Introduction
Poor adherence to asthma controller medications, such as inhaled corticosteroids (ICS), is frequent among asthma patients.1 This behavior may contribute to worsened clinical outcomes, including increased need for short courses of oral corticosteroids,2 increased risk of hospitalization,3 and increased risk of mortality from asthma.4
Conventional interventions for poor adherence include removing barriers to adherence, home visits, patient education, and school-based asthma care. However, in many patients these interventions may not be successful.5
Previously we tried to improve adherence in patients with high resource utilization by having a nurse make daily home visits to supervise controller medication administration.6 This reduced total hospitalization days for asthma from 70 in the year before intervention to 24 in the year after the intervention among seven children with very poor asthma control due to poor adherence to ICS. However Florida Medicaid and other third-party payers no longer cover payments for this method of intervention.
Accordingly we hypothesized that once or twice monthly administration of omalizumab (Xolair®, Genentech, Inc., South San Francisco, California, and Novartis Pharmaceuticals Corporation, East Hanover, New Jersey) would circumvent the challenge of daily adherence and, thus, improve outcomes in patients whose asthma was not well controlled because of poor adherence to ICS.
Omalizumab is an anti-immunoglobulin E (IgE) monoclonal antibody that binds circulating free IgE with a subsequent reduction in the number of high affinity receptors on mast cells and thereby decreases mast cell release of inflammatory mediators in asthma.7
Adenosine 5′-monophosphate (AMP) enhances the release of inflammatory mediators from activated mast cells and airway responsiveness to AMP is a marker of allergic airway inflammation.8,9 By using AMP as a surrogate of clinical effectiveness, we were able to improve the power of the study while minimizing the number of patients needed for a single center study. As an indicator of asthma control, the number of prednisone bursts required during the study was a secondary outcome measure.
Methods
Patients
This study included patients (ages 6–26 yr) with persistent asthma for whom ICS were prescribed for at least 3 months, either alone or in combination with a long-acting ß2-agonist or leukotriene modifier. They had poor asthma control (defined by any of the following: FEV1 < 80% predicted, short- acting ß-agonist use > 3 times/wk, nocturnal symptoms > 2 times/mo, exercise-induced bronchospasm from activities of daily living, unscheduled physician visits or hospitalization for asthma, or > 1 prednisone burst in previous 3 months). Other inclusion criteria were a pharmacy prescription refill history of < 50% of prescribed doses of ICS for ≥ 3 months; sensitization to one or more indoor allergens or outdoor altenaria; total IgE of 30 to 700 IU/ml for patients ≥ 12 years or up to 1,300 IU/ml for those 6 to 12 years; baseline FEV1 ≥ 60% predicted; and a 20% decrease in FEV1 after inhaling ≤ 60 mg/ml of AMP (i.e., PC20 FEV1 ≤ 60 mg/ml).
Patients were excluded if they had smoked in the past 12 months or had a smoking history of > 10 pack years, were pregnant or lactating, had a respiratory tract infection in the past 6 weeks, or had an omalizumab dosage requirement > 375 mg every 2 weeks.
This study was conducted under an investigator-sponsored Investigational New Drug Application approved by the US Food and Drug Administration (IND #70,241) for use of AMP challenge and study of children < 12 yr, and was approved by the University of Florida Institutional Review Board. All patients or parents gave written informed consent and children gave verbal assent.
Study Design
This was a randomized, double-blind, three-period, placebo-controlled, crossover study (Figure 1). Patients received omalizumab or placebo by subcutaneous injection every 2 or 4 weeks for 4 months, followed by a 3- to 4-month washout period and then 4 months of the opposite treatment. FEV1 was measured at each treatment visit; AMP PC20 was measured before and after each treatment period (see E-Supplement for details). Health care utilization was recorded at each visit and prescription refill histories were obtained from their pharmacies after the screening visit and upon discharge from the study. Patients were not asked to measure peak flow or record symptoms in a diary because they were poorly adherent to ICS and it was assumed that they would not reliably record in a diary.
Figure 1.
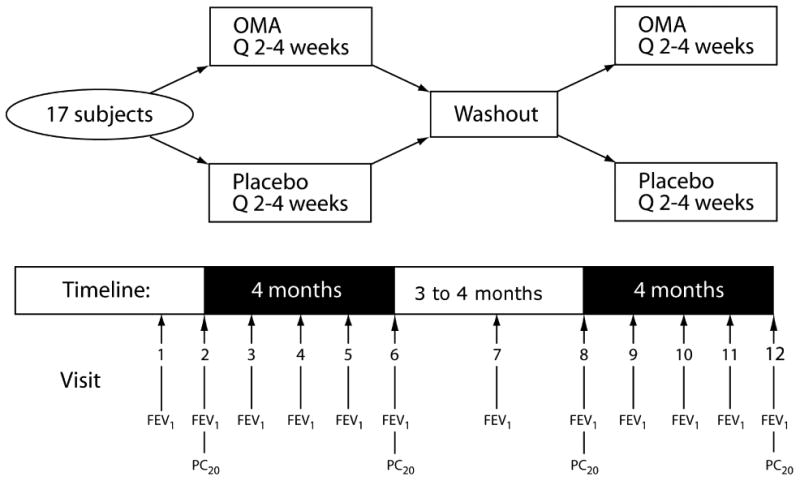
Placebo or OMA was administered every 2 or 4 weeks. FEV1 was measured at every study visit; PC20 FEV1 to adenosine-5′-monophosphate challenge was measured before and after each treatment period.
Statistical Analysis
A sample size of 16 patients was calculated (based on reported reproducibility of AMP PC20 in 13 subjects with asthma with a log standard deviation of 0.4)10 to provide 95% power to detect a 2-fold difference in PC20 between treatments.
The regression method of Shuster11 was used to compare the treatments. This method takes the period 2 less period 1 difference (irrespective of treatment assignment) and compares the two treatment orderings. The effect size estimate is superior to the one-sample t-test in that it is unbiased when the actual sample sizes assigned to the orderings differ, is more efficient, and adjusts for carryover effects. The dependent primary variable was the difference in the change in natural log final PC20 less baseline (period 2 less period 1). Note that two patients had post-dose values that could not be ascertained, except they were known to be above 200 mg/ml, the highest AMP concentration administered. These values were assigned 200 mg/ml. FEV1 was compared in the same way, except logs were not used. A fitted regression model was used to determine the prognostic importance of baseline PC20 and FEV1 on change in PC20 during active treatment. The number of steroid bursts was compared by the Friedman test. Median ICS use per month was assessed by the Sign test for obtaining 95% confidence intervals. A p value less than 0.05 was considered statistically significant.
Results
Patients
Of 104 patients screened, 17 were randomized and 15 completed both treatment periods (Table 1). The most common reasons for screen failure included no positive allergens detected by blood test for specific allergens (ImmunoCAP) (n = 16); FEV1 < 60% predicted (n = 12), and a combination of body weight and total IgE that would require an omalizumab dosage higher than 375 mg every 2 weeks (n = 12). The two randomized patients who failed to complete the study discontinued because they moved out of the area.
Table I. Patient Disposition.
| No. of Patients | |
|---|---|
| Total Screened | 104 |
| Total Screen Failures | 87 |
| Reasons for screen failure: | |
| No positive allergens | 16 |
| FEV1 < 60% predicted | 12 |
| Combination of total body weight and IgE that would require dosage of omalizumab > 375 mg Q 2 weeks | 12 |
| Unable to perform ATS acceptable and reliable spirometry | 9 |
| IgE < 30 IU | 9 |
| Lack of evidence for poor asthma control | 8 |
| PC20 > 60 mg/ml | 8 |
| Other* | 13 |
| Total randomized | 17 |
| Total completed both treatment periods | 15 |
| Discontinued because of relocation out of area | 2 |
Definition of abbreviations: IgE = immunoglobulin E; Q = every; ATS = American Thoracic Society.
Includes failure to return, abnormal electrocardiogram, positive result for illicit drugs, abnormal laboratory values, or inability to withhold medications as required by the protocol.
Of the 17 patients randomized to treatment (10 females, 7 males), the mean (± SD) age was 16.4 ± 5.5 years; five patients were aged 6 to 12 years. The mean baseline FEV1 was 83.7% predicted, geometric mean PC20 was 14.1 mg/ml, mean total IgE level was 427 IU (range 95–956), and doses of omalizumab ranged from 300 to 375 mg (Table 2).
Table II. Patient Demographics and Baseline Characteristics.
| Characteristic | Randomized Patients (n = 17) |
|---|---|
| Age, y (mean ± SD) | 16.4 ± 5.5 |
| Gender, n | |
| Female | 10 |
| Male | 7 |
| Race, n (%) | |
| Caucasian | 11 (64) |
| African American | 4 (24) |
| Asian | 1 (6) |
| Hispanic | 1 (6) |
| Weight, kg (mean ± SD) | 63.0 ± 20.2 |
| FEV1, % predicted (mean ± SD) | 83.7 ± 11.8 |
| PC20, mg/ml (Geometric mean [95% CI]) | 14.1 [10.8, 18.4] |
| Total IgE, IU (mean ± SD) | 427 ± 275 |
| ICS refills/mo* (median [95% CI]) | 0.17 (0.14,0.24) |
| Calculated omalizumab dose for study, mg | |
| Mean ± SD | 313 ± 38 |
| Frequency: Every 2 weeks, n | 10 |
| Every 4 weeks, n | 7 |
SD = standard deviation; CI = confidence interval; IgE = immunoglobulin E; ICS = inhaled corticosteroids.
12 months prior to study entry
Primary and Secondary Endpoints
In the 15 patients who completed the study, the geometric mean PC20 increased from 10.8 to 33.9 mg/ml during omalizumab treatment, while decreasing from 20.1 to 18.5 mg/ml during the placebo period (Table 3). Thus, the primary endpoint—geometric fold change in PC20 from baseline to end of treatment—was significantly improved with omalizumab versus placebo (3.1 vs. 0.9, p = 0.022; Figure 2). Based on the regression analysis, the point interval and 95% confidence interval for the ratio of fold changes (geometric means), omalizumab: placebo was 3.4 (1.23, 9.25). Per protocol, the washout period was extended 1 month when the PC20 did not return to baseline after washout. This occurred in five of the eight patients who received omalizumab treatment first, compared with two of the eight patients who received placebo first. Change in PC20 during omalizumab treatment showed no relationship with baseline values of either FEV1 or PC20. Also, there was no significant change in FEV1 during either treatment (the mean FEV1 value increased by 6% during both placebo and omalizumab treatment periods) (Table 3).
Table III. Individual and Mean Results for Spirometry and Adenosine Challenge (n =15 patients who completed both placebo and omalizumab treatment periods).
| Placebo | Omalizumab | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FEV1 (Liters) | PC20 | FEV1 (Liters) | PC20 | |||||||||
| Subject | Before | End | % Change | Before | End | Fold Change* | Before | End | % Change | Before | End | Fold Change* |
| Order = OMA/Placebo | ||||||||||||
| 1 | 1.85 | 1.96 | 6 | 146.4 | 19.93 | 0.1 | 1.90 | 1.72 | -9 | 10.3 | 182.34 | 17.8 |
| 4 | 3.68 | 3.96 | 8 | 60.8 | 72.09 | 1.2 | 4.02 | 3.98 | -1 | 39.8 | 30.46 | 0.8 |
| 9 | 2.08 | 2.65 | 27 | 17.1 | 20.36 | 1.2 | 2.47 | 2.43 | -2 | 12.2 | 35.74 | 2.9 |
| 14†† | 2.21 | 2.42 | 10 | 16.6 | 15.89 | 1.0 | 2.01 | 1.99 | -1 | 13.8 | 117.28 | 8.5 |
| 16 | 3.44 | 3.64 | 6 | 31.2 | 29.34 | 0.9 | 2.69 | 2.87 | 7 | 9.22 | 4.81 | 0.5 |
| 21 | 2.48 | 2.69 | 8 | 4.40 | 16.24 | 3.7 | 2.61 | 2.50 | -4 | 9.67 | 4.93 | 0.5 |
| 36 | 4.42 | 4.31 | -2 | 85.8 | 22.53 | 0.3 | 4.20 | 4.58 | 9 | 19.2 | 65.06 | 3.4 |
| Order = Placebo/OMA | ||||||||||||
| 3 | 2.63 | 2.89 | 10 | 19.7 | 54.23 | 2.8 | 2.70 | 2.73 | 1 | 65.2 | >200 | 3.1 |
| 6†† | 4.13 | 4.26 | 3 | 12.3 | 7.80 | 0.6 | 3.59 | 4.82 | 34 | 3.10 | 15.51 | 5.0 |
| 10†† | 2.55 | 2.81 | 10 | 10.9 | 5.23 | 0.5 | 2.39 | 3.22 | 35 | 2.45 | 5.93 | 2.4 |
| 13†† | 3.14 | 3.25 | 4 | 9.30 | 52.78 | 5.7 | 2.88 | 3.14 | 9 | 5.17 | 141.30 | 27.3 |
| 27†† | 2.81 | 2.60 | -7 | 5.50 | 2.64 | 0.5 | 2.27 | 2.40 | 6 | 1.05 | 4.45 | 4.2 |
| 30 | 3.23 | 3.19 | -1 | 40.8 | 158.09 | 3.9 | 3.23 | 3.35 | 4 | 27.8 | >200 | 7.2 |
| 34 | 2.44 | 2.26 | -7 | 15.4 | 2.91 | 0.2 | 2.54 | 2.47 | -3 | 20.2 | 90.51 | 4.5 |
| 38†† | 1.80 | 2.02 | 12 | 13.9 | 14.81 | 1.1 | 1.96 | 2.16 | 10 | 17.6 | 11.86 | 0.7 |
| Mean | 2.86 | 2.99 | 6 | 2.76 | 2.96 | 6 | ||||||
| SD | 0.80 | 0.76 | 9 | 0.71 | 0.91 | 13 | ||||||
| Geometric Mean | 20.1 | 18.5 | 0.9 | 10.8 | 33.9 | 3.1† | ||||||
| 95% CI | 11.7,34.6 | 9.8,35.1 | 0.5,1.7 | 5.9,19.7 | 15.0,76.8 | 1.6,6.2 | ||||||
Fold change = PC20 end/PC20 beginning of period. The log of these values was used in the statistical analysis.
Significantly greater than placebo (p = 0.022).
Received prednisone during placebo. Subject #10 required two courses.
Figure 2.
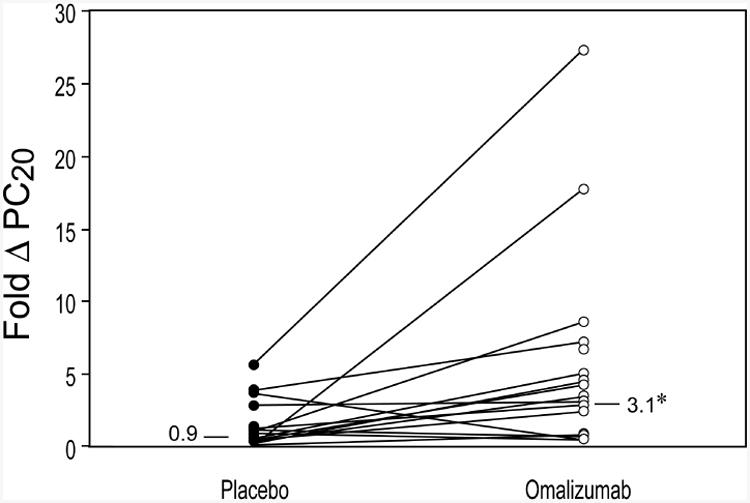
Geometric mean fold change (Δ) in adenosine PC20 from baseline to end of treatment period for omalizumab (○) versus placebo (●). *p = 0.022 for omalizumab versus placebo.
Six patients required at least one prednisone burst during placebo administration (five patients required one burst each, and one patient required two bursts during the 4-month period); however, none required prednisone during omalizumab treatment. One patient required an asthma-related emergency department visit while on placebo treatment (the same patient who required two prednisone bursts during the placebo period and one burst during washout). There were no emergency department visits during the omalizumab treatment period, and no asthma-related hospitalizations throughout the study. We did not observe a seasonal pattern to these exacerbations.
It is interesting to note that the median (95% CI) number of refills/mo for a 1-month supply of ICS was 0.15 (0.00, 0.33) throughout the study, similar to the 12 months prior to the study of 0.17 (0.12, 0.33). The median paired difference (during minus pre) was 0.04 (-0.17, +0.17), P=0.99, reflecting continued poor adherence to ICS therapy, in spite of instructions to continue ICS.
Adverse Events
Three patients reported serious adverse events resulting in emergency department visits: two during placebo administration (only one was asthma-related) and one during the washout period. There were no serious adverse events during treatment with omalizumab. Nonserious adverse events were reported by 10 patients during both treatment periods; two patients only during placebo administration, and four patients only during omalizumab treatment. None of these adverse events were considered to be related to study treatment. None of the patients spontaneously offered complaints about local injection site reactions. However, it is important to note that patients were asked open-ended questions at each visit rather than specific questions on whether they had experienced an injection site reaction from the previous visit.
Discussion
In this randomized, double-blind, crossover study in patients with poor asthma control and prior evidence of poor ICS adherence, omalizumab significantly increased adenosine PC20, a marker of airway inflammation, compared with placebo. In addition, none of the patients required prednisone for exacerbations of asthma during omalizumab treatment, whereas six patients required this intervention while on placebo. Interestingly, pharmacy refill rates for prescribed ICS therapy remained unchanged during the course of the study, indicating persistently poor adherence. It is noteworthy that 12 patients required one or more emergency department visits for asthma in the year prior to this study, whereas only one patient required an asthma-related emergency department visit during the study (occurring during the placebo period). This reduction may have been a result of providing a treatment plan which included supplying albuterol metered-dose inhalers and prednisone to keep on hand, along with telephone access to a study coordinator during week days and to a study physician during nights and weekends who initiated prednisone over the phone for bronchodilator-unresponsive symptoms.
FEV1 did not significantly improve during the treatment period with omalizumab which is consistent with other omalizumab clinical trials of similar duration.12,134 However, there was not much room for improvement since the mean baseline FEV1 was 84% predicted. It is not known whether the reduction in asthma exacerbations would decrease the rate of decline in lung function that may occur over time, however long-term studies would help answer this question.
Prieto et al compared the effects of omalizumab on airway responsiveness to methacholine and AMP in patients with mild to moderate allergic asthma in a randomized, placebo-controlled, parallel-group study.14 In that study, improvement in AMP PC20 was significantly greater in the omalizumab versus placebo group after 4 weeks of treatment (PC20 increased by 1.92 doubling concentrations in the omalizumab group vs. 0.41 doubling concentrations in the placebo group, p = 0.02). However, after 12 weeks of treatment, the increased PC20 in the omalizumab group was sustained, but improvements in the placebo group were such that the difference between groups was no longer significant (increased PC20 from baseline of 1.91 and 1.01 doubling concentrations in the omalizumab and placebo groups, respectively, p = 0.24). This lack of significant difference at 12 weeks may have been the result of too small of a sample size for the parallel design, in contrast to the crossover design of our current study which is statistically more powerful.
A potential limitation of this study is the observation that the AMP PC20 did not return to baseline after the washout period for five of the eight patients who received omalizumab first. Although a 3-month washout interval after omalizumab therapy was thought to be sufficient based on a previous report of airway responsiveness to acetylcholine,15 it appears that a longer washout period may have been needed. However, the use of AMP PC20 at the start of each treatment period to calculate the change in PC20 after 4 months and the regression analysis, which adjusts for a carryover effect, compensated for this. Also, patients were not asked to measure peak flow, or report daily symptoms or use of albuterol, all important measures of asthma control.16 It was our concern that data would be missing and make interpretation difficult. Rather, the emphasis was on collection of objective measures such as airway responsiveness to adenosine and FEV1 along with intervention with prednisone and other health care utilization as more reliable measures of impairment and risk. In retrospect, it would have been important to measure exhaled nitric oxide during the study, a marker of eosinophilic airway inflammation.17 However, we did not have that capability until the end of this study.
There are few proven methods of improving adherence to asthma medications. Some of them involve removing barriers, such as cost of medication or remembering to take the medication and others focus on changing patient behavior through interviews.5 Since omalizumab is extremely expensive ($12,000-$30,000/yr), this intervention should only be considered when conventional methods of dealing with poor adherence fail and the patients is at risk for severe outcomes. The goal is to circumvent the challenge of requiring daily adherence in order to decrease resource utilization and possibly even death in high risk patients. In this circumstance, the potential benefit far outweighs the risks. Local reactions at the injection site are uncommon; true anaphylaxis is rare18 and the most recent observation study (EXCELS) indicates that omalizumab does not increase the risk of malignancy.19 Nevertheless, a cost-benefit analysis of this alternative is needed.
In conclusion, omalizumab is an alternative therapy for patients with very poor asthma control who continue to have poor adherence to inhaled steroids after conventional interventions.
Supplementary Material
Acknowledgments
We thank Kathy Rice for word processing and editing the typescript and remember Carmen Lowell, RT, who performed the challenges but has since past away.
Funded by an investigator-initiated grant from Novartis Pharmaceuticals Corporation and NIH National Center for Research Resources, grant M01-RR00082 (Dr. Shuster) and NIH Research Facilities Construction Program C06, grant RR17568.
Footnotes
Clinicaltrials.gov #NCT00133042
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sherman J, Patel P, Hutson A, Chesrown S, Hendeles L. Adherence to oral montelukast and inhaled fluticasone in children with persistent asthma. Pharmacotherapy. 2001;21:1464–1467. doi: 10.1592/phco.21.20.1464.34485. [DOI] [PubMed] [Google Scholar]
- 2.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98:1051–1057. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 3.Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA. 1997;277:887–891. [PubMed] [Google Scholar]
- 4.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–336. doi: 10.1056/NEJM200008033430504. [DOI] [PubMed] [Google Scholar]
- 5.Apter AJ, Bender BG, Rand CS. Adherence. In: Adkinson NF, Bochner BS, Busse WW, Holgate ST, Lemanske RF, Simons FE, editors. Middleton's Allergy. 7th. Philadelphia, PA: Elsevier Inc; 2009. pp. 1473–1483. [Google Scholar]
- 6.Sherman JM, Baumstein S, Hendeles L. Intervention strategies for children poorly adherent with asthma medications; one center's experience. Clin Pediatr. 2001;40:253–258. doi: 10.1177/000992280104000503. [DOI] [PubMed] [Google Scholar]
- 7.Hendeles L, Sorkness CA. Anti-immunoglobulin E therapy with omalizumab for asthma. Ann Pharmacother. 2007;41:1397–1410. doi: 10.1345/aph.1K005. [DOI] [PubMed] [Google Scholar]
- 8.Van Den Berge M, Meijer RJ, Kerstjens HA, et al. PC20 adenosine 5′-monophosphate is more closely associated with airway inflammation in asthma than PC20 methacholine. Am J Respir Crit Care Med. 2001;163:1546–1550. doi: 10.1164/ajrccm.163.7.2010145. [DOI] [PubMed] [Google Scholar]
- 9.De Meer G, Heederik D, Postma DS. Bronchial responsiveness to adenosine 5′-monophosphate (AMP) and methacholine differ in their relationship with airway allergy and baseline FEV1. Am J Respir Crit Care Med. 2002;165:327–331. doi: 10.1164/ajrccm.165.3.2104066. [DOI] [PubMed] [Google Scholar]
- 10.Crimi N, Palermo F, Polosa R, et al. Effect of indomethacin on adenosine-induced bronchoconstriction. J Allergy Clin Immunol. 1989;83:921–925. doi: 10.1016/0091-6749(89)90106-1. [DOI] [PubMed] [Google Scholar]
- 11.Shuster JJ. Design and analysis of experiments. In: Ambrosius WT, editor. Topics in Biostatistics. Totowa, NJ: Humana Press; 2007. pp. 235–259. [Google Scholar]
- 12.Solèr M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 13.Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 14.Prieto L, Gutiérrez V, Colás C, et al. Effect of omalizumab on adenosine 5′-monophosphate responsiveness in subjects with allergic asthma. Int Arch Allergy Immunol. 2006;139:122–131. doi: 10.1159/000090387. [DOI] [PubMed] [Google Scholar]
- 15.Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Immunol. 2003;131:46–52. doi: 10.1159/000070434. [DOI] [PubMed] [Google Scholar]
- 16.National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. [Accessed April 14, 2014];Full Report. 2007 Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
- 17.Dweik RA, Boggs PB, Erzurum SC, et al. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox L, Platts-Mills TA, Finegold I, Schwartz LB, Simons FE, Wallace DV. American Academy of Allergy, Asthma & Immunology;American College of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2007;120:1373–1377. doi: 10.1016/j.jaci.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134:560–567. doi: 10.1016/j.jaci.2014.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


