Significance
ATP is highly concentrated in secretory vesicles. In vitro experiments suggest that the association of ATP with catecholamines reduces their osmotic forces, permitting the extraordinary accumulation of amines within chromaffin granules. However, this has yet to be proved in living cells. Because functional cells cannot be deprived of ATP, we manipulated the vesicular nucleotide carrier, demonstrating that the extent of vesicular ATP is closely linked to the quantum catecholamine size. This is particularly evident in newly synthesized vesicles, the first to be released. This is the in vivo demonstration that vesicular ATP is an essential factor in the accumulation of neurotransmitters, which may well be a wider mechanism supporting quantal transmission.
Keywords: exocytosis, purines, quantum size, secretory vesicles, VNUT
Abstract
The colligative properties of ATP and catecholamines demonstrated in vitro are thought to be responsible for the extraordinary accumulation of solutes inside chromaffin cell secretory vesicles, although this has yet to be demonstrated in living cells. Because functional cells cannot be deprived of ATP, we have knocked down the expression of the vesicular nucleotide carrier, the VNUT, to show that a reduction in vesicular ATP is accompanied by a drastic fall in the quantal release of catecholamines. This phenomenon is particularly evident in newly synthesized vesicles, which we show are the first to be released. Surprisingly, we find that inhibiting VNUT expression also reduces the frequency of exocytosis, whereas the overexpression of VNUT drastically increases the quantal size of exocytotic events. To our knowledge, our data provide the first demonstration that ATP, in addition to serving as an energy source and purinergic transmitter, is an essential element in the concentration of catecholamines in secretory vesicles. In this way, cells can use ATP to accumulate neurotransmitters and other secreted substances at high concentrations, supporting quantal transmission.
Virtually most, and possibly all, types of secretory vesicles found in cells contain ATP, which often accumulates at high concentrations and, commonly, in conjunction with different types of neurotransmitters. However, the reason for this widespread distribution of ATP remains a mystery. Although ATP is present in all animal species, including primitive life forms like Giardia lamblia that lack Golgi complexes and mitochondria, the detection of ATP in the secretory vesicles of sympathetic neurons was considered to be the first example of cotransmission (1). However, given the ubiquitous accumulation of ATP in secretory vesicles, it might instead be considered that it is the other neurotransmitters that coincide with ATP, rather than the other way around (2). Indeed, perhaps ATP should be considered as the first molecule used as a transmitter in primitive forms of life.
Astonishingly high concentrations of releasable species are stored inside secretory vesicles, far exceeding those in the cytosol (3, 4). For example, the catecholamine content of adrenal secretory granules (SGs), a type of large dense core secretory vesicles also known as chromaffin granules, was 0.8–1 M when measured directly in adrenal chromaffin cells using patch amperometry (5, 6). In addition, SGs from chromaffin cells contain ATP at ∼150 mM (7), calcium ∼40 mM (8), about 2 mM of granins, ascorbate, peptides, and other nucleotides, all in an acidic pH ∼5.5 environment.
ATP possesses intrinsic chemical characteristics that make it relevant to the accumulation of soluble substances in secretory vesicles. The formation of weak complexes between monoamines and ATP, the two main soluble compounds in chromaffin granules, has been demonstrated in vitro by NMR (9), ultracentrifugation (10), infrared spectroscopy, and calorimetry (11). This interaction was also demonstrated in isolated chromaffin granules (12), which are very similar structures to the large dense core vesicles present in many neurons and neuroendocrine cells. These vesicles are distinguishable from other vesicles through their size (∼200 nm), and through their granin (chromogranins and secretogranins), enzymes, and peptide content, which constitute the condensed vesicular core. However, in 1982 the interaction between these molecules and ATP was shown to behave as a nonideal solution in terms of osmotic pressure (13). It was suggested that the ring stacking of catechol and adenosine groups forms a sandwich with a catecholamine/ATP stoichiometry between 1:1 and 1:4. Moreover, disulphide bonds and electrostatic forces between the β-carbon and hydroxyl groups of catecholamines and the phosphate or nitrogen groups of purines, respectively, also contribute to the formation of a complex at the inner vesicular pH (∼5.5), providing osmotic stability to the complex (14).
The interaction of chromogranins (the most abundant vesicular proteins) with catecholamines was also proposed to be the mechanism responsible for reducing the osmotic forces that drive amine accumulation in the SGs of chromaffin cells (15). Indeed, we showed that complete ablation of chromogranins halved the amine content of SGs (16). However, even in the complete absence of chromogranins, the remaining amines are still theoretically hypertonic. Moreover, chromogranins are only present in dense core SGs and not in other organelles that are like synaptic vesicles, where the condensation of secretory products might rely on other mechanisms. For this reason we focused our attention on the colligative properties of ATP (13), a compound that accumulates strongly in SGs. The vesicular nucleotide carrier VNUT [or SLC17A9 (17–21)] uses the electrochemical gradient of protons inside SGs to exchange H+ with nucleotides (17).
Because cells cannot be deprived of ATP, our strategy in this study was to manipulate VNUT to evaluate the functional role of vesicular ATP in the storage and exocytosis of catecholamines, using bovine chromaffin cells as a secretory model.
Results
Endogenous VNUT Expression in Chromaffin Cells.
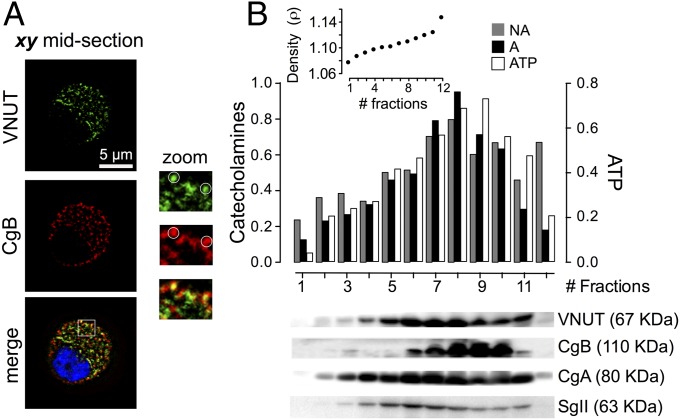
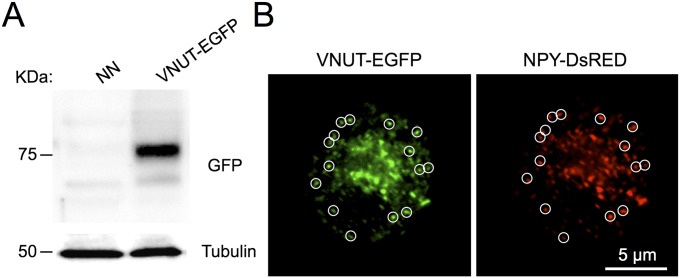
The presence of VNUT in SGs of chromaffin cells was confirmed using an antibody against the cytoplasmic N-terminal domain of this nucleotide transporter, and it was compared with the distribution of chromogranin B (CgB), a major and constitutive component of SGs of chromaffin cells (22). In confocal microscopy images, endogenous VNUT presented a characteristic punctuate distribution that mostly colocalized with CgB (∼75 ± 3%, mean ± SEM; n = 278 randomly taken spots from 7 different cells) (Fig. 1A). Furthermore, we confirmed that VNUT was specifically attached to SGs by transiently, yet simultaneously, expressing plasmids containing VNUT-EGFP and neuropeptide Y-coupled DsRed (NPY-DsRed). When analyzed 48 h after transfection, VNUT-EGFP and NPY-DsRed exhibited over 85% colocalization in confocal microscopy images (Fig. S1), showing that most of the newly synthesized VNUT was located in secretory vesicles.
Fig. 1.
(A) Endogenous VNUT codistributes with secretory chromaffin vesicles. Typical confocal microscopy images showing the cellular distribution of endogenous VNUT and CgB. The merged image also shows the DAPI staining. The 3× zoomed images show the colocalization of VNUT and CgB in specific structures. Quantification was performed as described in Materials and Methods. (B) Normalized distribution of several components in chromaffin secretory vesicles. Twelve fractions of 450 µL (#1 lighter, #12 heavier) were obtained by density gradient sedimentation. (Upper) Quantification of noradrenaline (NA), adrenaline (A), and ATP. The bars show pooled data from three independent gradients, and a typical chromaffin SG proteins distribution, data from one representative experiment (Lower). The Inset shows the iodixanol density profile of each fraction taken from a parallel ultracentrifuge tube. CgA, chromogranin A; CgB, chromogranin B; SgII, secretogranin II.
Fig. S1.
VNUT-EGFP sorts into chromaffin granules. (A) Western blot of VNUT-EGFP overexpressed in chromaffin cells. NN, nonnucleofected. (B) Confocal images show the codistribution of VNUT-EGFP with NPY-DsRED, indicating the sorting of the VNUT into chromaffin granules. White circles (0.9-µm diameter) were drawn around the ROI in the green channel and then transferred to the red channel. Only ROIs that were located at the periphery of the cells were analyzed and the results indicate an ∼85% colocalization of the two proteins (300 granules from 6 cells).
To provide further evidence of the association of VNUT to SGs, we purified chromaffin granules (23) and fractionated the preparation by ultracentrifugation on a continuous iodixanol (OptiPrep) gradient. The presence of chromogranins detected in immunoblots cofractionated with catecholamines indicates their selective incorporation into SGs. We then examined the profile of VNUT and compared its distribution to that of ATP and to the catecholamine content, along with that of other granins well characterized as vesicular proteins (Fig. 1B). VNUT, ATP, and catecholamines exhibited a similar distribution (particularly in fractions 4–11), and VNUT coeluted with other chromaffin granule-specific and enriched proteins like CgB, CgA, and SgII. In contrast, the presence of the cytochrome c oxidase I (a mitochondrial protein) was negligible in all fractions recovered from the gradient (24). Hence, this fractionation and cellular codistribution data indicated that SGs contain VNUT, a protein that governs the transport of nucleotides.
Interfering with VNUT Expression Drastically Reduces the Secretion of ATP.
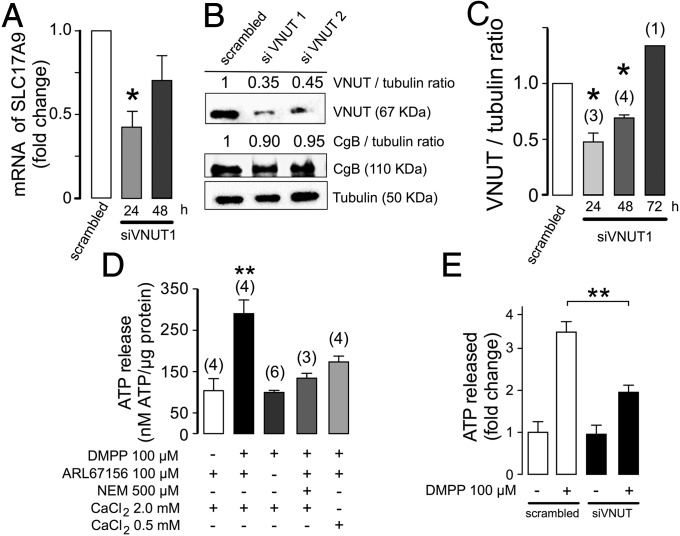
Because cells cannot be deprived of ATP, our strategy to prevent its uptake into SGs was to selectively silence VNUT. We designed two species-specific short-interfering RNA (siRNA) oligonucleotides (siVNUT1, siVNUT2) that target different sequences in bovine VNUT (SLC17A9). Transient transfection of these oligonucleotides in chromaffin cells efficiently silenced VNUT compared with a nontargeted siRNA (scrambled). The efficiency of transfection of the Cy3-siRNA was ∼40%. To establish the most appropriate experimental conditions, the expression of SLC17A9 mRNA was assayed 24 and 48 h after nucleofection by quantitative RT-PCR (qRT-PCR). The expression of these transcripts fell by ∼60% (siVNUT1) and ∼50% (siVNUT2) compared with the control cells (scrambled in Fig. 2A). A similar reduction in the total amount of VNUT was also detected in Western blots (Fig. 2B), where siVNUT reduced the amount of protein by ∼65 (siVNUT1) and ∼55% (siVNUT2). Conversely, the expression of CgB as shown in Fig. 2B was not modified by these siRNAs, indicating that our nucleofection procedure does not affect the expression of other components involved in the genesis and sorting of SGs. Because oligonucleotide siVNUT1 produced a stronger effect and the maximal inhibition was obtained 24 h after transfection (Fig. 2 A and C), we used these conditions for all further experiments.
Fig. 2.
Knockdown of the vesicular nucleotide transporter decreases ATP exocytosis. (A) VNUT mRNA assayed by qRT-PCR. The bars represent the mean expression ± SD (n = 3) and the values were compared using a one-way ANOVA followed by Bonferroni's multiple-comparison test: *P < 0.05. (B) Representative Western blots of VNUT expression carried out 24 h after siRNA transfection. The effects of nucleofection with the siVNUT1 and siVNUT2 oligonucleotides were compared with those of a nontargeted siRNA (scrambled). Equal amounts of protein (20 µg) were loaded per lane. (C) Quantitative analysis of the changes in VNUT expression at different times after transfection of siVNUT1, expressed as the VNUT/α-tubulin ratio in three different Western blots. Bonferroni's multiple-comparison test: *P < 0.05. (D) ATP release from chromaffin cells under resting conditions and after stimulation with the nicotinic agonist DMPP, in the presence or absence of the nucleotidase inhibitor ARL67156 or the exocytotic blocker NEM. (E) Decreased ATP release after VNUT knockdown. The experiments in D and E were performed at 37 °C and, unless specified, in the presence of 100 µM of ARL67156. **P < 0.01, Mann–Whitney U test.
ATP can be partially released by nonexocytotic mechanisms (25), so we quantified the exocytotic and nonexocytotic contributions to release. Cells seeded in 24-well plates (5 × 105 per well) were incubated for 10 min with the highly active plasmalemmal ectoATPase specific inhibitor, ARL 67156 (100 µM) (26), to avoid the rapid degradation of the ATP released that occurs in the absence of this inhibitor (Fig. 2D).
Because regulated exocytosis is a Ca2+-dependent process, this contribution to the overall ATP release was also blocked in the presence of N-ethylmaleimide (NEM) (Fig. 2D). The nonexocytotic, constitutive release, or basal secretion in the absence of stimuli, was 75 nM of ATP per microgram of total protein, which increased significantly (threefold) in the presence of 2 mM Ca2+ and the nicotinic agonist 1,1-dimethyl-4-phenyl-piperazinium (DMPP; 100 μM, 5 min), an increase that was attenuated when the Ca2+ concentration was reduced to 0.5 mM (Fig. 2D). These results show that triggered release of ATP is a Ca2+-dependent mechanism.
To validate the functional contribution of VNUT to the vesicular storage of ATP and its subsequent secretion, similar experiments were conducted to determine whether silencing VNUT expression affected ATP secretion. The evoked release of ATP measured between 24 and 48 h after transfection diminished about 40% in chromaffin cells nucleofected with siVNUT1, whereas the control with scrambled siRNA showed no such effect. Moreover, nonexocytotic secretion remained unaffected (Fig. 2E). Note that only the newly synthesized fraction of SGs would be affected by the siRNA, so previously existing vesicles would presumably carry intact VNUT. These data may underestimate the real reduction in the ATP content of vesicles lacking active VNUT.
Adrenaline Secretion Is Impaired When Secretory Vesicles Lack VNUT.
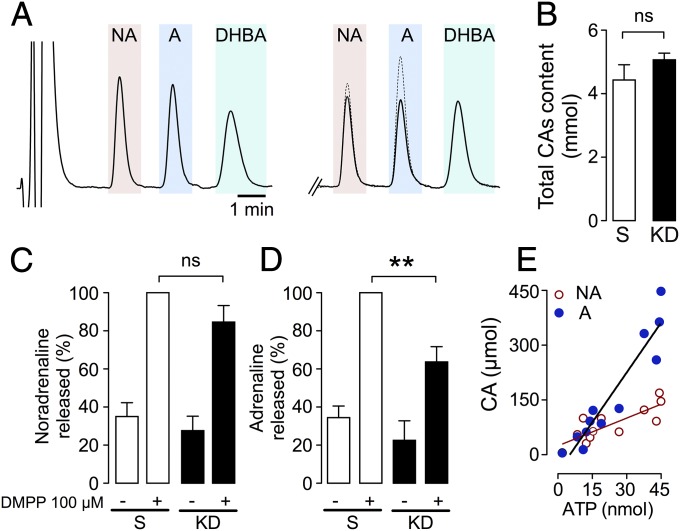
To evaluate how VNUT knockdown (VNUT-KD) affects catecholamine exocytosis, we analyzed the basal and DMPP-evoked catecholamine release in the same experiments described above using HPLC coupled with electrochemical detection. The catecholamines secreted by control cells in response to DMPP were typically 30–40% noradrenaline and 70–60% adrenaline (see representative chromatogram in Fig. 3A, Right). However, the reduction in intravesicular ATP when VNUT was silenced lowered DMPP-evoked catecholamine secretion in a manner that appeared to be somewhat selective for adrenaline (Fig. 3 A, C, and D). Conversely, neither the basal release (Fig. 3 C and D) nor the total amount of catecholamines in the lysates of control or VNUT-KD cells were affected (Fig. 3B).
Fig. 3.
Knockdown of VNUT preferentially impaired the vesicular exocytosis of adrenaline in chromaffin cells. (A) Typical HPLC chromatograms showing the detection of amines. (Left) External standard of noradrenaline (NA), adrenaline (A), and DHBA (internal standard). Cells were stimulated for 5 min with DMPP and the amines secreted into the media were analyzed. (Right) These traces show the catecholamines secreted from control cells (scrambled, dashed line) and VNUT-KD cells (solid line). (B) Average total catecholamine content in lysates from cells transfected with the scrambled (S) and siVNUT1 (KD) siRNAs. ns, not significant. (C and D) Basal and DMPP-evoked noradrenaline and adrenaline secretion from cells stimulated by DMPP, showing greater release of noradrenaline. The data are the means ± SEM of at least three independent cell cultures: *P < 0.05, **P < 0.01 (Dunnett’s paired t test). (E) Linear fit from the relation catecholamines/ATP obtained from purified SGs. The R2 values were 0.9 and 0.7 for ATP/adrenaline and ATP/noradrenaline, respectively. The slopes of the linear equations were 2.5 vs. 9.1, with values from an experiment from two independent gradients of isolated vesicles shown.
To better understand why the depletion of vesicular ATP preferentially modified adrenaline secretion, we separated purified SGs into 12 fractions using continuous gradients of iodixanol (Fig. 1B). The linear regression of both adrenaline and noradrenaline reflected a high linear dependency on ATP, yet the slope for adrenaline was more pronounced indicative of a tighter association between ATP and adrenaline (Fig. 3E). These results are consistent with those obtained when VNUT was silenced and ATP depletion was seen to mainly affect SGs containing adrenaline (Fig. 3 C and D).
Newly Synthesized Granules Are the First to Be Released.
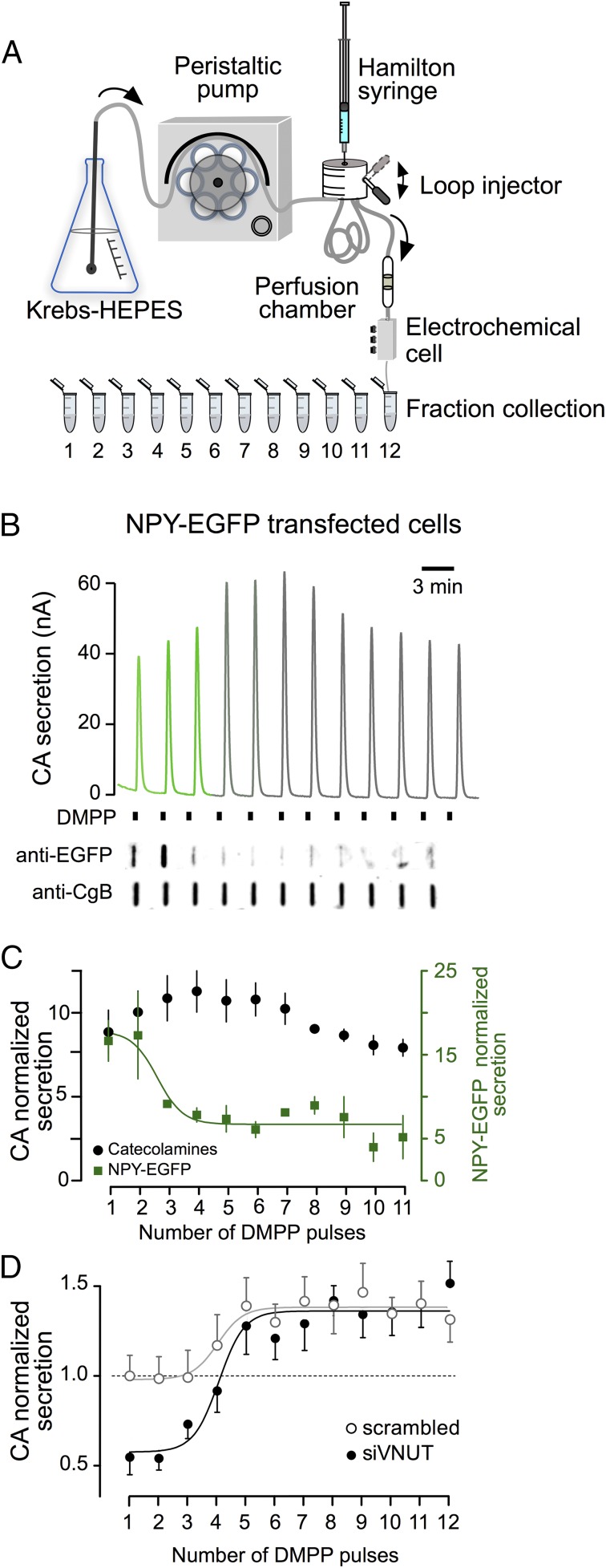
Because RNA interference is most likely to selectively affect newly synthesized SGs, we explored whether new vesicles are the first to be released. To measure secretion as a function of granule age we labeled one component of SGs (NPY-EGFP) and compared its release with an endogenous releasable protein (CgB). We nucleofected cells with a plasmid that encodes NPY-EGFP, as described in Materials and Methods, and the released EGFP was assayed by dot blot. The set-up used for the on-line analysis of catecholamine release by electrochemistry and the subsequent fraction collection of the perfusate from chromaffin cells trapped on a column is shown in Fig. 4A (27, 28).
Fig. 4.
VNUT knockdown predominantly affects catecholamine secretion from newly synthesized vesicles. The capacity of cells to recruit vesicles from the early- and late-releasable pools was studied using 12 successive DMPP stimulations. (A) The scheme shows the perfusion system used for the on-line electrochemical analysis of catecholamine release and the fraction collection for protein quantification. (B) Representative traces of the electrochemical recording from perfused chromaffin cells expressing NPY-EGFP. Each current peak indicates catecholamine secretion. A representative dot blot probed for NPY-EGFP and CgB obtained from the same experiment. (C) Time course of catecholamine and newly synthesized NPY (pooled results from four experiments). (D) Time course of catecholamine release from control (scrambled) and knockdown-VNUT (siVNUT) nucleofected chromaffin cells. To correct the bias of different cell batches, the data were normalized to the mean of the first secretory peak from the control cells (scrambled), considering the initial response as 1.
The cells were stimulated with repeated pulses of DMPP (10 µM, 20 s) applied at 3-min intervals, yet no autocrine effect was expected because the fast perfusion system washed out the released products (29). In this way, EGFP release will indicate the exocytosis of new vesicles, and quantification of the release of chromogranin B as an endogenous marker of exocytosis will reflect the release from new and old SGs. At the end of the experiment the cells were perfused with 0.1% Triton X-100 to quantify the total catecholamine content, which produced a big oxidation signal of about 4–4.5 µA, 75–80 µC, indistinguishable from that detected in scrambled cells.
In the example shown in Fig. 4B, the first stimulus caused a secretory response of about 25 nA, which was followed by a characteristic pattern of amperometric peaks that reflected the gradual growth in the size of the releasable pool during the first five pulses. This pattern was also observed in cells nucleofected with the scrambled oligonucleotide. In contrast, the EGFP secretory profile indicated that maximal release was achieved during the first two stimuli (note the clear differences in the time course of both secretory components in Fig. 4C). These data suggest that the youngest SGs are the first to undergo exocytosis.
Silencing VNUT Selectively Affects Catecholamine Release from Newly Synthesized Granules.
To test whether silencing VNUT synthesis affects the early releasable pool of vesicles distinctly, we analyzed the time course of catecholamine secretion in response to repetitive stimuli. Compared with the cells nucleofected with the scrambled siRNA, the silencing of VNUT had a selective impact on the catecholamine release from this newly created SG pool, whereas the older granules seem to remain unaffected (as summarized in Fig. 4D). Note that silencing VNUT halved the initial secretory responses compared with the control nucleofected cells.
VNUT-KD Affects the Rate of Granule Recruitment Inherent to DMPP–Induced-∆ [Ca2+].
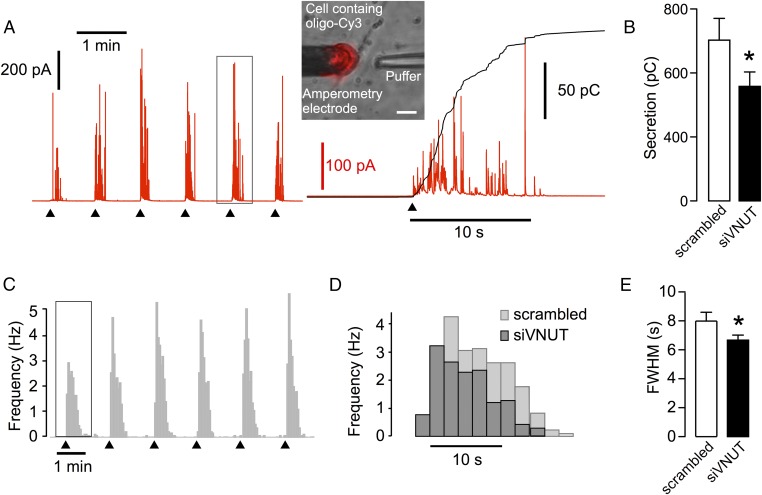
Because the reduction in catecholamine secretion observed after knockdown of VNUT could be caused by a reduction in the number of exocytotic events or the amount of amines released per quantum, we examined the effect of siVNUT on the frequency of exocytotic events. Transfected cells were identified for amperometry by using Cy3-tagged oligonucleotides (nucleofected cells were greater than three times more fluorescent than the SD of the background) (Fig. 5A, Inset) and these cells were stimulated for 10 s with DMPP (10 µM) using a repetitive protocol similar to that used for the on-line studies (Fig. 5A). The recordings were made on the same day using the same calibrated carbon-fiber electrode and alternating recordings from control and siRNA VNUT nucleofected cells to minimize the bias. The application of DMPP produced exocytotic bursts of ∼8 s that were significantly shorter in VNUT-KD cells (Fig. 5 D and E). Furthermore, and as expected given this effect, the integrated secretory response was weaker in silenced cells (defined as the catecholamine release per pulse) (Fig. 5B).
Fig. 5.
The number of exocytotic events is reduced by VNUT interference. (A) Representative amperometric recording from a control cell. Secretion was stimulated every minute by a 10-s pressure ejection of 10 µM DMPP from a micropipette placed 30 µm from the cell (triangles). After each stimulus the micropipette was moved 500-µm up using a motor-driven micromanipulator to avoid receptor desensitization caused by DMPP leakage. An amplified view of the fifth pulse is shown revealing the typical firing pattern of exocytosis (red trace) and the cumulative secretion obtained by integrating the amperometric signals (superimposed black trace). (Inset) A cell labeled with the fluorophore (Cy3-oligonucleotide) indicates successful nucleofection. The image shows the position of the carbon fiber electrode and the glass pipette used for DMPP injection (puffer, on the right). (Scale bar: 10 µm.) (B) Total secretion responses from control (scrambled) and siVNUT cells. The data represent the mean ± SE secretion pooled from six secretory responses elicited by 10-µM DMPP pulses. (C) Spike-frequency analysis (1-s bins). The image shows the frequency of exocytosis. Each bar represents the average spike frequencies from control cells (n = 10). The maximum number of spikes was reached 5 s after stimulation. (D) The first histogram is amplified and the spike frequency (recorded in 2-s bins) from the control (scrambled) and VNUT silenced cells superimposed. Note that spikes from VNUT-KD cells stopped firing before the control cells. (E) Pooled data from 10 cells showing the full width at half maximum (FWHM) from the sigmoidal fits of frequency histograms. *P < 0.05, Mann–Whitney U test.
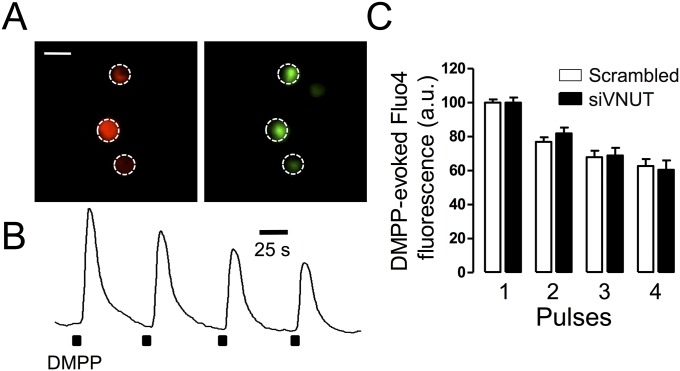
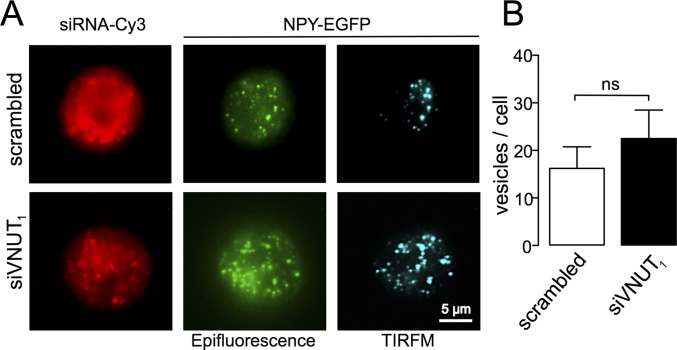
It is well known that regulated exocytosis is a Ca2+-dependent phenomenon and that the frequency of exocytosis is therefore mainly controlled by intracellular calcium. The release of less ATP could be lowering potential regulatory effects of purinergic autoreceptors. This possibility can be ruled out in the on-line perfusion experiments because the flow (1 mL/min) assures the fast washout of secreted products preventing autocrine effects on Ca2+ entry (29). When the time course of intracellular Ca2+ was analyzed by calcium-imaging, in perfused cells—in response to four successive stimulations with DMPP—no differences were evident in either the initial peaks or in the time course of Ca2+ signals (Fig. S2). Hence, the effects on the frequency of exocytosis did not appear to be a result of intracellular calcium dynamics. In addition, when we studied whether siVNUT affected the biogenesis of SGs by quantifying NPY-EGFP expression and their cellular distribution, this did not appear to be the case (Fig. S3). Thus, the reduction in the frequency of secretory spikes does not appear to be caused by changes in cytosolic calcium or vesicle biogenesis.
Fig. S2.
(A) siVNUT does not affect calcium dynamics. Cells nucleofected with siVNUT or scramble oligonucleotides tagged with Cy3 were loaded with the Fluo-4 calcium indicator. We only used red-tagged cells for the analysis. (Scale bar: 20 μm.) (B) The cells were stimulated with four successive DMPP pulses (see above) and the fluorescence was recorded continuously. Note the progressive reduction in intracellular calcium. (C) Pooled data represented as the mean ± SE from control (n = 14) and siVNUT1 (n = 11) nucleofected cells.
Fig. S3.
The NPY-EGFP expression remains unchanged in VNUT-KD cells. (A) Representative images of chromaffin cells obtained with conventional fluorescence showing siRNA oligonucleotides labeled with Cy3 and newly synthesized vesicles expressing NPY-EGFP (green). Newly formed vesicles closest to the membrane can be observed by total internal reflection fluorescence microscopy (TIRFM) (blue spots). (B) Pooled data from TIRFM images showing no differences in the number of vesicles expressing NPY-EGFP in control (scrambled) and siVNUT1-treated cells. The bars represent the means ± SD (n = 5). Values were compared using Mann–Whitney U test P = 0.458.
The Quantum Size of Exocytosis Is Drastically Reduced in VNUT-KD Cells.
The kinetic features of single exocytotic events were studied by single-cell amperometry. Secretion was elicited by a 5-s pressure ejection of 5 mM BaCl2, an especially useful secretagogue that does not require receptor activation and that produces a low frequency of exocytosis, enabling the quantification of individual secretory events. Indeed, the probability of two independent and undistinguished exocytotic overlapping events in our experimental conditions was very low (P = 0.018; n = 1,201 events).
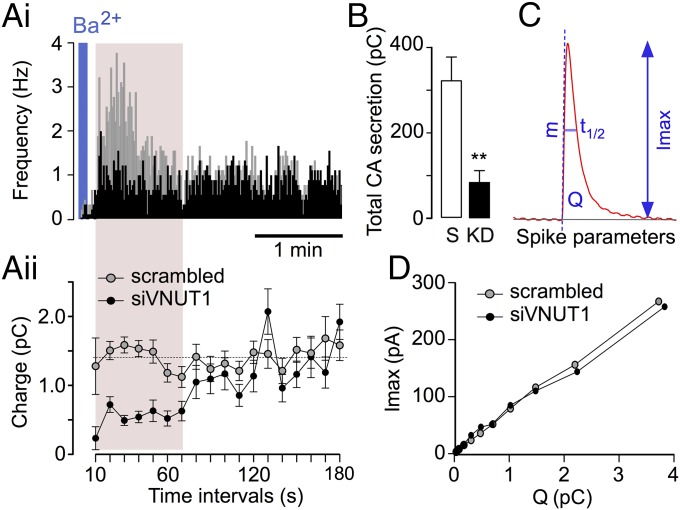
The distribution of spikes in control (Fig. 6A, scrambled, gray trace) and VNUT silenced chromaffin cells (Fig. 6A, siVNUT, black trace) was obtained from amperometric recordings. In our conditions, the typical profile of barium-evoked secretion from naïve cells is identical to that observed in control cells, whereas cells nucleofected with siVNUT do not show the initial spike burst that usually lasts for 1 min but that maintains a slow pace of about 0.8 Hz. The quantum size was drastically reduced during the 70 s after stimulation in cells nucleofected with siVNUT (Fig. 6 A, ii). The prespike feature “foot” duration, which has been assigned to the fusion pore stability, was significantly reduced in siVNUT cells. These differences in the kinetics of release and quantum size are more evident when considered in these two cell types after 70 s of recording (Table 1).
Fig. 6.
Quantum catecholamine size from VNUT-KD SGs. (A, i) Spike-frequency analysis of isolated cells nucleofected with the scrambled (gray traces) or siVNUT oligonucleotides (black traces) and stimulated for 5 s with 5 mM BaCl2. Each spike represents the catecholamine released from a single vesicle. Frequency bins are the average number of spikes in 1 s. (A, ii) Quantum size of the exocytotic events recorded in 10-s bins over 3-min intervals. Each open circle (scrambled) and solid circle (siVNUT1) represents the average charge (in picocoulombs, nine cells each). (B) Total secretion (mean ± SEM) comparing the control (scrambled, S) and siVNUT1 (KD). The cumulative charges were measured for 3 min after stimulation and they are expressed as picocoulombs. **P < 0.01, Student’s t test. (C) Parameters obtained from each secretory spike: Imax, maximum oxidation current; t1/2, spike width at the half-height; Q, net catecholamine charge; m, ascending slope of the spike. (D) Spike amplitude (Imax) versus quantum size (Q) of secretory spikes from siVNUT and control nucleofected cells: Mann–Whitney U test with the Bonferroni correction (the data are averaged from the experiment shown in Table 1).
Table 1.
Characteristics of the secretory spikes obtained from chromaffin cells nucleofected with scrambled or siVNUT1 oligonucleotides
| Time | Imax (pA) | Q (pC) | t1/2 (ms) | Mean (pA/ms) | Foot duration (ms) | n (cells) | n (spikes) |
| 0–70 s | |||||||
| Scrambled | 117.7 ± 15.1 | 1.68 ± 0.28 | 13.1 ± 1.2 | 36.9 ± 4.4 | 6.9 ± 0.3 | 9 | 990 |
| siVNUT1 | 55.2 ± 21.1* | 0.72 ± 0.28* | 8.4 ± 1.1* | 30.7 ± 5.9 | 4.9 ± 0.4** | 9 | 468 |
| Change | −53.1% | −57.1% | −64.1% | −19.8% | −29% | ||
| 70–180 s | |||||||
| Scrambled | 99.0 ± 17.8 | 1.49 ± 0.31 | 13.0 ± 1.5 | 20.5 ± 8.3 | 7.7 ± 0.3 | 9 | 866 |
| siVNUT1 | 68.7 ± 24.2 | 1.05 ± 0.47 | 10.3 ± 1.9 | 21.8 ± 5.9 | 6.9 ± 0.3** | 9 | 820 |
| Change | −30.6% | −29.5% | −20.7% | + 6.3% | −11% |
For explanation, see Materials and Methods and ref. 30. This is a representative experiment from four replicas carried out in different cell cultures, performed alternating scrambled and siVNUT cells. The data are expressed in the units in parentheses. *P < 0.05, **P < 0.01 (Mann–Whitney U test).
A consequence of the lower number of spikes is a drastic fall in the total amount of amines secreted by the siVNUT cells (Fig. 6B). Indeed, the reduction in the quantum size was accompanied with a parallel decrease in the Imax [maximum oxidation current, expressed in picoampere (pA)] and t1/2 (spike width at half height, expressed in milliseconds) (Fig. 6D), suggesting that the accumulation of ATP and catecholamines is linked. It is important to emphasize here that the reduction in both frequency of secretory events and quantum size were observed exclusively in the first spikes, which roughly coincided with the newly synthesized SGs that are the most strongly affected by VNUT-KD. These data are also consistent with the on-line secretion from the perfused cells.
The Overexpression of VNUT Increases Quantum Size of SGs.
To validate the above results indicating a specific role of intravesicular ATP as crucial factor in the accumulation of catecholamine, we conducted single-cell amperometric recordings on chromaffin cells overexpressing VNUT-EGFP. This approach also overrides the problems derived from double transfection in primary cultures of a cell like chromaffin cells when rescue strategies are required.
Chromaffin cells were nucleofected with VNUT-EGFP or EGFP (control cells) as described in Materials and Methods and the amperometry recordings were performed 48-h later. Secretory response was elicited by a 5-s pulse of BaCl2. Nevertheless, the quantum size increased ∼66% in cells that overexpressed VNUT. Table 2 summarizes these results. Note the quantitative differences between the control values from this and Table 1, as electrodes were different. In addition, control conditions are not identical (scrambled vs. EGFP overexpression) and cells came from different cultures and experimental conditions (30).
Table 2.
The overexpression of VNUT-EGFP increases the quantum size of exocytosis
| Experiment | Imax (pA) | Q (pC) | t1/2 (ms) | m (pA/ms) | n (cells) | n (spikes) |
| EGFP | 47.0 ± 8.4 | 1.18 ± 0.18 | 22.0 ± 1.6 | 5.2 ± 0.9 | 12 | 1,135 |
| VNUT-EGFP | 68.3 ± 14.9 | 1.96 ± 0.36* | 27.2 ± 3.4 | 7.5 ± 1.6 | 8 | 1,153 |
| Change | +45.3% | +66.1% | +23.6% | +44.2% |
This is a representative experiment from two experiments carried out in different cell cultures, performed alternating cells that either overexpressed EGFP (control cells) or VNUT-EGFP cells. The data are expressed in the units in parentheses. *P < 0.05 (Mann–Whitney U test).
Discussion
Phylogenetically speaking, ATP was probably the first neurotransmitter in the early forms of eukaryotic life and is now present in almost all of the SGs found in living organisms. Given this widespread distribution, the concept that ATP is costored with neurotransmitters should perhaps be reversed, such that almost all “secretable” substances are costored with ATP. The physiological significance of the high concentrations of ATP in SGs could reflect its intrinsic chemical properties. ATP has a high capacity for self-aggregation, behaving as a nonideal solute, which results in solutions with a far lower osmolarity than theoretically predicted. This physico-chemical interaction becomes even more evident when the osmotic behavior of ATP/catecholamine mixtures is studied (2, 13).
Because a cell cannot survive without cytosolic ATP, we diminished the expression of the vesicular nucleotide carrier, VNUT (20), to specifically reduce the ATP content of SGs without affecting its cytosolic concentration. Although the role of VNUT in ATP secretion from platelets has already been evaluated (31), to the best of our knowledge this is the first time that the influence of vesicular ATP on stored neurotransmitters has been studied in living cells. The lack of suitable antibodies or specific pharmacological blockers has impeded the characterization of VNUT. Moreover, many of the drugs widely used to study VNUT also have other effects, like the potent blocker DIDS (4,4'-diisothiocyano-2,2'-disulphonic stilbene acid) that is also a potent blocker of Cl− channels (32, 33)), or Evans’ blue (another widely used VNUT blocker) that is also an antagonist of P2X purinergic receptors (34). Moreover, the strong color of the latter prevents the use of the luciferase method to measure ATP. In addition, glyoxylate is another compound thought to act as a selective VNUT inhibitor (31), although it is well known to strongly interfere with the cellular production of ATP.
Although the estimated concentration of vesicular ATP in chromaffin cells varies among studies, it is generally found to be hundreds of millimolars. Furthermore, the gross averaged catecholamine/ATP ratios are also found to vary from 4:1–9:1 (7, 35, 36) depending on the study and the amine (adrenaline or noradrenaline) measured (see below). These astonishingly high concentrations of ATP make it a unique candidate to be an intravesicular factor that stoichiometrically associates with catecholamines and, probably, many other neurotransmitters. Chromogranins A and B are the main protein components of chromaffin granules and these large, dense core vesicles still retain half of their catecholamine content even after the complete ablation of chromogranins (16). The remaining amine content (∼400 mM) is still above theoretical isotonicity and, thus, the only remaining candidate to adsorb catecholamines must be ATP.
Here we silenced the expression of VNUT in bovine chromaffin cells and, as expected, the exocytotic component of ATP release was dramatically reduced. These data are consistent with those of earlier studies on PC12 cells (20) and hippocampal neurons (37). The reduction of vesicular ATP was accompanied by a decrease in catecholamine release without affecting noradrenaline, and with no measurable change in the total cell amine content. The explanation for this is that, even in the control situation only a fraction of the total SGs (roughly 30%) is releasable even by strong stimuli. From this population, only a small number of SGs are newly synthesized and consequently affected by siRNA. Because newly synthesized vesicles are released first (Fig. 4B), the siRNA affects only the first two to three stimuli and is not expected to measurably modify the total cellular amine content. When measuring the total cellular amine content we are underestimating the real extent of the role of VNUT (Fig. 5B). The fraction of SGs affected by VNUT silencing is smaller than that reported in the VNUT-KO mouse (21); these authors also reported a reduction in catecholamine secretion.
On density gradients we found ATP to be more tightly associated with adrenaline than noradrenaline (Fig. 3E). The most feasible explanation for this might be related to the different rates of the steps in adrenaline synthesis. Dopamine is taken up by SGs in a few minutes and there converted into noradrenaline. Some of the noradrenaline then slowly leaves the vesicle to become converted into adrenaline in the cytosol. The whole process ends in the uptake of adrenaline into SGs, requiring about 24 h (38). Because this last process is likely to be impaired, in the siVNUT-treated cells the expected concentration of released adrenaline must be smaller. This preferential effect on adrenaline is similar to that observed in the absence of chromogranins (16).
In the experiments of Fig. 3, cells were stimulated with a high concentration of the nicotinic agonist DMPP for a long period (5 min), whereby most nicotinic receptors should be desensitized and the secreted ATP is most likely to act on purinergic autoreceptors (39). To avoid these effects, rapid perfusion was used and VNUT-KD was seen to mainly affect only the first two to three secretory responses, the later secretory peaks remaining unaffected. Moreover, the selective presence of EGFP (from NPY-EGFP) in these two peaks demonstrated that the newly synthesized SGs are preferentially the first to be released and that these vesicles contain far fewer catecholamines and ATP than their controls (scrambled siRNA + NPY-EGFP–treated cells). These results support the “last synthesized/first released” theory postulated from evanescent wave microscopy (40, 41). However, our strategy using NPY-EGFP offers more direct evidence, given that the quantification in dot-blots can be compared with that of catecholamines and chromogranin B.
Total catecholamine secretion is reduced in VNUT-KD cells, in conjunction with a similar decrease in the frequency of secretory events, with VNUT-KD cells failing to maintain the number of secretory events as long as control cells. We do not have an explanation for this reduction in the number of spikes. One possible explanation is the presence of SGs with either no catecholamines or almost none, which is not detected by amperometry (42). In addition, the prefilled state of SGs has been recently proposed as a factor that modified the probability of exocytosis either in glutamate- (43) or GABA-neurons (44). It might be also argued that reduced activation of presynaptic purinergic receptors might occur because of the removal of feedback, although the intracellular calcium measurements suggest that purinergic regulation does not contribute to the reduction in spike frequency.
The overall effect of the reduction in vesicular ATP is a fall in the quantum release of catecholamines, particularly evident in the exocytotic events occurring within the first 70 s. As the quantum size of the spike feet decreases in parallel with the quantum size of spikes (45), it is expected that the number of spikes with foot and the foot duration was reduced (Table 1). Because Imax and Q [net spike charge, expressed in picocoulomb (pC)] are highly correlated parameters (Fig. 6D), we conclude that the reduction in quantum size resulted in scalable smaller spikes without any kinetic qualitative differences indicating a single phenomenon, contrary to that observed with other manipulations, like the suppression of chromogranins (16).
The main objective of this paper has been manipulate the intravesicular concentrations of ATP and study how these changes affected the accumulation of catecholamines inside SGs. Chromaffin cells are probably the best model to study neurosecretion using amperometry; however, they have some limitations when rescue protocols for siRNA of vesicular proteins are required. One such limitation is the restricted temporal window for obtaining a good number of amperometrical recordings. In addition, the efficiency of double transfection is very low. For these reasons we decided to promote the opposite effect: increase the vesicular ATP by overexpressing VNUT (46) in single-cell experiments. Table 2 clearly shows a substantial increase in the quantal amine release. Note that the overall parameters that described the kinetics of exocytosis also indicate the acceleration of the phenomenon, which is similar to that observed after catecholamine overload caused by l-DOPA (47, 48).
It should be noted that our data are probably underestimating the magnitude of the effect of ATP reduction in amine content as only a fraction of cells (35–40%) and only a fraction of SGs are affected by RNA interference. To the best of our knowledge, this is the first demonstration of the crucial role of vesicular ATP in the storage of a cotransmitter, probably based on its colligative properties (7).
The widespread distribution of vesicular ATP in most SGs from all known animal species has not received much attention from the scientific community. However, the participation of vesicular ATP in the storage and exocytosis of adrenal catecholamines raises new exciting questions regarding its possible participation in the homeostasis of other neurotransmitters.
Materials and Methods
A detailed description of the materials, antibodies, plasmid constructs, RNA isolation, cDNA synthesis, qRT-PCR, and SLC17A9 mRNA knockdown generation, as well as Western blotting procedure can be found in SI Materials and Methods.
Cell Culture and Transfection.
Bovine chromaffin cells were isolated by adrenal medulla digestion and cultured under standard conditions (SI Materials and Methods). To maximize the interference effect, we reduced the number of mature SGs by exposing isolated bovine chromaffin cells to a high K+ solution (35 mM) 5 min before transfection. A detailed description of the procedure in given in SI Materials and Methods.
Immunofluorescence and Confocal Microscopy.
Chromaffin cells (5 × 105 cultured cells) grown on 12-mm diameter poly-d-lysine–coated glass coverslips were fixed following a standard procedure. The analysis of codistribution was performed as described elsewhere (49); a detailed description can be found in SI Materials and Methods.
Isolation of Bovine Chromaffin Secretory Vesicles.
Adrenal medullas were triturated and vesicles isolated by ultracentrifugation in continuous gradient of iodixanol, as described in SI Materials and Methods.
ATP Secretion Assay.
Unless indicated, the selective inhibitor of ectoATPases ARL 67156 (100 µM) was present during the whole procedure. The ATP was measured in 50 µL of the supernatant using a luciferin-luciferase assay kit (Molecular Probes, Invitrogen) and quantified on sextuplicates in a standard luminometer (see a detailed description in SI Materials and Methods).
On-Line Analysis of Catecholamine Released from Perfused Bovine Chromaffin Cells.
Chromaffin cells (3 × 106 cells) plated in plastic Petri dishes (92-mm diameter) were nucleofected with siRNA oligos and cultured for 24 h in standard conditions. The cells were then gently removed from the bottom of the dish with a cell scraper, centrifuged, and packaged in a 100-µL microchamber sealed with a cigarette filter (cut to a 3-mm section). The cells were then perfused with Krebs-Hepes solution at the rate of 1 mL/min and the liquid emanated was conducted to an electrochemical detector (LC-4B, BioAnalytical Systems), connected to a PowerLab 8/30 (ADInstruments). The cells were stimulated every 3 min to secrete their catecholamines with 10-s pulses of a Krebs-Hepes solution containing DMPP (10 µM). Secretion was quantified by integration of the curves above basal levels.
Amperometric Detection of Exocytosis.
Chromaffin cells transfected with Cy3 tagged siRNA oligonucleotides were viewed by epi-fluorescence under an inverted microscope (Olympus IX51, using a 40×/0.60 NA objective). Excited light (mercury lamp, X-cite EXFO series, 120W) was band-pass–filtered (BP545/25) and the emitted light was passed through a dichroic 565-lpxr and band-pass filter (BP650/70; all filters were from Chroma Technology). Carbon-fiber microelectrodes (6-µm radius; Thornel P-55, Amoco) were prepared as described previously (30, 50), and electrochemical recordings were obtained using a VA-10X potentiostat (NPI Electronics) connected to a PowerLab 8/30 (ADInstruments) (see refs. 6 and 51, and SI Materials and Methods for details). All amperometric measurements were performed with a calibrated electrode gently touching the cell membrane. Cell release was stimulated by a 5-s pressure ejection of a BaCl2 solution (5 mM) or DMPP (10 µM) from a micropipette positioned 40 µm from the cell.
Amperometric signals were low-pass–filtered at 1 kHz and collected at 4 kHz. The data were analyzed using locally written macros for IGOR that extract the following parameters from each spike (Wavemetrics) (52): Imax, maximum oxidation current, expressed in picoampere; t1/2, spike width at half height, expressed in milliseconds; Q, net spike charge, expressed in picocoulombs; m, ascending slope of spike, expressed in nanoamperes per second (nA/s). The discrimination threshold was fixed at 2.5 SD of the basal noise of the first derivative of each recording. It usually includes spikes with an Imax over 2.5 pA. The kinetic parameters were calculated as mean values from at least 20 spikes per cell. To avoid the bias caused by the different number of spikes produced by each cell, the average values of the spike’s parameters recorded from each cell were considered as n = 1 (53).
HPLC Analysis of Catecholamines.
Chromaffin cells were triturated in ice-cold lysis buffer containing perchloric acid (0.05 N) and 3,4-dihydroxybenzylamine (DHBA, 200 nM) as internal standards. The homogenates were centrifuged and the cleared supernatants were analyzed by HPLC coupled to electrochemical detection (54).
Statistics.
The datasets are expressed as the means ± SE. The statistical significance of the data from groups of experiments was assessed with the nonparametric Mann–Whitney U test or Student’s t test as appropriate. Differences were considered significant at the level of P < 0.05. The data were analyzed with the Prism program (Graphpad Software).
The methods related to the measurement of cytosolic-free calcium are described in SI Materials and Methods.
SI Materials and Methods
Materials.
Culture plates were obtained from Nunc, and FBS (DE14-801F) and the basic nucleofector kit (VPI-1003) were from Lonza. Bicinchoninic acid, BSA (fraction V), DMPP, DMEM high glucose, Ham’s-F12 medium, Hepes, NEM, penicillin G, Coomassie blue, and Trypan blue were all purchased from Sigma-Aldrich. Collagenase type IA was obtained from Worthington and Sigma-Aldrich. The ARL 67156 inhibitor of ectoATPases was purchased from Tocris. Urografín was obtained from Schering España, and Percoll sterile solution was from GE Healthcare Life Sciences. Gentamicin (1405-41-0) was from Acofarma and the pEGFP-N1 vector was obtained from Clontech.
The primers used were synthesized by Integrated DNA Technologies and the siRNAs were purchased from Sigma-Aldrich. The restriction enzymes and the DNA ligase were obtained from Takara Bio. All other drugs were purchased from Sigma-Aldrich and all of the salts used for buffer preparation were reagent grade.
Antibodies.
Primary antibodies raised against the following proteins were used in this study: SLC17A9 (S-13) rabbit polyAb (anti-VNUT, sc-86312), GFP rabbit polyAb (sc-8334), chromogranin A (C-20) goat polyAb (sc-1488), chromogranin B (C-19) goat polyAb (sc-1489), and secretogranin II rabbit polyAb (sc-50290) were from Santa Cruz Biotechnology; VNUT, rabbit polyAb (ABN110, Millipore); and α-tubulin (T6074) and Actin (A3853) mouse mAbs (Sigma-Aldrich). Secondary horseradish peroxidase-conjugated antibodies against goat (P0160: Dakocitomotion), rabbit IgG (NA934) and mouse IgG (NA931: both from Amersham GE Healthcare) were used, as well as Alexa 488-conjugated anti-rabbit (A11008) and Alexa 568-conjugated anti-goat (A11057) from Molecular Probes, Invitrogen.
SLC17A9 mRNA Knockdown.
We designed specific short-interference oligonucleotides (siRNAoligos) for VNUT according to a previously reported rational-design protocol (55), targeting the following positions of the bovine SLC17A9 cDNA sequence (NM_001100378.2): siRNA-VNUT 1 (positions 783–801: sense, 5′-GGAGACAGCUCUUCCGAAA-3′-dTdT and antisense, 5′-UUUCGGAAGAGCUGUCUCC-3′dTdT); and siRNA-VNUT 2 (positions 310–329: sense, 5′-GGUCAUCCUGCUCUCGGCUU-3′-dTdT and antisense, 5′-AAGCCGAGAGCAGGAUGACC -3′-dTdT). The siRNA duplexes were tagged at the 5′ end of the sense strand with the red Cy3 dye. Specificity of the sequence was confirmed by a BLAST analysis for bovine VNUT. The sequences of the scrambled nontargeting siRNA used were: sense, 5′-GCGCGAUAGCGCGAAUAUA-3′-dTdT and antisense, 5′-UAUAUUCGCGCUUAUCGCGC-3′-dTdT.
Plasmid Constructs.
The vector to express full-length bovine SLC17A9 (VNUT: NCBI Reference Sequence: NM_001100378.2) was prepared by PCR using a high-fidelity polymerase (Expand High Fidelity PLUS PCR System; Roche) and cDNAs prepared from the total RNA isolated from purified bovine chromaffin cells as the template. The primers used for PCR were based on database sequences: forward, 5′-CTTCGAATTCTGGCCACCATGCAGCCGCCCCCAGAC-3′; reverse, 5′-CCGCGGTACCGTGAGGTCCTCATGGGCGGGGCTCAG-3′. The PCR products containing full coding sequences were cloned directly into pEGFP-N1 (Clontech, BD Bioscience) using the EcoRI and KpnI sites to produce VNUT-EGFP. The insert was sequenced and was confirmed by comparison with the bovine genome sequence. Circular plasmid cDNAs for transfection were prepared using a GE Healthcare midi-preparation kit.
The plasmids encoding neuropeptide Y (NPY) as a protein fusion with EGFP (NPY-EGFP) or DsRed (NPY-DsRed) were kindly provided by Wolfhard Almers (Vollum Institute, Oregon Health and Science University, Portland, OR) (56) and Zheng-Xing Wu (Institute of Biophysics & Biochemistry, College of Life Science & Technology, Huazhong University of Science & Technology, Wuhan, China), respectively.
Bovine Chromaffin Cell Culture and Transfection.
Bovine chromaffin cells were isolated by adrenal medulla digestion with collagenase IA and they were purified by centrifugation on discontinuous Urografin gradient, as described previously (57, 58). The cells were then suspended in DMEM and Ham’s-F12 medium (1:1) supplemented with 5% (vol/vol) FBS, 50 IU/mL–1 penicillin, and 50 mg/mL–1 gentamicin.
To maximize the interference effect, we reduced the number of mature SVs by exposing isolated bovine chromaffin cells to a high K+ solution (35 mM) 5 min before transfection. Subsequently, the cells were washed and centrifuged for 5 min at 900 × g and they were prepared for nucleofection. The high K+ solution was prepared by equiosmolar replacement of NaCl by KCl. Chromaffin cells (3 × 106 cells per cuvette) were transfected using a Nucleofector II device (Lonza), the protocol (O-005), and the specific Amaxa-kit with siRNA (0.72 µM) and DNA constructs (2 µg), performing experiments 24–48 h after transfection. This method yields ∼35–40% transfected bovine chromaffin cells, and it provides excellent and reproducible secretory responses 72 h after culture.
RNA Isolation, cDNA Synthesis, and qRT-PCR.
RNA was isolated from purified bovine chromaffin cells (7 × 106 cultured cells) using the RNAspin Mini RNA isolation kit (GE Healthcare). Genomic DNA contamination was avoided by adding DNase I and the RNA concentration was determined spectrophotometrically. Total RNA was used for first strand cDNA synthesis in a one-step reaction primed with oligodT probes, according to the manufacturer’s instructions (iScriptcDNA Synthesis; Bio-Rad).
The cDNAs were subjected to 30 cycles of qRT–PCR using the following sets of primers: forward, 5′-GGAAGCTCACTGGCATGGCCT-3′ and reverse, 5′-CGCCTGCTTCACCACCTTCTTG-3′, which amplifies 116 bp of GAPDH; 5′-GGAGGGGGCCGTCCTATGCA-3′ and 5′-GAGGCAGGGCTGGCTGTTGG-3′, which amplifies 95 bp of VNUT.
All qRT-PCR experiments were performed with the iQ SYBR Green Supermix and they were quantified by Mini Opticon Real-time PCR (Bio-Rad); each experimental condition was analyzed in triplicate for each probe used. VNUT expression was normalized to that of the housekeeping gene GAPDH and the relative expression of the genes of interest was determined by the δ−δ cycle threshold method (−ΔΔ cycle threshold) (59, 60). Primer amplification efficiency was assessed by amplifying serial dilutions of the template cDNA and it was found to be comparable among the probes.
Isolation of Bovine Chromaffin Secretory Vesicles.
Adrenal medullas were triturated (>50 strokes up-and-down) in a Dounce-type teflon homogenizer (10 mL) in an ice-cold buffer adjusted to pH 7.0 with KOH and containing: sucrose 250 mM, EDTA 1 mM, MgSO4 1 mM, Hepes 10 mM, KCl 10 mM, protease inhibitors (cOmplete, Roche), and DNase (1 µg/mL). The postnuclear supernatant was obtained after centrifugation at 1,000 × g for 10 min at 4 °C and the supernatant was centrifuged at 10,000 × g for 20 min at 4 °C to obtain a crude pellet enriched in large dense core vesicles. This pellet was resuspended in 100 μL of buffer containing 5% (vol/vol) iodixanol (OptiPrep) and then layered onto a continuous [8–26% (vol/vol)] isosmotic density iodixanol gradient of made using a Gradient Station former (BioComp Instruments). Vesicles were fractionated by centrifugation at 100,000 × g for 1 h at 4 °C (SW55Ti rotor, on a Beckman Optima L100 XP Ultracentrifuge, Beckman Coulter) and after centrifugation, 12 fractions were collected using a gradient station collector.
Iodixanol concentrations were determined by measuring the absorbance at 340 nm of each fraction and converting this to density using an iodixanol standard/calibration curves. Each fraction was then washed in PBS and centrifuged at 20,000 × g for 40 min at 4 °C to remove iodixanol. The pellets were resuspended in 50 μL of TENT for further analysis of the proteins, catecholamines and ATP.
ATP Secretion Assay.
Chromaffin cells (5 × 105 cells per well) were cultured in a 24-well plate and immediately before the start of the experiments, chromaffin cells were washed twice with a Krebs-Hepes buffer solution at pH 7.4 containing: NaCl 140 mM, KCl 5.9 mM, MgCl2 1.2 mM, CaCl2 2 mM, Hepes 10 mM, and glucose 11 mM. The cells were subsequently incubated for 10 min in this buffer at 37 °C, supplemented with 100 μM of ARL 67156, a selective inhibitor of ectoATPases. The cells were then exposed for 5 min to the Krebs/ARL 67156 solution containing DMPP (100 μM) to stimulate release. Aliquots of the bathing media (200 µL) were collected and the cells were then lysed by adding 200 µL of TENT buffer (Tris⋅HCl 50 mM, EDTA 5 mM, NaCl 150 mM, and 1% Triton X-100). The samples were subsequently centrifuged at 900 × g for 5 min at 4 °C and the supernatants were collected. The ATP was measured in 50 µL of the supernatant using a luciferine-luciferase assay kit (Invitrogen) and quantified on sextuplicates in a standard luminometer (Luminoskan Ascent, Thermo Fisher Scientific).
Western Blotting.
The extent of protein expression was assessed in Western blots of cell lysates. Control and siRNA transfected cells were lysed 24, 48, and 72 h after transfection by incubating them for 20 min at 4 °C in TENT lysis solution: Tris⋅HCl 50 mM, EDTA 5 mM, NaCl 150 mM, and 1% Triton X-100, with the protease inhibitor mixture Complete (11697498001, Roche Diagnostics). The samples were then sonicated for 10 s (Labsonic M) and stored at −80 °C. Equivalent amounts of protein (measured by the bicinchoninic method) were separated by SDS–PAGE on 10% (wt/vol) acrylamide gels and electroblotted onto 0.45-μm polyvinylidene difluoride membranes (Immobilon-P IPVH00010, Millipore). The membranes were then probed with specific antibodies and antibody binding was detected by ECL using a prime Western blotting detection reagent (RPN2232, GE Healthcare). The protein bands were analyzed using a ChemiDoc device and Image Lab software (Bio-Rad).
Immunofluorescence.
Chromaffin cells (5 × 105 cultured cells) grown on 12-mm diameter poly-d-lysine–coated glass coverslips were fixed for 10 min at room temperature in 4% (wt/vol) paraformaldehyde. The cells were then washed three times with PBS and permeabilized with 0.1% Tween 20 in PBS. The cells were quenched with 100 mM glycine in PBS and then blocked with 5% (vol/vol) FBS in permeabilization solution. The cells were exposed to the primary antibodies overnight at 4 °C in blocking solution and after washing, to the secondary antibodies for 1 h at room temperature. The coverslips were then mounted in Fluoroshield with DAPI (Sigma-Aldrich) and visualized in x-y middle sections on a FluoView FV1000 confocal microscope (Olympus). The final images were analyzed with Metamorph software (Universal Imaging).
Confocal Analysis of Codistribution.
As described previously (49), the overlap between different fluorescent molecules was determined from confocal images of 3 × 3 pixels that were low-pass–filtered using Metamorph. We plotted a circle of 0.8-µm diameter around each spot analyzed as a region of interest (ROI), and five circles outside this to calculate the local background. We drew the ROIs around VNUT spots and then transferred them into the image of the pair molecule at identical pixel locations, determining whether the new circle contained a fluorescent point concentric to within 0.15 µm to quantify the degree of colocalization with endogenous CgB. Colocalization was positive when the average fluorescence intensity was at least three times the SD of the background. The percentage of codistribution was determined in single cells after random codistribution subtraction and the average values were calculated from the total number of cells analyzed. Images were rotated 90° and molecule codistribution was again calculated as described above to ensure that the observed correlation was not a result of random signal overlap. If the observed colocalization were random, rotation of the image would not change the degree of signal overlap obtained before the rotation of the image.
Amperometric Detection of Exocytosis.
Chromaffin cells transfected with Cy3 tagged siRNA oligos were viewed by epi-fluorescence under an inverted microscope (Olympus IX51, using a 40×/0.60 NA objective). Excited light (mercury lamp, X-cite EXFO series, 120W) was band-pass–filtered (BP545/25) and the emitted light was passed through a dichroic 565-lpxr and band-pass filter (BP650/70: all filters were from Chroma Technology). Carbon fiber microelectrodes (6-µm radius; Thornel P-55: Amoco) were prepared as described previously (30, 50), and electrochemical recordings were obtained using a VA-10X potentiostat (NPI Electronics) connected to a PowerLab 8/30 (ADInstruments) (see refs. 6 and 51 for details). Microelectrode calibration was essential to ensure the reproducibility of the results, such that the electrodes were tested and accepted for cell studies when the application of noradrenaline (50 µM) resulted in an oxidation current of 300–400 pA, which should be reduced by 80–100 pA under stopping-flow conditions.
All amperometric measurements were performed with the electrode gently touching the cell membrane. Cell release was stimulated by a 5-s pressure ejection of a Ba2+ solution (5 mM) from a micropipette positioned 40 µm from the cell. To avoid any bias caused by unnoticed changes in the sensitivity of electrodes, all experiments were performed on the same day, using the same electrode and alternating recordings from siVNUT and scrambled cells.
Measurement of Cytosolic-Free Ca2+.
Chromaffin cells were seeded on 12-mm Ø coverslips in 24-well culture plates at a density of ∼100,000 cells per well. After 24 h the cells were incubated for 45 min at 37 °C with 2 µM fluo-4 (Molecular Probes, Invitrogen) in Krebs-Hepes. The cells were then washed for 45 min at room temperature and placed in a chamber mounted on the stage of a Zeiss Axiovert 200 microscope with continuous perfusion. Single-cell fluorescence was excited at 488 nm using an argon ion laser (100 ms of excitation every 1 s, 10-nm bandwidth; Lasos, Lasertechnik) and with a EC Plan-Neofluar 20×/0.50 objective (Zeiss). All measurements were taken at room temperature.
Images were collected using a 510-DCLP dichroic mirror and a D525/50 emission filter (Chroma Technology), and then recorded on a CCD camera (AxioCam MRm, Zeiss). Single-cell fluorescence records were plotted against time using the Metafluor software (Universal Imaging) and statistical analysis was carried out with the Student’s t test.
Acknowledgments
We thank the personnel of the “Matadero Insular de Tenerife” for providing cow adrenal glands. This work was supported by the Spanish Ministry of Economía y Competitividad (MINECO) Grants BFU2010-15822 and BFU2013-45253-P; J.E.-H. and A.G.-S. are recipients of PhD fellowships (MINECO); and J.D.M. held “Ramon y Cajal” Contract RYC2010-06256 (MINECO/FEDER).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600690113/-/DCSupplemental.
References
- 1.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27(3):166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Borges R. The ATP or the natural history of neurotransmission. Purinergic Signal. 2013;9(1):5–6. doi: 10.1007/s11302-012-9330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RG, Carlson NJ, Scarpa A. deltapH and catecholamine distribution in isolated chromaffin granules. J Biol Chem. 1978;253(5):1512–1521. [PubMed] [Google Scholar]
- 4.Morris SJ, Schultens HA, Schober R. An osmometer model for changes in the buoyant density of chromaffin granules. Biophys J. 1977;20(1):33–48. doi: 10.1016/S0006-3495(77)85535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albillos A, et al. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389(6650):509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- 6.Montesinos MS, et al. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci. 2008;28(13):3350–3358. doi: 10.1523/JNEUROSCI.5292-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler H, Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]
- 8.Santodomingo J, et al. Calcium dynamics in bovine adrenal medulla chromaffin cell secretory granules. Eur J Neurosci. 2008;28(7):1265–1274. doi: 10.1111/j.1460-9568.2008.06440.x. [DOI] [PubMed] [Google Scholar]
- 9.Granot J, Fiat D. NMR studies of catecholamines. Interactions with adenine nucleotides and divalent metal ions in aqueous solution. J Am Chem Soc. 1977;99(15):4963–4968. doi: 10.1021/ja00457a012. [DOI] [PubMed] [Google Scholar]
- 10.Berneis KH, Pletscher A, Da Prada M. Interaction of proteins with aggregates of catecholamines and nucleotides: Possible biological implications. Agents Actions. 1971;2(2):65–68. doi: 10.1007/BF01965384. [DOI] [PubMed] [Google Scholar]
- 11.Weder HG, Wiegand UW. The interaction of biogenic amines with adenosine-5′-triphosphate: A calorimetric study. FEBS Lett. 1973;38(1):64–66. doi: 10.1016/0014-5793(73)80514-9. [DOI] [PubMed] [Google Scholar]
- 12.Daniels A, Korda A, Tanswell P, Williams A, Williams RJ. The internal structure of the chromaffin granule. Proc R Soc Lond B Biol Sci. 1974;187(1088):353–361. doi: 10.1098/rspb.1974.0080. [DOI] [PubMed] [Google Scholar]
- 13.Kopell WN, Westhead EW. Osmotic pressures of solutions of ATP and catecholamines relating to storage in chromaffin granules. J Biol Chem. 1982;257(10):5707–5710. [PubMed] [Google Scholar]
- 14.Hillarp NA. Catecholamines: Mechanisms of storage and release. Acta Endocrinol Suppl (Copenh) 1960;(Suppl 50):181–185. doi: 10.1530/acta.0.xxxivs181. [DOI] [PubMed] [Google Scholar]
- 15.Helle KB, Reed RK, Pihl KE, Serck-Hanssen G. Osmotic properties of the chromogranins and relation to osmotic pressure in catecholamine storage granules. Acta Physiol Scand. 1985;123(1):21–33. doi: 10.1111/j.1748-1716.1985.tb07556.x. [DOI] [PubMed] [Google Scholar]
- 16.Díaz-Vera J, et al. Chromogranins A and B are key proteins in amine accumulation, but the catecholamine secretory pathway is conserved without them. FASEB J. 2012;26(1):430–438. doi: 10.1096/fj.11-181941. [DOI] [PubMed] [Google Scholar]
- 17.Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts. Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271(29):17132–17138. doi: 10.1074/jbc.271.29.17132. [DOI] [PubMed] [Google Scholar]
- 18.Luqmani YA. Nucleotide uptake by isolated cholinergic synaptic vesicles: Evidence for a carrier of adenosine 5′-triphosphate. Neuroscience. 1981;6(6):1011–1021. doi: 10.1016/0306-4522(81)90067-1. [DOI] [PubMed] [Google Scholar]
- 19.Gualix J, Alvarez AM, Pintor J, Miras-Portugal MT. Studies of chromaffin granule functioning by flow cytometry: Transport of fluorescent epsilon-ATP and granular size increase induced by ATP. Receptors Channels. 1999;6(6):449–461. [PubMed] [Google Scholar]
- 20.Sawada K, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105(15):5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto S, et al. Impairment of vesicular ATP release affects glucose metabolism and increases insulin sensitivity. Sci Rep. 2014;4:6689. doi: 10.1038/srep06689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosa P, et al. An antibody against secretogranin I (chromogranin B) is packaged into secretory granules. J Cell Biol. 1989;109(1):17–34. doi: 10.1083/jcb.109.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camacho M, Machado JD, Alvarez J, Borges R. Intravesicular calcium release mediates the motion and exocytosis of secretory organelles: A study with adrenal chromaffin cells. J Biol Chem. 2008;283(33):22383–22389. doi: 10.1074/jbc.M800552200. [DOI] [PubMed] [Google Scholar]
- 24.Díaz-Vera J, et al. Chromogranin B gene ablation reduces the catecholamine cargo and decelerates exocytosis in chromaffin secretory vesicles. J Neurosci. 2010;30(3):950–957. doi: 10.1523/JNEUROSCI.2894-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26(8-9):959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 26.Torres M, Pintor J, Miras-Portugal MT. Presence of ectonucleotidases in cultured chromaffin cells: Hydrolysis of extracellular adenine nucleotides. Arch Biochem Biophys. 1990;279(1):37–44. doi: 10.1016/0003-9861(90)90460-g. [DOI] [PubMed] [Google Scholar]
- 27.Herrera M, Kao LS, Curran DJ, Westhead EW. Flow-injection analysis of catecholamine secretion from bovine adrenal medulla cells on microbeads. Anal Biochem. 1985;144(1):218–227. doi: 10.1016/0003-2697(85)90109-5. [DOI] [PubMed] [Google Scholar]
- 28.Ballesta JJ, Borges R, García AG, Hidalgo MJ. Secretory and radioligand binding studies on muscarinic receptors in bovine and feline chromaffin cells. J Physiol. 1989;418:411–426. doi: 10.1113/jphysiol.1989.sp017849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernández-Guijo JM, Gandía L, Lara B, García AG. Autocrine/paracrine modulation of calcium channels in bovine chromaffin cells. Pflugers Arch. 1998;437(1):104–113. doi: 10.1007/s004240050754. [DOI] [PubMed] [Google Scholar]
- 30.Machado DJ, Montesinos MS, Borges R. Good practices in single-cell amperometry. Methods Mol Biol. 2008;440:297–313. doi: 10.1007/978-1-59745-178-9_23. [DOI] [PubMed] [Google Scholar]
- 31.Hiasa M, et al. Essential role of vesicular nucleotide transporter in vesicular storage and release of nucleotides in platelets. Physiol Rep. 2014;2(6):e12034. doi: 10.14814/phy2.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knauf PA, Fuhrmann GF, Rothstein S, Rothstein A. The relationship between anion exchange and net anion flow across the human red blood cell membrane. J Gen Physiol. 1977;69(3):363–386. doi: 10.1085/jgp.69.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camacho M, Machado JD, Montesinos MS, Criado M, Borges R. Intragranular pH rapidly modulates exocytosis in adrenal chromaffin cells. J Neurochem. 2006;96(2):324–334. doi: 10.1111/j.1471-4159.2005.03526.x. [DOI] [PubMed] [Google Scholar]
- 34.Bültmann R, Starke K. Evans blue blocks P2X-purinoceptors in rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1993;348(6):684–687. doi: 10.1007/BF00167248. [DOI] [PubMed] [Google Scholar]
- 35.Hillarp NA, Nilson B, Hogberg B. Adenosine triphosphate in the adrenal medulla of the cow. Nature. 1955;176(4491):1032–1033. doi: 10.1038/1761032a0. [DOI] [PubMed] [Google Scholar]
- 36.Kasai Y, Ohta T, Nakazato Y, Ito S. Release of dopamine and ATP from PC12 cells treated with dexamethasone, reserpine and bafilomycin A1. J Vet Med Sci. 2001;63(4):367–372. doi: 10.1292/jvms.63.367. [DOI] [PubMed] [Google Scholar]
- 37.Larsson M, et al. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb Cortex. 2012;22(5):1203–1214. doi: 10.1093/cercor/bhr203. [DOI] [PubMed] [Google Scholar]
- 38.Corcoran JJ, Wilson SP, Kirshner N. Flux of catecholamines through chromaffin vesicles in cultured bovine adrenal medullary cells. J Biol Chem. 1984;259(10):6208–6214. [PubMed] [Google Scholar]
- 39.Gandía L, García AG, Morad M. ATP modulation of calcium channels in chromaffin cells. J Physiol. 1993;470:55–72. doi: 10.1113/jphysiol.1993.sp019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duncan RR, et al. Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature. 2003;422(6928):176–180. doi: 10.1038/nature01389. [DOI] [PubMed] [Google Scholar]
- 41.Tsuboi T, Kitaguchi T, Karasawa S, Fukuda M, Miyawaki A. Age-dependent preferential dense-core vesicle exocytosis in neuroendocrine cells revealed by newly developed monomeric fluorescent timer protein. Mol Biol Cell. 2010;21(1):87–94. doi: 10.1091/mbc.E09-08-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabares L, Alés E, Lindau M, Alvarez de Toledo G. Exocytosis of catecholamine (CA)-containing and CA-free granules in chromaffin cells. J Biol Chem. 2001;276(43):39974–39979. doi: 10.1074/jbc.M106498200. [DOI] [PubMed] [Google Scholar]
- 43.Herman MA, Ackermann F, Trimbuch T, Rosenmund C. Vesicular glutamate transporter expression level affects synaptic vesicle release probability at hippocampal synapses in culture. J Neurosci. 2014;34(35):11781–11791. doi: 10.1523/JNEUROSCI.1444-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wojcik SM, et al. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50(4):575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Sombers LA, et al. The effects of vesicular volume on secretion through the fusion pore in exocytotic release from PC12 cells. J Neurosci. 2004;24(2):303–309. doi: 10.1523/JNEUROSCI.1119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menéndez-Méndez A, Díaz-Hernández JI, Miras-Portugal MT. The vesicular nucleotide transporter (VNUT) is involved in the extracellular ATP effect on neuronal differentiation. Purinergic Signal. 2015;11(2):239–249. doi: 10.1007/s11302-015-9449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colliver TL, Pyott SJ, Achalabun M, Ewing AG. VMAT-mediated changes in quantal size and vesicular volume. J Neurosci. 2000;20(14):5276–5282. doi: 10.1523/JNEUROSCI.20-14-05276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dominguez N, Estevez-Herrera J, Borges R, Machado JD. The interaction between chromogranin A and catecholamines governs exocytosis. FASEB J. 2014;28(11):4657–4667. doi: 10.1096/fj.14-249607. [DOI] [PubMed] [Google Scholar]
- 49.Barroso-González J, Machado JD, García-Expósito L, Valenzuela-Fernández A. Moesin regulates the trafficking of nascent clathrin-coated vesicles. J Biol Chem. 2009;284(4):2419–2434. doi: 10.1074/jbc.M805311200. [DOI] [PubMed] [Google Scholar]
- 50.Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112(2):119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- 51.Machado JD, Segura F, Brioso MA, Borges R. Nitric oxide modulates a late step of exocytosis. J Biol Chem. 2000;275(27):20274–20279. doi: 10.1074/jbc.M000930200. [DOI] [PubMed] [Google Scholar]
- 52.Segura F, Brioso MA, Gómez JF, Machado JD, Borges R. Automatic analysis for amperometrical recordings of exocytosis. J Neurosci Methods. 2000;103(2):151–156. doi: 10.1016/s0165-0270(00)00309-5. [DOI] [PubMed] [Google Scholar]
- 53.Colliver TL, Hess EJ, Pothos EN, Sulzer D, Ewing AG. Quantitative and statistical analysis of the shape of amperometric spikes recorded from two populations of cells. J Neurochem. 2000;74(3):1086–1097. doi: 10.1046/j.1471-4159.2000.741086.x. [DOI] [PubMed] [Google Scholar]
- 54.Borges R, Sala F, García AG. Continuous monitoring of catecholamine release from perfused cat adrenals. J Neurosci Methods. 1986;16(4):289–300. doi: 10.1016/0165-0270(86)90054-3. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds A, et al. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22(3):326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 56.Taraska JW, Almers W. Bilayers merge even when exocytosis is transient. Proc Natl Acad Sci USA. 2004;101(23):8780–8785. doi: 10.1073/pnas.0401316101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dominguez N, Rodriguez M, Machado JD, Borges R. Preparation and culture of adrenal chromaffin cells. In: Skaper SD, editor. Methods Mol Biol. New York: Humana Press; 2012. [DOI] [PubMed] [Google Scholar]
- 58.Moro MA, Lopez MG, Gandia L, Michelena P, Garcia AG. Separation and culture of living adrenaline- and noradrenaline-containing cells from bovine adrenal medullae. Anal Biochem. 1990;185(2):243–248. doi: 10.1016/0003-2697(90)90287-j. [DOI] [PubMed] [Google Scholar]
- 59.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 60.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]