Abstract
Endocananbnoid-system G-protein coupled receptors (GPCRs) and transient receptor potential (TRP) cation channels are critical components of cellular biosignaling networks. These plasma-membrane proteins are pleiotropic in their ability to interact with and engage structurally diverse ligands. The endocannabinoid and TRP signaling systems overlap in their recognition properties with respect to select naturally occurring plant-derived ligands that belong to the terpene and lipid chemical classes, the overlap establishing a physiological connectivity between these two ubiquitous cell-signaling systems. Identification and pharmacological profiling of phytochemicals engaged by cannabinoid GPCRs and/or TRP channels has inspired the synthesis of novel designer ligands that interact with cannabinoid receptors and/or TRP channel as xenobiotics. Functional interplay between the endocannabinoid and TRP-channel signaling systems is responsible for the antinocifensive action of some synthetic cananbinoids (WIN55,212-2 and AM1241), vasorelaxation by the endocannabinoid N-arachidonylethanolamide (anandamide), and the pain-relief afforded by the synthetic anandamide analogue N-arachidonoylaminophenol (AM404), the active metabolite of the widely used nonprescription analgesic and antipyretic acetaminophen (paracetamol). The biological actions of some plant-derived cannabinoid-receptor (e.g., Δ9-tetrahydrocannabinol) or TRP-channel (e.g,, menthol) ligands either carry abuse potential themselves or promote the use of other addictive substances, suggesting the therapeutic potential for modulating these signaling systems for abuse-related disorders. The pleiotropic nature of and therapeutically relevant interactions between cananbinergic and TRP-channel signaling suggest the possibility of dual-acting ligands as drugs.
Keywords: Drug discovery, endocananbinoid, G-protein coupled receptors, ion channels, ligands, phytochemicals, phytocannabinoid, signal transduction
Graphical abstract
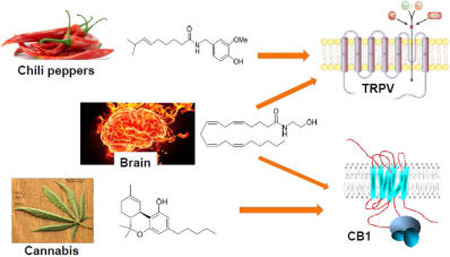
At the cellular level of biological organization, a fundamental paradigm for communication utilizes plasma-membrane proteins (receptors, ion channels, transporters, etc.) as components of organized signaling circuits that interact with molecules (ligands) for the purpose of transmitting information from the external milieu to the intracellular compartment, thus enabling the target-cell to respond to its environment.1 This core principle of signal transduction is well exemplified by two ubiquitous mammalian signaling systems, one of which employs cannabinoid (CB) receptors; the other, transient receptor potential (TRP) cation channels.
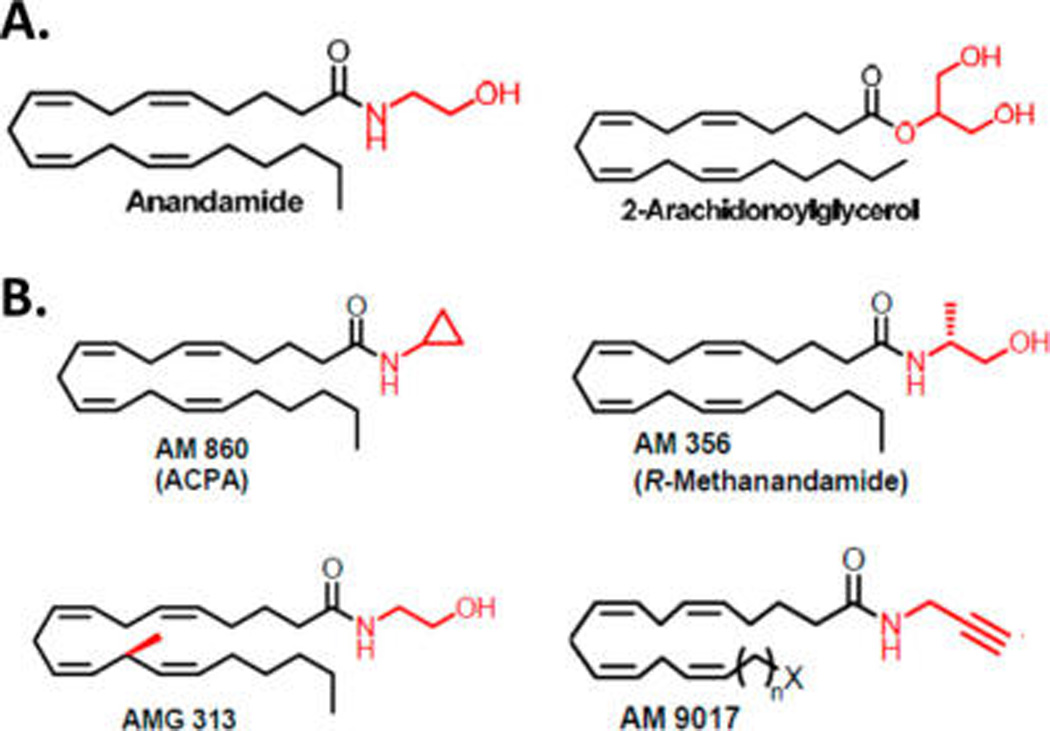
Discovery and molecular characterization of the first CB receptor to be cloned (CB1) was spurred by identification and synthesis of the major psychotrophic component of marijuana, (−)-Δ9-tetrahydrocannabinol (Δ9-THC), a plant-derived CB (“phytocannabinoid”) (Figure 1A). A second CB receptor, designated CB2, was subsequently identified through homology cloning, and other putative CB receptors have been suggested. Both CB1 and CB2 are class-A G-protein coupled receptors (GPCRs) featuring characteristic 7-transmembrane helical domains, significant homology with one another in their transmembrane domains, and distinct distributions: CB2 is found mainly in peripheral tissues (principally immuneassociated), whereas CB1 is a major GPCR in the central nervous system at presynaptic neurons and is also expressed in the periphery.2 CB1 and CB2, along with a growing family of their endogenous activators (“endocannabinoids”) and the enzymes that synthesize and inactivate those agonists, are constituents of the endocannabinoid system, a biosignaling network ubiquitous in mammals.3 The best-studied endocan-nabinoids, 2-arachidonoylglycerol (2-AG) and N-arachidonylethanolamide (or anandamide) (AEA), are lipid mediators derived from diacylglycerol and N-acylphophatidycholine, respectively (Figure 2A). They originate from membrane phospholipids by distinct enzymatic pathways and possess specific functional and pharmacological properties.4 In the central nervous system (CNS), the presynaptic serine hydrolase, monoacylglyceorol lipase (MGL), is primarily responsible for 2-AG inactivation in vivo along with the α,β-hydrolase domain-containing proteins 6 and 12 (ABHD6 and ABHD12), whereas AEA is inactivated by fatty acid amide hydrolase (FAAH) postsynaptically.5 Aside from its canonical role in the CNS as a key retrograde modulator of neurotransmitter release,6 the endocananibinoid system, either alone or in concert with other neuromodulatory signaling systems, is involved in a number of fundamental (patho)physiological processes, including energy balance, emotional processing, reward and motivation, immune function, and pain sensing.7–10
Figure 1.
Chemical structures of select plant-derived terpenoid cannabinoids (phytocannabinoids) (A) and select synthetic terpenoid cannabinoids (B) discussed in the text.
Figure 2.
Chemical structures of select lipid endocannabinoids (A) and structurally related synthetic cannabinergic agents (B) discussed in the text.
Across animal phyla, TRP channels constitute a superfamily of over 50 nonselective cation-channel membrane proteins that function as cellular sensors whose activity in response to external stimuli ultimately elicits a change in cell-membrane potential.11 All known TRP channels evidence six transmembrane segments (TMS1-TMS6) and a short, hydrophobic, cation-permeable pore domain between TMS5 and TMS6. TRP channels are polymodal: their ion permeability can be modulated by diverse mechanisms including G-protein coupled signaling, membrane depolarization, and direct ligand binding. The 28 distinct mammalian TRP channels identified have been classified on the basis of sequence homology into six subfamilies, each subfamily characterized by distinct gating mechanisms and cation selectivities.12 Of these subfamilies, the six vanilloid or “thermo” TRP channels (TRPV1–6) have garnered the most attention, with TRPV1 being the best-characterized and first-cloned TRPV due to its physiological role in nervous tissue as a molecular integrator of diverse noxious chemical and thermal stimuli, notably its extreme responsiveness to thermal (heat) activation from the pungent vanilloid capsaicin (8-methyl-N-vanillyl-6-nonenamide) (Figure 3A), an irritant found in hot chili peppers, as well as to other phytochemical toxins and acid.13 Reminiscent of the endocananbinoid system, TRP-channel signaling is involved in many (patho)physiological processes, for example, cell proliferation/differentiation, neurotransmitter release, chemical sensing, cell death, and inflammation.11,14,15 Indeed, both endocanabinoid-system proteins8–10 and TRP channels15–18 are aggressively being pursued as drug targets for indications including diabetes, pain, cancer, neurodegeneration, and substance-abuse disorders.
Figure 3.

Chemical structures of select plant-derived terpenoid TRP-channel ligands (A) and synthetic TRP-channel ligands (B) discussed in the text.
The goal of this Review is to summarize and discuss select concepts related to the most important ligands associated with the (in)activation of the CB and TRP-channel signaling systems. A particular focus will be on ligands belonging to the terpene or lipid chemical classes that interact with both systems.
COMMONALITIES BETWEEN CANNABINERGIC AND TRP BIOSIGNALING SYSTEMS
Aside from their basic physiological role as routes of cellular communication and focuses of contemporary drug discovery, CB-receptor- and TRP-channel-dependent signaling share several fundamental properties. Cannabinergic and TRP-signaling systems are both pleiotropic. Their pleiotropic, or “opportunistic”, nature is manifested in the ability of CB1, CB2, and TRPV1 to interact with and engage structurally diverse ligands belonging to a wide variety of chemical classes, some of which may also interact with other signaling systems. Most relevant to the present discussion, the endocannabinoid and TRP systems overlap in their ligand-recognition properties with respect to select naturally occurring ligands.19 The shared endogenous ligands are found in plants and animals and generally belong to the lipid and terpene chemical classes. The seminal discovery in this regard, demonstration in 1999 that the endocannabinoid AEA can activate TRPV1 channels,20 also established physiological connectivity between endocananbinoid-system and TRP-channel-mediated cellular signaling, a relationship particularly relevant in the CNS where CB1 and TRPV1 activation provides a framework for Δ9-THC’s psychobehavioral manifestations and the transmission and modulation of thermal and pain effects. The spectrum of natural cannabinergic and TRP-channel ligands has been greatly expanded by medicinal chemistry efforts that have produced designer synthetic ligands that interact as xenobiotics with CB receptors and/or TRP channels.9,21,22
PHYTOCANNABINOIDS AND LIGANDS BASED UPON A TERPENOID CHEMOTYPE
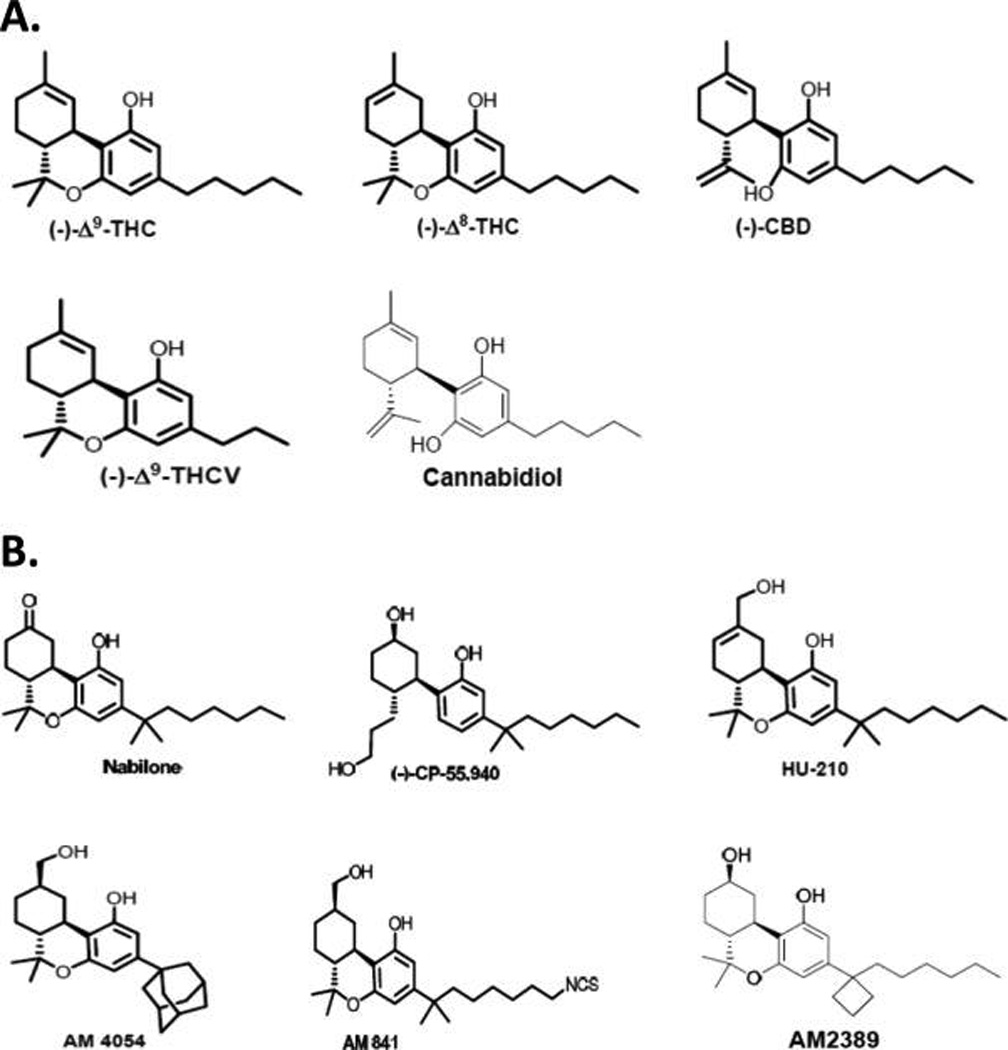
Among its approximately 500 endogenous phytochemicals,23 the cannabis plant contains some 70 unique cannabinoids (“phytocannabinoids”), of which the most well-studied are shown in Figure 1A. Δ9-THC is the archtypical “classical CB”, encompassing a fused-ring tricyclic terpenoid derivative incorporating a polar benzopyran ring with a terminal, hydrophobic alkyl (n-pentyl) side-chain, a characteristic lipophilic domain, and hydrogen-bonding phenolic group.24 Δ9 -THC can engage and activate both CB1 and CB2 receptors with low nanomolar affinity, although the action of Δ9-THC as a partial agonist at presynaptic CB1 receptors in the CNS is thought to account for its psychotropic activity as the main psychoactive cannabis phytocannabinoid. Δ9-THC and its much less prominent natural isomer, (−)-Δ9-tetrahydrocannabinol (Δ8-THC), are virtually equivalent as to CB-receptor affinity and pharmacological activity, although Δ8-THC is the more chemically stable isomer.25 As compared to Δ9 -THC, the phytocannabinoid (−)-cannabidiol (CBD) has significantly less affinity for CB receptors, modest CB2 selectivity, and negligible psychotropic activity.26,27 Another classical terpenoid phytocannabinoid, (−)-Δ9 -tetrahydrocannabivarin (Δ9 -THCV), is a shorter side-chain Δ9 -THC homologue with a similar CB-receptor affinity and selectivity profile. As a “neutral” CB1 antagonist in some systems, Δ9-THCV at low doses can antagonize the effects of Δ9-THC in a manner distinct from that of typical CB1 antagonists/inverse agonists and may also have CB receptor-independent pharmacological activities.27,28
The classic terpenoid phytocannabinoids THC, CBD, and Δ9-THCV have inspired the synthesis of several structurally related cannabinergic compounds that display varying degrees of selectivity as CB1/CB2 agonists and, generally, improved CB-receptor affinity versus Δ9-THC. Many of the synthetic CB agonists based on classic phytocannabinoids have been designed with therapeutic application in mind and, consequently, have been profiled preclinically for both their molecular pharmacology in vitro and potential salutary effects in disease models in vivo. Most prominent of these xenocannabinoids include nabilone, the first CB drug to be synthesized and used to treat chemotherapy-associated nausea, and CP-55,940, the first tritiated CB that, as a radiolabeled ligand, played a key role in the discovery of CB1.2,7,8 Other related xenocannabinoids include HU-210,29 AM4054,30 AM841,31 and AM238932 (Figure 1B. Of note, the isothiocyanate AM841 has been identified as an exceptionally potent “megagonist” at CB2 whose molecular mechanism of action involves covalent modification of a critical cysteine residue in the receptor’s transmembrane helix 6.33 Similarly, the analgesic potency of the novel, high-efficacy, CB1-selective agonist AM2389 is 1000-fold greater than that of morphine in the standard rat tail-flick model of pain.34
ENDOCANNABINOIDS AND RELATED LIPID LIGANDS
Endocannabinoid lipid mediators that activate CB1 and CB2 are exemplified by AEA and 2-AG (Figure 2A). AEA is a partial CB1 activator with modest affinity and a relatively weak CB2 ligand with low overall efficacy, whereas 2-AG is a full agonist at CB1 and CB2, albeit with lower affinity and greater efficacy relative to AEA.3,4 As is the case for synthetic phytocannabinoid analogues, several lipid cananbinergic ligands structurally related to AEA/2-AG have been synthesized by medicinal chemists for potential therapeutic application. These include arachidonoylcyclopropylamide (AM860, ACPA), a potent, selective CB1 agonist with anxiolytic and vasorelaxant properties in vivo;35 R-methanandamide (AM356), the first metabolically stable, chiral AEA analogue with partial CB1 efficacy and higher potency as compared to AEA itself that exerts therapeutic neuroprotective, antinociceptive, vasorelaxant, and anti-inflammatory effects;36 AMG313, the first AEA analogue with a chiral methyl arachidonoyl side chain;37 and AM9017, the first AEA analogue with high CB2 affinity (Whitten and Makriyannis, 2014, unpublished results) (Figure 2B).
PHYTO-TRPs AND RELATED TERPENOID LIGANDS
The prototypic, plant-derived TRPV1 agonist and homovanillic ester, capsaicin, is one of the five principal capsaicinoids present in Cayenne chili pepper (Capsicum annuum L.)38,39 (Figure 3A). Several other structurally diverse phytochemicals have been identified as naturally occurring TRP-channel modulators of various TRP channel subfamilies. The diterpene capsaicin analogue resiniferatoxin is an extremely potent TRPV1 agonist,40 and the phytocannabinoid cannabidiol is both a CB1/CB2 agonist as well as an activator of TRPV1, TRPV2, and TRPV341,42 (Figure 3A). Other phytochemical TRP-channel activators include the four transient potential receptor channel ankyrin 1 (TRPA1) agonists cinnamaldehyde (found in cinnamon),43 eugenol (found in cloves),44 gingerol (found in ginger),45 and umbellulone (found in California bay laurel, the “headache tree”)46 and the two marine sphingoids leucettamol-A and leucettamol-B, which activate TRPA1 and inhibit transient receptor potential channel melastatin 8 (TRPM8)47 (Figure 3A). Some plant-derived TRP-channel agonists have served as templates for medicinal chemistry efforts aimed at producing TRP-channel modulators with improved pharmacological profiles as potential drugs, for example, gingerol analogues.48
ENDOGENOUS TRP-CHANNEL AND RELATED LIPID LIGANDS
Several naturally occurring lipids act on TRP channels to modify cation flux through them.49–51 The endocannabinoid AEA modulates CB1/CB2 and also acts as a TRPV1 agonist20 (Figure 2A), while oleoylethanolamide (OEA) is a TRPV1 agonist that also binds to peroxisome proliferator-activated receptor alpha (PPAR-α) and the GPR119 CB-like receptor52 (Figure 4A). Other endogenous N-acyl-amide lipids structurally related to AEA include the FAAH inhibitor and TRPV1 antagonist N-arachidonoylserotonin53 and the CB1 and TRPV1 agonist N-arachidonoyldopamine (NADA).54 NADA was the first endogenous compound (“endovanilloid”) identified in mammalian nervous tissue with potency comparable to the phytochemical capsaicin at TRPV1 and is a long-chain fatty-acid amide, as are AEA and capsaicin.55 N-Acyl-amide lipids that act at TRP channels have inspired synthetic analogues including N-vanillylarachidonamide (“arvanil”), a TRPV1 agonist and CB1 partial agonist.56 Similarly, N-arachidonoylaminophenol (AM404), the first potent lipid-amide inhibitor of cellular AEA uptake, was subsequently shown to be a potent TRPV1 activator and cyclooxygenase-1/2 inhibitor as well57–59 (Figure 4B).
Figure 4.
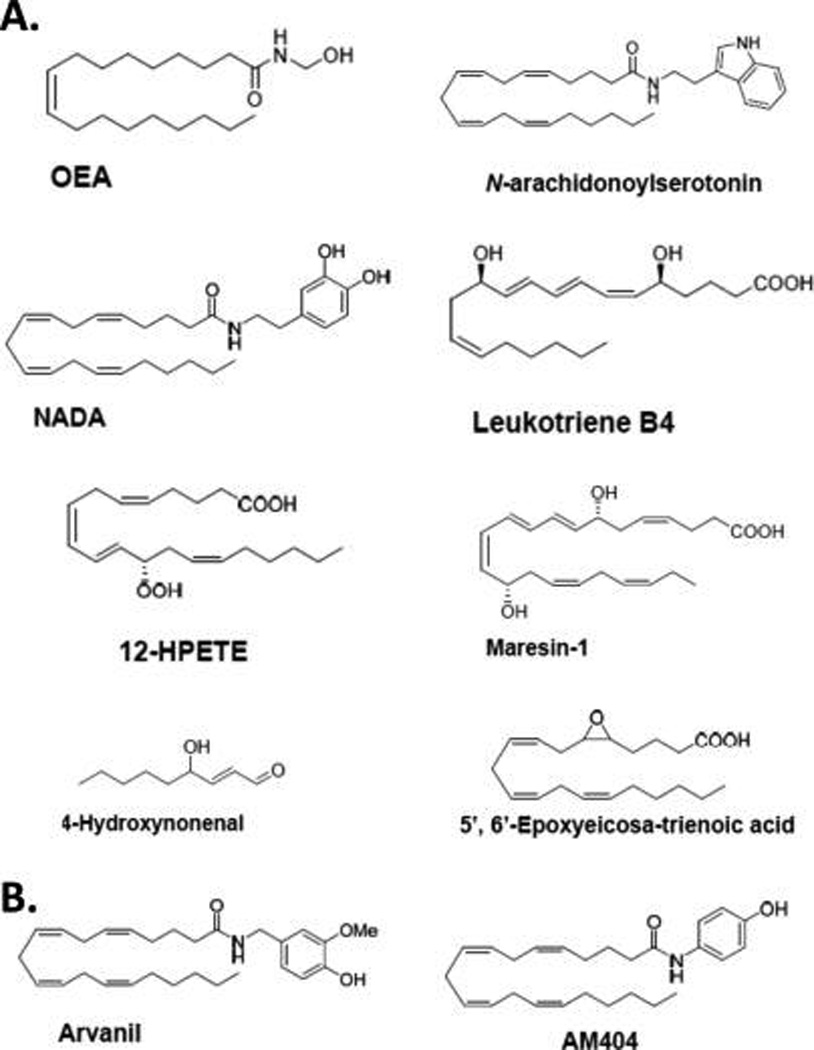
Chemical structures of select endogenous (A) and synthetic (B) lipid TRP-channel ligands discussed in the text.
Lipids produced by the lipoxygenase-mediated oxygenation of polyunsaturated 20-carbon fatty acids (especially arachidonic acid), including the eicosanoids leukotriene B4 (LTB4) and 12-hydroperoxyeicosatetraenoic acid (12-HPETE), are potent, endogenous TRPV1 activators60,61 (Figure 4A). Formed by macrophages in vivo, an endogenous lipid mediator involved in resolving inflammation, maresin-1 (4Z,7R,8E,10-E,12Z,14S,16Z,19Z)-7,14-dihydroxy-4,8,10,12,16,19-docosahexaenoic acid, is synthesized from docosahexaenoic acid lipoxygenation and acts as a TRPV1 antagonist62 (Figure 4A). Other endogenous lipid-derived mediators have been identified as modulators of TRP channels from nonvanilloid subfamilies or TRP channels in the vanilloid subfamily other than TRPV1; for example, 4-hydroxynonenal produced from polyunsaturated fatty acid peroxidation activates TRPA1,63 and epoxytrienoic acids (EETs), including 5′,6′-epoxyeicosatrienoic acid (5′,6′-EET) produced from epoxygenation of 20-carbon polyunsaturated fatty acids by cytochrome P450, activate TRPV1and TRPV464,65 (Figure 4A).
THERAPEUTICALLY RELEVANT FUNCTIONAL INTERPLAY BETWEEN CANNABINERGIC AND TRP-CHANNEL-MEDIATED SIGNALING
The ability of the synthetic aminoalkylindole cannabinoids R-(+)-WIN55,212-2 and AM1241 (Figure 5) to elicit peripherally mediated antinocieption and antihyperalgesia in acute pain models66 and alleviate capsaicin-induced hyperalgesia/allodynia67,68 prompted investigation of their mechanism of action. Particularly intriguing was the notion that both R-(+)-WIN55,212-2 and AM1241 can inhibit nociceptive sensory neurons while differing in their activation profiles at CB receptors: R-(+)-WIN55,212-2 is a full CB1 agonist,69 whereas AM1241 is a CB2-selective agonist.70 Cellular and in vivo animal data demonstrate that these CB agonists exert peripheral antinocifensive actions against the phytochemicals capsaicin and mustard oil by desensitizing TRPA1 and TRPV1 channels on sensory neurons.71 Thus, these cannabinergic compounds first act to activate TRPA1 and TRPV1, which is then followed by desensitization of these TRP channels.
Figure 5.
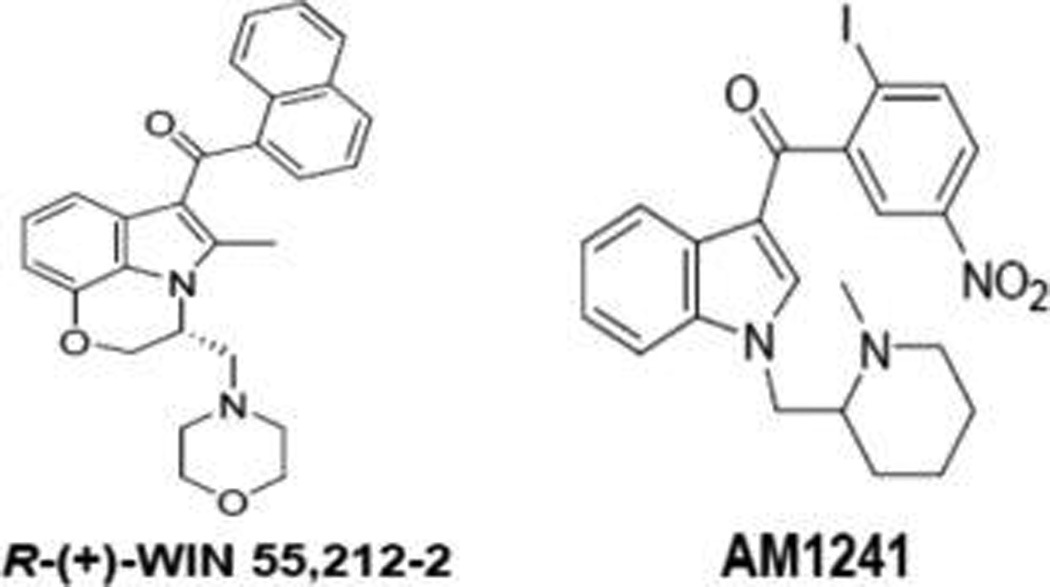
Chemical structures of synthetic aminoalkylindole cannabinoids discussed in the text.
Another level of interaction between endocananbinoid-system and TRP-channel signaling is illustrated by the metabolic cascade responsible for TRPV4 activation by the lipids AEA (Figure 2A) and 5′,6′-EET (Figure 4A). As elucidated by Watanabe et al.,72 enzymatic hydrolysis of the endocananbinoid AEA by FAAH produces arachidonic acid. This FAAH-dependent AEA hydrolysis is a metabolic conversion that is obligatory for AEA to activate TRPV4, since the arachidonic acid so produced serves as substrate for EET production through the cytochrome-P450 epoxygenase pathway. A resulting lipid epoxide product, 5′,6′-EET, is able to activate and open TRPV4, leading to calcium influx into the target cell, a phenomenon important to the physiological modulation of vascular tone and AEA’s vasorelaxant property.
A further example of the functional crosstalk between the endocananibnoid system and TRP channel-mediated information transduction has emerged from a series of laboratory studies in rodents on the mechanism of action of acetaminophen (N-acetyl-p-aminophenol), a widely used over-the-counter analgesic and antipyretic drug.59,73,74 Spanning a decade, these studies by Zygmunt and colleagues demonstrated that the antinociceptive effect of acetaminophen is dependent upon brain TRPV1 and that acetaminophen is biotransformed to the synthetic lipid AM404 through the action of the endocananbinoid-system enzyme, FAAH, in rat and mouse brain. The mechanism of acetaminophen’s TRPV1-mediated antinociception was demonstrated to reflect acetaminophen hepatic metabolism to p-aminophenol, which is subsequently conjugated with arachidonic acid in FAAH-containing neurons expressing TRPV1, leading to the formation of the TRPV1 activator AM404, which directly interacts with this TRP channel to elicit a therapeutic effect (analgesia, reduce fever). Notably, neither acetaminophen nor p-aminophenol interacts with TRPV1. Prior to this work, AM404 was shown to inhibit cellular AEA uptake and cyclooxygenase-1/2 and activate TRPV1.57,59 Thus, AM404’s potent analgesic activity in vivo may reflect its pleiotropic activity profile and effects on multiple endocananbinoid-system and TRP-channel targets.
INVOLVEMENT OF CANNABIBERGIC AND TRP-CHANNEL SIGNALING IN DRUG ABUSE
Potentiation of cannabinergic and TRP-channel signaling by phytochemicals has been linked to substance-abuse-related disorders, with great implications for human health. Stimulation of CB1 in the CNS by the phytocannabinoid Δ9-THC is generally accepted to be the basis for the negative cognitive effects of marijuana and its abuse liability,75 inviting novel medicinal chemistry approaches (e.g., CB1 agonists with limited CNS penetration76) for modulating CB1 activity without inviting adverse psychobehavioral events. Observations that changes in cannabinergic activity and/or endocannabinoid tone have been associated with a variety of physiological challenges and disease states involving the nervous system and most every peripheral organ suggest that the endocananbinoid system contributes to normal physiological conditions by responding to injurious or disease-provoking insults in order to attenuate or delay their potentially damaging consequences and help maintain homeostatic balance.77,78 Thus, modulation of endocannabinoid-system activity has been of great therapeutic interest with respect to two general modalities: (a) regulating endocananbinoid-system activity with an agent whose dosing regimen/molecular pharmacology does not itself invite adverse events; (b) enhancing cyto- and tissue-protective endocannabinoid-system activation in a time-, event-, and tissue/organ-specific manner so as to reduce the potential for adverse responses. Examples of the former modality are the successful introduction into the clinic in certain markets of Sativex, a mixture of Δ9-THC and the nonpsychoactive phytocannabinoid CBD (Figure 1A), for relief of neuropathic pain associated with multiple sclerosis, as an adjunctive pain reliever for advanced cancer, and for treating spasticity due to multiple sclerosis79 and CB1 (periphero-)neutral antagonists for cardiometabolic disease.80 The latter modality would include FAAH inhibitors that enhance the CNS endocannabinoid elevation observed after brain injury to therapeutic levels.10
The well-known cooling sensation of menthol (Figure 3A), a constituent of the wild mint plant (Mentha arvensis), reflects this phytochemical’s ability to trigger chemically the cold-sensitive TRPM8 receptors in cold-sensitive sensory neurons. Menthol also has complex olefactory- and somatosensory-stimulating properties and interacts with TRPA1, an irritant-sensing TRP channel expressed by nociceptors in the lung and respiratory tract.81 In humans, a common haplotype of the gene encoding for TRPA1 provides a functional TRPA1 channel associated with a preference for mentholated cigarettes among heavy smokers.82 This genetic and biological profile along with the ubiquitous presence of menthol as an additive in most commercial cigarettes, the preference for menthol-containing cigarettes during smoking initiation, and the lower smoking-cessation rates for menthol smokers have prompted investigation as to menthol’s potential role in promoting smoking behavior/nicotine addiction and its contribution to the incidence of tobacco-related diseases.83 Data in mice demonstrate that menthol, through TRPM8 activation, acts as a potent respiratory counterirritant to suppress the respiratory irritation caused by a wide variety of irritants in tobacco smoke.84 In this manner, menthol’s biological activity at TRPM8 may facilitate smoke inhalation and promote tobacco smoking/nicotine addiction, an enormous health problem as the leading cause of preventable death and illness underserved by current pharmacotherapeutic strategies.85 In the clinic, the CB1 antagonist/inverse agonist rimonabant has been shown to increase the likelihood that smokers will quit,86 and inhaled CBD reduces cigarette consumption, perhaps by modulating the craving-related salience of smoking cues.87 These aggregate in vivo and clinical data regarding menthol pharmacology support the proposition that TRP-channel and endocannabinoid-system signaling are involved in sustaining tobacco smoking and may be leveraged for therapeutic gain against nicotine addiction.
Perhaps instigated by menthol’s biological properties as related to tobacco smoking, the very potent synthetic TRPM8 agonist, icilin (Figure 3B), has been studied by the tobacco industry as a flavor enhancer for cigarettes.88 It is noteworthy, however, that icilin, but not menthol, requires calcium as a coagonist to attain maximal levels of TRPM8 activation, suggesting that discrete structural requirements must be fulfilled for ligand-induced TRP channel activation and degree of thermosensitivity.89–91 This proposition was indeed supported by mutagenesis experiments demonstrating that specific residues in the cytoplasmic loop interconnecting TMS2 and TMS3 (i.e., N799, D802, and G805) are critical for TRPM8’s icilin sensitivity, just as residues in analogous positions (i.e., Y511 and S512) are critical for activation of TRPV1 by capsaicin.91 Further evidence for ligand-sensitive functional domains in TRP channels comes from demonstration that many noxious TRPA1-activating compounds are electrophiles whose covalent modification of select reactive cysteine residues in this TRP channel is critical for the rapid signaling of potential tissue damage through the neural pain pathway.92 Despite their noxious and abuse-related properties, then, some phytochemicals have proven their value for interrogating the molecular mechanisms by which TRP channels are activated.
CONCLUSIONS
Both naturally occurring and synthetic terpenes and lipids stimulate cannabinergic or TRP channel-mediated signaling in mammals. Some of these signaling molecules are co-opted by both information systems and are able to act at both discrete CB and TRP-channel protein targets. Interaction of phytochemcials and synthetic ligands with both CB receptors and TRP channels and the significant degree to which CB1 and TRPV1 are coexpressed in several brain regions (including the hypothalamus, striatum, hippocampus and substantia nigra)93 carry implications for human health and disease treatment. For instance, demonstration that the competitive TRPV1 antagonist AMG9810 (Figure 3B) further reduces the inflammatory activation of human endothelial cells elicited by the synthetic CB R-(+)-WIN55,212-2 or the naturally occurring CB1 and TRPV1 agonist NADA, whereas TRPV1 inhibition with AMG9810 alone potentiated the inflammation suggests that cannabinergic and TRP-mediated signaling work in concert to regulate endothelial inflammatory sensitivity/homeostasis.94 The anticonvulsant effect of dual FAAH and TRPV1 blockade with N-arachidonoyl-serotonin depends upon potentiation of CB1 activity as a result of the increased AEA levels consequent to FAAH inhibition with a component of TRPV1 blockade against the neuroexcitatory effect of TRPV1 activation by AEA.95 Such findings suggest that discrete functional and pharmacological interactions between TRP channels and endocannabinoid-system proteins offer opportunities to develop novel, dual-acting ligands both as both probes for interrogation of their independent and integrative functionality and as drugs that modulate these two biosignaling systems for therapeutic gain.
Acknowledgments
We thank Mr. Mitchel Ayer for expert bibliographic assistance.
ABBREVIATIONS
- CB
cannabinoid
- TRP
transient receptor potential
- CB1
cannabinoid 1 receptor
- (−)-Δ9-THC
(−)-Δ9-tetrahydrocannabinol
- CB2
cannabinoid 2 receptor
- GPCR
G-protein coupled receptor
- 2-AG
2-arachidonoylglycerol
- AEA
anandamide
- CNS
central nervous system
- MGL
monoacylglycerol lipase
- ABHD6
α,β-hydrolase domain-containing protein 6
- ABHD12
α,β-hydrolase domain-containing protein 12
- FAAH
fatty acid amide hydrolase
- TMS
transmembrane segment
- TRPV
transient receptor potential vanilloid
- (−)-Δ8-THC
(−)-Δ8-tetrahydrocannabinol
- CBD
cannabidiol
- Δ9-THCV
Δ9-tetrahydrocannabivarin
- ACPA
arachidonoylcyclopropylamide
- TRPA1
transient potential receptor channel ankyrin 1
- TRPM8
transient receptor potential channel melastatin 8
- OEA
oleoylethanolamide
- PPAR-α
peroxisome proliferator-activated receptor alpha
- NADA
N-arachidonoyl dopamine
- LTB4
leukotriene B4
- 12-HPETE
12-hydroperoxyeicosatrie-noic acid
- EET
epoxytrienoic acid
- 5′,6′-EET
5′,6′- epoxytrienoic acid
Footnotes
Author Contributions
Both authors contributed to the writing of the manuscript.
Notes
The authors declare no competing financial interest.
REFERENCES
- 1.Machnicka B, Czogalla A, Hryniewicz-Jankowska A, Bogusławska DM, Grochowalaka R, Heger E, Sikorski AF. Spectrins: a structural platform for transporters and activation of membrane channels, receptors and transporters. Biochim. Biophys. Acta. 2014;1838(2):620–634. doi: 10.1016/j.bbamem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Console-Bram L, Marcu J, Abood ME. Cannabinoid receptors: nomenclature and pharmacological principles. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;38(1):4–15. doi: 10.1016/j.pnpbp.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharamcol. Res. 2009;60(2):77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Luchicchi A, Pistis M. Anandamide and 2-arachidonoylglycerol: pharmacological properties, functional features, and emerging specificities of the two major endocannabinoids. Mol. Neurobiol. 2012;46(2):374–392. doi: 10.1007/s12035-012-8299-0. [DOI] [PubMed] [Google Scholar]
- 5.Ueda N, Tsuboi K, Uyama T. Metabolism of endocannabinoids and related N-acylethanolamines: canonical and alternative pathways. FEBS J. 2013;280(9):1874–1894. doi: 10.1111/febs.12152. [DOI] [PubMed] [Google Scholar]
- 6.Ohno-Shosaku T, Tanimura A, Hashimotodani Y, Kano M. Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist. 2012;18(2):119–132. doi: 10.1177/1073858410397377. [DOI] [PubMed] [Google Scholar]
- 7.Yao B, Mackie K. Endocannabinoid receptor pharmacology. Curr. Top. Behav. Neurosci. 2009;1:37–63. doi: 10.1007/978-3-540-88955-7_2. [DOI] [PubMed] [Google Scholar]
- 8.Pacher P, Kunos G. Modulating the endocananbinoid system in human health and disease- successes and failures. FEBS J. 2013;280(9):1918–1943. doi: 10.1111/febs.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janero DR, Lindsley L, Vemuri VK, Makriyannis A. Cannabinoid 1 G protein-coupled receptor (periphero-)neutral antagonists: emerging therapeutics for treating obesity-driven metabolic disease and reducing cardiovascular risk. Expert Opin. Drug Discovery. 2011;6(10):995–1025. doi: 10.1517/17460441.2011.608063. [DOI] [PubMed] [Google Scholar]
- 10.Hwang J, Adamson C, Butler D, Janero DR, Makriyannis A, Bahr BA. Enhancement of endocannabinoid signaling by fatty acid amide hydrolase inhibition: a neuroprotective therapeutic modality. Life Sci. 2010;86(15–16):615–623. doi: 10.1016/j.lfs.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dadon D, Minke B. Cellular functions of transient receptor potential channels. Int. J. Biochem. Cell Biol. 2010;42(9):1430–1445. doi: 10.1016/j.biocel.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J. Molecular mechanism of TRP channels. Compr. Physiol. 2013;3(1):221–242. doi: 10.1002/cphy.c120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br. J. Pharmacol. 2012;165(4):787–801. doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson PL, Beck A, Cheng H. Transient receptor proteins illuminated: current views on TRPs and disease. Vet. J. 2011;187(2):153–164. doi: 10.1016/j.tvjl.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Wescott SA, Rauthan M, Xu XZ. When a TRP goes bad: transient receptor potential channels in addiction. Life Sci. 2013;92(8–9):410–414. doi: 10.1016/j.lfs.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moran MM, McAlexander MA, Biro T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discovery. 2011;10(8):601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 17.Quadid-Ahoidouch H, Dhennin-Duthille I, Gautoier M, Sevestere H, Ahidouch A. TRP channels: diagnostic markers and therapeutic targets for breast cancer? Trends Mol. Med. 2013;19(2):117–124. doi: 10.1016/j.molmed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Takeda Y, Numata T, Mori Y. Targeting TRPs in neurodegenerative disorders. Curr. Top. Med. Chem. 2013;13(3):322–334. doi: 10.2174/1568026611313030009. [DOI] [PubMed] [Google Scholar]
- 19.Di Marzo V, De Petrocellis L. Endocananbinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 2010;17(14):1430–1439. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- 20.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400(6743):452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 21.Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010;17(14):1360–1381. doi: 10.2174/092986710790980050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harteneck C, Klose C, Krautwurst D. Synthetic modulators of TRP channel activity. Adv. Exp. Med. Biol. 2011;704:87–106. doi: 10.1007/978-94-007-0265-3_4. [DOI] [PubMed] [Google Scholar]
- 23.Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol. 2006;147(Supplement 1):S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86(8):1646–1647. [Google Scholar]
- 25.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ8-tetrahydrocannabivarin. Br. J. Pharmacol. 2008;153(2):199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J. Clin. Pharmacol. 2002;42(11 Supplement):11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- 27.Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009;30(10):515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Raffa RB, Ward SJ. CB1-independent mechanisms of Δ9 -THCV, AM251 and SR141716. J. Clin. Pharm. Ther. 2012;37(3):260–265. doi: 10.1111/j.1365-2710.2011.01284.x. [DOI] [PubMed] [Google Scholar]
- 29.Ottani A, Giuliani D. HU 210: a potent tool for investigations of the cannabinoid system. CNS Drug Rev. 2001;7(2):131–145. doi: 10.1111/j.1527-3458.2001.tb00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thnakur GA, Bajaj S, Paronis C, Peng Y, Bowman AL, Barak LS, Caron MG, Parrish D, Deschamps JR, Makriyannis A. Novel adamantyl cannabinoids as CB1 receptor probes. J. Med. Chem. 2013;56(10):3904–3921. doi: 10.1021/jm4000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picone RP, Khanolkar AD, Ayotte LA, Thakur GA, Hurst DP, Abood ME, Reggio PH, Fournier DJ, Makriyannis A. (−)-7’-Isothiocyanato-11-hydroxy-1’,1’-dime-thylheptylhexahydrocannabinol (AM841), a high-affinity electrophilic ligand, interacts covalently with a cysteine in helix six and activates the CB1 cannabinoid receptor. Mol. Pharmacol. 2005;68(6):1623–1635. doi: 10.1124/mol.105.014407. [DOI] [PubMed] [Google Scholar]
- 32.Nikas SP, Alapafuja SO, Papanastasiou I, Paronis CA, Shukla VG, Papahatjis DP, Bowman AL, Halikhedkar A, Han X, Makriyannis A. Novel 1’,1’-chain substituted hexahydrocannabinols: 9β-hydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahy-drocannabinol (AM2389) a highly potent cannabinoid receptor 1 (CB1) agonist. J. Med. Chem. 2010;53(19):6996–7010. doi: 10.1021/jm100641g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szymanski DW, Papanastasiou M, Melchior K, Zvonok N, Mercier RW, Janero DR, Thanur GA, Cha S, Wu B, Karger B, Makriyannis A. Mass spectrometry-based proteomics of human cannabinoid receptor 2: covalent cysteine 6.47(257)-ligand interaction affording megagonist receptor activation. J. Proteome Res. 2011;10(10):4789–4798. doi: 10.1021/pr2005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma R, Nikas SP, Paronis CA, Wood JT, Halikhedkar A, Guo JJ, Thakur GA, Kulkarni S, Benchama O, Raghav JG, Gifford RS, Järbe TU, Bergman J, Makriyannis A. Controlled-deactivation cannabinergic ligands. J. Med. Chem. 2013;56(24):10142–10157. doi: 10.1021/jm4016075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, Murphy V, Pertwee RG, Campbell WB. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J. Pharmacol. Exp. Ther. 1999;289(3):1427–1433. [PubMed] [Google Scholar]
- 36.Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J. Med. Chem. 1994;37(12):1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- 37.Papahatjis DP, Nahmias VR, Nikas SP, Schimpgen M, Makriyannis A. Design and synthesis of (13S)-methyl-substituted arachidonic acid analogues: templates for novel endocannabinoids. Chemistry. 2010;16:4091–4099. doi: 10.1002/chem.200902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor—a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 39.Barbero GF, Ruiz AG, Liazid A, Palma M, Vera JC, Barroso CG. Evolution of total and individual capsaicinoids in peppers during ripening of the Cayenne pepper plant (Capsicum annuum L.) Food Chem. 2014;153:200–206. doi: 10.1016/j.foodchem.2013.12.068. [DOI] [PubMed] [Google Scholar]
- 40.Szallasi A, Blumberg PM. Resiniferatoxin and its analogs provide novel insights into the pharmacology of the vanilloid (capsaicin) receptor. Life Sci. 1990;47(16):1399–1408. doi: 10.1016/0024-3205(90)90518-v. [DOI] [PubMed] [Google Scholar]
- 41.De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG, Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011;163(7):1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Petrocellis L, Orlando P, Moriello AS, Aviello G, Stott C, Izzo AA, Di Marzo V. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2012;204(2):255–266. doi: 10.1111/j.1748-1716.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- 43.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 44.Chung G, Im ST, Kim YH, Jung SJ, Rhyu MR, Oh SB. Activation of transient receptor potential ankyrin 1 by eugenol. Neuroscience. 2014;261:153–160. doi: 10.1016/j.neuroscience.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 45.Dedov VN, Tran VH, Duke CC, Connor M, Christie MJ, Mandadi S, Roufogalis BD. Gingerols: a novel class of vanilloid receptor (VR1) agonists. Br. J. Pharmacol. 2002;137(6):793–798. doi: 10.1038/sj.bjp.0704925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong J, Minassi A, Prenen J, Taglialatela-Scafati O, Appendino G, Nilius B. Umbellulone modulates TRP channels. Pfluegers Arch. 2011;462(6):861–870. doi: 10.1007/s00424-011-1043-1. [DOI] [PubMed] [Google Scholar]
- 47.Chianese G, Fattorusso E, Putra MY, Calcinai B, Bavestrello G, Moriello AS, De Petrocellis L, Di Marzo V, Taglialatela-Scafati O. Leucettamols, bifunctionalized marine sphingoids, act as modulators of TRPA1 and TRPM8 channels. Mar. Drugs. 2012;10(11):2435–2447. doi: 10.3390/md10112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morera E, De Petrocellis L, Morera L, Moriello AS, Nalli M, Di Marzo V, Ortar G. Synthesis and biological evaluation of [6]-gingerol analogues as transient receptor potential channel TRPV1 and TRPA1 modulators. Bioorg. Med. Chem. Lett. 2012;22(4):1674–1677. doi: 10.1016/j.bmcl.2011.12.113. [DOI] [PubMed] [Google Scholar]
- 49.Bang S, Yoo S, Oh U, Hwang SW. Endogenous lipid-derived ligands for sensory TRP ion channels and their pain modulation. Arch. Pharm. Res. 2010;33(10):1509–1520. doi: 10.1007/s12272-010-1004-9. [DOI] [PubMed] [Google Scholar]
- 50.Palazzo E, Rossi F, de Novellis V, Maione S. Endogenous modulators of TRP channels. Curr. Top. Med. Chem. 2013;13(3):398–407. doi: 10.2174/1568026611313030014. [DOI] [PubMed] [Google Scholar]
- 51.Bradshaw HB, Raboune S, Hollis JL. Opportunistic activation of TRP receptors by endogenous lipids: exploiting lipidomics to understand TRP receptor cellular communication. Life Sci. 2013;92(8–9):404–409. doi: 10.1016/j.lfs.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syed SK, Bui HH, Beavers LS, Farb TB, Ficorilli J, Chesterfield AK, Kuo MS, Bokvist K, Barrett DG, Efanov AM. Regulation of GPR119 receptor activity with endocannabinoid-like lipids. Am. J. Physiol. Endocrinol. Metab. 2012;303(12):E1469–E1478. doi: 10.1152/ajpendo.00269.2012. [DOI] [PubMed] [Google Scholar]
- 53.Maione S, De Petrocellis L, de Novellis V, Moriello AS, Petrosino S, Palazzo E, Rossi FS, Woodward DF, Di Marzo V. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br. J. Pharmacol. 2007;150(6):766–781. doi: 10.1038/sj.bjp.0707145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sagar DR, Smith PA, Millns PJ, Smart D, Kendall DA, Chapman V. TRPV1 and CB(1) receptor-mediated effects of the endovanilloid/endocannabinoid N-arachidonoyl-dopamine on primary afferent fibre and spinal cord neuronal responses in the rat. Eur. J. Neursci. 2004;20(1):175–184. doi: 10.1111/j.1460-9568.2004.03481.x. [DOI] [PubMed] [Google Scholar]
- 55.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99(12):8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Marzo V, Griffin G, De Petrocellis L, Brandi I, Bisogno T, Williams W, Grier MC, Kulasegram S, Mahadevan A, Razdan RK, Martin BR. A structure/activity relationship study on arvanil, an endocannabinoid and vanilloid hybrid. J. Pharmacol. Exp. Ther. 2002;300(3):984–991. doi: 10.1124/jpet.300.3.984. [DOI] [PubMed] [Google Scholar]
- 57.Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. science. 1997;277(5329):1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- 58.Rawls SM, Ding Z, Cowan A. Role of TRPV1 and cannabinoid CB1 receptors in AM 404-evoked hypothermia in rats. Pharmacol. Biochem. Behav. 2006;83(4):508–516. doi: 10.1016/j.pbb.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 59.Högestätt ED, Jönsson BA, Ermund A, Andersson DA, Björk H, Alexander JP, Cravatt BF, Basbaum AI, Zygmunt PM. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J. Biol. Chem. 2008;280(36):31405–31412. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- 60.Fernandes ES, Vong CT, Quek S, Cheong J, Awal S, Gentry C, Aubdool AA, Liang L, Bodkin JV, Bevan S, Heads R, Brain SD. Superoxide generation and leukocyte accumulation: key elements in the mediation of leukotriene B4-induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB J. 2013;27(4):1664–1673. doi: 10.1096/fj.12-221218. [DOI] [PubMed] [Google Scholar]
- 61.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97(11):6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26(4):1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andrè E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. U.S.A. 2007;104(33):13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sisignano M, Park CK, Angioni C, Zhang DD, von Hehn C, Cobos EJ, Ghasemlou N, Xu ZZ, Kumaran V, Lu R, Grant A, Fischer MJ, Schmidtko A, Reeh P, Ji RR, Woolf CJ, Geisslinger G, Scholich K, Brenneis C. 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J. Neurosci. 2012;32(18):6364–6372. doi: 10.1523/JNEUROSCI.5793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ. Res. 2005;97(9):908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 66.Malan TP, Jr, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr. Opin. Pharmacol. 2003;3(1):62–67. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 67.Li J, Daughters RS, Bullis C, Bengiamin R, Stucky MW, Brennan J, Simone DA. The cannabinoid receptor agonist WIN 55,212-2 mesylate blocks the development of hyperalgesia produced by capsaicin in rats. Pain. 1999;81(1–2):25–33. doi: 10.1016/s0304-3959(98)00263-2. [DOI] [PubMed] [Google Scholar]
- 68.Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, Malan TP., Jr Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99(4):955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- 69.D’Ambra TE, Estep KG, Bell MR, Eissenstat MA, Josef KA, Ward SJ, Haycock DA, Baizman ER, Casiano FM, Beglin NC, Chippari SM, Grego JD, Kullnig RK, Daley GT. Conformationally restrained analogues of pravadoline: nanomolar potent, enantioselective, (aminoalkyl)indole agonists of the cannabinoid receptor. J. Med. Chem. 1992;35(1):124–135. doi: 10.1021/jm00079a016. [DOI] [PubMed] [Google Scholar]
- 70.Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93(3):239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 71.Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J. Neurosci. 2008;28(5):1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 73.Zygmunt PM, Chuang H, Movahed P, Julius D, Högestätt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur. J. Pharmacol. 2000;396(1):39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]
- 74.Barrière DA, Mallet C, Blomgren A, Simonsen C, Daulhac L, Libert F, Chapuy E, Etienne M, Högestätt ED, Zygmunt PM, Eschalier A. Fatty acid amide hydrolase-dependent generation of antinociceptive drug metabolites acting on TRPV1 in the brain. PLoS One. 2013;5(9):e12748. doi: 10.1371/journal.pone.0070690. DOI: doi:10.1371/journal.-pome.0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maldonado R, Berrendero F, Ozaita A, Robledo P. Neurochemical basis of cannabis addiction. Neuroscience. 2011;181:1–17. doi: 10.1016/j.neuroscience.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 76.Pryce G, Visintin C, Ramagopalan SV, Al-Izki S, De Faveri LE, Nuamah RA, Mein CA, Montpetit A, Hardcastle AJ, Kooij G, de Vries HE, Amor S, Thomas SA, Ledent C, Marsicano G, Lutz B, Thompson AJ, Selwood DL, Giovannoni G, Baker D. Control of spasticity in a multiple sclerosis model using central nervous system-excluded CB1 cannabinoid receptor agonists. FASEB J. 2014;28(1):117–130. doi: 10.1096/fj.13-239442. [DOI] [PubMed] [Google Scholar]
- 77.Miller LK, Devi LA. The highs and lows of cannabinoid receptor expression in disease: mechanisms and their therapeutic implications. Pharmacol. Rev. 2011;63(3):461–470. doi: 10.1124/pr.110.003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rani Sagar D, Burston JJ, Woodhams SG, Chapman V. Dynamic changes to the endocannabinoid system in models of chronic pain. Philos. Trans. R. Soc. London, Ser. B. 2012;367(1607):3300–3311. doi: 10.1098/rstb.2011.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.García-Merino A. Endocannabinoid system modulator use in everyday clinical practice in the UK and Spain. Expert Rev. Neurother. 2013;13(Supplement 1):9–13. doi: 10.1586/ern.13.4. [DOI] [PubMed] [Google Scholar]
- 80.Janero DR, Lindsley L, Vemuri VK, Makriyannis A. Cannabinoid 1 G protein-coupled receptor (periphero-)neutral antagonists: emerging therapeutics for treating obesity-driven metabolic disease and reducing cardiovascular risk. Expert Opin. Drug Discovery. 2011;6(10):995–1025. doi: 10.1517/17460441.2011.608063. [DOI] [PubMed] [Google Scholar]
- 81.Farco JA, Grundmann O. Menthol— pharmacology of an important naturally medicinal “cool”. Mini Rev. Med. Chem. 2013;13:124–131. [PubMed] [Google Scholar]
- 82.Uhl GR, Walther D, Behm FM, Rose JE. Menthol preference among smokers: association with TRPA1 variants. Nicotine Tob. Res. 2011;13(12):1311–1315. doi: 10.1093/ntr/ntr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson SJ. Menthol cigarettes and smoking cessation behaviour: a review of tobacco industry documents. Tob. Control. 2011;20(Supplement 2):49–56. doi: 10.1136/tc.2010.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 2011;25(12):4434–4444. doi: 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA, J. Am. Med. Assoc. 2014;311(2):193–194. doi: 10.1001/jama.2013.283787. [DOI] [PubMed] [Google Scholar]
- 86.Le Foll B, Forget B, Aubin HJ, Goldberg SR. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict. Biol. 2008;13(2):239–252. doi: 10.1111/j.1369-1600.2008.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict. Behav. 2013;38(9):2433–2436. doi: 10.1016/j.addbeh.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 88.RJ. Reynolds Tobacco Company Annual Performance Plan Review 1989 (890000) Available at: http://tobaccodocuments.org/rjr/507930836-0893.html?start_page=1&end_page=58.
- 89.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 90.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430(7001):748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 91.Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron. 2004;43(6):859–869. doi: 10.1016/j.neuron.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 92.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445(7127):541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 93.Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139(4):1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 94.Wilhelmsen K, Khakpour S, Tran A, Sheehan K, Schumacher M, Xu F, Hellman J. The endocannabinoid/endovanilloid N-arachidonoyl dopamine (NADA) and synthetic cannabinoid WIN55,212-2 abate the inflammatory activation of human endothelial cells. J. Biol. Chem. 2014;289:13079–13100. doi: 10.1074/jbc.M113.536953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vilela LR, Medeiros DC, de Oliveira AC, Moraes MF, Moreira FA. Anticonvulsant Effects of N-arachidonoyl-serotonin, a dual fatty acid amide hydrolase enzyme and transient receptor potential vanilloid Type-1 (TRPV1) channel blocker, on experimental seizures: The roles of cannabinoid CB1 receptors and TRPV1 channels. Basic Clin. Pharmacol. Toxicol. 2014 doi: 10.1111/bcpt.12232. DOI: 10.1111/bcpt.12232. [DOI] [PubMed] [Google Scholar]


