Significance
Anthrax toxin proteins engineered to require activation by tumor-associated proteases show high specificity and potency in suppression of solid tumor growth through actions on tumor endothelial cells. The toxin strongly inhibits proliferation of tumor endothelial cells. Importantly, an immunosuppressive regimen (pentostatin plus cyclophosphamide) not only prevents induction of toxin-neutralizing antibodies, allowing multiple courses of toxin treatment, but also has strong synergy with the toxin on solid tumors. The ability to give repeated doses of toxins, coupled with the specific targeting of tumor endothelium, suggests that our strategy should be efficacious for a wide range of solid tumors, meriting its clinical evaluation.
Keywords: anthrax toxin, tumor targeting, angiogenesis, CMG2, TEM8
Abstract
Engineered tumor-targeted anthrax lethal toxin proteins have been shown to strongly suppress growth of solid tumors in mice. These toxins work through the native toxin receptors tumor endothelium marker-8 and capillary morphogenesis protein-2 (CMG2), which, in other contexts, have been described as markers of tumor endothelium. We found that neither receptor is required for tumor growth. We further demonstrate that tumor cells, which are resistant to the toxin when grown in vitro, become highly sensitive when implanted in mice. Using a range of tissue-specific loss-of-function and gain-of-function genetic models, we determined that this in vivo toxin sensitivity requires CMG2 expression on host-derived tumor endothelial cells. Notably, engineered toxins were shown to suppress the proliferation of isolated tumor endothelial cells. Finally, we demonstrate that administering an immunosuppressive regimen allows animals to receive multiple toxin dosages and thereby produces a strong and durable antitumor effect. The ability to give repeated doses of toxins, coupled with the specific targeting of tumor endothelial cells, suggests that our strategy should be efficacious for a wide range of solid tumors.
Recognition that aberrant activation of the RAS and PI3K pathways is often the mechanism of human tumorigenesis has inspired development of many small molecule inhibitors of these pathways and has led to improved treatments in certain cancers (1). However, these therapies are effective only in patients having defects in the specific targets of these drugs, and the therapies are rarely curative due to the development of resistance through acquisition of additional oncogenic mutations (2). Therefore, strategies are needed that are effective against a broad spectrum of cancers and that act through features that are not subject to development of resistance. This unmet need has fostered continued interest in strategies that target host-derived tumor vasculature.
Anthrax toxin, a major virulence factor of Bacillus anthracis, consists of three individually nontoxic proteins: the cellular binding component, protective antigen (PA), and two enzymatic moieties, lethal factor (LF) and edema factor (EF) (3). PA binds to two host cell-surface integrin-like proteins: tumor endothelium marker-8 (TEM8) [also termed anthrax toxin receptor 1 (ANTXR1)] and capillary morphogenesis protein-2 (CMG2 or ANTXR2) (4, 5). Receptor-bound PA is processed by the ubiquitous cell-associated furin protease to a 63-kDa fragment (PA63), which then forms LF- and EF-binding competent PA oligomers. Three or four molecules of LF or EF bind to the PA oligomers, and the complexes are then endocytosed (6–8). The acidic pH within the endosomes causes the PA oligomer to form a pore in the endosomal membrane, allowing translocation of LF or EF into the cytosol of cells to exert their cytotoxic actions (9). Thus, LF plus PA forms lethal toxin and EF plus PA forms edema toxin, with both toxins playing essential roles in anthrax pathogenesis (10–12).
Interestingly, both TEM8 and CMG2 have been implicated in tumor angiogenesis and therefore have been considered potential targets for cancer therapy (13–17). Consequently, reagents aimed at targeting TEM8 and CMG2 have been developed and evaluated in experimental cancer therapy. As an example, TEM8 antibodies have been shown to be effective in treating several different tumors in mice (18).
In addition to being the cognate ligand for these proposed tumor endothelial markers, the anthrax toxin proteins have unique features that allow engineering to make them specific anticancer agents (19). One approach to achieving specificity for tumor cells has been to exploit the requirement that PA be proteolytically activated on the cell surface, together with the recognition that tumor cells overexpress cell-surface proteases such as matrix metalloproteinases (MMPs) and urokinase-type plasminogen activator (uPA). Thus, replacing the furin target sequence RKKR with MMP or uPA substrate sequences has yielded specific antitumor agents (20–22). To further increase tumor specificity, based on the fact that each LF-binding site on PA oligomers is located at the bridge region of adjacent PA63 monomers, we have previously generated intermolecular complementing PA variants dependent on the simultaneous presence of both MMPs and uPA for activation (23–25). Because both cancer cells and many tumor stromal cells overexpress MMPs and uPA (26–28), these PA variants are preferentially activated in solid tumors, thereby being able to selectively deliver effector proteins, such as LF or recombinant LF fusion proteins, to the cytosol of target cells to exert various cytotoxic effects.
Native LF is a zinc-metalloproteinase that inactivates mitogen-activated protein kinase kinases (MEKs), thereby shutting down the RAS-RAF-MEK-ERK pathway (29, 30). Because cancer-driving, oncogenic mutations in the RAS-RAF-MEK-ERK pathway occur frequently in human cancers (2), the intrinsic activity of LF toward this pathway is another unique feature of the engineered anthrax lethal toxins that increases their effectiveness in tumor targeting. Therefore, engineered anthrax lethal toxins have emerged as a novel class of potent reagents for targeted cancer therapy. However, the mechanisms responsible for the antitumor activities of the tumor-selective anthrax toxins remain elusive, as do the exact in vivo roles of TEM8 and CMG2 in tumor growth. Moreover, the high immunogenicity of the toxin proteins has prevented their repeated use, an issue that requires resolution if these candidate drugs are to attract wide clinical use.
To address all these questions, here we evaluated the antitumor activities of the engineered anthrax toxins in various tumors in TEM8- and CMG2-modified mice. We found, surprisingly, that TEM8 and CMG2 expressed on tumor stromal cells are not important for tumor growth. The potent antitumor activities of the engineered anthrax lethal toxins occur through direct effects on tumor endothelial cells, rather than on other types of cells present in tumor stromal compartments. The modified lethal toxin exhibits potent inhibitory effects on proliferation of isolated tumor endothelial cells. Finally, we found that pentostatin plus cyclophosphamide, a selective B-cell immune suppression regimen, completely prevents the induction of toxin-neutralizing antibodies against the engineered toxin, allowing multiple cycles of therapy. We demonstrate that the combined therapy of the engineered toxin and pentostatin plus cyclophosphamide has remarkable and prolonged antitumor effects.
Results
CMG2 and TEM8 in Tumor Stromal Compartments Are Not Essential for Tumor Growth.
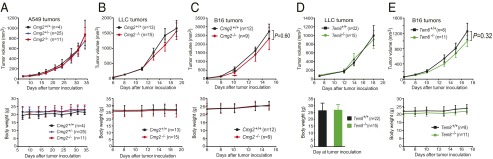
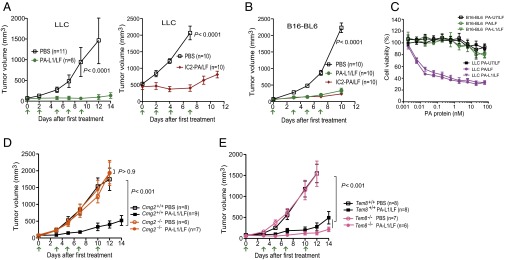
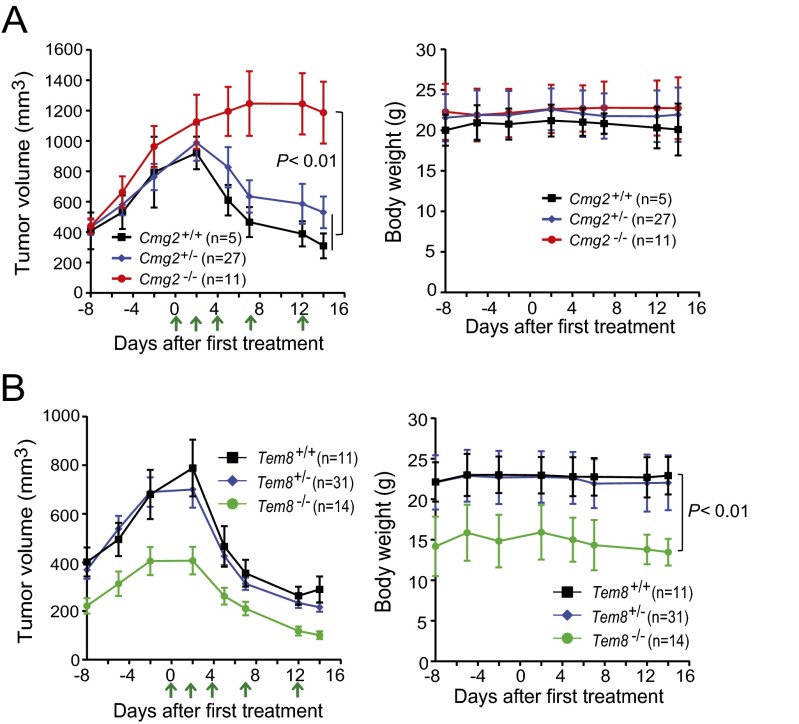
The angiogenic process is essential for tumor growth, and thus this process has attracted considerable attention in therapeutic development. The anthrax toxin receptors CMG2 and TEM8 have each been implicated in tumor angiogenesis, and thus each has been the subject of targeted therapies (13–16). To directly assess the roles of CMG2 and TEM8, we measured the growth rates of three different solid tumors in the TEM8- and CMG2-null mice that we previously described (31). The tumors evaluated were human lung carcinoma A549 xenografts and the syngeneic mouse Lewis lung carcinoma (LLC) and B16-BL6 melanoma (Fig. 1 A–C). Consistently, all three tumors grew as rapidly in CMG2-null mice as in their littermate control mice, indicating that CMG2 expression in tumor stromal compartments (e.g., endothelial cells, fibroblasts, and inflammatory cells, etc.) is not required for tumor growth (Fig. 1 A–C, Upper). No differences in body weight were observed between the tumor-bearing littermate Cmg2+/+, Cmg2+/−, and Cmg2−/− mice (Fig. 1 A–C, Lower).
Fig. 1.
Tumor growth rates in CMG2- and TEM8-null mice. (A) Littermate Cmg2+/+, Cmg2+/−, and Cmg2−/− athymic nude (Foxn1nu/nu) mice were injected intradermally with 1 × 107 per mouse A549 cells. (B and C) Littermate Cmg2+/+, Cmg2+/−, and Cmg2−/− immunocompetent C57BL/6J mice were inoculated with 5 × 105 per mouse LLC cells (B) or 5 × 105 per mouse B16-BL6 cells (C). (D and E) Soft food-fed (body weight-corrected) Tem8−/− mice and their littermate Tem8+/+ immunocompetent C57BL/6J mice were inoculated with 5 × 105 per mouse LLC cells (D) or 5 × 105 per mouse B16-BL6 cells (E). Tumor volumes (mean ± SE) and body weights of the mice (mean ± SD) were monitored. Student’s t test or one-way ANOVA (when ≥3 groups) did not detect significant differences in each panel.
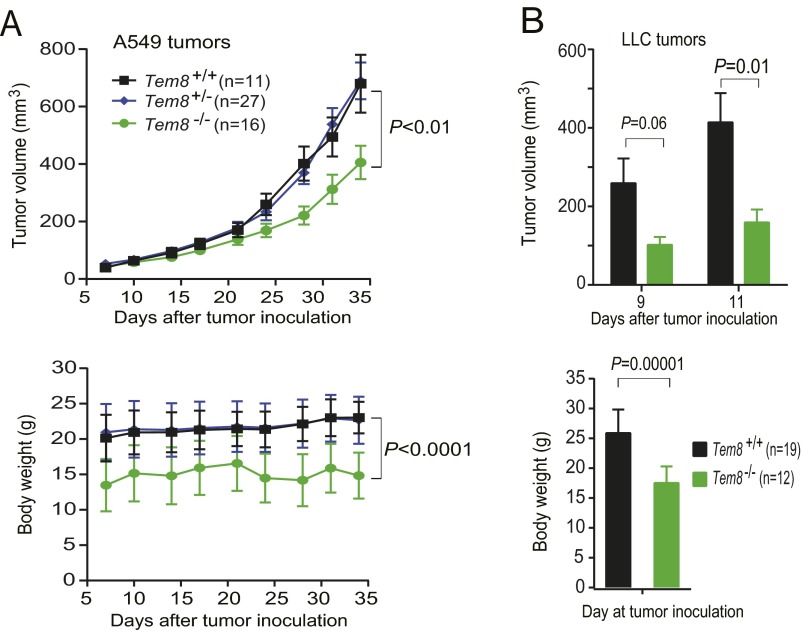
In preliminary studies, we observed that tumors grew more slowly in TEM8-null mice than in their littermate controls (Fig. S1). However, we found that nearly all these Tem8−/− mice had misaligned overgrown incisor teeth (malocclusion), causing these mice to have difficulty in chewing the hard food that was routinely provided. Consequently, the Tem8−/− mice became malnourished, reflected in lower body weights (Fig. S1). Interestingly, we found that the malnourished phenotypes, as well as the tumor growth rates of Tem8−/− mice, could be completely rescued after providing soft food (Nutra-Gel; Bio-Serv) (Fig. 1 D and E). Taken together, the results above demonstrate that expression of neither CMG2 nor TEM8 in stromal compartments is essential for tumor growth.
Fig. S1.
Malnourished phenotype correlates with slower tumor growth rates in TEM8-null mice. (A) Littermate Tem8+/+, Tem8+/−, and Tem8−/− athymic nude (Foxn1nu/nu) mice were injected intradermally with 1 × 107 per mouse human lung carcinoma A549 cells. Slower growth rates of A549 carcinomas accompanied by lower body weights of the Tem8−/− mice were observed. One-way ANOVA. (B) Littermate Tem8+/+ and Tem8−/− mice were injected intradermally with 5 × 105 per mouse LLC cells. Tumor volumes (means ± SE) at days 9 and 11 after tumor cell injection and body weights of the mice (means ± SD) at day 9 are shown. Slower growth rates of LLC carcinomas along with lower body weights of Tem8−/− mice were observed. One-way ANOVA.
Engineered Anthrax Lethal Toxins Block Tumor Growth Through Host-Derived CMG2.
We previously described a number of tumor-selective anthrax lethal toxins (having LF as the effector protein) that achieve tumor specificity through modification of the PA component so as to require activation by tumor-associated proteases, specifically MMPs and uPA. Here, we focus on the PA variants PA-L1 and IC2-PA. PA-L1 requires activation by MMPs to deliver the effector protein LF into the cytosol of cells (20). IC2-PA is the mixture of our recently generated intermolecular complementing PA variants PA-L1-I207R and PA-U2-R200A (32) and is an improved version of the previously described IC-PA combination consisting of PA-L1-I210A plus PA-U2-R200A (23, 24). These intermolecular complementing PA combinations display high tumor specificity when administered with LF, due to the requirement for the simultaneous presence of MMPs and uPA, two distinct tumor-associated proteases.
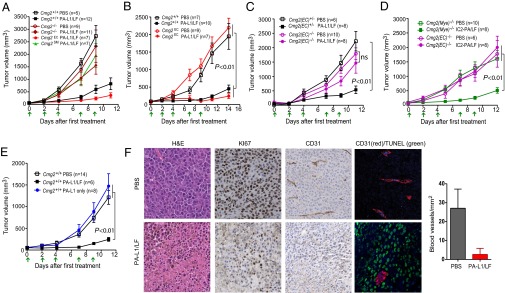
To investigate the antitumor mechanisms of these engineered lethal toxins, LLC carcinoma-bearing mice and B16-BL6 melanoma-bearing mice were treated systemically with PA-L1 plus LF or IC2-PA plus LF. Remarkably, these types of tumors were highly and equally sensitive to these engineered lethal toxins in vivo (Fig. 2 A and B). Interestingly, LLC cells were sensitive to the lethal toxins in the in vitro cytotoxicity assay whereas B16-BL6 cells were highly resistant (Fig. 2C). These results suggest that targeting certain cell types in tumor stromal compartments may play a dominant role in tumor responses to the toxins.
Fig. 2.
The engineered anthrax lethal toxins block tumor growth through the host-derived CMG2 receptor. (A and B) LLC carcinoma-bearing mice (A) and B16-BL6 melanoma-bearing mice (B) were treated intraperitoneally with 30 µg of PA-L1 plus 15 µg of LF, 30 µg of IC2-PA (15 µg of PA-L1-I207R plus 15 µg of PA-U2-R200A) plus 15 µg of LF, or PBS at the days indicated by the arrows with tumor volumes measured (mean ± SE). (C) LLC cells and B16-BL6 cells cultured in 96-well plates were incubated with various concentrations of PA, PA-L1, or PA-U7 in the presence of LF (5.5 nM = 500 ng/mL) for 72 h, and MTT assays were followed to evaluate cell densities relative to the nontoxin-treated cells. PA-U7 is a furin site mutated, activating protease-resistant PA variant. Data are shown as mean ± SD. (D and E) B16-BL6 melanoma-bearing Cmg2+/+ and Cmg2−/− mice (D) and B16-BL6 melanoma-bearing Tem8+/+ and Tem8−/− mice (E) were treated intraperitoneally with 25 µg of PA-L1 plus 12.5 µg of LF or PBS at the days indicated by the arrows with tumor volumes measured (mean ± SE). Student’s t test or one-way ANOVA was used to calculate differences between groups.
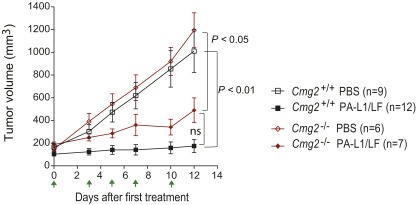
Because both CMG2- and TEM8-null mice are able to support normal tumor growth, these mice provide powerful genetic tools to dissect the mechanisms by which the engineered anthrax toxins control tumor growth. To determine the role of stromal compartments in the potent antitumor activities of the engineered anthrax lethal toxins, B16-BL6 tumor-bearing Cmg2−/− and Tem8−/− mice and their littermate control mice were treated with PA-L1/LF. Interestingly, whereas B16-BL6 tumors in Cmg2+/+ mice were highly sensitive to the toxin, the tumors growing in Cmg2−/− mice were completely resistant (Fig. 2D). However, the B16-BL6 tumors growing in Tem8−/− mice, as well as in their littermate control mice, were equally sensitive to the toxin treatments (Fig. 2E). These results clearly demonstrate that the antitumor activities of the engineered toxin involve targeting certain tumor stromal compartments through CMG2 rather than TEM8.
We also examined the responses of A549 tumors in Cmg2−/− and Tem8−/− mice. A549 tumor-bearing Cmg2−/− and Tem8−/− mice and their littermate control mice were treated with PA-L1/LF after tumors had grown to about 1 g. A549 cells contain WT BRAF and are insensitive to PA-L1/LF in in vitro cytotoxicity assays (Fig. S2 B and C). Consistently, whereas A549 tumors in Cmg2+/+ and Cmg2+/− mice, as well as in Tem8−/− and their littermate control mice, were very sensitive to the toxin, the tumors growing in Cmg2−/− mice were much less sensitive (Fig. S3), strengthening the notion that targeting tumor stromal compartments through the CMG2 receptor is the major mechanism for the toxin’s antitumor action. Additionally, the results shown in Fig. S3 revealed that, in the presence of stromal CMG2 expression, the engineered toxin was highly potent, showing efficacy even for tumors that were very large in size (∼5% of total body weight).
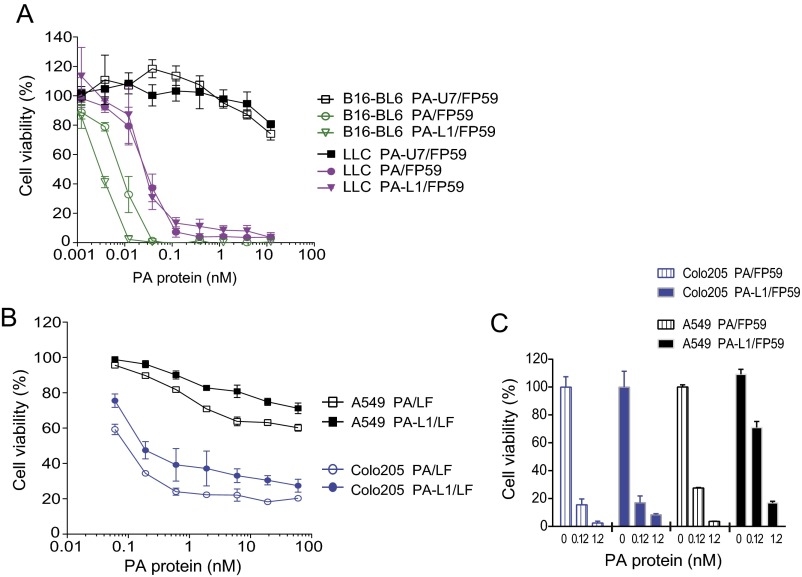
Fig. S2.
Cytotoxicity of anthrax toxins and their variants to tumor cells. (A) B16-BL6 cells and LL3 cells grown in 96-well plates were incubated with various concentrations of PA, PA-L1, or PA-U7 (protease-resistant PA variant) in the presence of (1.9 nM = 100 ng/mL) FP59 for 48 h, followed by MTT assay to determine cell viabilities as percentages of untreated control cells. FP59 is a fusion protein of LF amino acids 1–254 and the catalytic domain of Pseudomonas aeruginosa exotoxin A that kills all cells by ADP ribosylation of eEF2 after delivery to cytosol by PA or PA variants. Data are shown as mean ± SD. (B and C) Human lung carcinoma A549 and colorectal carcinoma Colo205 cells were incubated with various concentrations of PA or PA-L1 in the presence of (5.5 nM = 500 ng/mL) LF (B) or 1.9 nM FP59 (C) for 72 h, followed by MTT assay to determine cell viabilities. Data are shown as mean ± SD.
Fig. S3.
The engineered anthrax lethal toxins block A549 tumor growth through the host-derived CMG2 receptor. (A) Littermate Cmg2+/+, Cmg2+/−, and Cmg2−/− athymic nude (Foxn1nu/nu) mice were injected with 1 × 107 per mouse A549 cells. When A549 tumors reached ∼1 cm3, the tumor-bearing mice were treated intraperitoneally with five doses of 15 µg of PA-L1 plus 7.5 µg of LF as indicated by arrows. A slow and moderate response of A549 tumors on Cmg2−/− mice was likely due to the accumulated low toxin activity to A549 cells (Fig. S2B). (B) The A549 tumor-bearing littermate Tem8+/+, Tem8+/−, and Tem8−/− athymic nude (Foxn1nu/nu) mice were treated as in A. Note that the mice were fed the routine hard food and that the Tem8−/− mice exhibited lower body weight than their littermate controls. Tumor volumes, means ± SE; body weights, means ± SD. Student’s t test or one-way ANOVA was used to calculate differences between groups. In A, whereas the tumor volumes of the three groups were not significantly different before the toxin treatment, those of Cmg2+/+ and Cmg2+/− mice were significantly smaller than Cmg2−/− mice after the treatment; P < 0.01, using one-way ANOVA.
Targeting Tumor Endothelial Cells Is Responsible for the Antitumor Activities.
To determine which type of cells in the tumor stromal compartment is responsible for the antitumor action of PA-L1/LF, we inoculated the toxin-“insensitive” B16-BL6 tumor cells into three types of mice: Cmg2−/− mice, Cmg2−/− mice with a CMG2-transgene expressed only in endothelial cells (named Cmg2EC hereafter; see ref. 12 for a detailed description), and Cmg2−/− mice with a CMG2-transgene expressed only in vascular smooth muscle cells (Cmg2SM) (12). Interestingly, whereas the B16-BL6 tumors in Cmg2SM mice were, like in Cmg2−/− mice, insensitive to the toxin, the tumors in Cmg2EC mice were fully sensitive (Fig. 3 A and B). Thus, CMG2 endothelial expression is sufficient to mediate the antitumor activities of the toxin. To further evaluate the role of targeting tumor endothelial cells in cancer targeted therapy, B16-BL6 tumors were grown in endothelial cell-specific CMG2-null [termed Cmg2(EC)−/− hereafter; see ref. 12 for a detailed description] mice. Remarkably, the tumors in Cmg2(EC)−/− mice completely lost sensitivity to PA-L1/LF, as well as to IC2-PA/LF (Fig. 3 C and D), whereas the tumors in myeloid CMG2-specific CMG2-null [Cmg2(Mye)−/−; see ref. 33 for a detailed description] mice remained sensitive to IC2-PA/LF (Fig. 3D). As expected, no antitumor activity was observed when PA-L1 was used alone (Fig. 3E), confirming that the antitumor activity of these toxins requires the action of LF, the enzymatic moiety of the toxins.
Fig. 3.
Tumor endothelial cells are the major targets of the engineered anthrax lethal toxins in tumor therapy. (A–C) B16-BL6 melanoma-bearing mice with various CMG2 genotypes were treated intraperitoneally with 30 µg of PA-L1 plus 15 µg of LF at the days indicated by arrows. Cmg2EC, CMG2 receptor expressed solely in endothelial cells; Cmg2SM, CMG2 receptor expressed solely in vascular smooth muscle cells; Cmg2(EC)−/−, endothelial cell-specific CMG2-null; ns, nonsignificant different. (D) B16-BL6 melanoma-bearing endothelial cell-specific CMG2-null mice and myeloid-specific CMG2-null mice (Cmg2(Mye)−/−) and their littermate controls were treated intraperitoneally with 30 µg of IC2-PA (15 µg of PA-L1-I207R plus 15 µg of PA-U2-R200A) plus 10 µg of LF or PBS at the days indicated by the arrows. (E) B16-BL6 melanoma-bearing WT mice were treated intraperitoneally with 30 µg of PA-L1 plus 15 µg of LF, 30 µg of PA-L1 without LF, or PBS at the days indicated by the arrows. (F) B16-BL6 tumor-bearing mice were treated with PA-L1/LF (30 µg/15 µg) or PBS (n = 3 for each group) at days 0 and 2, and tumors were collected 24 h later, fixed, sectioned, and stained as indicated. Tumors were also costained for CD31 (red fluorescence) and TUNEL (green fluorescence). (Magnification: 200×.) Blood vessel densities were expressed as the means ± SE of CD31+ vasculatures in 10 fields from each group. Tumor volumes (means ± SE). One-way ANOVA was used to evaluate tumor size differences. In A, Cmg2+/+ PBS vs. Cmg2+/+ PA-L1/LF, P < 0.01; Cmg2−/− PBS vs. Cmg2EC PA-L1/LF, P < 0.01; Cmg2−/− PBS vs. Cmg2−/− PA-L1/LF or Cmg2SM PA-L1/LF, P > 0.05.
Taken together, the above results clearly demonstrate that the potent antitumor activities of the engineered anthrax lethal toxins are due to their unique toxicities to host-derived tumor endothelial cells rather than to other cell types in the tumor stromal compartments: e.g., vascular smooth muscle cells and myeloid lineage cells.
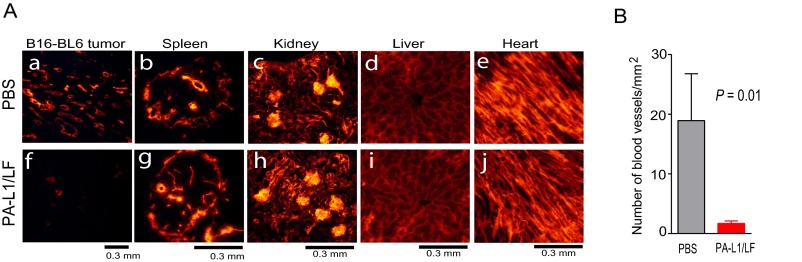
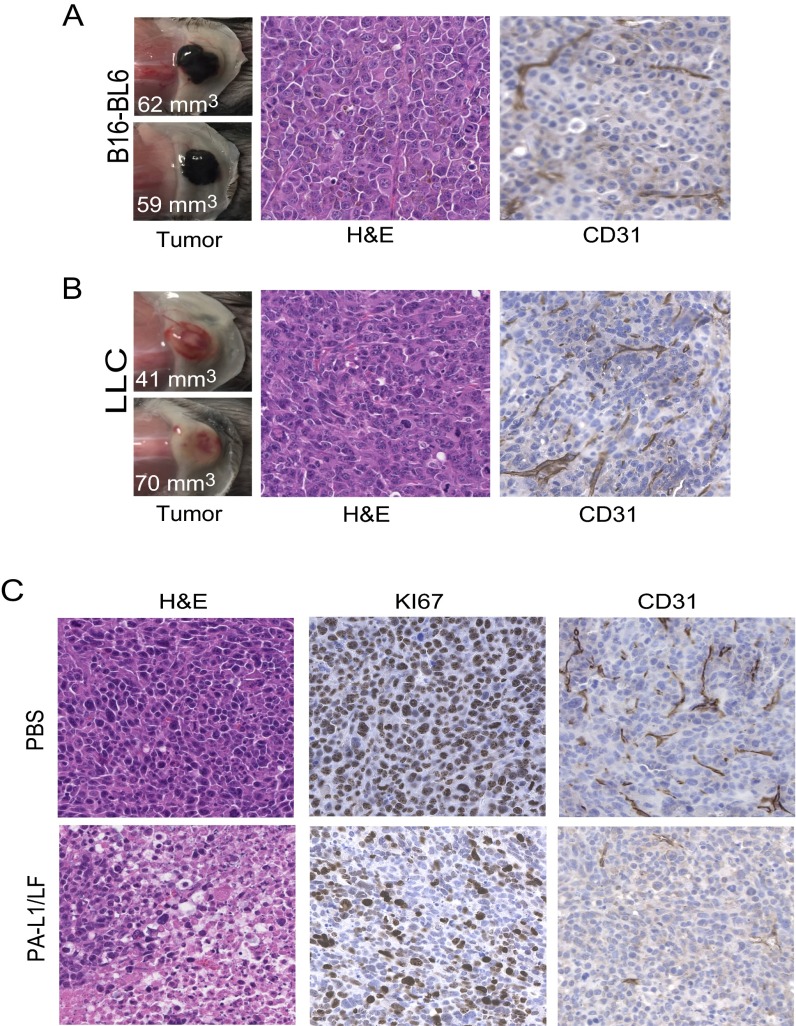
To further examine the toxin’s activities to tumor endothelium, blood vessels of B16-BL6 tumor-bearing mice treated with PBS or PA-L1/LF were labeled with the fluorescent lipophilic carbocyanine dye DiI by cardiac perfusion (34). During perfusion, DiI directly incorporates into endothelial cell membranes upon contact, allowing visualization by fluorescence microcopy of vascular structures within tumors and normal tissues. Remarkably, whereas blood vessels were abundant in the tumors treated with PBS, vessels in the tumors treated with PA-L1/LF were rarely detected (Fig. S4 A, a and f and Fig. S4B). Interestingly, no differences were detected in vasculature structures of various normal tissues, including the spleens, kidneys, livers, and hearts of the B16-BL6 tumor-bearing mice treated with PBS and the toxin (Fig. S4). B16-BL6 melanomas and LLC carcinomas were also sectioned and histologically analyzed after the tumor-bearing mice were treated with PA-L1/LF or PBS (Fig. 3F and Fig. S5). Extensive tumor necrosis (H&E staining) and decreases in cell proliferation (Ki67 staining) accompanied by loss of tumor vascular structures were readily detected in the toxin-treated B16-BL6 and LLC tumors (Fig. 3F and Fig. S5). These results support the notion that targeting tumor endothelial cells is the principal mechanism of the toxin’s antitumor activities. CD31 and TUNEL costaining was also performed on B16-BL6 tumors. Although extensive apoptotic tumor cell death was detected in PA-L1/LF–treated tumors, no apoptotic cell death was identified among the rarely detected tumor endothelial cells in the toxin-treated tumors (Fig. 3F), suggesting that the toxin may exert the antitumor effects through affecting endothelial cell proliferation rather than by inducing apoptosis (see below).
Fig. S4.
Visualization of vasculatures of tumor-bearing mice with a lipophilic DiI dye. (A) B16-BL6 tumor-bearing mice were treated intraperitoneally with PBS (a–e) (n = 3) or three doses of 30 µg of PA-L1 plus 15 µg of LF at days 0, 2, and 4 (f–j) (n = 3). The mice were then euthanized and perfused transcardially with DiI dye, and tissues were sectioned. Representative vasculatures in tumors (a and f), spleens (white pulps) (b and g), kidneys (glomeruli) (c and h), livers (d and i), and hearts (e and j) were visualized under fluorescence microscopy. (B) Quantifications of tumor blood vessels treated with PBS (n = 3) or PA-L1/LF (n = 3) in A. Blood vessels (means ± SD) in five random views from each tumor sample were counted. Student’s t test.
Fig. S5.
Tumor histology and immunohistochemistry before and after treatment with the engineered toxin. (A and B) B16-BL6 melanomas (A) and LL3 carcinomas (B) formed 6 d after tumor cell (5 × 105 per mouse) inoculation into midscapular subcutis of C57BL/6J mice. The appearance of representative tumors with sizes are shown. Note that tumor vasculatures were well-developed in both tumors, as shown by CD31 staining. (C) LL3 tumor-bearing mice were treated with PA-L1/LF (30 µg/15 µg) or PBS at days 0 and 2, and tumors were collected 24 h later, fixed, sectioned, and stained as indicated. (Magnification: 200×.)
Engineered Anthrax Lethal Toxins Inhibit Proliferation of Tumor Endothelial Cells.
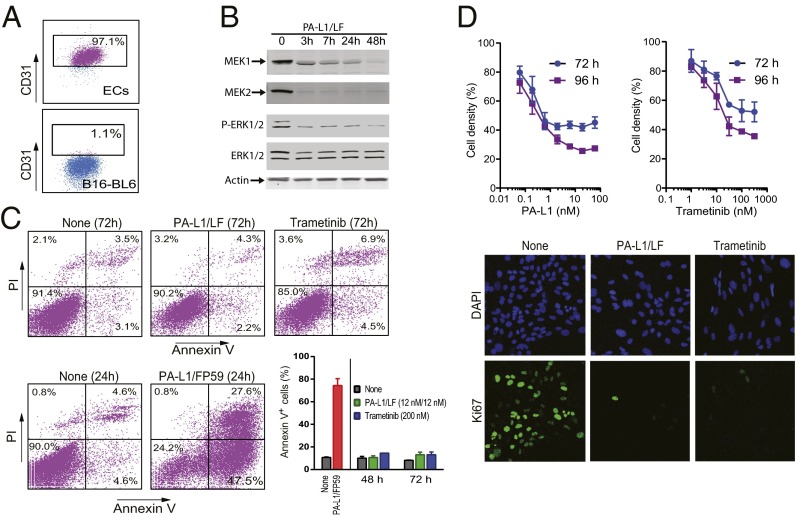
Tumor endothelial cells were isolated from B16-BL6 tumors through intermolecular adhesion molecule 2 (ICAM2) sorting to investigate the toxic effects of the engineered toxins on tumor endothelial cells. The purity of the isolated endothelial cells was confirmed by another endothelial marker, CD31 (Fig. 4A). As expected, delivery of LF into the cytosol of endothelial cells by PA-L1 was evidenced by the cleavage of MEK1 and MEK2, accompanied by a dramatic decrease in phosphorylation of ERK1/2 (Fig. 4B). Expression of the toxin-activating proteases by endothelial cells was also confirmed by the cells’ susceptibilities to the protease-activated PA variants in the presence of FP59 (Fig. S6). FP59 is a fusion protein of LF amino acids 1–254 and the catalytic domain of Pseudomonas aeruginosa exotoxin A that kills all cells by ADP ribosylation of eEF2 after delivery to cytosol by PA (35, 36). To examine the cytotoxic effects of the toxin on tumor endothelial cells, the cells were treated with PA-L1/LF for 48 h and 72 h, respectively, followed by annexin V plus propidium iodide (PI) staining to identify apoptotic cells by flow cytometry. Although PA-L1 plus FP59 could induce dramatic apoptotic cell death 24 h after incubation, PA-L1 plus LF could not do so even after 72 h incubation (Fig. 4C). Remarkably, although the engineered lethal toxin did not directly kill endothelial cells, the toxin displayed potent inhibitory effects on endothelial cell proliferation (Fig. 4D). Thus, Ki67 staining revealed that tumor endothelial cell proliferation nearly completely ceased after 72 h incubation with the toxin (Fig. 4D, Lower). Interestingly, the toxin’s effects on endothelial cells could be fully replicated by trametinib (although much higher molar concentrations were required) (Fig. 4 C and D), a small molecule inhibitor of MEK1/2 approved by the Food and Drug Administration for treating patients having metastatic melanoma with BRAFV600E mutation. These data suggest that the inhibitory effects of the engineered toxins were through disruption of the MEK-ERK pathway.
Fig. 4.
Effects of the engineered lethal toxin on tumor endothelial cell apoptosis and proliferation. (A) Flow cytometry analyses of tumor endothelial cells and B16-BL6 cells incubated with a CD31 antibody. (B) Tumor endothelial cells were incubated with PA-L1/LF (12 nM/11 nM = 1 µg/mL each) for various lengths of time, and then cell lysates were analyzed by Western blotting using MEK1, MEK2, ERK, and Phospho-ERK antibodies. (C) Tumor endothelial cells were incubated with or without PA-L1/LF (12 nM/11 nM) or trametinib (200 nM) for 72 h, or with PA-L1/FP59 (1.2 nM/1.9 nM) for 24 h. Then the cells were stained with PI and annexin V, followed by flow cytometry analysis. (D) Tumor endothelial cells cultured in 96-well plates were incubated with various concentrations of PA-L1 in the presence of LF (11 nM) or trametinib for 72 h or 96 h, and MTT assays were followed to evaluate cell numbers relative to the nontoxin-treated wells. Data are shown as mean ± SD. (Lower) Tumor endothelial cells were incubated with or without PA-L1/LF (12 nM/11 nM) or trametinib (200 nM) for 72 h followed by staining for DAPI (blue fluorescence) and Ki67 (green fluorescence). (Magnification: 200×.)
Fig. S6.
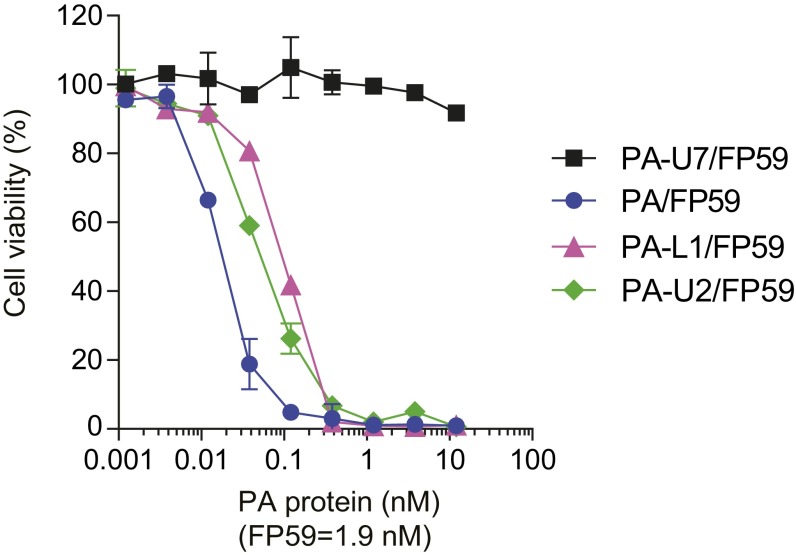
Expression of toxin-activating proteases by tumor endothelial cells. Tumor endothelial cells grown in 96-well plates were incubated with various concentrations of PA, PA-L1 (MMP-activated PA variant), PA-U2 (urokinase-activated PA variant), or PA-U7 (protease-resistant PA variant) in the presence of (1.9 nM) FP59 for 48 h, followed by MTT assay to determine cell viabilities as percentages of untreated control cells. Data are shown as mean ± SD.
Additional Benefit of the Toxin in Targeting Tumors Having the BRAF Mutation.
Due to their unique action on tumor endothelial cells, the tumor-associated protease-activated anthrax lethal toxins exhibit potent antitumor activities, even for tumors composed of cancer cells that are insensitive to the toxins (Fig. 2 B–E and Fig. S3). However, a subset of human cancer cells have oncogenic BRAF mutations, such as BRAFV600E, that make the tumor cells dependent on the RAF-MEK-ERK pathway for survival, while also making them exquisitely sensitive to anthrax lethal toxin (37). We hypothesized that engineered anthrax lethal toxins would have additional benefit in treatment of solid tumors having the BRAFV600E mutation. To test this hypothesis, human colorectal carcinoma Colo205 cells, which contain the oncogenic BRAFV600E mutation and are sensitive to PA-L1/LF in vitro (Fig. S2 B and C), were inoculated into littermate Cmg2−/− and Cmg2+/+ athymic nude mice and treated with PA-L1/LF. Significantly, Colo205 tumors in Cmg2−/− mice were sensitive to the toxin treatment although the response to the toxin treatment was lower than the strong response of the tumors growing in Cmg2+/+ mice (Fig. S7). These results suggest that, in the “toxin-sensitive” tumors, the antitumor activity of the toxin depends on targeting both tumor endothelial cells as well as the cancer cells.
Fig. S7.
Colo205 tumors grown in CMG2-null mice are sensitive to PA-L1/LF. Littermate Cmg2+/+ and Cmg2−/− athymic nude (Foxn1nu/nu) mice were inoculated intradermally with 1 × 107 per mouse human colorectal carcinoma Colo205 cells. The Colo205 tumor-bearing mice were treated intraperitoneally with PBS or 30 µg of PA-L1 plus 15 µg of LF at the days indicated by the arrows. Tumor volumes, means ± SE. One-way ANOVA.
Preventing Antibody Responses to the Engineered Toxin Allows Repeated Courses of Treatment.
Because of their high tumor specificity and high antitumor efficacy, the tumor-associated protease-activated anthrax toxins are strong candidates for further clinical development. However, these bacterial proteins are foreign antigens to mammalian hosts and are known to induce neutralizing antibodies that prevent long-term use. Therefore, strategies for preventing an immune response are essential. Recently, a combination of pentostatin and cyclophosphamide (PC), a regimen used to prevent host-versus-graft reactivity, has been used successfully to prevent neutralizing antibody production against SS1P, a Pseudomonas exotoxin A-based immunotoxin for targeting human mesotheliomas (38, 39).
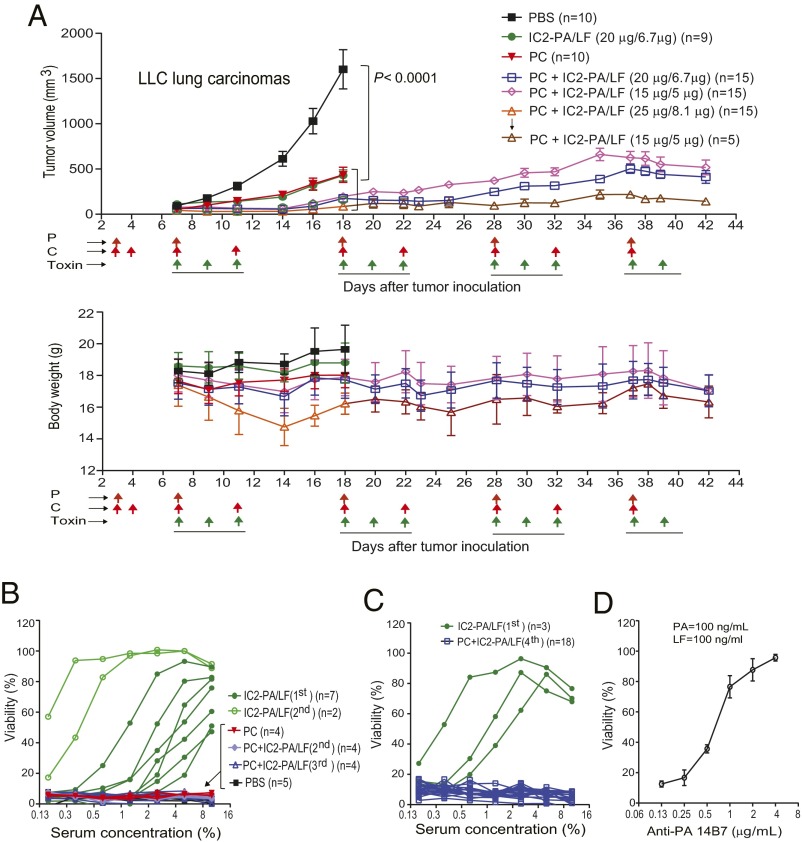
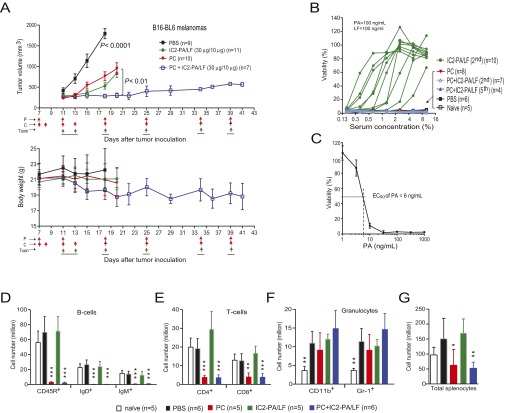
To examine whether a PC regimen blocks production of antibodies that neutralize engineered anthrax toxins, a trial was performed using the highly metastatic LLC (mouse) carcinomas established in syngeneic immunocompetent C57BL/6J mice. The tumor-bearing mice were treated with PBS, a PC regimen, IC2-PA/LF, or the combined therapy of the PC regimen and IC2-PA/LF, following the schedule shown in Fig. 5A. For the combined treatment groups, the tumor-bearing mice were prepared with doses of PC 3 and 4 d before the first toxin treatment. The combined treatment groups were treated with a total of four cycles of toxin and PC, with intervals of 5–7 d between cycles. As shown above (Fig. 2 A and B), IC2-PA/LF alone showed strong antitumor effects (Fig. 5A). Surprisingly, all of the combined treatments showed much higher antitumor efficacy at both early and late times, with the tumors remaining responsive to the treatments even after the fourth cycle of the therapy (Fig. 5A). Importantly, no mortality was observed in the low (15 µg of IC2-PA plus 5 µg of LF) and the medium (20 µg of IC2-PA plus 6.7 µg of LF) dose groups. In fact, the mice receiving the combined treatments were alive after 42 d, well after mice in the other groups had to be euthanized due to their high tumor burdens (Fig. 5A). Also of interest was that the PC regimen alone exhibited antitumor activities (Fig. 5A). As expected, neutralizing antibodies were detected in all of the mice treated with the toxin alone. Antibodies were detected as early as 10 d after the first treatment, the time at which the tumors began to show decreases in response to the toxin (only) treatment. Strikingly, no neutralizing antibodies were detected in the tumor-bearing mice of the combined therapy group, even after the fourth round of therapy (Fig. 5 B–D).
Fig. 5.
Remarkable antitumor efficacy of combined therapy with the engineered anthrax toxin and pentostatin plus cyclophosphamide regimen. (A) LLC lung carcinoma-bearing immunocompetent C57BL/6J mice were treated intraperitoneally with PBS, IC2-PA/LF (20 µg/6.7 µg), PC regimen (20 µg of pentostatin and 1 mg of cyclophosphamide), high (25 µg/8.1 µg), medium (20 µg/6.7 µg), or low (15 µg/5 µg) doses of IC2-PA/LF combined with PC regimen. Schedules for PC and the toxin treatments are indicated by the arrows. Tumor weights, mean ± SE; body weights, mean ± SD. Note that the high dose group was stopped due to deaths occurring after the first cycle treatment. The survivors in the high group were continued in the following cycles with the low dose of IC2-PA/LF. No deaths occurred in other groups during the courses of treatment. Most tumors of the toxin only and the PC only groups developed a necrotic core resulting in tumor ulceration, which required euthanization in compliance with the animal study protocol approved for the study. The subset of the IC2-PA/LF group that entered the second round therapy were shown not to respond to the treatments due to high titers of toxin-neutralizing antibody as shown in B. One-way ANOVA for tumor size differences: PBS vs. all other groups, P < 0.0001; PC plus IC2-PA/LF (20 µg/6.7 µg) vs. PC or IC2-PA/LF (20 µg/6.7 µg) (n = 9), P < 0.01. (B and C) RAW264.7 cells were incubated with PA/LF (100 ng/mL each) for 5 h in the presence of various dilutions of sera obtained from representative mice in A after the first, second, or third round of therapy (B) or after final round of therapy (fourth) (C). Cell viabilities were determined by MTT assay as described in Materials and Methods. Note that no neutralizing antibodies were detected in all of the cases from the PC and combined therapy groups. (D) As performed in B and C, 14B7 anti-PA monoclonal antibody was used as a positive control for neutralizing antibodies.
We extended this study to include therapy of another highly malignant syngeneic tumor, the B16-BL6 melanoma implanted in immunocompetent C57BL/6J mice. This experiment used a modified toxin and PC regimen as shown in Fig. S8A. The B16-BL6 melanoma-bearing mice were treated with PBS, IC2-PA/LF (30 µg/10 µg), a PC regimen, or the combined therapy of the PC regimen and IC2-PA/LF twice in the first week and weekly in the following weeks (Fig. S8A). Again, the PC alone regimen had a significant antitumor effect (Fig. S8A), and the combined treatment showed remarkable efficacy, with the tumors remaining responsive to the treatments even after the fifth cycle of the therapy (Fig. S8A). Consistently, no neutralizing antibodies against the engineered toxin were detected in mice treated with the combined PC and the toxin, even after the five cycles of therapy (Fig. S8 B and C).
Fig. S8.
Remarkable antitumor efficacy of the combined therapy of the engineered anthrax toxin and pentostatin plus cyclophosphamide regimen. (A) B16-BL6 melanoma-bearing immunocompetent C57BL/6J mice were treated intraperitoneally with PBS, IC2-PA/LF (30 µg/10 µg) (30 µg of IC2-PA = 15 µg of PA-L1-I207R plus 15 µg of PA-U2-R200A), PC regimen (20 µg of pentostatin and 1 mg of cyclophosphamide), combined IC2-PA/LF and PC regimen. Schedules for the treatments with PC regimen and the toxin are indicated by the arrows. Tumor volumes (means ± SE) and body weights of the mice (means ± SD) were monitored. Note that PC regimen alone had significant antitumor activity. One-way ANOVA for tumor size differences: PBS vs. all other groups, P < 0.0001; PC plus IC2-PA/LF vs. PC or IC2-PA/LF, P < 0.01. (B) RAW264.7 cells were incubated with PA/LF (100 ng/mL each) for 5 h in the presence of various dilutions of sera obtained from the representative mice in A after the second or fifth (final) round of therapy. Cell viabilities were determined by MTT assay as described in Materials and Methods. Note that no neutralizing antibodies were detected in all of the cases from PC, as well as the combined treatment groups. (C) RAW264.7 cells were incubated with various concentrations of PA in the presence of 100 ng/mL LF for 5 h, and cell viabilities were determined as in B. The EC50 (effective concentration required to kill 50% of the cells) of PA is 6 ng/mL in the presence of 100 ng/mL LF. (D–G) Effects of pentostatin plus cyclophosphamide regimen on immune cells. Splenocytes were isolated and counted from naive mice and the B16-BL6 melanoma-bearing mice after the second cycle of therapy with PBS, PC, IC2-PA/LF, or the combined PC and the toxin as shown in A and were subsequently stained with various fluorochrome-conjugated mAbs: anti-CD45R, anti-CD4, anti-CD8, anti-CD11b, and anti–Gr-1, anti-IgD, and anti-IgM. The cells were analyzed using a BD FACSCanto Flow Cytometer. Cell numbers positive for B-cell markers (D), T-cell markers (E), and granulocyte markers (F) were obtained by the following formula: total splenocytes × percentage of positive cells. B-cell markers were CD45R, IgD, and IgM; T-cell markers were CD4 and CD8; markers for granulocytes were CD11b and Gr-1. Data are shown as mean ± SD. (G) Total splenocyte counts of the mice analyzed. One-way ANOVA in D, E, and G, PBS vs. treated groups, *P < 0.05, **P < 0.01, ***P < 0.001.
Taken together, the above results reveal that the combined toxin and PC therapy has remarkable and prolonged antitumor effects by blocking neutralizing antibody production.
Effects of Pentostatin and Cyclophosphamide on Immune Cells.
To investigate the effects of the PC regimen on immune cells, we isolated splenocytes from naive mice and B16-BL6 melanoma-bearing mice from various treatment groups after the second round of treatments as shown in Fig. S8A. Flow cytometry analyses revealed that B-cell populations (CD45R+, IgM+, and IgD+ cells) were nearly completely depleted in the PC as well as in the combined therapy groups (Fig. S8D). To a lesser extent, T-cell populations (CD4+ and CD8+ cells) were also reduced in these treatment groups (Fig. S8E). Interestingly, the PC regimen and the combined PC and toxin treatments did not affect granulocyte populations (CD11b+ and Gr-1+ cells). In fact, we found that the numbers of CD11b+ and Gr-1+ granulocytes were significantly increased in the tumor-bearing mice compared with the numbers in naive mice, regardless of treatment type, suggesting the existence of innate immune responses to the tumors (Fig. S8F). The IC2-PA/LF alone did not significantly affect these cell populations (Fig. S8 D–G). Therefore, the above results clearly demonstrate that the PC regimen efficiently depletes lymphocytes, in particular B-cells, while sparing innate immunity. In agreement with PC’s effects on lymphocytes, the total splenocytes of the mice treated with PC regimen alone or in combination with the toxin were also significantly decreased (Fig. S8G). Therefore, the absence of a humoral immune response to the engineered toxins was due to the B-cell depletion caused by the PC regimen.
Discussion
As the major virulence factor of B. anthracis, anthrax toxin has been the subject of intensive research, through which it has become one of the best characterized systems for delivery of polypeptides into cells (19, 40). This sophisticated system can be modified in multiple ways to achieve specific delivery of therapeutic effector proteins to certain cell types, including cancer cells (19, 24, 25, 41–43). In particular, PA protein variants engineered to require activation by tumor-associated proteases have provided the basis for a novel class of potent antitumor agents. Interestingly, the cognate receptors used by the toxins to gain entry into target cells are TEM8 and CMG2, two putative tumor endothelium markers, which have themselves been considered as candidates for tumor-targeted therapy (16).
In this work, we first investigated the roles of TEM8 and CMG2 in tumor growth using TEM8- and CMG2-null mice. Surprisingly, all solid tumors inoculated into either CMG2-null mice or TEM8-null mice (the latter strain needed to be fed soft food so as to compensate for their difficulty in eating) had growth rates equal to those in their littermate control mice. Therefore, expression of these two receptors in tumor stromal compartments (e.g., tumor endothelial cells) is not essential for tumor growth. However, these results cannot exclude the possibility of functional redundancy of these proteins, as well as the potential importance of them in tumor growth when expressed on tumor cells. Antitumor effects have been shown by using TEM8 antibodies and an uncleavable variant of PA (18, 44); thus it is also possible that TEM8 and CMG2 blockade and their deletion may have different effects on endothelial cells.
Identification of the specific cell type in tumor stroma that is responsible for mediating the antitumor activities came from inoculating murine B16-BL6 tumors into Cmg2−/− mice having CMG2 transgenes expressed only in endothelial cells (Cmg2EC mice) or in vascular smooth muscle cells (Cmg2SM mice). Remarkably, the tumors in Cmg2EC mice were highly sensitive to the toxin treatments whereas the tumors in Cmg2−/− and Cmg2SM mice were insensitive. In parallel, we found that tumors in endothelial cell-specific CMG2-null mice completely lost sensitivity to the treatments. These gain- and loss-of-function studies revealed that targeting of tumor endothelial cells rather than other cell types (e.g., myeloid-lineage cells) in tumor stromal compartments is responsible for the tumor responses to the toxin. Interestingly, advanced tumors of large size were still responsive to low doses of the toxin (PA-L1/LF, 15 µg/7.5 µg) (Fig. S3). This high efficacy can be attributed to the ease with which the systemically administered toxin can reach the tumor endothelial cells and explains, in part, the highly favorable therapeutic index of these agents (45). It should also be noted that the genetic stability of host-derived tumor endothelial cells will greatly limit the ability of tumors to develop resistance to this antitumor strategy.
Some human cancers, in particular the 70% of human cutaneous melanomas and the lower percentage of other human cancers that have the BRAFV600E activating mutation, are directly sensitive to anthrax lethal toxin. These tumors are dependent on the MEK-ERK pathway for survival and thus are sensitive to inhibitors targeting the MEK-ERK pathway (37, 46, 47). We found that our modified toxin has an additional benefit for this group of tumors (such as human Colo205 tumors), through targeting both the cancer cells and the tumor endothelial cells.
Protein toxin-based agents (“immunotoxins”) have been studied for decades, but their clinical and commercial development has been severely limited by the inevitable induction of antibodies to these foreign proteins. Recently, a PC regimen, which was used clinically to treat chronic B-cell leukemia (48, 49), was shown to be effective in preventing induction of antibodies against a P. aeruginosa exotoxin A-based immunotoxin in human mesothelioma patients (38, 39). Strikingly, we found that a similar PC regimen could prevent an immunogenic response to the engineered anthrax toxins. Tumor-bearing immunocompetent C57BL/6J mice treated with the PC regimen were severely depleted for lymphocytes, in particular B-cells, while sparing innate immune activity, allowing the engineered toxin to be used in multiple cycles. The combined toxin and PC regimen exhibited extraordinary antitumor effects on highly malignant and metastatic syngeneic murine lung carcinomas and melanomas, greatly exceeding the performance of the toxin and PC regimens used separately, indicating synergistic antitumor effects.
In summary, the engineered, tumor-selective anthrax lethal toxins have the following attractive features, reasonably predicting that they may provide benefit to cancer patients: (i) CMG2 and TEM8, as the cognate toxin receptors and putative tumor endothelial markers, are inherently targeted by the modified toxins, with CMG2 being the key receptor mediating the tumor targeting; (ii) the engineered toxin selectively delivers LF into the cytosol of tumor endothelial cells, as well as cancer cells, achieving high tumor specificity; (iii) the intrinsic action of LF in targeting the RAS-RAF-MEK-ERK pathway profoundly inhibits proliferation of genetically stable tumor endothelial cells, thus curtailing tumor angiogenesis; (iv) LF can directly kill cancer cells with the BRAFV600E mutation; and (v) the immunogenicity of the toxin can be overcome by a PC regimen, allowing multiple rounds of toxin therapy. Therefore, we propose the clinical development of the combined therapeutic strategy using the tumor-associated protease-activated anthrax lethal toxins and a PC regimen to target solid tumors. A broad spectrum of solid tumors are expected to be responsive, but cancer patients having BRAF mutation-positive tumors may derive additional benefit, as discussed above.
Materials and Methods
Proteins and Reagents.
Recombinant PA variants and LF proteins were purified from supernatants of BH480, an avirulent, sporulation-defective, protease-deficient B. anthracis strain, as described previously (50, 51). PA-L1 is an MMP-activated PA variant, in which the furin-cleavage sequence RKKR (residues 164–167) is replaced with an MMP substrate sequence GPLGMLSQ (20). PA-U7 is a protease-resistant variant with a furin-cleavage sequence changed to PGG (21). IC2-PA is the mixture of our recently generated intermolecular complementing PA variants PA-L1-I207R and PA-U2-R200A (the furin site is replaced with an artificial uPA substrate sequence PGSGRSA) (32) and is an improved version of the previously described IC-PA combination (23). FP59 is a fusion protein of LF amino acids 1–254 and the catalytic domain of P. aeruginosa exotoxin A that kills cells by ADP ribosylation of eEF2 after delivery to cytosol by PA (35). The LF and FP59 used here contain the native N-terminal sequence AGG (50). MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and pentostatin (SML0508) were from Sigma. Cyclophosphamide (NDC10019-957-01) was from Baxter Healthcare. Trametinib was from Selleck Chemicals (S2673).
Cells and Cytotoxicity Assay.
All cultured cells were grown at 37 °C in a 5% CO2 atmosphere. Murine B16-BL6 melanoma cells and Lewis lung carcinoma (LLC) cells (52) were originally from Judah Folkman, Harvard Medical School, Boston, and human lung carcinoma A549 cells and colorectal carcinoma Colo205 cells were from the NCI-60 cell set. All these tumor cells were cultured in DMEM supplemented with 10% (vol/vol) FBS.
Mouse endothelial cells and tumor endothelial cells from B16-BL6 melanomas were isolated following the protocol for lung endothelial cell isolation (53). Briefly, mouse lungs and B16-BL6 tumors were digested with type I collagenase and plated on gelatin and collagen-coated flasks. The cells were then subjected to sequential negative sorting by magnetic beads coated with a sheep anti-rat antibody using an Fc Blocker (rat anti-mouse CD16/CD32, cat. 553142; BD Pharmingen) to remove macrophages and to positive sorting by magnetic beads using an anti-ICAM2 (or CD102) antibody (cat. 553326; BD Pharmingen) to isolate endothelial cells. Nonendothelial cells from lungs (defined as the ICAM2− cells) were also isolated. Isolation of tumor endothelial cells was facilitated when mice containing a mutated allele of eEF2 (eEF2+/G717R) were used (36). The endothelial cells isolated from these mice are resistant to PA/FP59, allowing efficient removal of the contaminated B16-BL6 cells by treating with PA/FP59. Endothelial cells were cultured in DMEM supplemented with 20% FBS, endothelial cell growth supplement (30 mg in 500 mL of DMEM) (E2759; Sigma), and heparin (50 mg in 500 mL of DMEM) (H3149-100 KU; Sigma).
For cytotoxicity assays, cells grown in 96-well plates (50% confluence) were incubated with various concentrations of PA or PA variant proteins combined with 500 ng/mL LF or 100 ng/mL FP59, or various concentrations of trametinib for 48 or 72 h. Cell viabilities were then assayed by MTT as described previously (54) and are expressed as the percentage of MTT signals of untreated cells. For Ki67 staining, cells grown on gelatin and collagen-coated glass slides were incubated with PA-L1/LF (1.2 nM/1.1 nM) or trametinib (200 nM) for 72 h followed by Ki67 staining using an anti-Ki67 antibody (ab16667, 1:100 dilution; Abcam).
Western Blotting.
Tumor endothelial cells grown in 12-well plates were incubated with or without PA-L1 plus LF for various lengths of time at 37 °C and then washed three times with Hanks’ balanced salt solution (Biofluids). Cells were then lysed in modified radioimmunoprecipitation assay (RIPA) lysis buffer containing protease inhibitors, and lysates were subjected to SDS/PAGE and Western blotting using anti-MEK1 (N terminus, cat. 07–641; EMD Millipore), anti-MEK2 (N terminus, sc-524; Santa Cruz Biotechnology), anti-ERK (cat. 4695), and antiphospho-ERK (cat. 4370; Cell Signaling Technology) antibodies.
Mice and Tumor Studies.
TEM8- and CMG2-null mice were generated previously (31). TEM8- and CMG2-null mice were also crossed with C57BL/6J nude (Foxn1nu/nu) mice (The Jackson Laboratory). The resulting TEM8+/−/Foxn1+/nu mice and CMG2+/−/Foxn1+/nu mice were intercrossed to generate TEM8−/−, CMG2−/− and their littermate control athymic nude (Foxn1nu/nu) mice, which were used to establish human tumor xenografts. The tissue-specific CMG2-null mice, including Cmg2(EC)−/− and Cmg2(Mye)−/− mice, and the tissue-specific CMG2-expressing mice, including Cmg2EC and Cmg2SM mice, were generated as described previously with C57BL/6J background (12, 31, 33) (see the Fig. 3 legend for descriptions of the genotypes). For tumor studies, 10- to 14-wk-old male and female mice were used. To grow syngeneic tumors, 5 × 105 cells per mouse B16-BL6 melanoma cells or LLC lung carcinoma cells (52) were injected in the midscapular subcutis of the preshaved mice with C57BL/6J background and indicated genotypes. Visible B16-BL6 and LLC tumors (about 50 mm3) usually formed 5–6 d after inoculation. For human tumor xenografts, 1 × 107 cells per mouse human Colo205 colorectal carcinoma cells or A549 lung carcinoma cells were injected intradermally into athymic nude mice having the indicated genotypes. Visible Colo205 and A549 tumors usually formed 12–14 d after inoculation. Tumors were treated when they became visible or at later stages and measured with digital calipers (FV Fowler). Tumor volumes were estimated with the length, width, and height of tumor dimensions using formulas: tumor volume (mm3) = 1/2(length in mm × width in mm2) or 1/2(length in mm × width in mm × height in mm). Tumor-bearing mice were randomized into groups and injected intraperitoneally following schedules indicated in the figures, with PBS, the engineered toxins, a PC regimen, or a combined therapy. Mice were weighed and the tumors were measured before each injection.
Visualization of Blood Vessels with Lipophilic Carbocyanine Dye DiI.
The visualization procedure was previously described (34). In brief, B16-BL6 tumor-bearing mice treated with three doses of 30 µg of PA-L1 plus 15 µg of LF or PBS were euthanized by CO2 inhalation, followed immediately by sequential cardiac perfusion using PBS, DiI dye (Sigma), and 4% paraformaldehyde. Frozen tissue sections were then prepared for fluorescence microscopy to visualize vasculatures of tumors and various normal tissues. For tumor blood vessel quantifications, we counted blood vessels in five random views (11 mm2 per view) from each tumor sample (n = 3 for each treatment group).
Histology and Immunohistochemistry.
B16-BL6 tumor-bearing mice treated with two doses of 30 µg of PA-L1 plus 15 µg of LF or PBS were euthanized by CO2 inhalation. Tumors were harvested, fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin, sectioned, and hematoxylin/eosin (H&E) stained. Unstained sections were stained with a goat polyclonal anti-mouse CD31 (1:500 dilution) (cat. sc-1506; Santa Cruz Biotechnology) and a rabbit monoclonal anti-Ki67 antibody (1:500 dilution) (cat. ab16667; Abcam) to reveal blood vessel density and alterations in cellular proliferation. Unstained sections were also costained with an anti-mouse CD31 (sc-1506; Santa Cruz Biotechnology) and TUNEL (using the DeadEnd Fluorometric TUNEL System, G3250; Promega). All of the images were captured by a Zeiss 780 Confocal Microscope. For quantification of blood vessel densities in tumor samples, 10 fields-of-view (708 μm × 708 μm) were analyzed.
Measurement of Toxin-Neutralizing Antibodies.
B16-BL6 or LLC tumor-bearing mice from various treatment groups were terminally bled, and sera were prepared. To titrate toxin-neutralizing antibodies in the sera, RAW264.7 cells grown in 96-well plates were incubated with 100 ng/mL PA plus 100 ng/mL LF (amounts that kill >95% of the cells) in the presence of various dilutions of the sera for 5 h, followed by an MTT assay to determine cell viabilities as described above.
Flow Cytometry.
Spleens from naive mice and the B16-BL6 melanoma-bearing mice from the groups treated with PBS, PC regimen, IC2-PA/LF, or the combined PC and the toxin were dissected and weighed after the second round treatments as shown in Fig. S8A. Splenocytes were isolated, counted, and stained with fluorochrome-conjugated mAbs anti-CD45R APC-Cy7 (cat. 552094; BD Pharmingen), anti-CD4 APC (cat. 553051; BD Pharmingen), anti-CD8 PE (cat. 553033; BD Pharmingen), anti-CD11b PerCP-Cy5 (cat. 550993; BD Pharmingen), and anti–Gr-1 FITC (cat. 553127; BD Pharmingen), or anti-IgD FITC (cat. 553439; BD Pharmingen) and anti-IgM PE (cat. 553409; BD Pharmingen). The cells were analyzed using a BD FACSCanto Flow Cytometer, and percentages of each cell population positive for the indicated immune cell markers were obtained. Cell numbers positive for each immune cell marker were obtained by the following formula: total splenocytes × the percentage of the marker-positive cells.
For propidium iodide (PI) and annexin V staining, endothelial cells treated with or without toxins were collected (including the cells in medium supernatants) and resuspended in 1× binding buffer (BD Biosciences) at a concentration of 1 × 106 cells per milliliter. Then, 100 μL of the solution was stained with 5 μL each of annexin V (BD Biosciences) and 50 μg/mL PI (Invitrogen), with incubation at room temperature for 15 min. The cells were analyzed using a BD FACSCanto Flow Cytometer, and percentages of each cell population were obtained.
Statistics.
Statistical significances of differences were calculated using the two-tailed Student's t test or one-way ANOVA when more than two groups were compared. Survival curves were compared using a Log-rank test. P < 0.05 was considered as a significant difference.
Study Approval.
All animal studies were carried out in accordance with protocols approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
Acknowledgments
We thank Mahtab Moayeri for helpful discussions. This research was supported with funds from the Divisions of Intramural Research of the National Institute of Allergy and Infectious Diseases, the National Heart, Lung, and Blood Institute, and the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.K.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600982113/-/DCSupplemental.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug Discov. 2014;13(12):928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Moayeri M, Leppla SH. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol. 2014;22(6):317–325. doi: 10.1016/j.tim.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414(6860):225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 5.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci USA. 2003;100(9):5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mogridge J, Cunningham K, Lacy DB, Mourez M, Collier RJ. The lethal and edema factors of anthrax toxin bind only to oligomeric forms of the protective antigen. Proc Natl Acad Sci USA. 2002;99(10):7045–7048. doi: 10.1073/pnas.052160199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham K, Lacy DB, Mogridge J, Collier RJ. Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc Natl Acad Sci USA. 2002;99(10):7049–7053. doi: 10.1073/pnas.062160399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feld GK, et al. Structural basis for the unfolding of anthrax lethal factor by protective antigen oligomers. Nat Struct Mol Biol. 2010;17(11):1383–1390. doi: 10.1038/nsmb.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moayeri M, Leppla SH, Vrentas C, Pomerantsev AP, Liu S. Anthrax pathogenesis. Annu Rev Microbiol. 2015;69:185–208. doi: 10.1146/annurev-micro-091014-104523. [DOI] [PubMed] [Google Scholar]
- 10.Moayeri M, et al. The heart is an early target of anthrax lethal toxin in mice: A protective role for neuronal nitric oxide synthase (nNOS) PLoS Pathog. 2009;5(5):e1000456. doi: 10.1371/journal.ppat.1000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112(5):670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, et al. Key tissue targets responsible for anthrax-toxin-induced lethality. Nature. 2013;501(7465):63–68. doi: 10.1038/nature12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Croix B, et al. Genes expressed in human tumor endothelium. Science. 2000;289(5482):1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 14.Carson-Walter EB, et al. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61(18):6649–6655. [PubMed] [Google Scholar]
- 15.Bell SE, et al. Differential gene expression during capillary morphogenesis in 3D collagen matrices: Regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114(Pt 15):2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 16.Cryan LM, Rogers MS. Targeting the anthrax receptors, TEM-8 and CMG-2, for anti-angiogenic therapy. Front Biosci (Landmark Ed) 2011;16:1574–1588. doi: 10.2741/3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves CV, Dufraine J, Young JA, Kitajewski J. Anthrax toxin receptor 2 is expressed in murine and tumor vasculature and functions in endothelial proliferation and morphogenesis. Oncogene. 2010;29(6):789–801. doi: 10.1038/onc.2009.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhary A, et al. TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer Cell. 2012;21(2):212–226. doi: 10.1016/j.ccr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Schubert RL, Bugge TH, Leppla SH. Anthrax toxin: Structures, functions and tumour targeting. Expert Opin Biol Ther. 2003;3(5):843–853. doi: 10.1517/14712598.3.5.843. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Netzel-Arnett S, Birkedal-Hansen H, Leppla SH. Tumor cell-selective cytotoxicity of matrix metalloproteinase-activated anthrax toxin. Cancer Res. 2000;60(21):6061–6067. [PubMed] [Google Scholar]
- 21.Liu S, Bugge TH, Leppla SH. Targeting of tumor cells by cell surface urokinase plasminogen activator-dependent anthrax toxin. J Biol Chem. 2001;276(21):17976–17984. doi: 10.1074/jbc.M011085200. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Aaronson H, Mitola DJ, Leppla SH, Bugge TH. Potent antitumor activity of a urokinase-activated engineered anthrax toxin. Proc Natl Acad Sci USA. 2003;100(2):657–662. doi: 10.1073/pnas.0236849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, et al. Intermolecular complementation achieves high-specificity tumor targeting by anthrax toxin. Nat Biotechnol. 2005;23(6):725–730. doi: 10.1038/nbt1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer JM, et al. Efficient targeting of head and neck squamous cell carcinoma by systemic administration of a dual uPA and MMP-activated engineered anthrax toxin. PLoS One. 2011;6(5):e20532. doi: 10.1371/journal.pone.0020532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips DD, et al. Engineering anthrax toxin variants that exclusively form octamers and their application to targeting tumors. J Biol Chem. 2013;288(13):9058–9065. doi: 10.1074/jbc.M113.452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: A moving target for therapeutic intervention. J Clin Invest. 1999;103(9):1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 28.Danø K, et al. Cancer invasion and tissue remodeling: Cooperation of protease systems and cell types. APMIS. 1999;107(1):120–127. doi: 10.1111/j.1699-0463.1999.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 29.Duesbery NS, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280(5364):734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 30.Vitale G, et al. Anthrax lethal factor cleaves the N-terminus of MAPKKS and induces tyrosine/threonine phosphorylation of MAPKS in cultured macrophages. J Appl Microbiol. 1999;87(2):288. doi: 10.1046/j.1365-2672.1999.00893.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, et al. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci USA. 2009;106(30):12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wein AN, et al. An anthrax toxin variant with an improved activity in tumor targeting. Sci Rep. 2015;5:16267. doi: 10.1038/srep16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, et al. Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice. Cell Host Microbe. 2010;8(5):455–462. doi: 10.1016/j.chom.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, et al. Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat Protoc. 2008;3(11):1703–1708. doi: 10.1038/nprot.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arora N, Klimpel KR, Singh Y, Leppla SH. Fusions of anthrax toxin lethal factor to the ADP-ribosylation domain of Pseudomonas exotoxin A are potent cytotoxins which are translocated to the cytosol of mammalian cells. J Biol Chem. 1992;267(22):15542–15548. [PubMed] [Google Scholar]
- 36.Liu S, et al. Diphthamide modification on eukaryotic elongation factor 2 is needed to assure fidelity of mRNA translation and mouse development. Proc Natl Acad Sci USA. 2012;109(34):13817–13822. doi: 10.1073/pnas.1206933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abi-Habib RJ, et al. BRAF status and mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 activity indicate sensitivity of melanoma cells to anthrax lethal toxin. Mol Cancer Ther. 2005;4(9):1303–1310. doi: 10.1158/1535-7163.MCT-05-0145. [DOI] [PubMed] [Google Scholar]
- 38.Mossoba ME, et al. Pentostatin plus cyclophosphamide safely and effectively prevents immunotoxin immunogenicity in murine hosts. Clin Cancer Res. 2011;17(11):3697–3705. doi: 10.1158/1078-0432.CCR-11-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hassan R, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5(208):208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verdurmen WP, Luginbühl M, Honegger A, Plückthun A. Efficient cell-specific uptake of binding proteins into the cytoplasm through engineered modular transport systems. J Control Release. 2015;200:13–22. doi: 10.1016/j.jconrel.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Bachran C, et al. Cytolethal distending toxin B as a cell-killing component of tumor-targeted anthrax toxin fusion proteins. Cell Death Dis. 2014;5:e1003. doi: 10.1038/cddis.2013.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCluskey AJ, Collier RJ. Receptor-directed chimeric toxins created by sortase-mediated protein fusion. Mol Cancer Ther. 2013;12(10):2273–2281. doi: 10.1158/1535-7163.MCT-13-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCluskey AJ, Olive AJ, Starnbach MN, Collier RJ. Targeting HER2-positive cancer cells with receptor-redirected anthrax protective antigen. Mol Oncol. 2013;7(3):440–451. doi: 10.1016/j.molonc.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers MS, et al. Mutant anthrax toxin B moiety (protective antigen) inhibits angiogenesis and tumor growth. Cancer Res. 2007;67(20):9980–9985. doi: 10.1158/0008-5472.CAN-07-0829. [DOI] [PubMed] [Google Scholar]
- 45.Peters DE, et al. Comparative toxicity and efficacy of engineered anthrax lethal toxin variants with broad anti-tumor activities. Toxicol Appl Pharmacol. 2014;279(2):220–229. doi: 10.1016/j.taap.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, et al. Matrix metalloproteinase-activated anthrax lethal toxin demonstrates high potency in targeting tumor vasculature. J Biol Chem. 2008;283(1):529–540. doi: 10.1074/jbc.M707419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamanna N, et al. Pentostatin, cyclophosphamide, and rituximab is an active, well-tolerated regimen for patients with previously treated chronic lymphocytic leukemia. J Clin Oncol. 2006;24(10):1575–1581. doi: 10.1200/JCO.2005.04.3836. [DOI] [PubMed] [Google Scholar]
- 49.Kay NE, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood. 2007;109(2):405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta PK, Moayeri M, Crown D, Fattah RJ, Leppla SH. Role of N-terminal amino acids in the potency of anthrax lethal factor. PLoS One. 2008;3(9):e3130. doi: 10.1371/journal.pone.0003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pomerantsev AP, et al. A Bacillus anthracis strain deleted for six proteases serves as an effective host for production of recombinant proteins. Protein Expr Purif. 2011;80(1):80–90. doi: 10.1016/j.pep.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Reilly MS, et al. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79(2):315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds LE, Hodivala-Dilke KM. Primary mouse endothelial cell culture for assays of angiogenesis. Methods Mol Med. 2006;120:503–509. doi: 10.1385/1-59259-969-9:503. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, Leppla SH. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J Biol Chem. 2003;278(7):5227–5234. doi: 10.1074/jbc.M210321200. [DOI] [PubMed] [Google Scholar]