Significance
This study focuses on a molecular machine (Xer/dif/FtsK) involved in circular chromosome processing during the bacterial cell cycle. Xer site-specific recombinases are well known to act at the chromosomal dif (dimer resolution) sites for chromosome dimer resolution (CDR). The Xer/dif recombination machine is, however, highly versatile and is also implicated in integration and excision of mobile genetic elements (MGE). Whereas CDR depends on the FtsK DNA translocase, MGE mobility somehow escapes this control. Focusing on the case of the gonococcal genetic island found in pathogenic Neisseria species, we reveal how FtsK distinguishes a Xer/dif complex involved in vertical genetic transfer (CDR) from one involved in horizontal gene transfer (MGE mobility).
Keywords: XerCD, dif, FtsK, GGI, IMEX
Abstract
In bacteria, the FtsK/Xer/dif (chromosome dimer resolution site) system is essential for faithful vertical genetic transmission, ensuring the resolution of chromosome dimers during their segregation to daughter cells. This system is also targeted by mobile genetic elements that integrate into chromosomal dif sites. A central question is thus how Xer/dif recombination is tuned to both act in chromosome segregation and stably maintain mobile elements. To explore this question, we focused on pathogenic Neisseria species harboring a genomic island in their dif sites. We show that the FtsK DNA translocase acts differentially at the recombination sites flanking the genomic island. It stops at one Xer/dif complex, activating recombination, but it does not stop on the other site, thus dismantling it. FtsK translocation thus permits cis discrimination between an endogenous and an imported Xer/dif recombination complex.
In all organisms, the processing of chromosome ends or termini relies on specific activities for replication and segregation. In eukaryotes, telomeres are often targeted by mobile genetic elements, which may even substitute for telomeric functions (1). Circular chromosomes found in prokaryotes have no telomeres but harbor chromosome dimer resolution sites, called dif sites, on which dedicated Xer recombinases (XerC and XerD in most cases) act (2, 3). Besides their role in chromosome maintenance, dif sites are targeted by numerous mobile genetic elements, referred to as integrating mobile element exploiting Xer (IMEX) (4). How IMEXs integrate into dif without inactivating its cellular function and how they are stably maintained in their integrated state has remained unclear despite study over the past decade (4–7). Here we answer these questions by studying the gonococcal genomic island (GGI), an IMEX stably integrated into the dif site of pathogenic Neisseria species that encodes crucial functions for gene exchange and virulence (8, 9).
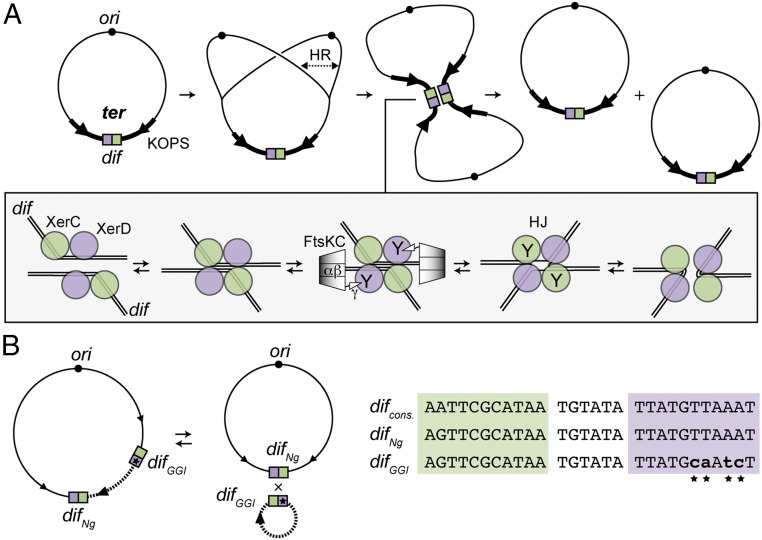
In Escherichia coli, chromosome dimers form by homologous recombination during replication and are resolved by site-specific recombination between sister dif sites catalyzed by the XerC and XerD recombinases (Fig. 1) (3). The 28-bp dif site carries binding sites for each recombinase, separated by a 6-bp central region at the border of which strand exchanges are catalyzed. After assembly of the recombination complex (synapse), one pair of strands is exchanged by the XerD monomers, leading to a branched DNA intermediate (Holliday junction, HJ) subsequently resolved by XerC. Dimer resolution is integrated into the general processing of the terminal region of the chromosome (ter region) during cell division (10). FtsK, a DNA translocase associated with the division apparatus, segregates this region at the onset of cell division (10, 11). The translocation motor, FtsKαβ, is located in the C terminal of FtsK (12). Translocation is oriented toward the dif site located at the center of the ter region via a direct interaction between the extreme C-terminal subdomain of FtsK, FtsKγ, and the KOPS DNA motifs (13). Upon reaching the XerCD/dif complex, FtsK stops translocating and activates recombination via direct interaction with XerD (14, 15) (Fig. 1). The mechanisms of translocation arrest and of recombination activation are poorly understood but they both involve FtsKγ. However, these activities appear to be distinct from each other because FtsKγ can activate recombination in vivo and in vitro when isolated from the FtsKαβ motor or fused to XerC or XerD (16).
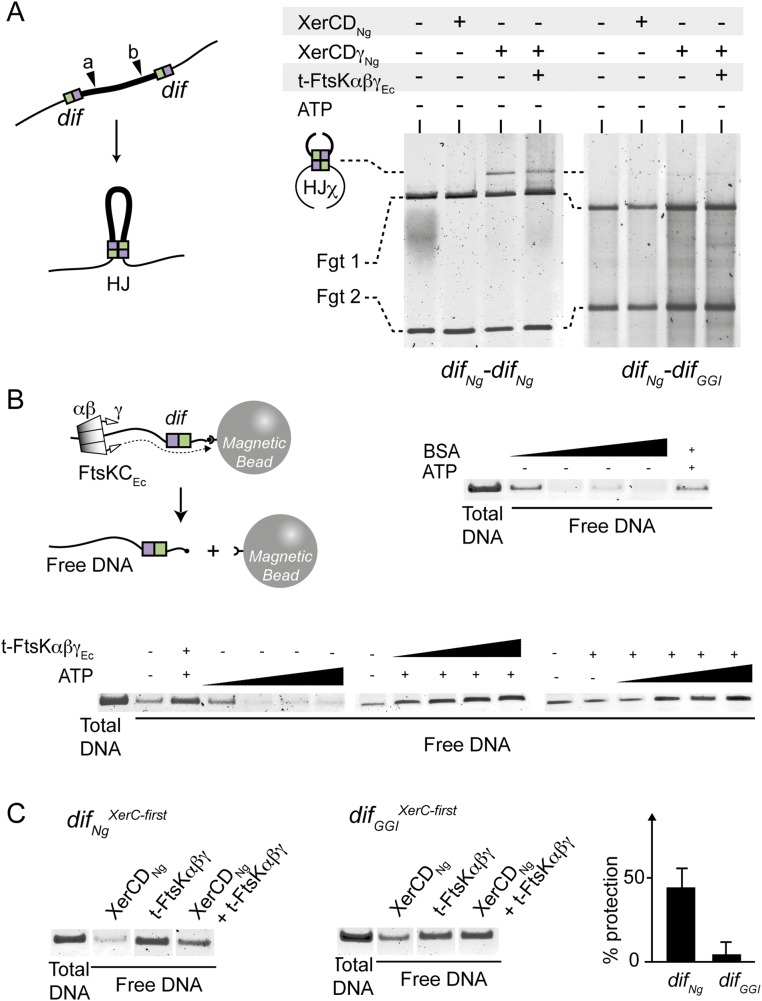
Fig. 1.
The XerCD/dif recombination. (A) Chromosome dimer formation by homologous recombination (HR) during replication and resolution by site-specific recombination between the two dif sites. The dif site is represented as green and purple boxes for the XerC-binding and the XerD-binding sites, respectively. ori (black circle), some KOPS motifs (arrows), and the ter domain (thick line) are represented. The mechanism of XerCD/dif recombination is represented in the box. XerC (green circles) and XerD (purple circles) bind two distant dif sites to create a synapse. Hexamers of the FtsK C-terminal domain [FtsKC: FtsKαβ: (diamonds) + FtsKγ: (triangle) contacting XerD] translocate toward dif and contact XerD. This activates XerD (Y indicates the active recombinases), which catalyzes the first-strand exchange. This process leads to the formation of an HJ intermediate within which XerC is active and catalyzes the second-strand exchange (3). (B) Integration and excision of the GGI (dotted line) by XerCD catalysis. KOPS, difNg, and difGGI sites are represented as in A. An alignment of difNg, difGGI and consensus dif sequence (27, 28) is shown on the left. Substituted positions in difGGI are represented as lowercase characters and highlighted by stars.
In numerous bacteria, the XerCD/dif system is hijacked by IMEXs, which integrate their host genome into dif sites by using XerCD-mediated catalysis (4). In all of the reported cases, integration of IMEXs recreates a bona fide dif site, thereby not interfering with chromosome dimer resolution, which would lead to their counter-selection. The best-described examples are Vibrio cholerae IMEXs, which carry crucial virulence determinants (5–7, 17). These IMEXs have developed different strategies to integrate and to remain stably integrated, although the mechanisms ensuring their stable maintenance are not fully understood. Neisseria species contain an unusually long IMEX called the gonococcal genomic island (GGI) (8). In Neisseria gonorrheae, the GGI is 57 kb long and encodes a type IV secretion system that exports the chromosomal DNA of its host, rendering it available to neighboring cells for gene exchange by genetic transformation (8, 18). The GGI carries a dif site, difGGI, consisting of a XerC-binding site, a central region homologous to the Neisseria dif site, difNg, and a divergent XerD-binding site (Fig. 1B). Comparison of N. gonorrheae strains harboring or lacking the GGI, together with functional data, indicates that the GGI integrates by XerCD-dependent recombination (9). The nonreplicative excised circular form of the GGI can be detected and the GGI can also be lost, showing that excision occurs, although at low frequencies (9). Although the GGI was identified over a decade ago, it has remained unclear how DNA flanked by two Xer recombination sites is stably maintained at a chromosomal locus processed by FtsK during each cell cycle. In this study, we have combined in vitro and in vivo approaches to show that difGGI is indeed an active Xer recombination site at which the Neisseria Xer recombinases catalyze recombination when activated by FtsKγ. However, we find that recombination between difNg and difGGI is inhibited by translocating FtsK. Inhibition is a result of the absence of translocation arrest at XerCDNg/difGGI complexes that most likely precludes recombination activation, an absence that causes the complex to dismantle. We conclude that, depending on the sequence of the recombination site, Xer recombination complexes have the intrinsic capacity to be activated or inhibited by FtsK.
Results
Xer Recombination Complexes Readily Form at difNg and difGGI.
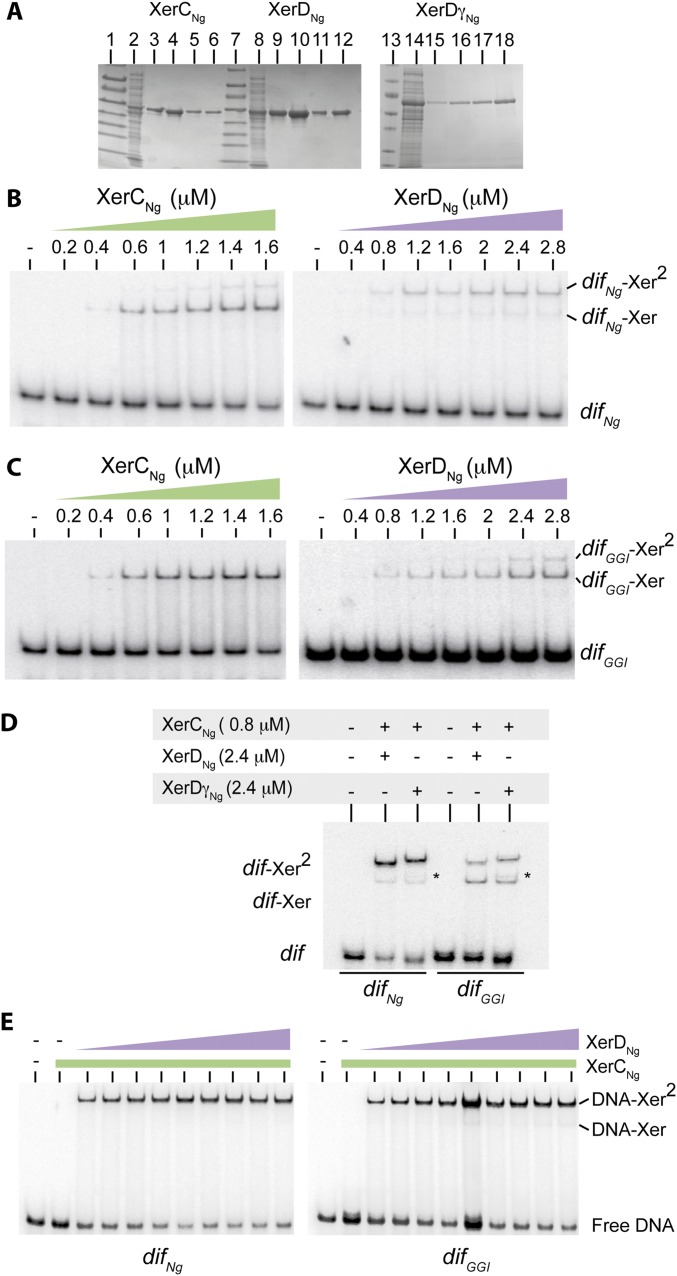
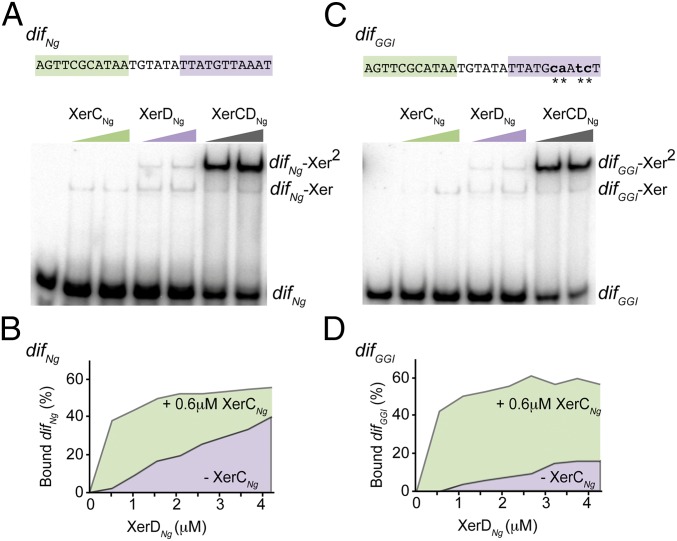
N. gonorrheae encodes XerC and XerD homologs as well as two FtsK homologs (19). We cloned and purified tagged versions of XerCNg and XerDNg (Methods, SI Text, and Fig. S1A) and used the two proteins in EMSA experiments. XerCNg or XerDNg alone formed two complexes with either radiolabeled difNg, or difGGI (Fig. 2, SI Text, and Fig. S1 B and C). Comparison with results from the E. coli Xer system suggests that the first complex corresponds to the binding of one recombinase monomer (dif-Xer), and the second to the binding of two recombinases monomers to both sides of the recombination sites (dif-Xer2) (20). The ratios of these two complexes were different between the difNg and difGGI sites (SI Text and Fig. S1 B and C). However, the overall efficiency of either XerCNg or XerDNg binding was similar on the two sites. As in the case in E. coli, XerCNg and XerDNg bound cooperatively to difNg (Fig. 2B and Fig. S1E). Similar efficiencies of complex formation were obtained with difGGI (Fig. 2D and Fig. S1E). We concluded that XerDNg readily binds to difGGI despite the four base changes of its predicted binding site compared with difNg (Fig. 1). In addition, XerCDNg/difGGI complexes formed as efficiently as XerCDNg/difNg complexes by cooperative binding of the two recombinases.
Fig. S1.
XerCNg, XerDNg, and XerDγNg purification and DNA binding. (A) SDS/PAGE analysis (Coomassie blue staining) of the different purification steps. Lanes 1, 7, and 13: prestain protein marker (New England Biolabs). Lanes 2, 8, and 14: soluble fractions. Lanes 3, 9, and 15: His-Trap elution. Lanes 4, 10, and 16: Heparin elution. Lanes 5, 11, and 17: size-exclusion elution. Lanes 6, 12, and 18: concentrated stocks (Methods). (B) EMSA (Methods) with an increasing concentration of purified tagged XerCNg or XerDNg, and a DNA molecule containing difNg. Unbound DNA (difNg), complexes with one recombinase bound (difNg–Xer), and complexes with two recombinases bound (difNg–Xer2) are represented. (C) Idem with a DNA molecule containing difGGI. (D) EMSA with XerCNg + XerDNg or XerDγNg and a DNA molecule containing difNg or difGGI. Stars (*) highlight DNA–protein complexes containing XerDγNg, which migrate slower than XerDNg. (E) EMSA with 0.6 μM of purified tagged XerCNg and increasing concentration of purified tagged XerDNg (0.4 μM to 4.2 μM) and a DNA molecule containing difNg or difGGI. Unbound DNA (free DNA), complexes with one recombinase bound (DNA–Xer), and complexes with two recombinases bound (DNA–Xer2) are represented. Quantifications of these EMSA are presented Fig. 2 B and D.
Fig. 2.
XerCNg and XerDNg bind to difNg and difGGI. EMSA experiment showing the interaction between an increasing concentration of XerCNg (0.4 and 0.6 μM) and XerDNg (1.4 and 1.8 μM) and a 28-bp DNA fragment containing either difNg (A) or difGGI (C). The color code used is the same as in Fig. 1. Unbound DNA (dif), complexes with one recombinase bound (dif–Xer), and complexes with two recombinases bound (dif–Xer2) are represented. In C, substituted positions in difGGI are represented as lowercase characters and highlighted by stars. (B and D) Titration experiment of difNg (B) or difGGI (D) by XerDNg. The experiment was done in presence (underlined with green) or in absence (underlined with purple) of XerCNg (see also Fig. S1E).
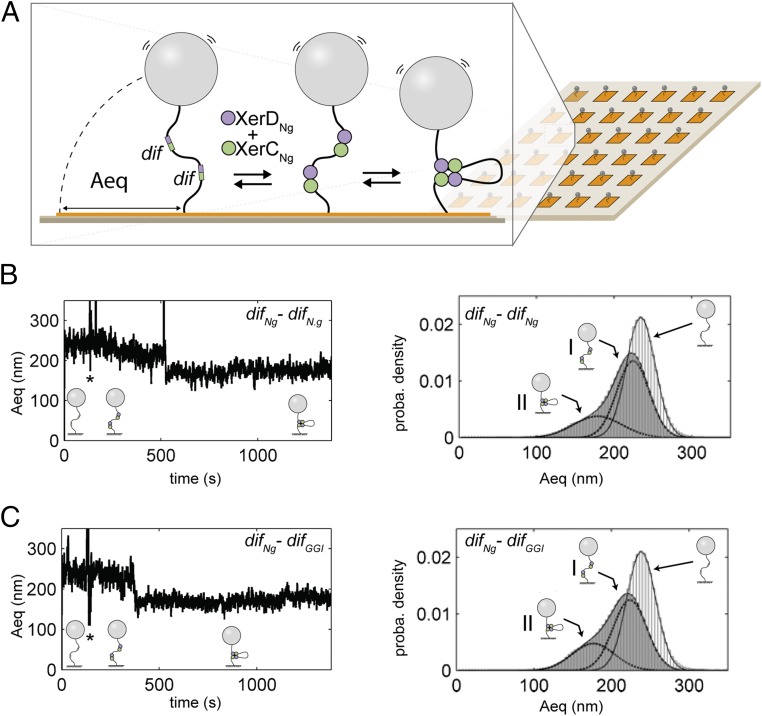
Once assembled, XerCD-dif complexes come together in a recombination-proficient complex containing two monomers of each recombinase gathering two recombination sites (Fig. 1A). We used tethered particle motion (TPM) (21, 22), a single-molecule technique that involves tracking beads attached at one end of the DNA molecules while the other extremity of the DNA is tethered to a coverslip (Methods and Fig. 3A). The amplitude of motion at equilibrium of the bead (Aeq) directly depends on the apparent length of the DNA (22, 23). We constructed two 2,311-bp long DNA molecules, containing either two difNg sites or a difNg and a difGGI site separated by 945 bp, and recorded their Aeq with a recently developed multiplexed version of the TPM [high-throughput (HT)-TPM] (Methods and Fig. 3A) (22). Addition of XerCNg and XerDNg to either DNA molecule resulted in a displacement toward smaller values and a broadening of Aeq distribution well fitted by two Gaussian peaks (Fig. 3B, Right). The first peak (I: 70% of the probability density) was shortened by 10 nm compared with the naked DNA. This shortening was too small to be a result of formation of a recombination complex and was more likely because of XerDNg binding to the recombination sites, as previously observed with E. coli XerD (21). The second peak (II: 30% of the probability density) was shortened by 60 nm. Considering the TPM calibration equation [ΔAeq (in nm) = 0.0623 L (in bp) + 92.3; measured in these very same experimental conditions (21)], this shortening was consistent with the formation of a recombination complex (Aeq = 0.0623 × 945 ∼ 59 nm). No difference was detected between the DNA containing either two difNg or one difNg and one difGGI (Fig. 3 B and C). We concluded that XerCNg and XerDNg form recombination complexes between difNg sites. Most importantly, equivalent complexes also formed between difNg and difGGI, suggesting that the base changes present in difGGI do not affect the formation of recombination complexes.
Fig. 3.
HT-TPM measurement of recombination complex formation. (A) A glass coverslip (pale brown) is coated with neutravidin (orange). A DNA molecule is attached to this surface by biotin bound to one of its 5′ end. A latex bead coated with antidigoxigenin is bound to the other extremity of the DNA carrying digoxigenin at its 5′ end. XerCNg and XerDNg (green and purple circles) bind dif sites (green and purple boxes) and the formation of a complex between two dif sites significantly decreases the amplitude of bead motion (Aeq). (B and C, Left) Typical traces [Aeq = f(time)] observed for DNA molecules containing either two difNg sites (B) or one difNg one difGGI sites (C). Stars (*) indicate the times of XerCDNg mix injection. Schemes of the inferred DNA structures of the DNA are shown underneath each trace. (Right) Probability distributions of Aeq before protein addition (light gray) and during the 20 min following XerCDNg addition (dark gray). The Gaussian fitting curves, used to characterize the different DNA subpopulations contributing to the Aeq distributions, are superimposed to the distributions as well as the schemes of the inferred DNA structures for each subpopulation (I refers to the first peak and II to the second).
Both difNg and difGGI Are Active Xer Recombination Sites.
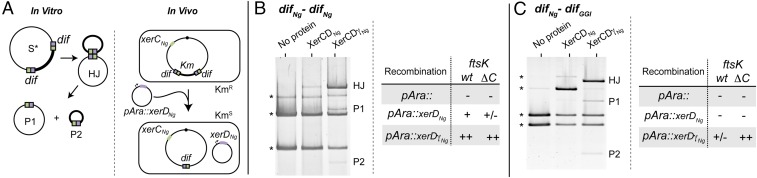
To directly monitor recombination, we constructed two reporter-cassettes, difNg-difNg and difNg-difGGI, containing a kanamycin-resistance gene between the two dif sites, and inserted them on a plasmid (Fig. 4A). In vitro assays performed by incubating the difNg-difNg plasmid with XerCNg and XerDNg resulted in the appearance of a faint quantity of branched DNA containing a HJ [Fig. 4B, Left (Center column); see SI Text and Fig. S2 for HJ characterization], an intermediate in Xer recombination (16). These HJ-containing forms were not detected with the difNg-difGGI plasmid [Fig. 4C, Left (Center column)]. We next constructed a constitutively active version of XerDNg, XerDγNg, by fusing the FtsKγ subdomain of N. gonorrheae to the C terminus of XerDNg (Methods and Figs. S1 A and D and S2) (16). Substituting XerDNg for XerDγNg yielded increased levels of HJ and recombination products for both difNg-difNg– and difNg-difGGI–containing plasmids [Fig. 4 B and C, Left (Right columns)]. In these reactions, XerDγNg likely catalyzes the initial strand exchange because the HJ were not detected using a catalytically inactive variant of this protein (SI Text and Fig. S2). We conclude that FtsKγNg activates Xer recombination by activating XerDNg-mediated catalysis and that FtsKγNg can activate recombination between difNg and difGGI to the same level as recombination between difNg sites.
Fig. 4.
In vivo and In vitro GGI excision. (A) Schemes of the in vitro (Left) and in vivo (Right) assays used. The color code is as in Fig. 1. (B) In vitro recombination reactions using plasmids containing the difNg-difNg cassette. After DNA restriction, substrate (*) and products (HJ, P1, and P2) are separated by electrophoresis. In vivo recombination reactions using E. coli strains containing the difNg-difNg cassette inserted at the dif locus. Recombination was scored as the appearance of kanamycin-sensitive colonies after production of either XerDNg or XerDγNg. The experiment was done in wt and ΔC ftsK strains (Methods). –, Less than 2%; ±, 2–10%; +, 10–50%; ++, 50–100% kanamycin colonies (see also Table S1). (C) Same as B but for difNg-difGGI cassettes.
Fig. S2.
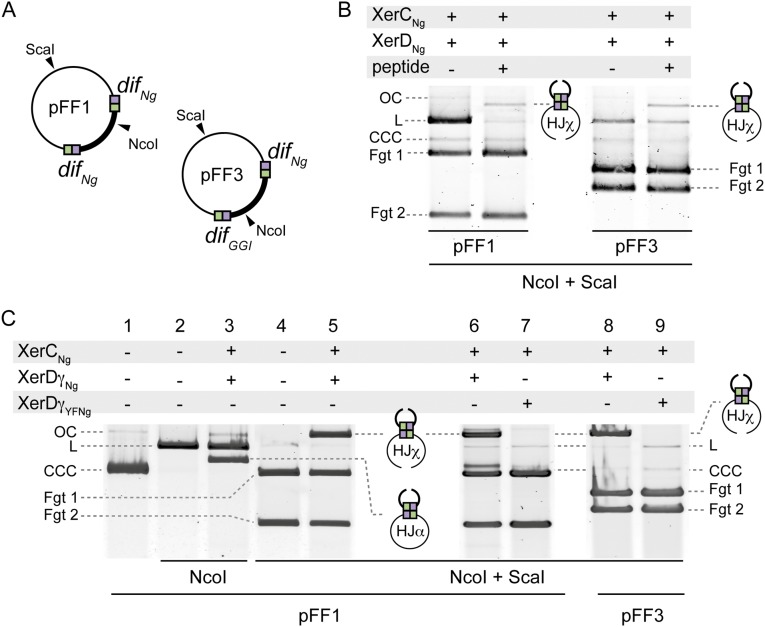
In vitro and in vivo recombination. (A) Schematic map of pFF1 and pFF3 plasmids, which are used as substrates in the in vitro recombination experiments. Restriction cleavage sites used for product simplification are represented. (B) HJ intermediate captures. The recombination reactions (Methods) were supplemented with the peptide WRWYCR (10 μM). CCC, OC, and L forms of the substrate plasmids are represented. Fgt1 and Fgt2 correspond to the DNA fragments obtained after Nco1 and ScaI digestion. (C) HJ intermediates characterization. Lanes 1–5: The recombination reactions (Methods) were either digested with NcoI (forms HJα; lane 3) or with NcoI and ScaI (forms HJχ; lane 5). Lanes 6–9: Purified XerDγYFNg (Methods) (catalytically defective variant of XerDγNg) was used in the HJ intermediate formation assay (Methods).
Table S1.
Recombination percentages
| Recombination (%) | difNg-difNg | difNg-difGGI |
| ftsKwt, xerCNg n = 1 | 1 | 1 |
| ftsKwt, xerCDNg n > 3 | 25.3 (12–38) | 1 (1–1) |
| ftsKwt, xerCDγNg n > 3 | 99.7 (99–100) | 5.5 (3–8) |
| ftsKwt, xerCDγYFNg n = 3 | 2 (2–2) | 0.25 (0–1) |
| ftsKΔC, xerCDNg n = 3 | 7.3 (1–18) | 0.5 (0–1) |
| ftsKΔC, xerCDγNg n = 3 | 90 (87–96) | 85 (81–88) |
Recombination was scored as the percentage of kanamycin-sensitive colonies: mean (minimum – maximum) (Methods).
To assess the activity of XerCDNg in vivo, we then placed these reporter cassettes into the E. coli chromosome in place of the natural dif site (Methods and Fig. 4A). In the strains carrying the cassettes on their chromosome, the endogenous xerC gene was replaced by xerCNg, whereas the endogenous xerD was deleted (Methods). Recombination was induced by transformation with a plasmid containing xerDNg under the control of an arabinose-inducible promoter (Methods). In E. coli, XerCNg and XerDNg promoted recombination between difNg sites, which partly depended on E. coli FtsK (FtsKEc) (Fig. 4B, Right). Recombination was increased and became independent of FtsKEc when XerDNg was replaced with XerDγNg (Fig. 4B, Right). Recombination between difNg and difGGI differed from difNg-difNg recombination in several ways. Recombination was barely detected between difNg and difGGI with XerCNg and XerDNg (Fig. 4C, Right), consistent with the absence of the HJ in vitro (Fig. 4C, Left). Recombination between difNg and difGGI is thus less efficient than recombination between two difNg. Substituting XerDNg by XerDγNg increased recombination, showing that, as observed in vitro (Fig. 4C, Right), the Neisseria FtsKγNg domain activates recombination between difNg and difGGI. This finding confirms that difGGI is an active site that recombines with difNg when activated by FtsKγNg.
The FtsKC Motor Inhibits GGI Excision.
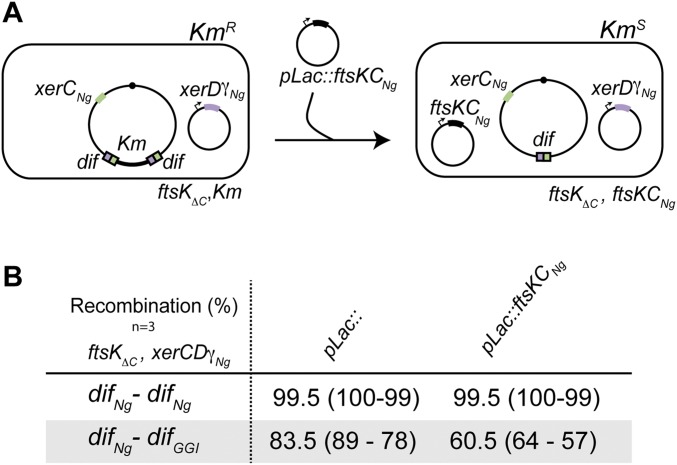
Surprisingly, XerCNg-XerDγNg catalyzed recombination between difNg and difGGI strongly increased in a strain deleted for the whole C-terminal part of FtsKEc (FtsKαβγEc) (Fig. 4C, Right) (ΔC) and reached frequencies equivalent to those of the difNg-difNg recombination in the same strain (compare Fig. 4 B and C, Right). Thus, the whole E. coli FtsK C-terminal domain (FtsKCEc) inhibits difNg-difGGI recombination in these conditions. This observation prompted us to explore the effect of the C-terminal part of Neisseria FtsK (FtsKCNg) on XerCNg-XerDγNg–driven recombination between difNg and difGGI. Production of FtsKCNg from a plasmid in an otherwise ΔC strain (SI Text and Fig. S3) yielded 27% inhibition of XerCNg and XerDγNg catalyzed recombination between difNg and difGGI (Fig. S3). FtsKCNg thus inhibited recombination between difNg and difGGI, although to a lower extent compare with FtsKEc. This difference might be because of a poor activity of FtsKCNg in these conditions. Importantly, recombination between two difNg sites was neither inhibited by FtsKEc nor by FtsKCNg. This result shows that FtsKC specifically inhibits recombination between the difNg and difGGI sites.
Fig. S3.
In vivo recombination reaction. (A) Schematic of the assay. The color code is as in Fig. 1. (B) In vivo recombination reactions using E. coli strains containing the difNg-difNg or difNg-difGGI cassette inserted at the dif locus. Recombination was scored as the percentage of kanamycin-sensitive colonies: mean (minimum – maximum) (Methods).
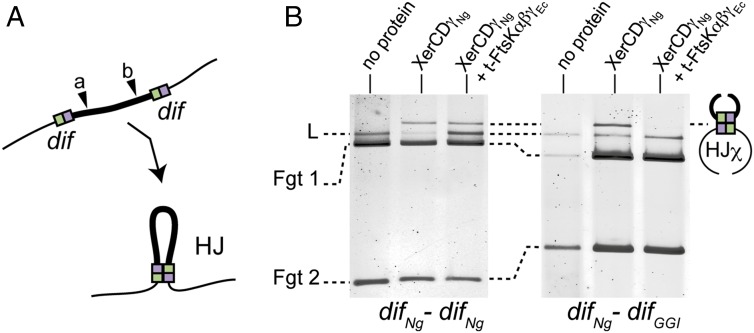
To assay the role of the FtsK motor in vitro, we used purified trimeric FtsKαβγEc (t-FtsKαβγEc) constructed from E. coli FtsK, which is known to translocate efficiently (24). We also used linear instead of supercoiled DNA substrates to lower the efficiency of recombination and ease the detection of inhibitory effects (Methods and Fig. 5A). In these conditions, both difNg-difNg and the difNg-difGGI reporter cassettes formed HJ intermediates in equivalent amounts in the presence of XerCNg and XerDγNg but no complete duplex products were detected (Fig. 5B). Addition of t-FtsKαβγEc and ATP to reactions containing XerCNg and XerDγNg did not alter HJ formation between difNg sites (Fig. 5B, Left). Conversely, addition of t-FtsKαβγEc lowered HJ quantity detected between difNg and difGGI (Fig. 5B, Right). This effect was ATP-dependent (Fig. 5B and Fig. S4A), suggesting it was caused by the translocation activity of t-FtsKαβγEc. Although we cannot completely exclude that t-FtsKαβγEc stimulates HJ resolution back to its initial substrate form, we inferred that t-FtsKαβγEc inhibits HJ formation. Taken together, these results strongly suggest that recombination between difNg and difGGI is inhibited by the translocase activity of t-FtsKαβγEc.
Fig. 5.
In vitro HJ formation. (A) Schematic of the in vitro HJ formation assay using a color code as in Fig. 1. NcoI restriction site is represented: (a) for difNg-difNg cassette and (b) for difNg-difGGI cassette. (B) HJ formation for difNg-difNg (Left) or difNg-difGGI (Right) cassettes. As indicated, DNA substrates were incubated with XerDNg and XerDγNg ± t-FtsKαβγEc. After DNA restriction, HJχ were separated from substrate DNA by electrophoresis: linear (L, partial restriction) and fragments (Fgt 1 and Fgt 2, total restriction).
Fig. S4.
FtsK translocation and HJ inhibition. (A, Left) Schematic of the in vitro HJ formation assay using a color code as in Fig. 1. The HJ formation experiment was performed with difNg-difNg (Left) or difNg-difGGI (Right) cassettes. NcoI restriction site is represented: (a) for difNg-difNg cassette and (b) for difNg-difGGI cassette. (Right) As indicated, DNA substrates were incubated with XerCNg and XerDNg or XerDγNg with or without t-FtsKαβγEc, but without ATP. After DNA restriction, HJχ were separated from substrate DNA by electrophoresis: fragments 1 and 2 (Fgt 1 and Fgt 2). (B, Upper Left) Schematic of the FtsK Translocation assay using a color code as in Fig. 1. (Upper Right) Free DNA obtained after incubation of the DNA-beads complexes with an increasing amount of BSA (from 5 to 15 mg/mL) with or without ATP (5 mM). (Lower) Free DNA obtained after incubation of the DNA-beads complexes with: an increasing concentration of ATP (from 1 mM to 5 mM) without t-FtsKαβγEc; an increasing concentration of t-FtsKαβγEc (from 0.5 mM to 5 mM) and ATP (5 mM); with t-FtsKαβγEc (0.5 μM) and an increasing concentration of ATP (from 1 mM to 5 mM). (C) FtsK stoppage on XerCD/dif complexes with t-FtsKαβγEc contacting XerCNg in first. Each gel represents the analysis of the free DNA obtained after incubation of the substrate (DNA containing difNg or difGGI) with XerCDNg, t-FtsKαβγEc, or both. The “total DNA” control was obtained by heat denaturation of the different DNA-bead complexes. The “% protection” represents the difference of free DNA obtained after t-FtsKαβγEc incubation in absence and presence of XerCDNg (mean of at least three independent experiments with SDs).
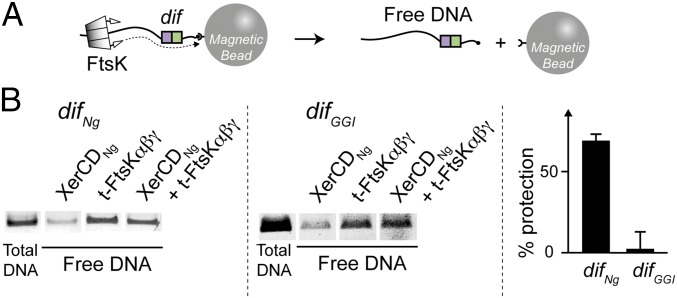
FtsK Translocation Stops on XerCDNg/difNg but Not on XerCDNg/difGGI.
FtsK is a powerful translocase able to displace proteins bound to DNA in vitro (14, 15). In contrast, FtsK specifically stops on XerCD/dif complexes, which is likely a prerequisite for the activation of recombination (14–16, 25). Because FtsK appears to inhibit the first steps of recombination between difNg and difGGI, we tested its capacity to stop translocating when encountering XerCDNg/difGGI complexes. We took advantage of the fact that t-FtsKαβγEc translocation was shown to break a biotin–streptavidin link placed at the end of a DNA molecule (24). One extremity of a DNA molecule containing a recombination site (difNg or difGGI) was attached to a magnetic bead by a biotin/streptavidin link. t-FtsKαβγEc should only break the biotin/streptavidin link and dissociate the DNA from the bead if a XerCD/dif complex was unable to stop translocation (Fig. 6A). The quantity of free DNA yielded after t-FtsKαβγEc action is thus inversely proportional to the capacity of a XerCD/dif complex to stop translocation. Results are shown in Fig. 6B, with the total DNA (after heat denaturation) presented beside the free DNA recovered in the supernatant after magnetic pull-down. Incubation of the DNA-bead complexes with t-FtsKαβγEc (and ATP) led to an increase in the quantity of free DNA recovered compared with incubation with XerCDNg. Dissociation of the DNA–bead complexes depended on the concentration of t-FtsKαβγEc and ATP, suggesting that it is a result of translocation of t-FtsKαβγEc (SI Text and Fig. S4B). Addition of XerCNg and XerDNg in absence of t-FtsKαβγEc did not dissociate the DNA–bead complexes (Fig. 6B). When XerCNg, XerDNg, and t-FtsKαβγEc were incubated together with the difNg-containing DNA–bead complexes, the quantity of free DNA decreased, showing that t-FtsKαβγEc translocation stops at XerCDNg/difNg complexes (Fig. 6B). The difference of free DNA recovered in the absence and in the presence of the recombinases (percent protection) (Fig. 6B) showed significant although not total arrest of FtsK. This result is consistent with the binding experiment (Fig. 2), which did not show 100% difNg binding by XerCDNg, and with previous FtsK arrest experiments (14). This protection effect was independent of the orientation of the difNg site (i.e., FtsK reaching the XerCDNg/difNg complex by its XerCNg or XerDNg side) (Fig. S4C). In contrast to this finding, incubation of XerCNg, XerDNg, and t-FtsKαβγEc with difGGI-containing DNA–bead complexes did not alter levels of free DNA compared with incubation with t-FtsKαβγEc, suggesting that t-FtsKαβγEc did not stop at difGGI recombination complexes (Fig. 6B). Again, the orientation of the difGGI site was unimportant (Fig. S4C). We conclude that XerCDNg/difGGI complexes cannot stop t-FtsKαβγEc translocation efficiently and are thus dissociated by t-FtsKαβγEc. These results may explain how t-FtsKαβγEc inhibited HJ formation between difNg and difGGI but not between two difNg (Fig. 5B). Indeed, it is tempting to postulate that t-FtsKαβγEc dissociates XerCDNg/difGGI complexes before HJ formation.
Fig. 6.
FtsK stoppage on XerCD/dif complexes. (A) Schematic of the translocation assay using a color code as in Fig. 1. The dotted line represents FtsK translocation, which releases the DNA from the bead (biotine/streptavidin link breakage), allowing its recovery in the bead-free supernatant after bead pull down (Methods). (B) Each gel represents the analysis of free DNA obtained after incubation of the substrate (DNA containing difNg or difGGI) with XerCDNg, t-FtsKαβγEc or both. The “total DNA” control was obtained by heat denaturation of the different DNA-bead complexes. The “% protection” represents the difference of free DNA obtained after t-FtsKαβγEc incubation in absence and presence of XerCDNg (mean of at least three independent experiments with SDs).
Discussion
Mobile genetic elements need to accurately balance their stability and transfer. This is most pertinent in the case of IMEXs that use the host Xer machine for mobility. Indeed, FtsK may induce Xer recombination between dif sites at each generation. Consistent with this view, segregation of the region surrounding the dif site is accomplished by FtsK, whether the chromosomes are dimeric or monomeric (10). IMEXs are nevertheless rarely excised and lost. The GGI is present in 80% of N. gonorrheae strains and the excised form is barely detected (8, 9). We have shown here that the integrated form of the GGI is flanked by two active Xer sites. Recombination between these sites is induced by the FtsKγNg activating domain, with XerDNg mediating exchange of the first pair of strands as in the resolution of chromosome dimers. This finding suggests that a classic FtsK-controlled XerCD/dif reaction promotes GGI excision, which should thus be very efficient. However, recombination is not activated when a translocation-proficient form of FtsK is added (either FtsKEc or FtsKCNg in vivo or t-FtsKαβγEc in vitro) (Figs. 4 and 5). We resolved this apparent paradox by showing that the difGGI site, bound by Xer recombinases, is not recognized as a bona fide dif site by the FtsK motor. Indeed, the XerCDNg/difGGI complex does not stop t-FtsKαβγEc translocation; this most probably leads to disassembly of the complex and precludes recombination activation. Such an unsuspected level of control by which FtsK activates or represses Xer recombination in response to subtle changes in the recombination complex provides a clue to the question of how GGI-type IMEXs can be stably maintained in host dif sites.
The Neisseria Xer recombinases and dif site, difNg, function similarly to their E. coli counterparts. The XerCDNg/difNg complex can form HJs but no complete duplex recombination products. Complete recombination requires FtsKγNg (Fig. 4). E. coli FtsK can activate XerCDNg/difNg recombination, although inefficiently, suggesting that activation is partly species-specific, as previously reported for E. coli and Haemophilus influenzae (26). The difGGI site differs from difNg at four positions all included in the XerD-binding site (Fig. 1B). These positions correspond to variable positions in the dif consensus (27, 28) and do not preclude the assembly of a recombination complex that can be activated for catalysis by FtsKγEc (Figs. 2–4). Clearly, the XerCDNg/difGGI complex assembles with the same efficiency as the XerCDNg/difNg complex. In addition, these two complexes form recombination complexes as efficiently as two XerCD/difNg complexes. These findings may appear surprising, given the high conservation of the XerD-binding sites of dif sites, suggesting a particular selection pressure on the sequence of this part of dif (27, 28). However, we show that XerCDNg/difGGI complexes do not stop t-FtsKαβγEc translocation. The selection pressure on the XerD-binding site may then reside in the capacity of the dif site to assemble a complex able to stop FtsK, which would require a particular interaction of XerD with its binding site involving bases not directly involved in catalysis. Using single-molecule FRET, it has recently been shown that XerCD/dif complexes may adopt different conformations (25). The nucleotides modified within difGGI may favor one of these conformations that would not be recognized by FtsK.
Following KOPS directionality, FtsK preferentially translocates toward dif (13, 15). Translocation is powerful enough to strip bound proteins off DNA (14, 15). Recent experiments using t-FtsKαβγEc reported that the stripping efficiency and outcome depends on the affinity for DNA of the protein to displace (15). The stripping activity is proposed to have functional implications in releasing MatP/matS-mediated cohesion between the ter regions of the sister chromosome during cytokinesis in E. coli (10). Exceptions to the stripping effect are XerCD/dif complexes, whether they are synapsed or not, at which t-FtsKαβγEc stops and resides for a very short time, during which a single round of recombination can be induced (25). Our results suggest an additional role of the stripping activity in stabilizing IMEXs. In these cases, FtsK would inhibit excision by dismantling the recombination complexes assembled at one of the dif sites flanking the IMEX. The stoppage or stripping-off choice thus appears crucial in differentiating chromosome dimer resolution sites from other Xer sites.
The recombination activation activity of FtsK can be separated from translocation, for example by fusing FtsKγ to XerD. However, translocation appears to be a prerequisite for activation in the natural situation (i.e., when FtsKγ is linked to the FtsK motor). This can be inferred from the incapacity of FtsK mutants defective in translocation (i.e., unable to hydrolyze ATP) to induce recombination (29, 30). We further show that an active FtsK motor also fails to activate recombination if unable to stop at the recombination complex. This failure can be observed in vitro and in vivo even when recombination is constitutively activated using a XerD–FtsKγ fusion (Figs. 4 and 5). Thus, both translocation and programmed stoppage are required for the activation of recombination, providing a tight control of this process.
The rapidly increasing number of known IMEXs have been classified following the structure of the Xer site they carry, specifying types of integration and excision mechanisms (4). These Xer sites carry at least an intact binding site, either for XerC or for XerD, resulting in the reformation of an active chromosome dimer resolution site after integration, which guarantees that integration is harmless for chromosome segregation. Excision then needs to be prohibited or tightly controlled. Most described IMEXs use a mechanism involving first-strand exchange by XerC without a catalytic role for XerD, thereby escaping activation by FtsK. The GGI, as well as the V. cholerae IMEX, called TLC (6), do not follow this paradigm but use a chromosome dimer resolution-like mechanism, with XerD exchanging the first pair of strands followed by XerC catalysis. In the case of the GGI, FtsK directly inhibits excision by stripping off the recombinases from one of the two dif sites. The Xer recombination complexes thus have the intrinsic capacity to be activated or inhibited by FtsK depending on the sequence of the recombination site. Understanding the molecular basis of this dual control urgently calls for structural studies of the Xer recombination machine.
Methods
Strain and Plasmids.
N. gonorrheae xerCNg and xerDNg genes (NGO0035 and NGO0329), were synthesized by Genscript and cloned in pET32b (Navagen) to give pROUT008 and pROUT011 plasmids. PROUT008 was used to construct xerDγNg (pFF011). N. gonorrheae xerCNg and xerDNg genes were also cloned into pBAD18 to give the pCP127 and pCP128, respectively, and pCP128 was used to introduce xerDγNg into pBAD18 (pFF013). For in vitro analysis, synthetic Kanamycin-resistant cassettes (pFF01: difNg-Kn-difNg, pFF03: difNg-Kn-difGGI) were generated on pUC57 and transferred into E. coli (DS941: AB1157recF143 lacIq Δ(lacZ)M15). In E. coli CP1088 [LN2666: W1485 F-leu thyA thi deoB or C supE rpsl (StR); xerD::frt; xerC::frt], the dif site was substituted by one of the two possible cassettes, using a previously described insertion/deletion procedure (31) (CP1106, CP1108). To produce XerCNg in these strains, xerCNg was inserted at the xerC::frt locus (31): CP1182 (CP1106, xerCNg); CP1184 (CP1108, xerCNg). To produce XerDNg or XerDγNg, these strains were transformed by pBAD18 derivatives carrying xerDNg or xerDγNg.
Protein Purification.
Expression plasmids (pROUT008 or pROUT011 or pFF011) were used to transform the E. coli BL2-DE3 strain. Xer proteins were purified as described previously (21). The purification of t-FtsKαβγEc was performed as in ref. 24.
EMSA.
The 28-bp 5′-end-labeled [γ32P] DNA fragments carrying difNg and difGGI sites were used in a binding reaction carried out as in ref. 32 and analyzed with a typhoon TRIO GE.
Multiplexed Tethered Particle Motion Analysis.
The overall HT-TPM procedure, including data analysis, has been described previously (22, 33). The 2,311-bp long DNA molecules were produced by PCR from pFF01, pFF03, and purified as previously described (21). Data acquisitions were performed at 22 °C for 25 min. The initial step of 2 min corresponds to the tracking of the DNA–beads complexes in the reaction buffer (10 mM Tris⋅HCl pH 8, 160 mM NaCl, 1 mM MgCl2, and 1 mg/mL Pluronic F-127). It is followed by the injection of a mix of 50 nM XerDNg and 50 nM XerCNg diluted in the same buffer.
Recombination Assays.
In vivo recombination experiments were performed using CP1182, or CP1184 strains transformed with pCP128 or pFF03, as previously described (32). In vitro recombination reactions were performed as previously described (16). The final concentration of each Xer protein was 0.8 μM. The concentration of substrate plasmid used was 300 ng per reaction. After 1 h at 30 °C, phenol/chloroform extraction and ethanol precipitation, products were digested by NcoI and ScaI (Fermentas), analyzed on 0.8% agarose gels, and visualized by Sybr Green coloration using Typhoon-Trio-GE. For HJ detection, 50 ng of linear DNA [NdeI (Fermentas) digestion of pFF01 or pFF03] was incubated with proteins (160 nM XerCNg + 160 nM XerDγNg ± 1 μM t-FtsKαβγEc) in a buffer containing 25 mM Tris⋅HCl pH 7.5, 10 mM MgCl2, 0.5% glycerol, 0.02 mM EDTA, 0.02 mM DTT, 0.1% PEG 8000, 75 mM NaCl, and 6.25 mM ATP. After incubation at room temperature for 20 min, DNA was purified as described for the in vitro recombination assay (see above), digested by NcoI (Fermentas), and finally analyzed as described for the in vitro recombination assay (see above).
Translocation Test.
DNA molecules were obtained by PCR on pFF01 or pFF02. These DNA molecules were mixed with streptavidin-coated magnetic beads (Streptavidin MagneSphere, Promega), at a ratio of 15 ng of DNA for 1 mL of the commercial beads solution, in 25 mM Tris⋅HCl pH 7.5, 10 mM MgCl2 buffer. After 30-min incubation at room temperature, beads were precipitated and washed to eliminate most unbound DNA. Translocation reactions were carried out in 25 mM Tris⋅HCl pH 7.5, 10 mM MgCl2, 10% (vol/vol) glycerol, 2 mM EDTA, 2 mM DTT, 250 mM NaCl, 5 mM ATP. Reactions contained 45 ng of DNA and 500 nM of each protein present: t-FtsKαβγEc and/or XerDNg and XerCNg. Reactions were incubated at room temperature for 1 min and beads were precipitated. Supernatant was collected and placed at 42 °C for 15 min with buffer containing 10% SDS, 2 mg/mL proteinase K, 0.01 mg/mL biotin. Products were analyzed by electrophoresis, as described above.
SI Text
Binding of XerCNg, XerDNg and XerDγNg to difNg and difGGI.
NGO0035 (xerCNg) and NGO0329 (xerDNg) from Neisseira gonorrheae (NC-002946) were cloned and purified as tagged version (Methods and Fig. S1A). These proteins were used in EMSA experiments (Methods, Fig. 2, and Fig. S1 B and C) to detect Xer/dif complexes (20). In the concentration range we used, XerCNg formed two complexes with difNg but only one with difGGI. In the same experiment, XerDNg alone formed two complexes with either difNg or difGGI. Results suggest that XerCNg binds DNA more efficiently than XerDNg and that XerDNg forms directly difNg–Xer2 complexes but not difGGI–Xer2 ones. However, the overall efficiency of binding of XerCNg or XerDNg to difNg and difGGI was not significantly different.
To test XerDNg activation by FtsKNg, we constructed a fusion between XerDNg and the γ domain of N. gonorrheae FtsK, as previously done with Escherichia coli proteins (16). Alignment of FtsK homologs pointed the protein of the NGO0590 gene as the FtsK homolog most likely involved in activating Xer recombination in N. gonorrheae (19). We therefore used the part corresponding to the ftsKγ subdomain (192 nt, 64 aa) (Methods) to construct xerDγNg. XerDγNg was purified and shown to bind both difNg and difGGI (Methods and Fig. S1 A and D).
HJ Intermediates Characterization.
In vitro assays performed by incubating, on a long enough period (1 h) (Methods), difNg-difNg containing plasmids (pFF1) with XerCNg and XerDNg resulted in the appearance of a faint quantity of branched DNA, predicted to contain a HJ intermediate (Fig. 4B, Left). To test this hypothesis, the recombination reactions where performed on shorter times (10 min) but supplemented with 10 μM of the WRWYCR peptide, which is known to block XerCD-dif recombination at the HJ step (34).
Results presented in Fig. S2B show that a faint quantity of branched DNA is only observed in presence of the peptide. This finding suggests that this branched DNA accumulates in presence of the peptide, consistent with the fact that it contains a HJ intermediate. Note that the same result was obtained with a difNg-difGGI containing plasmid (pFF3), although no HJ was observed in absence of peptide (Fig. 4C), suggesting a very weak HJ formation activity on this substrate (or a rapid backward reaction). To further demonstrate the presence of HJ, we performed a differential restriction analysis (Fig. S2C). In this experiment, we used XerDγNg and 20-min incubation times at 37 °C to increase HJ formation. When NcoI was used to digest the product of the reaction, the branched DNA migrated between the linear and the supercoiled forms of pFF1 (Fig. S2C, lane 3, HJα). However, when NcoI and SacI where used together to digest the product of the reaction, the branched DNA migrated more slowly (Fig. S2C, lane 5, HJχ). Again, this result is consistent with HJ formation. Finally, to test if this HJ is an intermediate of the XerCDNg–dif recombination, we used a catalytically defective variant of XerDγNg (XerDγYFNg) in which the catalytic tyrosine is replaced by a phenylalanine. As predicted, this variant is unable to form HJ intermediate (Fig. S2, compare lane 6 with lane 7 and lane 8 with lane 9). Taken together, these results show that the species identified as branched DNA effectively contains a HJ intermediates and that HJs are mostly formed by FtsKγNg-induced XerDNg catalysis.
Translocation Stoppage Controls.
To control that the magnetic pull-down assay we are using (Fig. 6) is specific to the translocation activity of t-FtsKαβγEc, we performed the experiment: (i) with increasing amounts of BSA, (ii) with increasing amounts of ATP, and (iii) with increasing amounts of t-FtsKαβγEc (Fig. S4B). Results show that free DNA only increased in presence of ATP and t-FtsKαβγEc. Furthermore, the amount of free DNA increased as the concentration of t-FtsKαβγEc or ATP increased in the reaction. These results are consistent with the fact that the increasing of free DNA is because of t-FtsKαβγEc translocation activity.
Acknowledgments
We thank all the members of our teams, F.-X. Barre, and C. Midonet for helpful discussions; M. Sahli and B. Laisnez for technical assistance; A. Segall for providing peptides; and C. Johnston for help with the manuscript. This work was funded by the CNRS, University of Toulouse 3–Paul Sabatier and ANR Contracts 11NANO01003 and 14CE10000701; and a fellowship from the “Ministère de l’Enseignement Supérieur et de la Recherche” (to F.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523178113/-/DCSupplemental.
References
- 1.Levin HL, Moran JV. Dynamic interactions between transposable elements and their hosts. Nat Rev Genet. 2011;12(9):615–627. doi: 10.1038/nrg3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blakely G, et al. Two related recombinases are required for site-specific recombination at dif and cer in E. coli K12. Cell. 1993;75(2):351–361. doi: 10.1016/0092-8674(93)80076-q. [DOI] [PubMed] [Google Scholar]
- 3.Crozat E, Fournes F, Cornet F, Hallet B, Rousseau P. Resolution of multimeric forms of circular plasmids and chromosomes. Microbiol Spectr. 2014;2(5) doi: 10.1128/microbiolspec.PLAS-0025-2014. [DOI] [PubMed] [Google Scholar]
- 4.Das B, Martínez E, Midonet C, Barre F-X. Integrative mobile elements exploiting Xer recombination. Trends Microbiol. 2013;21(1):23–30. doi: 10.1016/j.tim.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Bischerour J, Spangenberg C, Barre F-X. Holliday junction affinity of the base excision repair factor Endo III contributes to cholera toxin phage integration. EMBO J. 2012;31(18):3757–3767. doi: 10.1038/emboj.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Midonet C, Das B, Paly E, Barre F-X. XerD-mediated FtsK-independent integration of TLCϕ into the Vibrio cholerae genome. Proc Natl Acad Sci USA. 2014;111(47):16848–16853. doi: 10.1073/pnas.1404047111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Val M-E, et al. The single-stranded genome of phage CTX is the form used for integration into the genome of Vibrio cholerae. Mol Cell. 2005;19(4):559–566. doi: 10.1016/j.molcel.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Dillard JP, Seifert HS. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol Microbiol. 2001;41(1):263–277. doi: 10.1046/j.1365-2958.2001.02520.x. [DOI] [PubMed] [Google Scholar]
- 9.Domínguez NM, Hackett KT, Dillard JP. XerCD-mediated site-specific recombination leads to loss of the 57-kilobase gonococcal genetic island. J Bacteriol. 2011;193(2):377–388. doi: 10.1128/JB.00948-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stouf M, Meile J-C, Cornet F. FtsK actively segregates sister chromosomes in Escherichia coli. Proc Natl Acad Sci USA. 2013;110(27):11157–11162. doi: 10.1073/pnas.1304080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crozat E, Rousseau P, Fournes F, Cornet F. The FtsK family of DNA translocases finds the ends of circles. J Mol Microbiol Biotechnol. 2014;24(5-6):396–408. doi: 10.1159/000369213. [DOI] [PubMed] [Google Scholar]
- 12.Löwe J, et al. Molecular mechanism of sequence-directed DNA loading and translocation by FtsK. Mol Cell. 2008;31(4):498–509. doi: 10.1016/j.molcel.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigot S, et al. KOPS: DNA motifs that control E. coli chromosome segregation by orienting the FtsK translocase. EMBO J. 2005;24(21):3770–3780. doi: 10.1038/sj.emboj.7600835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham JE, Sivanathan V, Sherratt DJ, Arciszewska LK. FtsK translocation on DNA stops at XerCD-dif. Nucleic Acids Res. 2010;38(1):72–81. doi: 10.1093/nar/gkp843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JY, Finkelstein IJ, Arciszewska LK, Sherratt DJ, Greene EC. Single-molecule imaging of FtsK translocation reveals mechanistic features of protein-protein collisions on DNA. Mol Cell. 2014;54(5):832–843. doi: 10.1016/j.molcel.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grainge I, Lesterlin C, Sherratt DJ. Activation of XerCD-dif recombination by the FtsK DNA translocase. Nucleic Acids Res. 2011;39(12):5140–5148. doi: 10.1093/nar/gkr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das B, Bischerour J, Barre F-X. VGJphi integration and excision mechanisms contribute to the genetic diversity of Vibrio cholerae epidemic strains. Proc Natl Acad Sci USA. 2011;108(6):2516–2521. doi: 10.1073/pnas.1017061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton HL, Domínguez NM, Schwartz KJ, Hackett KT, Dillard JP. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol Microbiol. 2005;55(6):1704–1721. doi: 10.1111/j.1365-2958.2005.04521.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen C-C, et al. Draft genome sequence of a dominant, multidrug-resistant Neisseria gonorrhoeae strain, TCDC-NG08107, from a sexual group at high risk of acquiring human immunodeficiency virus infection and syphilis. J Bacteriol. 2011;193(7):1788–1789. doi: 10.1128/JB.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blakely GW, Davidson AO, Sherratt DJ. Binding and cleavage of nicked substrates by site-specific recombinases XerC and XerD. J Mol Biol. 1997;265(1):30–39. doi: 10.1006/jmbi.1996.0709. [DOI] [PubMed] [Google Scholar]
- 21.Diagne CT, et al. TPM analyses reveal that FtsK contributes both to the assembly and the activation of the XerCD-dif recombination synapse. Nucleic Acids Res. 2014;42(3):1721–1732. doi: 10.1093/nar/gkt1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plénat T, Tardin C, Rousseau P, Salomé L. High-throughput single-molecule analysis of DNA-protein interactions by tethered particle motion. Nucleic Acids Res. 2012;40(12):e89. doi: 10.1093/nar/gks250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manghi M, et al. Probing DNA conformational changes with high temporal resolution by tethered particle motion. Phys Biol. 2010;7(4):046003. doi: 10.1088/1478-3975/7/4/046003. [DOI] [PubMed] [Google Scholar]
- 24.Crozat E, et al. Separating speed and ability to displace roadblocks during DNA translocation by FtsK. EMBO J. 2010;29(8):1423–1433. doi: 10.1038/emboj.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May PFJ, Zawadzki P, Sherratt DJ, Kapanidis AN, Arciszewska LK. Assembly, translocation, and activation of XerCD-dif recombination by FtsK translocase analyzed in real-time by FRET and two-color tethered fluorophore motion. Proc Natl Acad Sci USA. 2015;112(37):E5133–E5141. doi: 10.1073/pnas.1510814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yates J, Aroyo M, Sherratt DJ, Barre F-X. Species specificity in the activation of Xer recombination at dif by FtsK. Mol Microbiol. 2003;49(1):241–249. doi: 10.1046/j.1365-2958.2003.03574.x. [DOI] [PubMed] [Google Scholar]
- 27.Kono N, Arakawa K, Tomita M. Comprehensive prediction of chromosome dimer resolution sites in bacterial genomes. BMC Genomics. 2011;12(1):19. doi: 10.1186/1471-2164-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carnoy C, Roten C-A. The dif/Xer recombination systems in proteobacteria. PLoS One. 2009;4(9):e6531. doi: 10.1371/journal.pone.0006531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham JE, Sherratt DJ, Szczelkun MD. Sequence-specific assembly of FtsK hexamers establishes directional translocation on DNA. Proc Natl Acad Sci USA. 2010;107(47):20263–20268. doi: 10.1073/pnas.1007518107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigot S, Corre J, Louarn J-M, Cornet F, Barre F-X. FtsK activities in Xer recombination, DNA mobilization and cell division involve overlapping and separate domains of the protein. Mol Microbiol. 2004;54(4):876–886. doi: 10.1111/j.1365-2958.2004.04335.x. [DOI] [PubMed] [Google Scholar]
- 31.Cornet F, Louarn J, Patte J, Louarn JM. Restriction of the activity of the recombination site dif to a small zone of the Escherichia coli chromosome. Genes Dev. 1996;10(9):1152–1161. doi: 10.1101/gad.10.9.1152. [DOI] [PubMed] [Google Scholar]
- 32.Nolivos S, Pages C, Rousseau P, Le Bourgeois P, Cornet F. Are two better than one? Analysis of an FtsK/Xer recombination system that uses a single recombinase. Nucleic Acids Res. 2010;38(19):6477–6489. doi: 10.1093/nar/gkq507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunet A, et al. Probing a label-free local bend in DNA by single molecule tethered particle motion. Nucleic Acids Res. 2015;43(11):e72. doi: 10.1093/nar/gkv201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanhooff V, Normand C, Galloy C, Segall AM, Hallet B. Control of directionality in the DNA strand-exchange reaction catalysed by the tyrosine recombinase TnpI. Nucleic Acids Res. 2010;38(6):2044–2056. doi: 10.1093/nar/gkp1187. [DOI] [PMC free article] [PubMed] [Google Scholar]