Abstract
BACKGROUND
Isolated systolic hypertension (ISH), defined as systolic blood pressure (SBP) ≥140 mm Hg and diastolic blood pressure (DBP) <90 mm Hg, in younger and middle-aged adults is increasing in prevalence.
OBJECTIVE
The aim of this study was to assess the risk for cardiovascular disease (CVD) with ISH in younger and middle-aged adults.
METHODS
CVD risks were explored in 15,868 men and 11,213 women 18 to 49 years of age (mean age 34 years) at baseline, 85% non-Hispanic white, free of coronary heart disease (CHD) and antihypertensive therapy, from the Chicago Heart Association Detection Project in Industry study. Participant classifications were as follows: 1) optimal-normal blood pressure (BP) (SBP <130 mm Hg and DBP <85 mm Hg); 2) high-normal BP (130 to 139/85 to 89 mm Hg); 3) ISH; 4) isolated diastolic hypertension (SBP <140 mm Hg and DBP ≥90 mm Hg); and 5) systolic diastolic hypertension (SBP ≥140 mm Hg and DBP ≥90 mm Hg).
RESULTS
During a 31-year average follow-up period (842,600 person-years), there were 1,728 deaths from CVD, 1,168 from CHD, and 223 from stroke. Cox proportional hazards models were adjusted for age, race, education, body mass index, current smoking, total cholesterol, and diabetes. In men, with optimal-normal BP as the reference stratum, hazard ratios for CVD and CHD mortality risk for those with ISH were 1.23 (95% confidence interval [CI]: 1.03 to 1.46) and 1.28 (95% CI: 1.04 to 1.58), respectively. ISH risks were similar to those with high-normal BP and less than those associated with isolated diastolic hypertension and systolic diastolic hypertension. In women with ISH, hazard ratios for CVD and CHD mortality risk were 1.55 (95% CI: 1.18 to 2.05) and 2.12 (95% CI: 1.49 to 3.01), respectively. ISH risks were higher than in those with high-normal BP or isolated diastolic hypertension and less than those associated with systolic diastolic hypertension.
CONCLUSIONS
Over long-term follow-up, younger and middle-aged adults with ISH had higher relative risk for CVD and CHD mortality than those with optimal-normal BP.
Keywords: cardiovascular risk, long-term follow-up, younger adults
Isolated systolic hypertension (ISH), defined as systolic blood pressure (SBP) ≥140 mm Hg and diastolic blood pressure (DBP) <90 mm Hg, is highly prevalent in older adults but less so in younger and middle-aged adults (1–4). Data from the National Health and Nutrition Examination Survey indicate that among younger and middle-aged adults (<40 years of age) in the United States, the overall prevalence of ISH between 1988 and 1994 of 0.7% more than doubled between 1999 and 2004 to 1.6% (2). From 1988 to 1994, of patients with untreated hypertension <50 years of age in the National Health and Nutrition Examination Survey, 20% to 30% had ISH (1), which increased to 40% from 1999 to 2004 (2,4).
The clinical consequences of ISH in younger and middle-aged adults remain uncertain (5). Whether ISH in younger adults is “pseudo” or “spurious” hypertension is still being debated (5–7). Data from a nested case-control study (insurance actuarial data) and from United States and Swedish nationwide cohort investigations indicate that higher SBP and/or DBP is associated with higher risk for CVD mortality in younger adults (8–15). None of these studies, however, examined the risk by hypertension subtype: ISH, isolated diastolic hypertension (IDH), and systolic diastolic hypertension (SDH). Because the Chicago Heart Association Detection Project in Industry (CHA) study enrolled a large number of younger and middle-aged adults and their prospective follow-up encompasses more than 30 years (16,17), it provides a unique opportunity to investigate these issues.
Using the CHA study data, we assessed whether ISH in younger and middle-aged adults (18 to 49 years of age) is associated with higher risk for cardiovascular disease (CVD) mortality compared with normal blood pressure (BP).
METHODS
STUDY SAMPLE
Between 1967 and 1973, the CHA study recruited 39,441 participants from Chicago-area companies and organizations. Details of the study design and methods have been described (16–18). Trained staff members obtained a single casual supine BP measurement using a standard mercury sphygmomanometer. DBP was recorded as Korotkoff phase V. Heart rate was recorded by electrocardiography. Nonfasting serum total cholesterol levels were measured using the Levine-Zak method. Diabetes was defined as clinically diagnosed by a personal physician or the use of antihyperglycemic medication (18,19). Questionnaires were used to collect information on demographics, smoking, medical history, and medication use. All participants gave written informed consent. The study protocol has received periodic institutional review board approval and, as described by the Health Insurance Portability and Accountability Act, the institutional review board granted a waiver before commencement of the present project.
For this study, we identified participants originally between 18 and 49 years of age with ascertained vital status during follow-up (n = 28,238). We excluded those who had pre-existing coronary heart disease (CHD), defined as electrocardiographic evidence of myocardial infarction (n = 20); those on antihypertensive drugs at baseline (n = 599); and 538 additional participants with missing or incomplete baseline BP data and/or covariates. As a result, 27,081 participants were eligible for inclusion.
OUTCOMES ASCERTAINMENT
Vital status was ascertained through 2003, with an average follow-up period of 31.1 ± 5.5 years (842,600 person-years). As previously reported (16,17), before 1979, follow-up was pursued by direct mail, telephone, contact with employer, and matching records with Social Security Administration files. From 1979 on, the National Death Index was used to identify deaths. Death certificates were obtained and coded by trained research staff members for multiple causes according to the International Classification of Diseases-Eighth Revision (ICD-8), and the International Classification of Diseases-Ninth Revision (ICD-9). Mortality from CVD was defined as ICD-8 and ICD-9 codes 400 to 445, CHD mortality was defined as ICD-8 and ICD-9 codes 410 to 414, and stroke mortality was defined as ICD-8 and ICD-9 codes 430 to 438.
BP CLASSIFICATION
Participants were stratified into 5 mutually exclusive BP categories: 1) optimal-normal BP (SBP <130 mm Hg and DBP <85 mm Hg); 2) high-normal BP (SBP 130 to 139 mm Hg and DBP 85 to 89 mm Hg, SBP 130 to 139 mm Hg and DBP <85 mm Hg, or SBP <130 mm Hg and DBP 85 to 89 mm Hg); 3) ISH (SBP ≥140 mm Hg and DBP <90 mm Hg); 4) IDH (SBP <140 mm Hg and DBP ≥90 mm Hg); and 5) SDH (SBP ≥140 mm Hg and DBP ≥90 mm Hg) (20). In a prior CHA report on the association of BP categories (i.e., optimal BP, normal BP, high-normal BP, and stages 1 to 3 hypertension) with 25-year CVD mortality in young adults (16), there was no difference in CVD mortality risk between optimal BP and normal BP. Hence, we combined optimal BP and normal BP into a single BP category, which we defined as the reference group.
STATISTICAL ANALYSES
All statistical analyses were performed with SPSS version 18.0J software (SPSS, Chicago, Illinois). The demographic and clinical characteristics of the participants by sex and hypertension subtype were compared by analysis of variance and by chi-square tests. We used sex-specific Cox proportional hazards models to examine the associations between hypertension subtype and risk for mortality from CVD, CHD, and stroke. Unadjusted and multivariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated for each hypertension subtype. Our analyses were performed with sequential adjustment. In the first step, we carried out unadjusted analyses (model 1). In model 2, we added age, race/ethnicity (non-Hispanic white, African American, Hispanic, Asian, and others), and educational attainment (years) as adjustment covariates. In model 3, we further adjusted for body mass index (BMI), smoking, serum total cholesterol, and diabetes. Statistical significance was defined by a p value <0.05 by 2-sided t test.
RESULTS
DESCRIPTIVE STATISTICS
Of the 27,081 participants between 18 and 49 years of age, 59% were men, 85% were non-Hispanic white, the mean age was 33.6 ± 9.1 years, and 39% had hypertension at baseline. Demographic and clinical characteristics of the included participants by sex are shown in Tables 1 and 2. The percents of participants with optimal-normal BP, high-normal BP, ISH, IDH, and SDH were 26.9%, 24.3%, 25.3%, 3.7%, and 19.8% in men and 52.9%, 21.6%, 12.9%, 2.9%, and 9.7% in women. In both men and women, those with ISH were less educated and had a higher proportion of current smoking, higher mean BMI, higher mean heart rate, and higher mean total cholesterol level than those with optimal-normal BP.
TABLE 1.
Participant Characteristics According to Hypertension Subtype in Men (n = 15,868)
| Total | Optimal-Normal BP (n = 4,261) | High-Normal BP (n = 3,854) | ISH (n = 4,015) | IDH (n = 589) | SDH (n = 3,149) | p Value | |
|---|---|---|---|---|---|---|---|
| Age, yrs | 34.3 ± 8.4 | 33.4 ± 8.1 | 33.6 ± 8.3 | 33.1 ± 8.4 | 37.4 ± 7.7 | 37.4 ± 8.1 | <0.001 |
|
| |||||||
| Ethnicity | <0.001 | ||||||
| Non-Hispanic whites | 88.6 | 87.5 | 88.7 | 90.2 | 86.9 | 88.2 | |
| African American | 7.4 | 7.4 | 7.3 | 6.2 | 8.5 | 8.7 | |
| Hispanic | 2.5 | 2.8 | 2.5 | 2.2 | 2.9 | 2.2 | |
| Asian | 0.4 | 0.6 | 0.3 | 0.3 | 0.8 | 0.2 | |
| Other | 1.1 | 1.5 | 1.1 | 1.1 | 0.8 | 0.7 | |
|
| |||||||
| Body mass index, kg/m2 | 26.3 ± 3.6 | 25.0 ± 3.1 | 25.8 ± 3.2 | 26.5 ± 3.5 | 27.1 ± 3.6 | 28.2 ± 4.1 | <0.001 |
|
| |||||||
| Current smoker | 46.4 | 44.5 | 47.2 | 48.2 | 43.1 | 46.5 | 0.004 |
|
| |||||||
| Education, yrs | 13.7 ± 2.7 | 14.1 ± 2.7 | 13.7 ± 2.6 | 13.4 ± 2.6 | 13.3 ± 2.6 | 13.2 ± 2.7 | <0.001 |
|
| |||||||
| Diabetes | 1.5 | 1.6 | 1.1 | 1.4 | 3.1 | 1.7 | 0.003 |
|
| |||||||
| Serum total cholesterol, mg/dl | 196.3 ± 37.8 | 190.2 ± 36.1 | 193.2 ± 36.6 | 195.7 ± 37.4 | 202.6 ± 36.7 | 208.1 ± 39.3 | <0.001 |
|
| |||||||
| BP, mm Hg | |||||||
| SBP | 135.5 ± 16.1 | 117.5 ± 6.0 | 130.5 ± 3.0 | 145.0 ± 7.6 | 129.7 ± 4.8 | 154.6 ± 14.6 | <0.001 |
| DBP | 79.5 ± 10.8 | 71.3 ± 7.1 | 76.2 ± 6.9 | 77.8 ± 6.4 | 90.8 ± 2.2 | 94.6 ± 7.2 | <0.001 |
|
| |||||||
| Heart rate, beats/min* | 75.8 ± 12.2 | 71.5 ± 10.7 | 75.0 ± 11.4 | 77.9 ± 11.9 | 76.0 ± 13.4 | 80.1 ± 13.0 | <0.001 |
Values are mean ± SD or %. The p values were obtained by analysis of variance or chi-square tests among hypertension subtypes.
Heart rate was measured by electrocardiography in 13,670 participants.
BP = blood pressure; DBP = diastolic blood pressure; IDH = isolated diastolic hypertension; ISH = isolated systolic hypertension; SBP = systolic blood pressure; SDH = systolic and diastolic hypertension.
TABLE 2.
Participant Characteristics According to Hypertension Subtype in Women (n = 11,213)
| Total | Optimal-Normal BP (n = 5,935) | High-Normal BP (n = 2,419) | ISH (n = 1,446) | IDH (n = 328) | SDH (n = 1,085) | p Value | |
|---|---|---|---|---|---|---|---|
| Age, yrs | 32.6 ± 10.0 | 30.8 ± 9.5 | 32.6 ± 9.9 | 35.0 ± 10.4 | 34.8 ± 9.5 | 39.0 ± 8.9 | <0.001 |
|
| |||||||
| Ethnicity | <0.001 | ||||||
| Non-Hispanic whites | 78.6 | 76.7 | 80.4 | 84.7 | 73.5 | 78.2 | |
| African American | 18.1 | 19.2 | 17.5 | 12.9 | 21.6 | 19.1 | |
| Hispanic | 2.2 | 2.6 | 1.6 | 1.4 | 3.0 | 2.0 | |
| Asian | 0.6 | 0.8 | 0.1 | 0.8 | 0.9 | 0.5 | |
| Other | 0.5 | 0.7 | 0.5 | 0.2 | 0.9 | 0.2 | |
|
| |||||||
| Body mass index, kg/m2 | 23.4 ± 4.2 | 22.3 ± 3.4 | 23.6 ± 3.9 | 24.6 ± 4.5 | 24.5 ± 4.6 | 26.6 ± 5.8 | <0.001 |
|
| |||||||
| Current smoker | 43.1 | 43.4 | 43.0 | 44.1 | 43.3 | 39.9 | 0.25 |
|
| |||||||
| Education, yrs | 12.7 ± 2.1 | 12.9 ± 2.1 | 12.7 ± 2.1 | 12.4 ± 2.0 | 12.3 ± 1.9 | 12.2 ± 2.0 | <0.001 |
|
| |||||||
| Diabetes | 1.2 | 0.9 | 0.9 | 1.8 | 2.1 | 2.1 | <0.001 |
|
| |||||||
| Serum total cholesterol, mg/dl | 189.4 ± 36.8 | 185.1 ± 35.8 | 189.1 ± 36.3 | 194.5 ± 37.0 | 197.8 ± 37.5 | 204.5 ± 37.6 | <0.001 |
|
| |||||||
| BP, mm Hg | |||||||
| SBP | 126.1 ± 15.7 | 114.8 ± 7.1 | 130.2 ± 3.1 | 144.0 ± 7.1 | 128.4 ± 5.3 | 153.9 ± 14.8 | <0.001 |
| DBP | 74.6 ± 10.9 | 68.9 ± 7.9 | 75.6 ± 7.2 | 77.8 ± 6.3 | 90.4 ± 1.7 | 94.8 ± 7.0 | <0.001 |
|
| |||||||
| Heart rate, beats/min* | 78.9 ± 12.0 | 76.6 ± 11.2 | 80.3 ± 11.7 | 82.7 ± 12.9 | 80.5 ± 11.6 | 83.3 ± 13.4 | <0.001 |
Values are mean ± SD or %. The p values were obtained by analysis of variance or chi-square tests among hypertension subtype.
Heart rate was measured by electrocardiography in 10,040 participants.
Abbreviations as in Table 1.
BASELINE BP STATUS AND LONG-TERM CVD MORTALITY
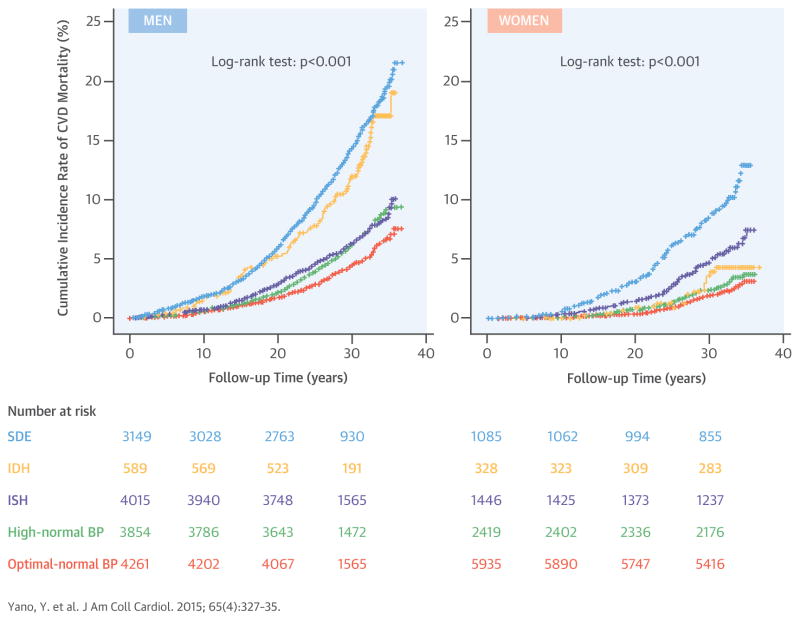
In men, during an average follow-up period of 30.8 years (489,393 person-years), deaths numbered 1,318 (269 per 100,000 person-years) from CVD, 925 (189 per 100,000 person-years) from CHD, and 145 (30 per 100,000 person-years) from stroke. In women, during an average follow-up period of 31.5 years (353,206 person-years), deaths numbered 410 (116 per 100,000 person-years) from CVD, 243 (69 per 100,000 person-years) from CHD, and 78 (22 per 100,000 person-years) from stroke. The sex-specific Kaplan-Meier cumulative incidence of CVD mortality, stratified by hypertension subtype, is shown in Central Illustration. In both sexes, cumulative CVD mortality was lowest for those with optimal-normal BP. In men, the cumulative incidence rate of CVD mortality in those with ISH was higher compared with those with optimal-normal BP and was lower than in those with IDH or SDH. In women, the cumulative incidence rate of CVD mortality in those with ISH was higher compared with those with optimal-normal BP or IDH and was lower than in those with SDH.
CENTRAL ILLUSTRATION. Hypertension Subtype and Cardiovascular Mortality: Kaplan-Meier Curves of the Cumulative Incidence of CVD Mortality by Sex.
Sex-specific cumulative incidence rate of cardiovascular disease (CVD) mortality for each hypertension subtype is shown. The definition of each color line is as follows: periwinkle, systolic diastolic hypertension (systolic blood pressure [SBP] ≥140 mm Hg and diastolic blood pressure [DBP] ≥90 mm Hg); gold, isolated diastolic hypertension (SBP <140 mm Hg and DBP ≥90 mm Hg); violet, isolated systolic hypertension (SBP ≥140 mm Hg and DBP <90 mm Hg); green, high-normal blood pressure (BP) (SBP 130 to 139 mm Hg and DBP 85 to 89 mm Hg, SBP 130 to 139 mm Hg and DBP <85 mm Hg, or SBP <130 mm Hg and DBP 85 to 89 mm Hg); salmon, optimal-normal BP (SBP <130 mm Hg and DBP <85 mm Hg). The log-rank was used to calculate p values. IDH = isolated diastolic hypertension; ISH = isolated systolic hypertension; SDH = systolic diastolic hypertension.
Results from Cox proportional hazards models suggest that among men, ISH was associated with a higher risk for CVD and CHD mortality compared with optimal-normal BP (model 1, Table 3). Adjustment for demographic variables attenuated the associations (model 2), but ISH remained significantly associated with CVD and CHD mortality with adjustment also for clinical characteristics including BMI, smoking, total serum cholesterol, and diabetes (model 3). The adjusted relative risk for CVD mortality was highest for SDH (HR: 1.77; 95% CI: 1.49 to 2.09), followed by IDH (HR: 1.68; 95% CI: 1.29 to 2.17), high-normal BP (HR: 1.25; 95% CI: 1.05 to 1.50), and ISH (HR: 1.23; 95% CI: 1.03 to 1.46). The adjusted HR for CVD mortality associated with ISH in men under 40 years of age (n = 2,938) was 1.27 (95% CI: 0.97 to 1.67; p = 0.09), and the HR in men 40 years of age or older (n = 1,077) was 1.18 (95% CI: 0.93 to 1.50; p = 0.17); no significant interactions between age (<40 or ≥40 years) and ISH in association with CVD mortality risk were found (p = 0.41).
TABLE 3.
Sex-Specific Unadjusted and Multivariate-Adjusted HRs (95% CIs) for Risk for CVD Mortality, CHD Mortality, and Stroke Mortality by Hypertension Subtype
| Men (n = 15,868)
|
Women (n = 11,213)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Optimal-Normal BP |
High-Normal BP |
ISH | IDH | SDH | Optimal-Normal BP |
High-Normal BP |
ISH | IDH | SDH | |
| CVD mortality | ||||||||||
| Person-yrs of follow-up | 134,247 | 120,694 | 124,588 | 17,741 | 92,124 | 188,288 | 76,827 | 45,054 | 10,198 | 32,839 |
| Number of events (incidence per 100,000 person-yrs) | 221 (165) | 276 (229) | 282 (226) | 79 (445) | 460 (499) | 139 (74) | 73 (95) | 82 (182) | 13 (127) | 103 (314) |
| Relative risk: HR (95% CI) | ||||||||||
| Model 1 (unadjusted) | 1 (reference) | 1.39 (1.16–1.66)‡ | 1.39 (1.16–1.65)‡ | 2.75 (2.13–3.56)‡ | 3.16 (2.69–3.71)‡ | 1.00 (reference) | 1.28 (0.96–1.70) | 2.49 (1.90–3.27)‡ | 1.73 (0.98–3.06) | 4.37 (3.39–5.64)‡ |
| Model 2 | 1 (reference) | 1.31 (1.10–1.57)† | 1.36 (1.14–1.63)† | 1.93 (1.49–2.49)‡ | 2.16 (1.83–2.54)‡ | 1.00 (reference) | 1.04 (0.78–1.38) | 1.66 (1.26–2.19)‡ | 1.21 (0.68–2.13) | 2.13 (1.64–2.77)‡ |
| Model 3 | 1 (reference) | 1.25 (1.05–1.50)* | 1.23 (1.03–1.46)* | 1.68 (1.29–2.17)‡ | 1.77 (1.49–2.09)‡ | 1.00 (reference) | 1.00 (0.75–1.33) | 1.55 (1.18–2.05)† | 1.05 (0.59–1.85) | 1.79 (1.36–2.37)‡ |
|
| ||||||||||
| CHD mortality | ||||||||||
| Number of events (incidence per 100,000 person-yrs) | 152 (113) | 200 (166) | 203 (163) | 55 (310) | 315 (342) | 71 (38) | 39 (51) | 60 (133) | 9 (88) | 64 (194) |
| Relative risk: HR (95% CI) | ||||||||||
| Model 1 (unadjusted) | 1.00 (reference) | 1.46 (1.18–1.81)‡ | 1.45 (1.18–1.79)† | 2.78 (2.04–3.79)‡ | 3.13 (2.58–3.79)‡ | 1.00 (reference) | 1.34 (0.91–1.98) | 3.57 (2.53–5.04)‡ | 2.35 (1.18–4.71)* | 5.33 (3.80–7.47)‡ |
| Model 2 | 1.00 (reference) | 1.38 (1.12–1.70)† | 1.42 (1.15–1.75)† | 1.94 (1.43–2.65)‡ | 2.13 (1.75–2.59)‡ | 1.00 (reference) | 1.09 (0.73–1.61) | 2.35 (1.66–3.33)‡ | 1.62 (0.81–3.25) | 2.58 (1.83–3.66)‡ |
| Model 3 | 1.00 (reference) | 1.32 (1.07–1.63)* | 1.28 (1.04–1.58)* | 1.71 (1.25–2.33)† | 1.75 (1.43–2.14)‡ | 1.00 (reference) | 1.03 (0.70–1.52) | 2.12 (1.49–3.01)‡ | 1.33 (0.66–2.68) | 1.97 (1.36–2.85)‡ |
|
| ||||||||||
| Stroke mortality | ||||||||||
| Number of events (incidence per 100,000 person-yrs) | 24 (18) | 26 (22) | 26 (21) | 6 (34) | 63 (68) | 28 (15) | 17 (22) | 14 (31) | 1 (10) | 18 (55) |
| Relative risk: HR (95% CI) | ||||||||||
| Model 1 (unadjusted) | 1.00 (reference) | 1.20 (0.69–2.09) | 1.18 (0.68–2.05) | 1.93 (0.79–4.72) | 4.05 (2.53–6.49)‡ | 1.00 (reference) | 1.48 (0.81–2.70) | 2.10 (1.11–3.99)* | 0.66 (0.90–4.86) | 3.77 (2.09–6.82)‡ |
| Model 2 | 1.00 (reference) | 1.14 (0.66–1.99) | 1.17 (0.67–2.04) | 1.32 (0.54–3.24) | 2.74 (1.70–4.41)‡ | 1.00 (reference) | 1.22 (0.66–2.23) | 1.47 (0.77–2.81) | 0.47 (0.06–3.44) | 1.92 (1.04–3.53)* |
| Model 3 | 1.00 (reference) | 1.12 (0.64–1.95) | 1.08 (0.62–1.90) | 1.15 (0.47–2.83) | 2.40 (1.46–3.94)† | 1.00 (reference) | 1.23 (0.67–2.25) | 1.46 (0.76–2.82) | 0.45 (0.06–3.30) | 1.91 (1.01–3.62)* |
Sex-specific unadjusted and adjusted HRs (95% CI) for risk of CVD mortality, CHD mortality, and stroke mortality among each hypertension subtype are shown. As adjustment factors, model 2 includes demographic variables (age at baseline, race, and education), and model 3 includes demographic variables plus body mass index, current smoking, total cholesterol, and diabetes. Statistical significance was defined as p < 0.05.
p < 0.05;
p < 0.01;
p < 0.001.
CHD = coronary heart disease; CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio; other abbreviations as in Table 1.
In women, ISH was associated with a higher risk for CVD and CHD mortality compared with optimal-normal BP (model 1, Table 3). Adjustment for demographic variables attenuated the associations (model 2), but ISH remained significantly associated with CVD and CHD mortality with adjustment also for clinical characteristics (model 3). The relative risk for CVD mortality was highest for SDH (HR: 1.79; 95% CI: 1.36 to 2.37), followed by ISH (HR: 1.55; 95% CI: 1.18 to 2.05). The adjusted HR for CVD mortality risk for ISH in women under 40 years of age (n = 816) was 1.15 (95% CI: 0.63 to 2.10; p = 0.65), and the HR in women 40 years of age or older (n = 630) was 1.68 (95% CI: 1.22 to 2.31; p = 0.002); no significant interactions were found between age (<40 or ≥40 years) and ISH in association with CVD mortality risk (p = 0.59). In the overall analyses (n = 27,081), there were no significant interactions between sex and ISH in association with CVD mortality risk (p = 0.13) and CHD mortality risk (p = 0.29).
In both men and women, ISH was not associated with stroke risk, with HRs of 1.1 (95% CI: 0.6 to 1.9) and 1.5 (95% CI: 0.8 to 2.8), respectively. Only SDH was associated with a higher risk for stroke mortality independent of demographic and clinical characteristics, with HRs of 2.4 (95% CI: 1.5 to 3.9) in men and 1.9 (95% CI: 1.0 to 3.6) in women. We analyzed the association of BP as a continuous variable with CVD risk. In men, both SBP and DBP were associated with a higher risk for CVD mortality independent of one another, while only SBP was associated with CVD mortality risk in women (Table 4).
TABLE 4.
Sex-Specific Unadjusted and Multivariate-Adjusted HR (95% CIs) for BP as a Continuous Variable for Risk for CVD Mortality, CHD Mortality, and Stroke Mortality
| Model | Men (n = 15,868)
|
Women (n = 11,213)
|
||
|---|---|---|---|---|
| SBP, 10 mm Hg | DBP, 5 mm Hg | SBP, 10 mm Hg | DBP, 5 mm Hg | |
| CVD mortality | ||||
| Model 1 (unadjusted) | 1.26 (1.23–1.30)‡ | 1.25 (1.22–1.28)‡ | 1.37 (1.31–1.43)‡ | 1.28 (1.23–1.33)‡ |
| Model 2 | 1.18 (1.15–1.21)‡ | 1.15 (1.13–1.18)‡ | 1.19 (1.13–1.25)‡ | 1.14 (1.09–1.19)‡ |
| Model 3 | 1.13 (1.10–1.17)‡ | 1.12 (1.09–1.15)‡ | 1.15 (1.09–1.21)‡ | 1.11 (1.07–1.17)‡ |
| Model 4 | 1.06 (1.01–1.10)† | 1.08 (1.05–1.12)‡ | 1.10 (1.02–1.19)* | 1.05 (0.99–1.12) |
|
| ||||
| CHD mortality | ||||
| Model 1 (unadjusted) | 1.25 (1.20–1.29)‡ | 1.24 (1.20–1.27)‡ | 1.41 (1.33–1.49)‡ | 1.30 (1.24–1.37)‡ |
| Model 2 | 1.16 (1.12–1.20)‡ | 1.14 (1.11–1.17)‡ | 1.23 (1.15–1.31)‡ | 1.16 (1.10–1.23)‡ |
| Model 3 | 1.11 (1.07–1.16)‡ | 1.10 (1.07–1.14)‡ | 1.17 (1.10–1.25)‡ | 1.12 (1.06–1.19)‡ |
| Model 4 | 1.05 (0.997–1.10) | 1.08 (1.03–1.12)‡ | 1.14 (1.03–1.25)† | 1.04 (0.96–1.12) |
|
| ||||
| Stroke mortality | ||||
| Model 1 (unadjusted) | 1.39 (1.29–1.51)‡ | 1.38 (1.29–1.47)‡ | 1.35 (1.22–1.50)‡ | 1.27 (1.16–1.39)‡ |
| Model 2 | 1.29 (1.19–1.40)‡ | 1.27 (1.19–1.36)‡ | 1.19 (1.06–1.33)† | 1.14 (1.03–1.26)† |
| Model 3 | 1.26 (1.16–1.37)‡ | 1.25 (1.17–1.35)‡ | 1.19 (1.06–1.34)† | 1.14 (1.03–1.26)* |
| Model 4 | 1.08 (0.96–1.23) | 1.19 (1.07–1.33)† | 1.13 (0.95–1.34) | 1.06 (0.92–1.22) |
Sex-specific unadjusted and adjusted HRs (95% CI) of BP (continuous variable) for risk for CVD mortality, CHD mortality, and stroke mortality are shown. As adjustment factors, model 2 includes demographic variables (age at baseline, race, and education) plus SBP (or DBP), model 3 includes demographic variables plus clinical characteristics (body mass index, current smoking, total cholesterol, and diabetes) plus SBP (or DBP), and model 4 includes demographic variables plus clinical characteristics plus SBP plus DBP. Statistical significance was defined as p < 0.05.
p < 0.05;
p < 0.01;
p < 0.001.
DISCUSSION
Our main findings are that younger and middle-aged adults (mean age 34 years) with ISH had higher relative risk for CVD and CHD mortality over 31 years of follow-up compared with those with optimal-normal BP. In men, the risks associated with ISH were similar to those in men with high-normal BP and not as great as those associated with IDH and SDH. In women, the risks associated with ISH were higher than those associated with high-normal BP and IDH and not as great as those associated with SDH.
The proportion of ISH among younger to middle-aged participants varies by cohort (2% to 8% in all persons and 14% to 40% in patients with hypertension) (1–5,21,22). The variation largely relates to differences in population characteristics, such as age, race/ethnicity, and obesity. Our data show that the prevalence of ISH was 25% in men and 13% in women, and that one-half of younger men and women with hypertension had ISH. This high prevalence may be because our cohort included middle-aged adults who underwent only 1 BP measurement (23). Consistent with prior reports (1,2,4,5,22,24,25), those with ISH showed a higher proportion of current smoking, less education, higher BMI, higher heart rate, and higher serum total cholesterol levels compared with subjects with optimal-normal BP.
At least 3 studies have shown prospective associations between SBP and DBP in young men and future risk for CVD events; evidence pertaining to younger women is scarce. A nested case-control study of more than 45,000 students (mean age 19 years, 93% men) identified higher SBP (≥130 mm Hg) as a predictor of CHD mortality over the subsequent 50 years (9). Multivariate adjustments were not performed. In the second study, of 8,354 male students (mean age 21 years), a 10 mm Hg higher SBP was associated with a 14% higher risk for CVD mortality over a median follow-up period of 41 years (10). The third study, of a Swedish nationwide cohort of more than 1.2 million military men (mean age 18 years), found that higher SBP or higher DBP was associated with a higher risk for CVD mortality over a median follow-up period of 24 years (13).
Few studies have assessed CVD risk by hypertension subtype among younger and middle-aged adults. Strandberg et al. (26) examined the impact of ISH on CVD mortality among Finnish men between 30 and 45 years of age (n = 3,267, 32-year follow-up), but only 17 participants were classified as having ISH. Rutan et al. (27) reported that among 317,871 United States white men between 35 and 57 years of age (34% were 50 years of age or older), ISH, defined as SBP ≥160 mm Hg and DBP <90 mm Hg, was associated with significantly increased risk for CHD mortality (HR: 2.7; p < 0.001) over 6 years of follow-up, compared with the reference group (those with SBP/ DBP <160/90 mm Hg). The definition of ISH is not applicable to current clinical practice, and the reference group included those with SBP of 140 to 159 mm Hg.
Reasons for the different CVD risks in men with ISH compared with those with IDH or SDH remain uncertain. We found that men with ISH were younger than those with IDH or SDH, suggesting that men with ISH might have had shorter overall exposure to high BP. The hemodynamic pattern of ISH has been shown to vary by individual, involving higher stroke volume and/or aortic stiffness with normal peripheral vascular resistance, whereas IDH and SDH had higher peripheral vascular resistance (3). Hemodynamic alterations of ISH in younger adults may be indicative of a comparatively early clinical stage of hypertension, that is, a hyperdynamic circulation preceding the development of higher peripheral vascular resistance (28,29). These hemodynamic characteristics of ISH may relate to the differences in CVD risks from those associated with IDH or SDH among men. The similar CVD mortality risks between high-normal BP, a predecessor of full-blown hypertension that is nonetheless associated with atherosclerosis (30), and ISH in the present study may be interpreted as supporting the concept of ISH in men as a comparatively early clinical stage of hypertension.
Consistent with prior nationwide United States survey data (2), 25% of younger and middle-aged adults with ISH were women. Mechanisms of ISH among young women are poorly understood. In general, women have higher central (aortic) BP, reflecting the pressure experienced by perfused target organs (e.g., brain, heart, and kidneys), which may therefore be a better predictor of CVD compared with brachial BP (31). This reflects greater central arterial wave augmentation and smaller pulse pressure amplification than in men (32). Brachial pulse pressure is high for ISH, so the central BP for ISH, particularly in women, is expected to be high. Unlike in men, the CVD mortality risk of ISH in women was greater than that of high-normal BP in the present study. It remains uncertain if this phenomenon is driven by the nonsignificant risk for high-normal BP compared with optimal-normal BP in women or if the clinical implications of ISH differ by sex. Unlike in men, high-normal BP in young and middle-aged women was not more correlated with CVD mortality risk than optimal-normal BP in the present study. In the Framingham Heart Study, although the association of high-normal BP (vs. optimal BP) with CVD risk was significant in both men and women, this association was attenuated in women, but not in men, after adjustments for comorbidities (33). To assess whether the association of high-normal BP with CVD risk differs by sex, especially for younger adults, etiopathophysiological studies and further analysis using long-term follow-up studies are warranted.
The CVD mortality risk of ISH was significantly increased in women 40 years of age or older, but not in those under 40 years of age. The nonsignificant results with wider 95% CIs in those <40 years of age are probably related to their low event rate. The direction of the association between ISH and CVD mortality risk was the same between those older and younger than 40 years of age (HRs: 1.68 and 1.15). The interaction term between age (<40 or ≥40 years) and ISH in association with CVD mortality risk did not achieve statistical significance; this difference was modest.
There was no significant association of ISH and IDH with risk for stroke mortality. Other cohort studies also reported a lack of association between higher BP and future risk for stroke mortality in young adults (11,14). The numbers of stroke deaths were small compared with those for CHD mortality, limiting the statistical power of the analyses. In addition, CHD and stroke may compete as outcomes. In other words, some individuals would not experience fatal stroke events, because CHD death had already occurred.
We analyzed the association of BP as a continuous variable with CVD risk. In men, both SBP and DBP were independently associated with a higher risk for CVD mortality, whereas only SBP was associated with CVD mortality risk in women. These results are consistent with the findings on hypertension phenotype risks; that is, ISH, IDH, and SDH are associated with a higher risk for CVD mortality in men, and ISH and SDH are associated with a significantly higher risk for CVD mortality in women. The clinical implications of SBP and DBP and their relative importance (subsequently, ISH and IDH) in younger adults may differ between men and women (31,32,34). Replication in different studies and cohorts and further etiopathophysiological studies are warranted.
STUDY LIMITATIONS
First, BP values were only from baseline measurements in the supine position, with the possibility of misclassification and regression-dilution bias. DBP is especially affected by body posture, though the influence of the supine position on BP is heterogeneous (35,36). In addition, we could not assess the impact of BP changes and use of antihypertensive medication during follow-up. In aggregate, these factors would tend to underestimate the true association between BP and CVD risk.
Second, participants from the CHA study were recruited between 1967 and 1973, before the onset of the obesity epidemic, so the results do not illuminate the impact of that epidemic on BP and BP-related risks.
Third, because the follow-up duration of the present study is very long, competing effects of non-CVD deaths may be significant.
Fourth, the covariate set used in the present study was limited, and residual confounding (e.g., socioeconomic status, diet, alcohol intake, and psychological factors) (2,37–39) may exist.
Finally, our sample consisted largely of non-Hispanic white participants, so our findings may or may not be generalizable to other racial/ethnic groups.
CONCLUSIONS
Among younger and middle-aged adults, those with ISH were at higher relative risk for CVD and CHD mortality compared with those with optimal-normal BP over long-term follow-up. The benefits of drug treatment for ISH among the elderly have been proved (40), but such evidence does not exist for younger and middle-aged adults (41). Our data indicate that ISH in younger and middle-aged adults is not an innocuous condition. We cannot definitively infer whether the excess CVD risk from ISH in younger and middle-aged adults warrants antihypertensive drug therapy or whether only lifestyle modification treatment is necessary. Further research is needed, including clinical trials and studies seeking better ways (e.g., central BP monitoring, biomarkers) to identify younger and middle-aged adults with ISH who are at especially greater risk for developing CVD events.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
The prevalence of ISH in young and middle-aged adults is increasing in the United States and is associated with an increased risk for cardiovascular mortality during long-term follow-up.
TRANSLATIONAL OUTLOOK
Further studies of ISH in young and middle-aged adults are warranted to determine whether antihypertensive drug therapy could lower cardiovascular risk more than lifestyle modification without medication.
Acknowledgments
This study was supported by the American Heart Association and its Chicago and Illinois affiliates; grants R01-HL 15174, R01-HL 21010, and R01-HL 03387 from the National Heart, Lung, and Blood Institute; the Northwestern Memorial Foundation; and the Goldberg Family Charitable Trust.
ABBREVIATIONS AND ACRONYMS
- BMI
body mass index
- BP
blood pressure
- CHA
Chicago Heart Association Detection Project in Industry
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HR
hazard ratio
- ICD-8
International Classification of Diseases-Eighth Revision
- ICD-9
International Classification of Diseases-Ninth Revision
- IDH
isolated diastolic hypertension
- ISH
isolated systolic hypertension
- SBP
systolic blood pressure
- SDH
systolic diastolic hypertension
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Franklin SS, Jacobs MJ, Wong ND, et al. Predominance of isolated systolic hypertension among middle-aged and elderly U.S. hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–74. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 2.Grebla RC, Rodriguez CJ, Borrell LN, et al. Prevalence and determinants of isolated systolic hypertension among young adults: the 1999–2004 U.S. National Health and Nutrition Examination Survey. J Hypertens. 2010;28:15–23. doi: 10.1097/HJH.0b013e328331b7ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEniery CM, Yasmin, Wallace S, et al. for the ENIGMA Study Investigators. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46:221–6. doi: 10.1161/01.HYP.0000165310.84801.e0. [DOI] [PubMed] [Google Scholar]
- 4.Franklin SS, Barboza MG, Pio JR, et al. Blood pressure categories, hypertensive subtypes, and the metabolic syndrome. J Hypertens. 2006;24:2009–16. doi: 10.1097/01.hjh.0000244950.72664.02. [DOI] [PubMed] [Google Scholar]
- 5.Franklin SS, Wilkinson IB, McEniery CM. Unusual hypertensive phenotypes: what is their significance? Hypertension. 2012;59:173–8. doi: 10.1161/HYPERTENSIONAHA.111.182956. [DOI] [PubMed] [Google Scholar]
- 6.O’Rourke MF, Adji A. Guidelines on guidelines: focus on isolated systolic hypertension in youth. J Hypertens. 2013;31:649–54. doi: 10.1097/HJH.0b013e32835d8230. [DOI] [PubMed] [Google Scholar]
- 7.McEniery CM, Franklin SS, Wilkinson IB, et al. Isolated systolic hypertension in the young: a need for clarity. J Hypertens. 2013;31:1911–3. doi: 10.1097/HJH.0b013e3283635315. [DOI] [PubMed] [Google Scholar]
- 8.Paffenbarger RS, Jr, Notkin J, Krueger DE, et al. Chronic disease in former college students. II. Methods of study and observations on mortality from coronary heart disease. Am J Public Health Nations Health. 1966;56:962–71. doi: 10.2105/ajph.56.6.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paffenbarger RS, Jr, Wing AL. Chronic disease in former college students. XI. Early precursors of nonfatal stroke. Am J Epidemiol. 1971;94:524–30. doi: 10.1093/oxfordjournals.aje.a121351. [DOI] [PubMed] [Google Scholar]
- 10.McCarron P, Smith GD, Okasha M, et al. Blood pressure in young adulthood and mortality from cardiovascular disease. Lancet. 2000;355:1430–1. doi: 10.1016/S0140-6736(00)02146-2. [DOI] [PubMed] [Google Scholar]
- 11.McCarron P, Okasha M, McEwen J, et al. Blood pressure in early life and cardiovascular disease mortality. Arch Intern Med. 2002;162:610–1. doi: 10.1001/archinte.162.5.610. [DOI] [PubMed] [Google Scholar]
- 12.Lew EA. Blood pressure and mortality: life insurance experience. In: Stamler J, Stamler R, Pullman TN, editors. The Epidemiology of Hypertension. New York, NY: Grune & Stratton; 1967. pp. 392–7. [Google Scholar]
- 13.Sundström J, Neovius M, Tynelius P, et al. Association of blood pressure in late adolescence with subsequent mortality: cohort study of Swedish male conscripts. BMJ. 2011;342:d643. doi: 10.1136/bmj.d643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray L, Lee IM, Sesso HD, et al. Blood pressure in early adulthood, hypertension in middle age, and future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study) J Am Coll Cardiol. 2011;58:2396–403. doi: 10.1016/j.jacc.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neaton JD, Wentworth D for the Multiple Risk Factor Intervention Trial Research Group. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Arch Intern Med. 1992;152:56–64. [PubMed] [Google Scholar]
- 16.Miura K, Daviglus ML, Dyer AR, et al. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med. 2001;161:1501–8. doi: 10.1001/archinte.161.12.1501. [DOI] [PubMed] [Google Scholar]
- 17.Mosley WJ, II, Greenland P, Garside DB, et al. Predictive utility of pulse pressure and other blood pressure measures for cardiovascular outcomes. Hypertension. 2007;49:1256–64. doi: 10.1161/HYPERTENSIONAHA.106.083592. [DOI] [PubMed] [Google Scholar]
- 18.Stamler J, Dyer AR, Shekelle RB, et al. Relationship of baseline major risk factors to coronary and all-cause mortality, and to longevity: findings from long-term follow-up of Chicago cohorts. Cardiology. 1993;82:191–222. doi: 10.1159/000175868. [DOI] [PubMed] [Google Scholar]
- 19.Stamler J, Rhomberg P, Schoenberger JA, et al. Multivariate analysis of the relationship of seven variables to blood pressure: findings of the Chicago Heart Association Detection Project in Industry 1967–1972. J Chronic Dis. 1975;28:527–48. doi: 10.1016/0021-9681(75)90060-0. [DOI] [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, et al. for the National Heart, Lung and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 21.Mallion JM, Hamici L, Chatellier G, et al. Isolated systolic hypertension: data on a cohort of young subjects from a French working population (IHPAF) J Hum Hypertens. 2003;17:93–100. doi: 10.1038/sj.jhh.1001506. [DOI] [PubMed] [Google Scholar]
- 22.Hulsen HT, Nijdam ME, Bos WJ, et al. Spurious systolic hypertension in young adults; prevalence of high brachial systolic blood pressure and low central pressure and its determinants. J Hypertens. 2006;24:1027–32. doi: 10.1097/01.hjh.0000226191.36558.9c. [DOI] [PubMed] [Google Scholar]
- 23.Franklin SS. The importance of diastolic blood pressure in predicting cardiovascular risk. J Am Soc Hypertens. 2007;1:82–93. doi: 10.1016/j.jash.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Saladini F, Dorigatti F, Santonastaso M, et al. for the HARVEST Study Group. Natural history of hypertension subtypes in young and middle-age adults. Am J Hypertens. 2009;22:531–7. doi: 10.1038/ajh.2009.21. [DOI] [PubMed] [Google Scholar]
- 25.Sorof JM. Prevalence and consequence of systolic hypertension in children. Am J Hypertens. 2002;15(2 Pt 2):57S–60S. doi: 10.1016/s0895-7061(01)02303-2. [DOI] [PubMed] [Google Scholar]
- 26.Strandberg TE, Salomaa VV, Vanhanen HT, et al. Isolated diastolic hypertension, pulse pressure, and mean arterial pressure as predictors of mortality during a follow-up of up to 32 years. J Hypertens. 2002;20:399–404. doi: 10.1097/00004872-200203000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Rutan GH, Kuller LH, Neaton JD, et al. Mortality associated with diastolic hypertension and isolated systolic hypertension among men screened for the Multiple Risk Factor Intervention Trial. Circulation. 1988;77:504–14. doi: 10.1161/01.cir.77.3.504. [DOI] [PubMed] [Google Scholar]
- 28.Messerli FH, Frohlich ED, Suarez DH, et al. Borderline hypertension: relationship between age, hemodynamics and circulating catecholamines. Circulation. 1981;64:760–4. doi: 10.1161/01.cir.64.4.760. [DOI] [PubMed] [Google Scholar]
- 29.Julius S, Pascual AV, London R. Role of parasympathetic inhibition in the hyperkinetic type of borderline hypertension. Circulation. 1971;44:413–8. doi: 10.1161/01.cir.44.3.413. [DOI] [PubMed] [Google Scholar]
- 30.Pimenta E, Oparil S. Prehypertension: epidemiology, consequences and treatment. Nat Rev Nephrol. 2010;6:21–30. doi: 10.1038/nrneph.2009.191. [DOI] [PubMed] [Google Scholar]
- 31.McEniery CM, Cockcroft JR, Roman MJ, et al. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35:1719–25. doi: 10.1093/eurheartj/eht565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shim CY, Park S, Choi D, et al. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J Am Coll Cardiol. 2011;57:1226–33. doi: 10.1016/j.jacc.2010.09.067. [DOI] [PubMed] [Google Scholar]
- 33.Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson IB, Franklin SS, Hall IR, et al. Pressure amplification explains why pulse pressure is unrelated to risk in young subjects. Hypertension. 2001;38:1461–6. doi: 10.1161/hy1201.097723. [DOI] [PubMed] [Google Scholar]
- 35.Netea RT, Smits P, Lenders JW, et al. Does it matter whether blood pressure measurements are taken with subjects sitting or supine? J Hypertens. 1998;16:263–8. doi: 10.1097/00004872-199816030-00002. [DOI] [PubMed] [Google Scholar]
- 36.Netea RT, Lenders JW, Smits P, et al. Both body and arm position significantly influence blood pressure measurement. J Hum Hypertens. 2003;17:459–62. doi: 10.1038/sj.jhh.1001573. [DOI] [PubMed] [Google Scholar]
- 37.Moore TJ, Conlin PR, Ard J, et al. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension. 2001;38:155–8. doi: 10.1161/01.hyp.38.2.155. [DOI] [PubMed] [Google Scholar]
- 38.Beilin LJ, Puddey IB. Alcohol and hypertension: an update. Hypertension. 2006;47:1035–8. doi: 10.1161/01.HYP.0000218586.21932.3c. [DOI] [PubMed] [Google Scholar]
- 39.Harburg E, Julius S, Mcginn NF, et al. Personality traits and behavioral patterns associated with systolic blood pressure levels in college males. J Chronic Dis. 1964;17:405–14. doi: 10.1016/0021-9681(64)90101-8. [DOI] [PubMed] [Google Scholar]
- 40.Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996;276:1886–92. [PubMed] [Google Scholar]
- 41.Mancia G, Fagard R, Narkiewicz K, et al. for the Task Force Members. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]