IgG oligoclonal bands (OCB) are detected in the cerebrospinal fluid (CSF) of more than 90% of multiple sclerosis (MS) patients and are considered the immunological hallmark that supports MS diagnosis. OCB are not unique to MS but are also observed in chronic CNS infections, where they target their causative agents (1–3). However, the target specificities of the IgG within OCB in MS have remained a mystery. Identification of those antigens recognized by OCB antibodies is thought to hold fundamental clues to the pathogenesis of MS. In a recent PNAS publication, Brändle et al. (4) provide evidence that OCB in MS target ubiquitous intracellular antigens released in cellular debris.
In 1942, Kabat et al. (5) established the diagnostic value of quantitative determinations of CSF gamma globulins in clinical neurology, and particularly in MS. They observed that changes in CSF IgG were independent of those in serum, suggesting that this Ig production was compartmentalized to the CNS. Advances in CSF analytics and gel electrophoresis led to the identification of OCB in 1959 (6). CSF OCB in MS patients are persistent, which is thought to be a reflection of both ongoing CNS inflammation and immunologic memory. Understanding the specificity of OCB has since captivated the interest of clinical neurologists and scientists alike. It has been assumed that the OCB target antigens are relevant to MS pathogenesis. The most popular theory contends that IgG within OCB target myelin autoantigens and/or viruses that may elicit CNS damage directly or indirectly via molecular mimicry. Some earlier studies that evaluated whole CSF IgG from MS patients identified antibodies to several different viruses, such as measles, varicella zoster, human T-lymphocytic virus 1, and human hepatitis virus 6 (7), whereas other investigations found antibodies targeting major myelin proteins, myelin basic protein (MBP) and myelin oligodencrocyte glycoprotein (MOG) (8, 9) as well as glycolipids, fatty acids, and neurofilament proteins (10). Similarly, more recent investigations that have applied single-cell PCR cloning to individual CSF B cells in MS have detected antibodies to certain viruses or myelin proteins (11–13). However, it has been impossible to match specificity of antibodies identified in CSF to OCB by studying whole CSF IgG or recombinant antibodies constructed from rearranged Ig heavy- and light-chain genes in individual B cells.
Dornmair and coworkers used a combination of new biochemical, proteomic, and transcriptomic approaches (4, 14) to examine the specificity of antibodies in MS OCB. Disulfide-linked IgG heavy- (IgH) and IgG light- (IgL) chain complexes were purified from single OCB spots using affinity chromatography and two-dimensional gel electrophoresis. Those antibody (IgH2IgL2) complexes were then analyzed by mass spectrometry to generate patient-specific Ig peptidomes. In parallel, IgH and IgL genes, including the unique complementarity-determining region 3, from CSF B cells isolated from the corresponding patient were sequenced to generate patient-specific IgH and IgL transcriptomes. Alignment of patient-specific Ig peptidomes to the corresponding patient-specific Ig transcriptomes produced full-length sequences of matching IgG heavy and light chains, therefore representing distinct antibody species originating from one of the OCB. Using an expression system, Dornmair and coworkers produced recombinant OCB antibodies for target antigen characterization using a protein microarray that displayed over 9,400 full-length recombinant human proteins. As validation of this methodology, these investigators demonstrated that a recombinant OCB antibody from a patient with Lyme disease, an infectious CNS disorder caused by the bacterium Borrelia burgdorferi, recognized a Borrelia antigen. They also used the commercially available anti-MOG antibody (clone r8-18C5) as a control to demonstrate specificity and sensitivity for binding of a myelin protein. Six different OCB recombinant antibodies (rAb) from the four MS patients were produced. Three of those rAb, originating from two of the four MS patients, recognized three different autoantigens. As expected, the control anti-MOG r8-18C5 antibody specifically bound MOG. However, the three OCB rAb recognized neither CNS-specific proteins (e.g., MOG, MBP, and proteolipid protein) nor suspected pathogens associated with MS. Instead, the recombinant OCB antibodies were directed against three different ubiquitous (i.e., not CNS-specific) intracellular proteins: MAP kinase-interacting serine/threonine kinase 1/2 (MKNK1/2), family with sequence similarity 84 member A (FAM84A), and A-kinase anchor protein 17A (AKAP17A). Collectively, these results demonstrated that those antibodies from OCB in MS patients are directed against intracellular antigens, suggesting they may result from a secondary immune response to cellular damage (Fig. 1).
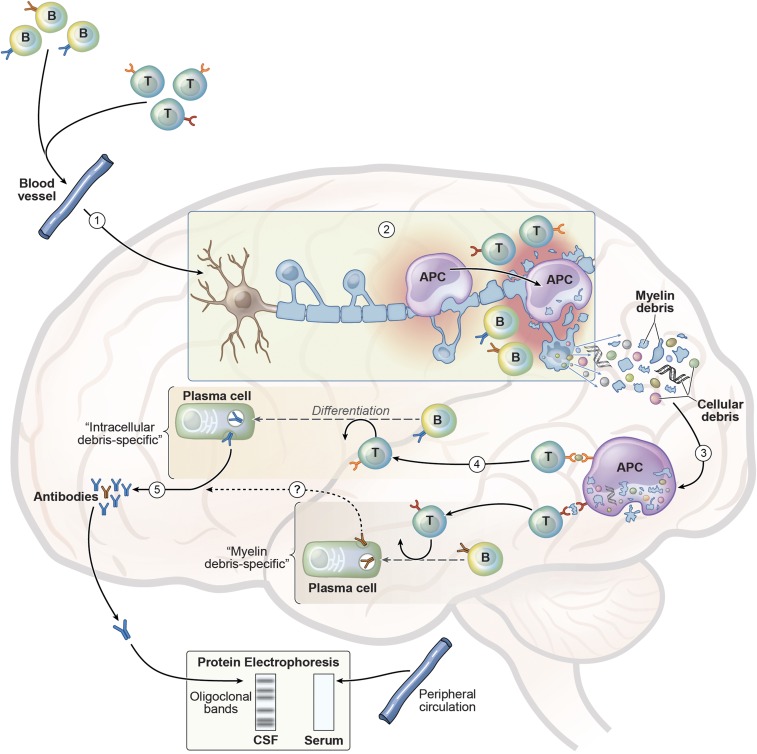
Fig. 1.
Model for development of secondary intracellular debris-specific antibodies in CSF OCB. (1) Upon activation in peripheral secondary lymphoid tissues, myelin-specific T cells, including T cells that recognize cross-reactive epitopes of infectious organisms (i.e., molecular mimicry), enter the blood, traffic to the CNS, cross the blood–brain barrier, and infiltrate the parenchyma. Similarly, B cells may comigrate with the activated T cells. (2) Within the CNS, myelin-specific T cells may initiate focal inflammation by cytokine production and activation of resident microglia, which may serve as APC to those T cells. This initial CNS inflammatory response leads to recruitment of infiltrating macrophages and dendritic cells that can also serve as APC, as well as other immune cells, culminating in the destruction and release of debris from myelin and the myelin-forming oligodendrocytes. (3) Intracellular and myelin debris are phagocytosed and processed by APC, then presented to infiltrating T cells that may recognize those neoantigens. (4) Activated antigen-specific T cells (e.g., T follicular helper cells) help infiltrating B cells that may recognize intracellular debris differentiate into antibody-producing plasma cells. (5) These clonally expanded plasma cells produce IgG in the CSF, which are depicted as discrete “oligoclonal” bands when analyzed by protein gel electrophoresis. Because this immune response is compartmentalized to the CNS, the corresponding bands are not detected by serum protein electrophoresis. Brändle et al. (4) demonstrated that IgG antibodies within three separate bands recognize ubiquitous intracellular antigens (i.e., MKNK1/2, FAM84A, and AKAP17A). Other investigators have identified clonally expanded CSF plasma cells that recognize myelin proteins (11). Currently, it is not known whether antibodies produced by those myelin debris-specific B cells also contribute to formation of discrete bands (indicated by a question mark in step 4). Image courtesy of Xavier Studio.
The demonstration by Brändle et al. (4) that antibodies in MS OCB target ubiquitous intracellular proteins is a surprise, especially because decades of research evaluating CSF B cells and OCB has focused on the identification of candidate viruses and myelin antigens. The inability to identify and confirm potential targets led Arnason and his colleagues to question whether the antibodies in OCB are “nonsense” and therefore may be irrelevant to MS pathogenesis (15). These new findings raise several questions. Are intracellular debris-specific IgG in CSF OCB pathogenic? Except for the view that the humoral response in MS is compartmentalized, one might question why targeting intracellular proteins does not lead to systemic autoimmunity (e.g., systemic lupus erythematosus). Conversely, is it possible that a CNS secondary humoral response to intracellular target antigens is beneficial? In light of Grabar’s “debris hypothesis” in autoimmunity (16), it could be possible that OCB antibodies targeting intracellular proteins may promote clearance of degradation products. However, the possibility that OCB antibodies have a protective role is difficult to imagine because OCB in MS patients predict a more severe course (17).
There is one clear limitation to the study by Brändle et al. (4). Although they generated six OCB rAbs from four MS patients, they were successful in identifying the specificity of only three antibodies originating from two patients. It is therefore possible that by examining more OCB from additional MS patients some of those OCB antibodies might recognize myelin or neuronal proteins. One might also question the importance of identifying the specificity of OCB antibodies in MS. Clinical trials have shown that the anti-CD20 B-cell-depleting antibody ocrelizumab is remarkably effective in treatment of relapsing–remitting (18) and primary progressive MS (19). However, such clinical benefit occurs soon after B-cell depletion without evident changes in antibodies, and therefore likely reflects a loss in B-cell antigen-presenting cell (APC) function (20). Whether long-term B-cell depletion influences OCB is not yet clear. Regardless of whether OCB could serve as a surrogate marker of therapeutic response in MS, elucidating the specificity of those antibodies will be critical for determining if they participate in MS pathogenesis. Dornmair and coworkers (4) have provided the tools to solve this conundrum.
Footnotes
The authors declare no conflict of interest.
See companion article on page 7864.
References
- 1.Vandvik B, Norrby E, Nordal HJ, Degré M. Oligoclonal measles virus-specific IgG antibodies isolated from cerebrospinal fluids, brain extracts, and sera from patients with subacute sclerosing panencephalitis and multiple sclerosis. Scand J Immunol. 1976;5(8):979–992. doi: 10.1111/j.1365-3083.1976.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 2.Porter KG, Sinnamon DG, Gillies RR. Cryptococcus neoformans-specific oligoclonal immunoglobulins in cerebrospinal fluid in cryptococcal meningitis. Lancet. 1977;1(8024):1262. doi: 10.1016/s0140-6736(77)92473-4. [DOI] [PubMed] [Google Scholar]
- 3.Hansen K, Cruz M, Link H. Oligoclonal Borrelia burgdorferi-specific IgG antibodies in cerebrospinal fluid in Lyme neuroborreliosis. J Infect Dis. 1990;161(6):1194–1202. doi: 10.1093/infdis/161.6.1194. [DOI] [PubMed] [Google Scholar]
- 4.Brändle SM, et al. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc Natl Acad Sci USA. 2016;113:7864–7869. doi: 10.1073/pnas.1522730113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabat EA, Moore DH, Landow H. An electrophoretic study of the protein components in cerebrospinal fluid and their relationship to the serum proteins. J Clin Invest. 1942;21(5):571–577. doi: 10.1172/JCI101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmøy T. The discovery of oligoclonal bands: A 50-year anniversary. Eur Neurol. 2009;62(5):311–315. doi: 10.1159/000235944. [DOI] [PubMed] [Google Scholar]
- 7.Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4(3):195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao BG, Linington C, Link H. Antibodies to myelin-oligodendrocyte glycoprotein in cerebrospinal fluid from patients with multiple sclerosis and controls. J Neuroimmunol. 1991;31(2):91–96. doi: 10.1016/0165-5728(91)90014-x. [DOI] [PubMed] [Google Scholar]
- 9.Link H, et al. Persistent anti-myelin basic protein IgG antibody response in multiple sclerosis cerebrospinal fluid. J Neuroimmunol. 1990;28(3):237–248. doi: 10.1016/0165-5728(90)90017-h. [DOI] [PubMed] [Google Scholar]
- 10.Blauth K, et al. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid cause demyelination of spinal cord explants. Acta Neuropathol. 2015;130(6):765–781. doi: 10.1007/s00401-015-1500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Büdingen HC, Harrer MD, Kuenzle S, Meier M, Goebels N. Clonally expanded plasma cells in the cerebrospinal fluid of MS patients produce myelin-specific antibodies. Eur J Immunol. 2008;38(7):2014–2023. doi: 10.1002/eji.200737784. [DOI] [PubMed] [Google Scholar]
- 12.Sotelo J, Ordoñez G, Pineda B. Varicella-zoster virus at relapses of multiple sclerosis. J Neurol. 2007;254(4):493–500. doi: 10.1007/s00415-006-0402-x. [DOI] [PubMed] [Google Scholar]
- 13.Cepok S, et al. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. J Clin Invest. 2005;115(5):1352–1360. doi: 10.1172/JCI23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obermeier B, et al. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med. 2008;14(6):688–693. doi: 10.1038/nm1714. [DOI] [PubMed] [Google Scholar]
- 15.Mattson DH, Roos RP, Arnason BG. Isoelectric focusing of IgG eluted from multiple sclerosis and subacute sclerosing panencephalitis brains. Nature. 1980;287(5780):335–337. doi: 10.1038/287335a0. [DOI] [PubMed] [Google Scholar]
- 16.Grabar P. “Self”and “not-self” in immunology. Lancet. 1974;1(7870):1320–1322. doi: 10.1016/s0140-6736(74)90685-0. [DOI] [PubMed] [Google Scholar]
- 17.Joseph FG, et al. CSF oligoclonal band status informs prognosis in multiple sclerosis: A case control study of 100 patients. J Neurol Neurosurg Psychiatry. 2009;80(3):292–296. doi: 10.1136/jnnp.2008.150896. [DOI] [PubMed] [Google Scholar]
- 18.Kappos L, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: A phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378(9805):1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- 19.Steinman L, Zamvil SS. Beginning of the end of two-stage theory purporting that inflammation then degeneration explains pathogenesis of progressive multiple sclerosis. Curr Opin Neurol. 2016;29(3):340–344. doi: 10.1097/WCO.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 20.Molnarfi N, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med. 2013;210(13):2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]