 2005;67:841–845. doi: 10.1097/01.psy.0000190615.20656.83. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer's disease. Journal of the American Medical Association. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Rajan KB, Barnes LL, Hebert LE, Mendes de Leon CF, Evans DA. Cognitive aging and rate of hospitalization in an urban population of older people. Journals of Gerontology, Series A. Biological Sciences and Medical Sciences. 2014;69:447–454. doi: 10.1093/gerona/glt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Bentler SE, Hockenberry J, Jones MP, Weigel PA, Kaskie B, Wallace RB. A prospective cohort study of long-term cognitive changes in older Medicare beneficiaries. BMC Public Health. 2011;11:710. doi: 10.1186/1471-2458-11-710. [DOI] [PMC free article] [PubMed] [Google Scholar]