Significance
Oligoclonal bands (OCBs) of the cerebrospinal fluid (CSF) are a hallmark of multiple sclerosis (MS). They are expanded antibody species that are detectable in >95% of patients. Because several OCB and polyclonal antibodies are present in a CSF sample, it was for technical reasons thus far not possible to isolate distinct OCBs and identify their antigens. Here we combined refined biochemical analysis, proteomics, and transcriptomics to molecularly characterize distinct OCB antibodies. We produced six recombinant OCB antibodies and characterized three autoantigens. All of them were ubiquitous intracellular proteins, not specific to brain tissue. This finding indicates that in MS, part of the OCBs do not directly mediate tissue destruction, but rather, indicate a secondary immune response.
Keywords: multiple sclerosis, oligoclonal bands, proteomics, transcriptomics, autoantigens
Abstract
Oligoclonal Ig bands (OCBs) of the cerebrospinal fluid are a hallmark of multiple sclerosis (MS), a disabling inflammatory disease of the central nervous system (CNS). OCBs are locally produced by clonally expanded antigen-experienced B cells and therefore are believed to hold an important clue to the pathogenesis. However, their target antigens have remained unknown, mainly because it was thus far not possible to isolate distinct OCBs against a background of polyclonal antibodies. To overcome this obstacle, we copurified disulfide-linked Ig heavy and light chains from distinct OCBs for concurrent analysis by mass spectrometry and aligned patient-specific peptides to corresponding transcriptome databases. This method revealed the full-length sequences of matching chains from distinct OCBs, allowing for antigen searches using recombinant OCB antibodies. As validation, we demonstrate that an OCB antibody from a patient with an infectious CNS disorder, neuroborreliosis, recognized a Borrelia protein. Next, we produced six recombinant antibodies from four MS patients and identified three different autoantigens. All of them are conformational epitopes of ubiquitous intracellular proteins not specific to brain tissue. Our findings indicate that the B-cell response in MS is heterogeneous and partly directed against intracellular autoantigens released during tissue destruction. In addition to helping elucidate the role of B cells in MS, our approach allows the identification of target antigens of OCB antibodies in other neuroinflammatory diseases and the production of therapeutic antibodies in infectious CNS diseases.
Multiple sclerosis (MS) is a severe inflammatory disease of the central nervous system (CNS) with a presumed autoimmune pathogenesis (1–4). Oligoclonal bands (OCBs) (5–7) of the cerebrospinal fluid (CSF) are the only established immunological biomarker of MS that has a diagnostic and prognostic relevance. OCBs are expanded Ig species that contain abundant somatic hypermutations, supposedly in response to sustained antigen stimulation (8–14). They are visualized by analytical immunoblotting on isoelectric focusing (IEF) gels, but so far, no particular target antigens could be assigned to distinct OCBs (15), because OCB quantities in diagnostic lumbar punctures are too low for direct antigen searches using biochemical techniques. Furthermore, OCBs are superimposed on a background of polyclonal antibodies, precluding differentiation between signals from OCB and background antibodies. Antibody cloning from single CSF B cells (16, 17) is an elegant approach for obtaining matching heavy (H) to light (L) chains of antibodies, but it is impossible to know whether a particular antibody relates to an OCB or to the polyclonal background. Candidate target antigens detected with unfractionated CSF or with recombinant antibodies cloned from single CSF-resident B cells included lipids, proteins, combinatorial peptides, and viral products (18–23). Of note, none of these studies assigned a defined antigen to a distinct OCB antibody.
We combined refined biochemical analysis, proteomics, and transcriptomics to characterize distinct OCB antibodies for recombinant expression and antigen searches. Although we could previously relate OCB proteomes and transcriptomes showing that CSF-resident B cells produce OCBs (24), we could not assign IgG-H to -L chains of distinct OCB antibodies. Here, we overcame this limitation by copurifying disulfide-linked IgG-H and -L chains from distinct OCBs and analyzing the matching chains together by mass spectrometry. This method allowed us to relate patient-specific Ig peptides, which contained unique amino acid substitutions introduced by somatic hypermutation and V(D)J recombination, to the corresponding full-length Ig transcriptomes obtained from CSF B cells of the same patients (24). This strategy revealed full-length cDNA sequences of matching Ig chains and allowed us to produce recombinant OCB (rOCB) antibodies. As proof of concept, we show that an OCB antibody from a patient with neuroborreliosis (NB) binds to a protein from Borrelia (25). Then, we characterized six rOCB antibodies from four MS patients and identified three different OCB target autoantigens. Their common feature is that they are intracellular proteins that are expressed in many cell types, suggesting that part of the OCB antibodies in MS are directed to antigens released through secondary tissue destruction.
Results
Characterization of Distinct OCB Immunoglobulins.
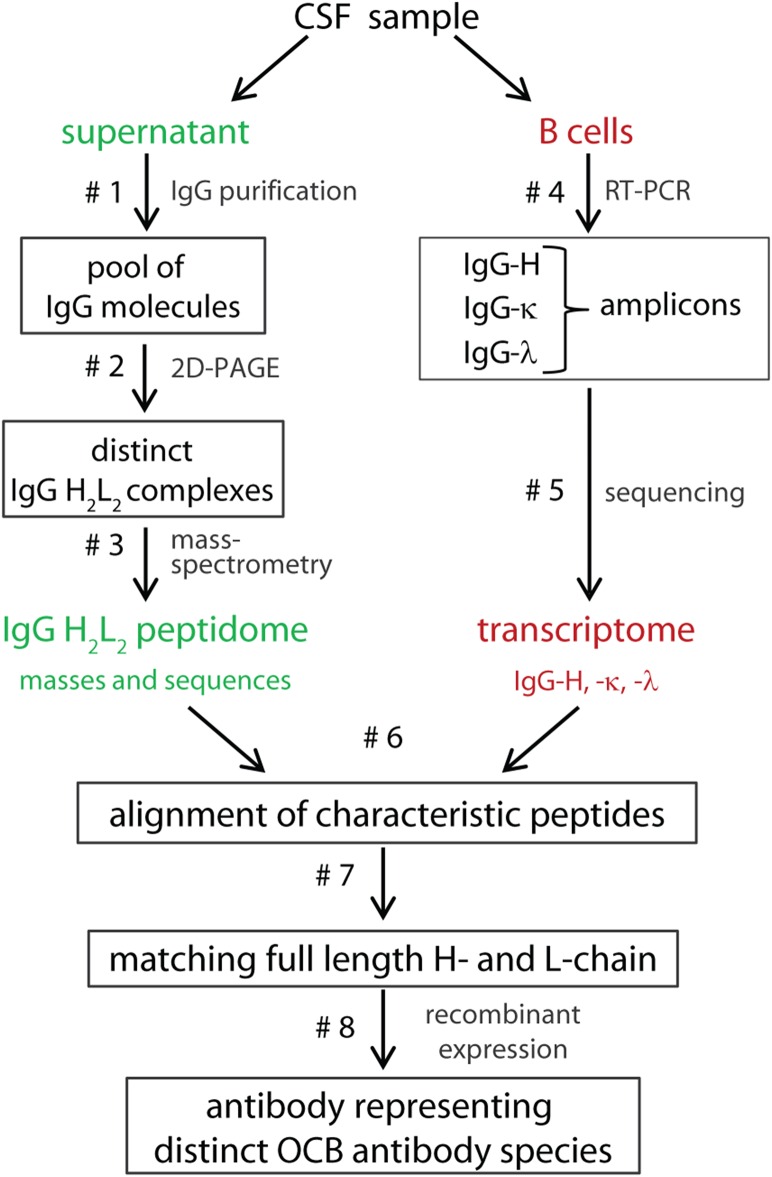
A key step in the improved technology used here (Fig. 1) is the copurification of disulfide-linked IgG-H and -L chains from single OCB spots by affinity chromatography and two-dimensional (2D) gel electrophoresis under nonreducing conditions. This method yields intact H2L2 complexes. Although the amount of distinct OCB antibodies obtained was by far too small for direct antigen searches, it was sufficient for identifying several peptides from both chains by mass spectrometry. These peptides do not need to cover the full-length IgG-H and -L sequences. It is sufficient that some of them contain highly specific mutations introduced by somatic hypermutation and V(D)J recombination (referred to as “characteristic peptides”) (24, 26). We aligned characteristic peptides to patient-specific Ig transcriptomes from CSF-resident B cells, which contain unique nucleotide mutations that correspond to the amino acid mutations of the characteristic peptides. Thus, few peptides that covered only parts of the sequences permitted unequivocal reconstruction of full-length, matching IgG-H and -L chains. Recombinant expression then yielded sufficient amounts of antibodies representing distinct OCBs for antigen searches.
Fig. 1.
Flowchart for the molecular characterization of distinct OCB antibody species. From a diagnostic CSF sample, the Ig proteome was revealed from the supernatant (Left; green) and the Ig transcriptome from the B cells in the pellet (Right; red). A pool of all IgG molecules was purified by protein G affinity chromatography and deglycosylated to yield a more homogeneous preparation (step 1). Next, a modified 2D gel electrophoresis was performed, comprising an improved IEF electrophoresis using polyacrylamide gels followed by SDS/PAGE (step 2). All steps were performed under nonreducing conditions to prevent disassembly of the IgG-H and -L chain heterodimers (H2L2) as far as possible. However, because of the high electrical field required for the polyacrylamide IEF gels, some disassembly of H2L2 complexes was still observed. H2L2 complexes were separated from complexes where one or more chains were dissociated (H2L, HL, and H2) by a second dimension of nonreducing SDS/PAGE. Spots that contained H2L2 complexes were excised from the stained 2D gels and in-gel digested with trypsin, and the tryptic peptides were analyzed by mass spectrometry (step 3). In parallel, the transcriptomes of IgG-H and -L chains were determined. To this end, B-lineage cells were collected, IgG-H and -L transcripts were amplified by RT-PCR (step 4), and either conventionally cloned and sequenced or subjected to next-generation sequencing (step 5). The characteristic peptides identified by mass spectrometry were then aligned to the specific Ig transcriptome database (step 6). Only when unambiguous alignments of characteristic peptides were possible (step 7), matching IgG-H and -L chains representing distinct OCB antibody species were expressed in a recombinant expression system (step 8).
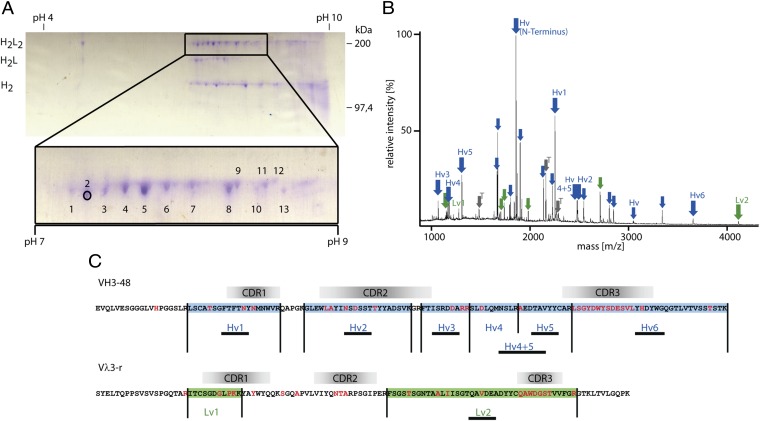
We characterized six distinct H2L2 complexes from four patients with MS and one from a patient with NB. From a nonreducing 2D gel (Fig. 2A), we isolated H2L2 complexes, in-gel digested the chains with trypsin, and subjected the fragments to MALDI-TOF/TOF mass spectrometry (Fig. 2B). The amino acid sequences of >50% of the peptides were verified by tandem mass spectrometry. We then aligned the masses and sequences of the identified peptides to patient-specific Ig-transcriptome databases, which we had generated in parallel from CSF cells (Fig. 2C). Characteristic peptides allowed for unambiguous assignments. We expressed rOCB antibodies from MS patients MS1 (rOCB-MS1-s2, -s8, and -s9), MS2 (rOCB-MS2-s5), MS3 (rOCB-MS3-s1), and MS4 (rOCB-MS4); rOCB-NB1-s13 from NB patient NB1; and the control antibody r8-18C5, which recognizes myelin oligodendrocyte glycoprotein (MOG) (27) as full-length humanized antibodies in HEK293EBNA cells (28) (Fig. S1). For purification and detection, the IgG-H chains were extended by His6 and V5 tags. rOCB-MS2-s5 was toxic to HEK cells and was therefore expressed as single-chain Fv (scFv) fragment in Escherichia coli (rOCB-scFv-MS2-s5).
Fig. 2.
Analysis of matching H and L chains of OCB from patient MS1 yielding rOCB-MS1-s2. (A) Nonreducing 2D gel electrophoresis of antibodies from a CSF sample. For the first dimension, an IEF gel was run, and for the second dimension, SDS/PAGE was performed. Most spots were detected between pH 7.0 and 9.0 (see detail). The positions of the H2L2 heterodimers, the H2L, and H2 complexes (left) and a mass scale (right) are indicated. Single spots of H2L2 heterodimers were excised, digested with trypsin, and analyzed by mass spectrometry. Spot 2 is indicated by a circle. (B) MALDI-TOF spectrum of spot 2 from A. The relative peak intensities are plotted as a function of the mass-to-charge ratio m/z. Peptides from the IgG-H (blue arrows) and IgG-L (green arrows) chains are indicated. Peaks representing peptides from VN(D)NJ-regions are termed “Hv” and “Lv” followed by a number. Peaks from the constant regions are indicated by arrows only. Peaks from trypsin are indicated in gray. (C) Deduced amino acid sequences of the H and L chains as obtained by cDNA sequencing. Peptides identified by mass spectrometry are numbered and highlighted in blue and green. Peptide sequences verified by tandem mass spectrometry are underlined. Amino acids introduced by somatic hypermutation or V(D)J recombination are highlighted in red letters. The putative positions of the complementarity determining regions CDR1, CDR2, and CDR3 regions are indicated.
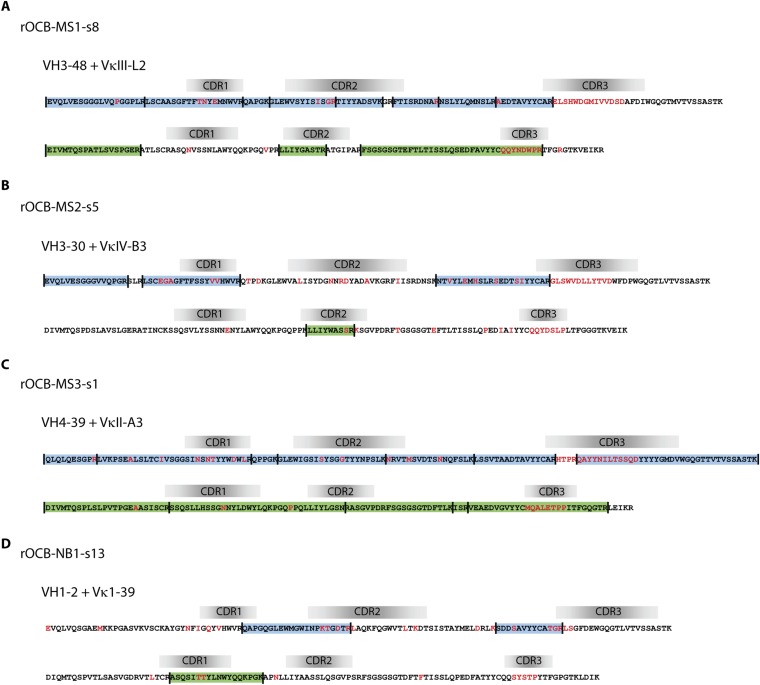
Fig. S1.
Amino acid sequences of the VN(D)NJ-regions of the IgG-H and -L chains of OCB antibodies OCB-MS1-s8 (A), OCB-MS2-s5 (B), OCB-MS3-s1 (C), and OCB-NB1-s13 (D) are shown. See legend to Fig. 2C for details.
An OCB from a Patient with NB Recognizes a Borrelia Antigen.
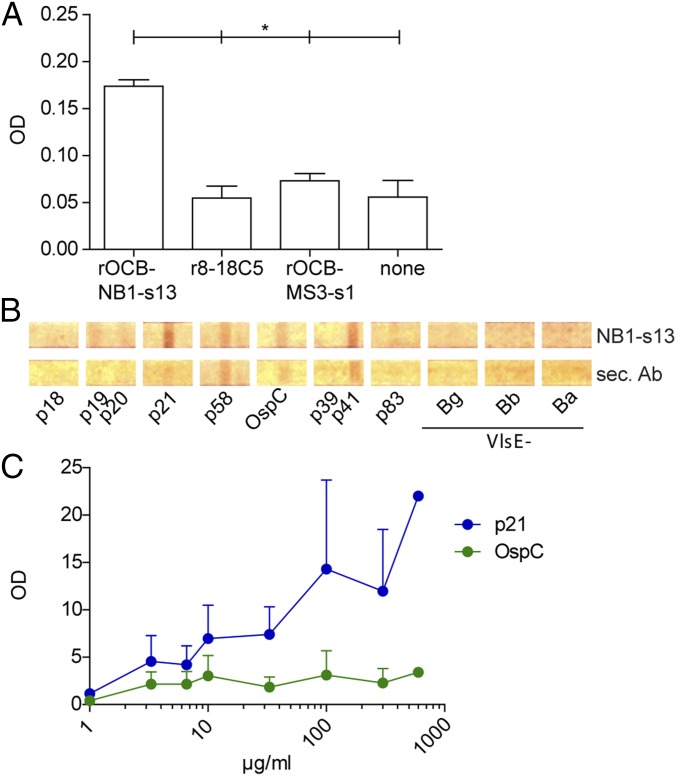
For proof of concept, we used rOCB-NB1-s13 from a patient with NB. As expected, rOCB-NB1-s13 recognized lysates from Borrelia in ELISA, whereas the control antibodies r8-18C5 and rOCB-MS3-s1 yielded background signals (Fig. 3A). The specific signal of rOCB-NB1-s13 is relatively low because the ELISA format used here is designed for clinical routine analysis, where unfractionated CSF samples from NB patients are used to screen crude Borrelia lysates. Such CSF samples contain many different Borrelia-specific antibodies, and the lysates contain many different Borrelia antigens, resulting in a high overall signal. By contrast, a monoclonal antibody as used here binds only to one antigen, which is diluted in the crude lysate and, therefore, expectedly yields a lower signal. Experiments using commercially available immunoblots with different Borrelia antigens indicated that rOCB-NB1-s13 recognizes an antigen termed p21 by the manufacturer, which corresponds to a 21-kDa fragment of the protein BBK53 (29) from Borrelia burgdorferi (Fig. 3 B and C).
Fig. 3.
rOCB-NB1-s13 isolated from CSF of a patient with NB recognizes a Borrelia antigen. (A) Lysates from Borrelia were tested in a clinical routine ELISA format. rOCB-NB1-s13, the control antibodies r8-18C5 and rOCB-MS3-s1, and no antibody (none) were diluted in CSF samples from four patients with NINDs. *P ≤ 0.05 using the Mann–Whitney u test. Data represent mean signal of four individual experiments. (B) Representative immunoblot of 12 Borrelia protein antigens used in clinical routine. The purified antigens contain common Borrelia proteins p18-21, p58, outer surface protein C (OspC), p39, p41 (flagellin), p83, and the expressed variable major protein-like sequence (VlsE) specific for garinii (-Bg), burgdorferi (-Bb), and afzelii (-Ba) strains. Some antigens showed relatively high signals with the polyclonal secondary antibody alone. Only p21 showed a significantly higher signal with rOCB-NB1-s13 compared with the secondary antibody. The concentration of rNB1-s13 was 300 µg/mL. (C) Titration of rOCB-NB1-s13 with the candidate antigen p21 and the control OspC in the range from 1 to 600 µg/mL The intensities of the spots were determined by densitometry. Signals were normalized to the secondary antibody. The assays were performed in triplicate, except for the highest concentration. Error bars indicate SEM.
Target Antigens of OCB from MS Patients.
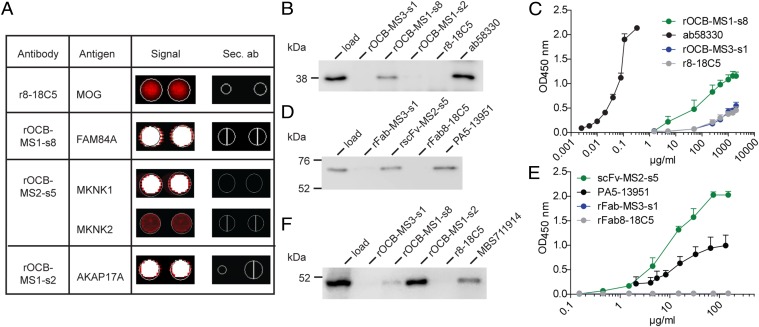
Several initial attempts to identify the target antigens of rOCB from MS patients were negative. rOCB or rFab fragments did not recognize suspected causative pathogens (Epstein–Barr, herpes simplex, measles, rubella, and zoster viruses and Chlamydia) or nuclear or neutrophil cytoplasmic antigens in clinical routine assays (EUROIMMUN). Similarly, tests for ssDNA, dsDNA, lipopolysaccharide, and insulin (30) and for lipid reactivity (21), Western blotting on denaturing 2D gels of human brain homogenates, and attempts to stain brain tissue by immunohistochemistry failed to yield consistent results. Owing to fixation, disulfide-reduction, and chaotropic agents, the above methods likely destroy protein secondary structures. Therefore, it may not be surprising, in retrospect, that these tests were negative, because we later found that at least rOCB-MS1-s2 and -s8 and rOCB-MS2-s5 recognize strictly conformational epitopes. Finally, we applied an unbiased searching strategy with antigen arrays as pioneered by Robinson et al. (31). We hybridized the rOCB to protein microarrays (ProtoArray), which display ∼9,400 full-length recombinant human proteins produced in insect cells. The positive control antibody r8-18C5 hybridized to its cognate antigen MOG; rOCB-MS2-s5 hybridized to isoforms of MAP kinase-interacting serine/threonine kinase (MKNK1 and MKNK2); rOCB-MS1-s8 hybridized to family with sequence similarity 84 member A (FAM84A); and rOCB-MS1-s2 hybridized to A-kinase anchoring protein 17A (AKAP17A) (Fig. 4A). Characteristics of these proteins are given in the SI Materials and Methods. The three other rOCBs did not show consistent reactivity with any protein contained in the array.
Fig. 4.
Identification and validation of antigens of OCB antibodies from patients with MS. (A) Hybridization of the control antibody r8-18C5 and three rOCB antibodies to ProtoArrays. Spots are shown in duplicate. The color code ranges from black (no reactivity) to red (medium reactivity) and white (strong reactivity). Compilation of rOCBs (left column), their antigens (second column), the signals from array hybridization (third column), and the corresponding signals from hybridization of secondary antibodies alone (fourth column) is shown. The anti-MOG antibody r8-18C5 showed an intermediate, but specific, signal with MOG (second row); rOCB-MS1-s8 showed strong signal with FAM84A (third row); rOCB-MS2-s5 showed a strong signal with MKNK1 and an intermediate signal with the highly homologous isoform MKNK2 (fourth and fifth rows); and rOCB-MS1-s2 showed strong signals with AKAP17A (sixth row). All secondary antibodies alone (sec. Ab) showed no reactivity (fourth column). (B) Validation of FAM84A recognition by rOCB-MS1-s8 by immunoprecipitation. Recombinant FAM84A produced in HEK293 cells was specifically precipitated by rOCB-MS1-s8 and the commercial anti-FAM84A antibody ab58330, but not by control antibodies rOCB-MS3-s1, rOCB-MS1-s2, and r8-18C5. Lane 1 shows the loading control. The blot is representative of three independent experiments. (C) Titration of FAM84A recognition by rOCB-MS1-s8 analyzed by ELISA. Recombinant FAM84A produced in E. coli was specifically recognized by rOCB-MS1-s8 in a dose-dependent manner. The commercial monoclonal anti-FAM84A antibody ab58330 recognized FAM84A at considerably lower concentrations, indicating higher avidity. The negative control antibodies rOCB-MS3-s8 and r8-18C5 bound only very weakly at very high concentrations. The secondary antibody alone yielded background signal. Error bars indicate SD of the mean. Data are representative of four independent experiments. (D) Validation of MKNK1 recognition by rOCB-MS2-s5 by immunoprecipitation. Recombinant MKNK1 produced in HEK293 cells was specifically precipitated by rOCB-MS2-s5 and the commercial anti-MKNK1 antibody PA5-13951, but not by control antibodies rOCB-MS3-s1 and r8-18C5. Lane 1 shows the loading control. The blot is representative of three independent experiments. (E) Titration of MKNK1 recognition by rscFv-MS2-s5 analyzed by ELISA. Recombinant MKNK1 produced in HEK293 cells was specifically recognized by rscFv-MS2-s5 and with a slightly higher avidity by the commercial anti-MKNK antibody PA5-13951, but not by control Fab fragments rFab-MS3-s1 and rFab-8-18C5. Error bars indicate SD of the mean. Data are representative of four independent experiments. (F) Validation of AKAP17A recognition by rOCB-MS1-s2 by immunoprecipitation. AKAP17A produced in HEK293 EBNA cells was precipitated by rOCB-MS1-s2 and -s8 and the commercial anti-AKAP17A antibody MBS711914, but not by the control antibodies rOCB-MS3-s1 and r8-18C5. Lane 1 shows the loading control. The blot is representative of five independent experiments.
Next, we validated the ProtoArray results by immunoprecipitation and ELISA—i.e., by methods that rely on high-affinity interaction—and used antigens that were produced in organisms different from insect cells. Thus, rOCB-MS1-s8 immunoprecipitated full-length FAM84A produced in HEK293 cells (Fig. 4B). In a parallel experiment, rOCB-MS1-s8 recognized FAM84A from E. coli in an ELISA in a concentration-dependent manner (Fig. 4C). The control antibody ab58330 yielded a much stronger signal than rOCB-MS1-s8, presumably because only clones with high avidity were selected for commercial production. None of the control antibodies rOCB-MS3-s1, rOCB-MS1-s2, or r8-18C5 recognized FAM84A by immunoprecipitation or ELISA.
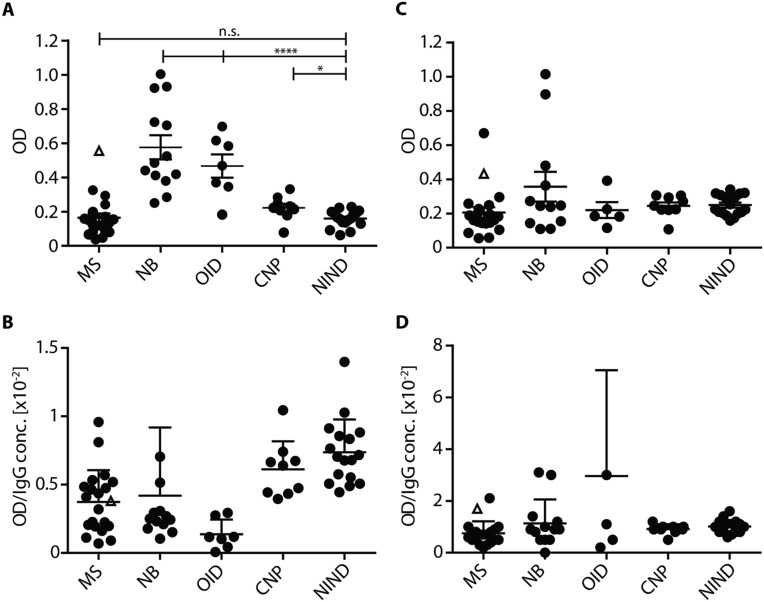
In an earlier study (22), FAM84A was recognized just below significant levels by unfractionated CSF from MS patients. Therefore, we examined its recognition by CSF samples from patients with MS and inflammatory and noninflammatory CNS diseases (Fig. S2). Confirming the earlier results (22), MS1 was the only MS patient recognizing FAM84A. However, we detected higher levels of anti-FAM84A antibodies in patients with acute inflammatory CNS diseases compared with MS or noninflammatory diseases, concomitant to increased overall IgG levels. This finding is consistent with the presence of anti-FAM84A antibodies in patients with inflammatory bowel disease (32) and indicates that FAM84A is an intracellular antigen that might elicit strong secondary immune reactivity when released as cellular debris.
Fig. S2.
Reactivity of unfractionated CSF (A and B) and serum (C and D) from patients with different neurological diseases to FAM84A. We tested samples from five different cohorts of patients with MS (n = 21), NB (n = 13), other (bacterial and viral) infectious diseases of the CNS (OID; n = 7), cranial nerve palsy (CNP; n = 9), and NINDs (n = 17) by ELISA. Data are given as optical density (OD). Data from MS patient MS1 are given as △; other data as ●. (A) Unfractionated CSF was tested directly at 25 µL per test. No significant difference was seen between samples form patients with MS and noninflammatory diseases (CNP and NIND). Of note, the sample from MS1 (△), showed increased reactivity compared with other patients with MS, suggesting private reactivity. Samples from patients with acute inflammatory diseases (NB and OID) yielded significantly higher signals compared with the cohorts with MS, CNP, and NIND. ****P ≤ 0.0001; *P ≤ 0.1; n.s., not significant. (B) Same data as shown in A, but OD values were divided by the IgG concentration, yielding specific OD values (OD/[IgG]). No significant difference was seen between the different cohorts. Comparison of the OD values to (OD/[IgG]) reveals that the differences between the cohorts seen in A is due to the higher total Ig concentrations under acute inflammatory conditions. (C) Serum of the same patients as in A was tested at 25 µL per test. No significant difference was seen between the different cohorts. (D) Specific OD values (OD/[IgG]) of serum samples from different patient cohorts. No significant differences were observed between the different cohorts. Data represent mean OD values of two independent experiments. Error bars indicate SD. Comparison of MKNK1 recognition between MS and NIND patients by ELISA revealed baseline signals from all patients.
rOCB-scFv-MS2-s5 and the commercial anti-MKNK1 antibody PA5-13951, but no other rOCB-Fab fragments tested, immunoprecipitated MKNK1 produced in human HEK293 cells (Fig. 4D), confirming the specific recognition found by array hybridization. Titration of MKNK1 by rOCB-scFv-MS2-s5 and PA5-13951 revealed dose-dependent recognition (Fig. 4E).
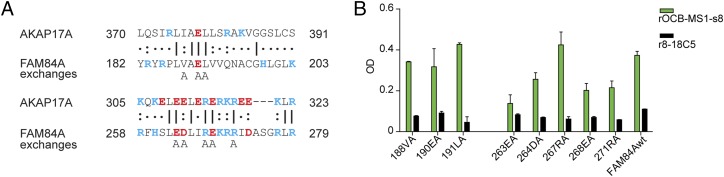
AKAP17A from HEK293 EBNA cells was immunoprecipitated by rOCB-MS1-s2 and a commercial anti-AKAP17A antibody, but not by r8-18C5 and rOCB-MS3-s1. However, although rOCB-MS1-s8 recognized FAM84A, but not AKAP17A, on the microarray, it immunoprecipitated both proteins (Fig. 4F), whereas rOCB-MS1-s2 recognized only AKAP17A but not FAM84A (Fig. 4 B and C). To explain this cross-reactivity, we compared the sequences of AKAP17A and FAM84A and found two regions of high homology (Fig. 5A). We mutated individual amino acids in the putative epitopes of FAM84A to alanine and found that mutations of amino acids 188, 190, 191, and 267 did not change recognition by rOCB-MS1-s8 in ELISA, whereas exchanges of 263, 264, 268, and 271 considerably reduced recognition (Fig. 5B). This finding indicated that the homologous regions between amino acids 263–271 in FAM84A and 310–318 in AKAP17A are shared epitopes of rOCB-MS1-s8. Because rOCB-MS1-s8 did not recognize AKAP17A on the ProtoArray, we surmise that the epitope is conformation-dependent.
Fig. 5.
Cross-reactivity of rOCB-MS1-s8 between highly homologous epitopes of FAM84A and AKAP17A. (A) Comparison of amino acid sequences of 370–391 (Upper) and 305–323 (Lower) of AKAP17A (upper lines) and 182–203 and 258–279 of FAM84A (middle lines). Identical amino acids are indicated by a dash, highly homologous amino acids by a colon, and similar amino acids by a dot. Positively and negatively charged amino acids are depicted in blue and red letters, respectively. The lowest line indicates which of the FAM84A amino acids were individually replaced by alanine. (B) Recognition of wild-type (FAM84wt) and the eight mutated FAM84A molecules by rOCB-MS1-s8 and the control antibody r8-18C5 as measured by ELISA. Recognition of FAM84A with exchanges of amino acids V188, E190, L191, and R267 to alanine was comparable to the wild-type protein, but considerably reduced by exchanges of amino acids E263, D264, E268, and R271. Data are representative of four independent experiments. Error bars indicate SD.
SI Materials and Methods
Analysis of CSF Ig Transcriptomes.
Transcriptome and proteome data of patient MS4 were published (24) (therein termed “NS-52”). IgG-H and -L chain transcriptomes from CSF B cells of all other patients were obtained as described (24), with the following exceptions. First, cDNA from patient MS3 was obtained according to Nohra et al. (41). Second, we added PCRs with the following primers to the second round of the nested PCR: (i) VκII A3/A19 with the outer forward primer 5′-ATGAGGCTCCCTGCTCAGC-3′ and the inner forward primer 5′-TATTGTGATGACTCAGTCTCC-3′ (patient MS3 only); (ii) VH3 with the inner forward primer 5′-GTGCAGCTGGTGGAGTCTG-3′ (patient MS1 only); and (iii) VκIII L2/L16 with the inner forward primer 5′-TGATGACGCAGTCTCCAGC-3′ (patient MS1 only).
CSF samples from patients MS2 and NB1 were additionally analyzed by pyrosequencing as described (12). Although the total number of sequences obtained by pyrosequencing was higher for factors of 10–20, compared with Sanger sequencing, the deduced databases were very similar—i.e., only few additional sequences were detected by pyrosequencing. Furthermore, the distribution of different chains was similar—i.e., chains that were detected frequently by Sanger sequencing were also detected frequently by pyrosequencing.
Purification and Deglycosylation of CSF IgG Molecules.
We purified IgG antibodies from CSF supernatant by Protein G Dynabeads (Life Technologies). To this end, we incubated 397 µg of IgG (patient MS1) with 600 µL of bead suspension; 95 µg of IgG (patient MS2) with 600 µL of bead suspension; 128 µg of IgG (patient MS3) with 300 µL of bead suspension; and 259 µg of IgG (patient NB1) with 520 µL of bead suspension, for 1 h on a slow shaker. Then we washed the beads three times with 0.1 M Na-acetate, 150 mM NaCl (pH 5.0), and the IgG molecules were eluted in 1% SDS in 10 mM Na-phosphate buffer, 150 mM NaCl (pH 7.4) after 2-min incubation at 37 °C. The final volumes were 75 µL (patient MS1), 60 µL (patient MS2), 50 µL (patient MS3), and 50 µL (patient NB1). To increase homogeneity on IEF gels, we deglycosylated the Ig molecules by heating the eluates to 95 °C for 1 min and incubating them for 3 h at 37 °C in the presence of 1% (wt/vol) MEGA-10 at pH 7.2 with 100 U/mL N-glycosidase F recombinant (Roche). Then, we dialyzed the samples in a D-tube Dialyzer Mini MWCO 12-14 kDa (Novagen) against 6 M urea for 2 h on a stirrer at room temperature (RT), and then for 15 min at 50 V in a flatbed gel electrophoresis chamber to remove residual SDS.
IgG Separation by 2D Gel Electrophoresis.
After dialysis, we added 50 µL of 6 M urea, 2 M thiourea, 10% (vol/vol) glycerol, and bromphenol blue to the eluates and loaded them onto rehydrated 24-cm Immobiline DryStrip pH 3–10 IEF gels (GE Healthcare). We used a 3100 OFFGEL Fractionator (Agilent) in the in-gel mode according to the manufacturer’s recommendations, but with two exceptions. First, instead of rehydrating the IEF strips together with the eluates, we placed a loading cup (8 × 2 mm, conical) onto the rehydrated strip at position pH 4.5. We chose this pH because we found that this prevents to a great extent the disassembly of H and L chains, which occurs upon loading the gel at extreme pH values like pH 3 or 10. Further, because most OCBs have isoelectric points above pH 6.0, they enter the gel more easily at pH 4.5. Second, we modified the default focusing method to a slower voltage-increase and extended the duration of electrophoresis to 120 kVh. The IEF strips were equilibrated for 20 min on a slow shaker in 6 M urea, 4% (wt/vol) SDS, 50 mM Tris, and 30% (vol/vol) glycerol. Nonreducing SDS/PAGE was performed on 9% (wt/vol) acrylamide gels by using a cooled electrophoresis chamber. We stained the gels in 0.1% Coomassie Brilliant Blue R-250.
Mass Spectrometry and Identification of Matching IgG-H and -L Chains.
We excised spots that contained the H2L2 heterodimer and in-gel digested them by trypsin (Roche) as described (24). Mass spectra were acquired with a Proteomics Analyzer 4700 (MALDI-TOF/TOF) spectrometer (Applied Biosystems) and sequenced by tandem mass spectrometry, if possible (24). Patient-specific transcriptomes obtained by cDNA analysis of CSF B cells served as databases for the identification of peptide masses using the program MASCOT (Matrix Science) (24). Matching H and L chains could only be identified in some of the spots. The criterions were that only one dominant H and one dominant L chain were unambiguously detected, with only very minor contaminations of other Ig-chains. Peaks that could be assigned to trypsin or keratin were ignored. Peptides known to derive from the constant Ig regions served as internal standard for peak intensities.
Cloning of H- and L-Chain Constructs, Expression, and Characterization of Antibodies and Antibody Fragments.
We generated full-length recombinant antibodies of the H2L2-chain pairs of OCBs MS1-s2, MS1-s8, MS1-s9, NB1-s13, MS3-s1, and MS4. As a control, we expressed the MOG-specific antibody 8-18C5. cDNA of all chains was obtained either from Sanger sequencing-based transcriptome analysis or by gene synthesis (Geneart). For all rOCBs, we used the leader sequences of VκI-O12 and VH4-04. For the constant regions, we used the human IgG1 and κ regions. The IgG-H chains, which were extended with either His6 and V5 tags or with a His6 tag only, and the IgG-L chains were cloned into the expression plasmid pTT5, and antibodies were expressed in HEK293EBNA cells in serum-free medium (28).
For some antigen-search experiments, we generated recombinant Fab-fragments. The IgG-H chains were extended by a His6 tag that allowed for dimerization by the anti-His6 antibody HIS.H8 (Abcam).
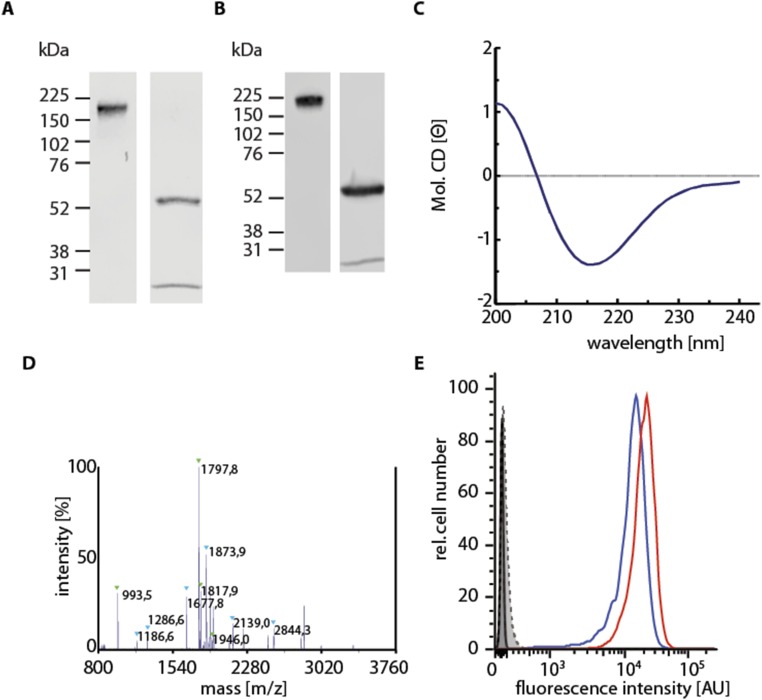
The antibodies or Fab fragments were purified by immobilized metal affinity chromatography using Ni-NTA Agarose (Qiagen), eluted with 200 mM imidazole, and dialyzed against PBS. H2L2 heterodimers were detected on nonreducing SDS/PAGE gels and Western blotting. The sequences were confirmed by mass spectrometry and the secondary structure by circular dichroism spectroscopy. The r8-18C5 antibody and Fab fragment recognized MOG expressed on the surface of TE671 cells (42) as determined by flow cytometry (Fig. S3).
Fig. S3.
Biochemical characterization of purified recombinant antibodies exemplarily shown for the MOG-reactive antibody r8-18C5. (A) Coomassie-stained SDS/PAGE of nonreduced (Left) and reduced (Right) r8-18C5. As expected, the undissociated complexes migrate at ∼180 kDa, the heavy chain at 57 kDa, and the light chain at 28 kDa. (B) Western blots of the samples shown in A. (C) Circular dichroism spectroscopy revealed relative contents of 47 ± 3% of β-sheets and <3% α-helix, which fits to the expected values. (D) MALDI-TOF/TOF mass spectrometry revealed approximately equal amounts of IgG-H and -L chains. (E) Flow cytometry confirmed that r8-18C5 is functional. r8-18C5 (red curve) and 8-18C5 isolated from hybridoma cells (blue curve) both recognized specifically their antigen MOG on MOG-transfected TE671 cells. The control antibody rOCB-MS3-s1, the isotype control antibody 349040, and the secondary antibodies A21455 and A21236 alone did not recognize MOG (gray curves). Untransfected TE671 cells were not recognized by r8-18C5 and 8-18C5 (gray curves). All other recombinant antibodies and Fab fragments were characterized comparably.
The IgG-H and -L chain pair of MS2-s5 was additionally expressed as scFv in inclusion bodies in E. coli. The scFv construct contains the IgG-H and -L chain variable regions connected via a (GGGGS)3-linker and C-terminal His6 and V5 tags. Inclusion bodies were prepared and dissolved in 8 M guanidinium chloride, 5 mM DTT, 5 mM EDTA, and 50 mM Tris/HCl (pH 7.0). Soluble scFv fragments were refolded by rapid dilution 1:100 in PBS under vigorous stirring. The refolded scFv fragments were dialyzed against PBS and concentrated to 0.1 mg/mL, and proper folding was confirmed by CD spectroscopy (10% α-helix, 36% β-sheet).
Detection of Borrelia Antigens.
For detection of Borrelia-specific antigens by rOCB-NB1-s13, the Borrelia afzelii + VlsE IgG ELISA Testkit (Virotech, lot no. 333-01) was used according to the instructions of the manufacturer. It contains a mixture of lysates of B. afzelii strain PKo, Borrelia garinii stain PBr, and B. burgdorferi strain ZS7. rOCB-NB1-s13 and control antibodies r8-18C5 and rOCB-MS3-s1 were diluted in equal amounts in CSF samples from four patients with NINDs.
As a qualitative tool to measure Borrelia-specific antibody reactivities, the anti-Borrelia-EUROLINE-RN-AT immunoblot (EUROIMMUN, lot no. D151208AH) was used, according to the instructions supplied by the manufacturer. The antibody rOCB-NB1-s13 was diluted at concentrations from 1 to 600 µg/mL in 1% BSA in PBS. A polyclonal alkaline phosphatase coupled goat–anti-human IgG secondary antibody (EUROIMMUN) was used for staining at a dilution of 1:10. The intensities of the bands were quantified by densitometry.
Attempts to Identify Target Antigens of MS-Related rOCBs by Immunohistochemistry and 2D Western Blotting.
Clinical routine assays were used to test whether our rOCB antibodies or rFab fragments might recognize suspected causative pathogens such as Epstein–Barr, herpes simplex, measles, rubella, and zoster viruses or Chlamydia by EUROIMMUN assays FI2799, FI2531, FI2610, FI2590, FI2650, and FI2191, respectively. Recognition of nuclear or neutrophil cytoplasmic antigens was investigated by using EUROIMMUN assays FB1510 and FA1201.
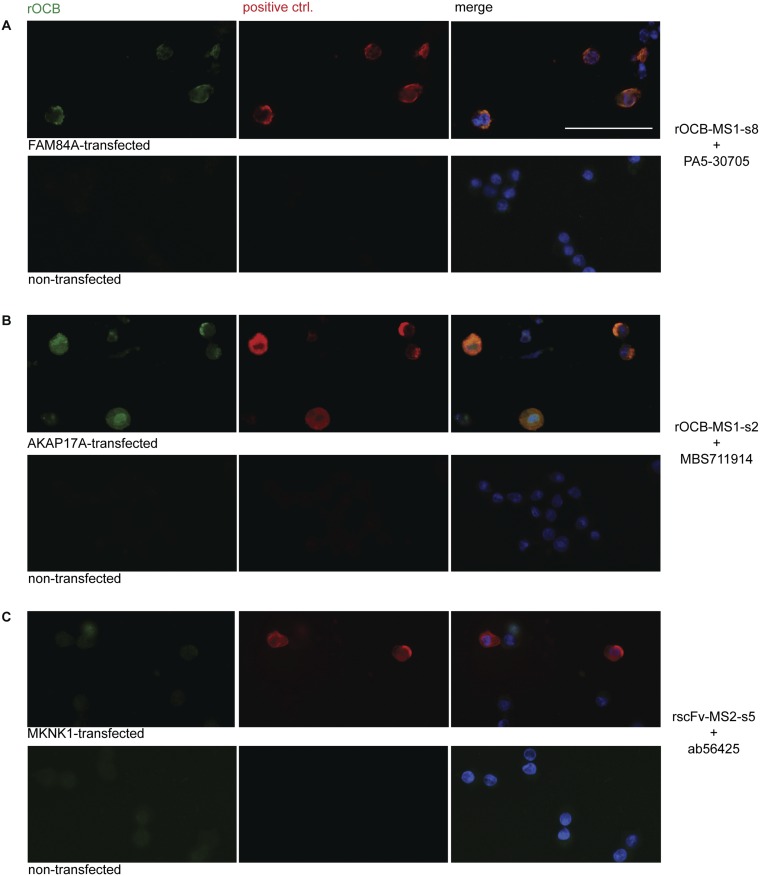
Human brain tissue was fixed by 4% (vol/vol) formaldehyde and stained as described (16). We could not detect specific staining with any of the rOCB antibodies. After identification of the antigens, intracellular staining was observed with all antibodies except rscFv-MS2-s5 in antigen-overexpressing HEK293 cells that were fixed mildly by ethanol. However, no staining was observed in wild-type HEK293 cells (Fig. S4).
Fig. S4.
Reactivity of rOCBs to antigen-transfected and nontransfected ethanol-fixed HEK293EBNA cells. We show triple-staining for FAM84A (A), AKAP17A (B), or MKNK1 (C) by rOCB antibodies (green; Left) and commercial antibodies (red; Center). Right shows the merged images. Nuclei are stained by DAPI. We compare HEK293EBNA cells that were transfected with the respective antigens (Upper) to untransfected HEK293EBNA cells (Lower). We used the following antibodies: rOCB-MS1-s2 and PA5-30705 for FAM84A-staining (A), rOCB-MS1-s8 and MBS711914 for AKAP17A staining (B), and scFv-MS2-s5 and Ab56425 for MKNK1 staining (C). All antibodies, except scFv-MS2-s5, stained antigen-transfected cells, but not untransfected cells, indicating that the endogenous expression levels are too low. (Scale bar: 50 µm.)
For 2D gel electrophoresis, human brain samples were delipidated by chloroform–methanol extraction, subjected to IEF and SDS/PAGE under reducing conditions, blotted onto nitrocellulose membranes, and tested by rOCB antibodies or rFab fragments.
Identification of Antigens of OCB Antibodies from MS Patients by Protein Microarray Hybridization.
ProtoArrays (Version 5.0; Human Protein Microarrays, Invitrogen, PAH0525101, lots HA20259 and HA20302) were used to identify candidate antigens of the OCB antibodies. The arrays were treated according to the manufacturer’s instructions, and all procedures were carried out at 4 °C. rOCB antibodies were added at a concentration of 10 µg/mL in wash buffer (1× synthetic block in PBS, 0.05% Tween-20) and incubated for 90 min. The secondary anti-V5 antibody conjugated with Alexa 647 (45-1098, Life Technologies) was added at 1 µg/mL concentration in wash buffer and incubated for 90 min. The array was dried and stored in the dark at 4 °C until analyzed with the Microarray Scanner GenePix 4000B (Axon Instruments) at λ = 635 nm. Each of the ∼9,400 proteins on the array is present in duplicate. Analysis was performed by using the ProtoArray Prospector program (Version 5.2.1; Life Technologies). Positive signals were defined as duplicate signals five times higher than the mean signal of the array and three times higher than the signal produced by the secondary antibody and an isotype control antibody (e.g., r8-18C5). Positive signals were sorted by signal intensity. All signals were visually confirmed.
Recombinant Target Proteins and Site-Directed Mutagenesis.
MKNK1 (UniProt Q9BUB5-1) is an intracellular protein that is expressed in all tissues. The mRNA expression rate is low with listed reads per kilobase per million mapped reads (RPKM) between 4.5 and 14 (www.gtexportal.org/home/). It is induced in response to environmental stress and cytokines. The HEK-derived protein carrying a Myk/DDK-tag was obtained commercially (amsbio).
AKAP17A (UniProt Q02040-3, also termed SF17A) is a splicing factor that regulates alternative splice site selection. It acts as a scaffolding protein, binding tightly to many different proteins. This property is probably the reason why it could not be purified and coated to ELISA plates. It is expressed in all tissues at low levels of 7–18 RPKM. AKAP17A carrying a His6 tag was expressed intracellularly in HEK293 EBNA cells. The yield was 2 mg/g HEK cells wet weight. Cells were stored at −20 °C until lysis.
FAM84A (UniProt Q96KN4-1) is an intracellular protein of unknown function that is expressed in most tissues at very low levels of 0.2–2 RPKM. We used a commercial preparation (Origene) from HEK293 cells for immunoprecipitation. For ELISA, FAM84A carrying a V5 and a His6 tag was produced soluble in the cytosol of E. coli. Bacteria were lysed by sonication, and FAM84A was purified from the supernatant by immobilized metal chelate affinity chromatography. Native folding was confirmed by circular dichroism spectroscopy (20.5% α-helix, 25.4% β-sheet). The yield of folded FAM84A was 5.5 mg/g bacterial wet weight.
To screen for homologous regions, FAM84A and AKAP17A amino acid sequences were locally aligned—i.e., stretches of 20 amino acids of the FAM84A-sequence were aligned to the full AKAP17A-sequence. This method identified regions AA182-203 and AA258-279 as homologous. The wild-type FAM84A-sequence was mutated at amino acids Val-188, Glu-190, Leu-191, Glu-263, Asp-264, Arg-267, Glu-268, and Arg-271 individually to Ala. We used PCR primers with appropriate nucleotide exchanges that spanned unique restriction sites for fragment exchanges. For exchanges between amino acids 182 and 203, we used a forward primer covering the unique NdeI restriction site and a reverse primer covering the unique MscI-site. For exchanges between amino acids 258 and 279, we used a forward primer covering the MscI restriction site and a reverse primer covering a newly introduced silent Bsu36I restriction site at nucleotide position 820 (corresponding to AA273/274). All mutated proteins were produced and purified as the wild type.
Immunoprecipitation.
AKAP17A-transfected HEK293 EBNA cells were resuspended in 10 volumes of 450 mM NaCl, 160 mM NaH2PO4 (pH 7.5) and sonified for 5 min on ice. The lysate was centrifuged for 10 min at 3,400 × g and 10 min at 12,000 × g at 4 °C. To preclear the supernatant from compounds that might interact with the surface of protein G Dynabeads, 6 mg of protein G Dynabeads were first incubated with “synthetic buffer” (Life Technologies) in PBS for 1 h and then with 5 mL of supernatant. In parallel, 750 µg of protein G Dynabeads were incubated with 6 µg of rOCB-MS1-s2, rOCB-MS1-s8, rOCB-MS3-s1, r8-18C5, or the commercial polyclonal anti-AKAP17A antibody MBS711914 (MyBioSource). Coupling of these antibodies to protein G Dynabeads was carried out according to the manufacturer’s recommendations by cross-linking with BS3 reagent (Pierce). Beads were washed three times in PBS and incubated with synthetic buffer in PBS for 1 h. Then, 1 mL of the lysate supernatant was incubated with 750 µg of beads for 30 min. Beads were washed five times with PBS/0.05% Tween-20, and antigens were eluted with 30 µL of reducing SDS/PAGE loading buffer (4 mM Tris⋅HCl, pH 7.5; 4.5 mg/mL glycerol; 1.5 mg/mL SDS; 15 mM β-mercaptoethanol; and 0.01 mg/mL bromophenol blue) at 95 °C for 10 min and analyzed by Western blotting.
A total of 2 µg of the FAM84A purified from HEK293 was incubated with 25 µg of either rOCB-MS1-s8, rOCB-MS3-s1, rOCB-MS1-s2, r8-18C5, or the positive control mouse anti-FAM84A antibody ab58330 (Abcam) for 30 min at RT. Then, 1 mg of protein G Dynabeads, blocked with synthetic buffer as described above, was added and incubated with the antigen–antibody complexes for 10 min. Antigens were eluted with 60 µL of reducing SDS/PAGE loading buffer at 95 °C for 10 min and analyzed by Western blotting.
rscFv-MS2-s5, rFab-MS3-s1, rFab8-18C5, and the positive control mouse anti-MKNK1 antibody ab56425 (Abcam) were coupled to tosyl-activated magnetic beads (Thermo Fisher Scientific) according to the manufacturer’s recommendations. Beads were blocked in 1% BSA in PBS-T for 1 h. A total of 0.7 µg of MKNK1 (amsbio) from HEK293 cells was added and incubated for 30 min at RT. Antigens were eluted with 60 µL of reducing SDS/PAGE loading buffer at 95 °C for 5 min and analyzed by Western blotting.
ELISA.
For analyzing reactivity of rOCB and of CSF samples to FAM84A, Costar Half Area 96 Well Assay Plates (Corning 3690) were coated with the rabbit anti-V5 antibody AB3792 (Millipore) at a concentration of 10 µg/mL by incubation at 4 °C overnight. All incubations thereafter were at 37 °C. Plates were blocked using 2% BSA in PBS/0.05% Tween-20 for 2 h. E. coli-derived FAM84A that carried a V5 tag was added at a concentration of 16 µg/mL in 0.5% BSA in the above buffer containing 70 µM DTT. For titration experiments, rOCB-MS1-s8 and the control antibodies rOCB-MS3-s1 and r8-18C5 were used in concentrations ranging from 5 µg/mL to 2 mg/mL The positive control antibody ab58330 was used in concentrations of 2.5 to 330 ng/mL 0.5% BSA in PBS-T. Samples were incubated for 30 min. To test reactivity of CSF and matching serum samples of five different patient cohorts (Clinical Samples), 25 µL of CSF was used directly, and serum samples were diluted 1/400 in PBS. In both types of experiments, the plates were then washed five times with PBS/0.05% Tween-20. For detection and development, the secondary rabbit anti-human HRP-coupled antibody ab97160 (Abcam) was diluted 1:100,000, in case of the ab58330 the rabbit–anti-mouse-HRP antibody R21455 (Life Technologies) was diluted 1:10,000. The 3,3′,5,5′-tetramethylbenzidine reaction was detected at 450 nm by using a Wallac 1420 Victor Multilabel counter (PerkinElmer).
To validate recognition of MKNK1, commercial HEK293 cell-derived MKNK1 protein (TP301149; amsbio) was coated to the assay plate at a concentration of 16 µg/mL in PBS at 4 °C overnight. The plate was blocked with 1% BSA in PBS for 2 h at 37 °C. For titration experiments, rscFv-MS2-s5, the control Fab fragments rFab-MS3-s1 and rFab8-18C5, and the positive control antibody PA5-13951 (ThermoScientific) were used in concentrations ranging from 150 to 0.5 µg/mL in 0.5% BSA in PBS/0.05% Tween-20 and incubated for 40 min at 37 °C. The HRP-coupled secondary anti-human IgG H+L chain antibody and anti-rabbit IgG antibody were diluted 1:100,000 and 1:5,000, respectively, and incubated for 20 min at 37 °C. Between incubation steps, the plate was washed five times using PBS/0.05% Tween-20, and signals were detected as described above.
Western Blotting and Circular Dichroism Spectrometry.
Immunoprecipitated V5-tagged FAM84A was detected by Western blotting using the mouse anti–V5-HRP (1:5,000; R96125) antibody. AKAP17A was detected with rabbit anti-AKAP17A (1:2,000; MBS711914) and the secondary HRP-coupled donkey anti-rabbit antibody (1:10,000; ab16284). Bands were developed with enhanced chemiluminescence and detected on X-ray films or the LI-COR Odyssey System. For circular dichroism spectroscopy, protein concentrations were adjusted to 0.1–0.2 mg/mL in PBS. Spectra were acquired by using the J-715 (Jasco) and analyzed with the Spectra Manager CDPro Analysis program using the Contin SMP56 reference data set.
Discussion
OCBs have been regarded as a perennial conundrum in MS since their first description in 1959 (7, 33, 34). They are also observed in infectious CNS disorders, where it is known that the OCBs recognize the relevant infectious agent (25). In MS, however, no such infectious agent could be identified (35), but, nevertheless, >95% of MS patients have OCBs in their CSF. Assuming that the OCBs are directed against self-antigen(s), there are two main possibilities. First, the OCB might recognize a pathogenetically relevant, perhaps even unifying autoantigen—e.g., an “encephalitogenic” autoantigen identified in animal models (36). Second, the OCB response might occur as a secondary reaction to tissue injury. To address this question, we set out to isolate and produce antibodies from distinct OCBs.
In the past, mainly technical reasons precluded identification of OCB antigens from MS patients. Firstly, the small amount of CSF obtained during a diagnostic lumbar puncture precludes direct biochemical analysis of distinct OCBs. Secondly, the CSF contains not only OCB Ig, but also many polyclonal antibodies. Therefore, when whole CSF is used for analysis, it is not known whether a signal arises from this polyclonal background or from an OCB. Thirdly, when antibodies are cloned from single B cells and expressed recombinantly (16, 17), it will again not be known whether a particular B cell has produced an OCB or contributed to the polyclonal background.
We have overcome these technical challenges by combining refined biochemical analysis, proteomics, and transcriptomics. As a crucial initial step, we copurified disulfide-linked IgG-H and -L chains from distinct OCBs for concurrent mass spectrometry, revealing characteristic patient- and OCB-specific peptides (24). From these “fingerprints,” the full-length sequences of matching chains can be deduced by aligning the peptides to Ig transcriptomes. This method allowed us to express distinct OCB antibodies recombinantly and to search for their target antigens.
We could produce six recombinant antibodies from distinct OCBs of four MS patients and characterize three different target autoantigens, whereas the other three OCB antibodies did not yield reproducible signals. The three antigens are ubiquitous intracellular proteins that are not specific to brain tissue. The observed diversity and heterogeneity of the OCB response and the intracellular localization of their target autoantigens indicate that, in MS, part of the OCB response represents a secondary reaction to cellular debris, as postulated by Grabar decades ago as a general concept (37). Such reactivity might not be specific to MS, but could be a more general feature of (neurological) autoimmune and inflammatory diseases. As shown by immunoprecipitation and site-directed mutagenesis, one OCB recognized an epitope shared by two different intracellular autoantigens. Such cross-reactivity would seem consistent with the “debris hypothesis.” In future studies, it will be interesting to see whether “public” antigens can be detected in informative groups of patients. Furthermore, it will be important to assess the frequencies of antigen-specific B cells in CSF and blood and, ideally, to investigate the CSF B-cell response over time.
It should be noted that none of our OCBs showed the classic “polyreactivity” pattern described by Wardemann et al. (30). Although we did not observe any consistent antilipid or antiviral reactivity of our OCB antibodies, we cannot rule out the possibility that some OCBs in MS recognize nonself or nonprotein antigens (18, 21, 23, 38, 39) based on the small sample of OCBs that we could investigate in such detailed manner. For the same reason, we cannot exclude the possibility that some OCB antibodies recognize antigens exposed at the surface of brain-resident cells and, therefore, might contribute to the pathogenesis. Thus, OCB antigens may be quite heterogeneous, not only in their structure and function, but also regarding their possible pathogenic role. Moreover, cross-reactivity between autoantigens and microbial antigens—i.e., molecular mimicry—cannot be excluded (reviewed in ref. 15).
Beyond MS, our approach may allow the identification of a priori unknown target antigens from diagnostic CSF samples, other body fluids, or tissues, where expanded antibodies are detectable along with B cells. For future therapeutic purposes, it will be especially helpful that such antibodies are directly “produced” by human patients who have already mounted an immune response to fight pathogens. This aspect may be important in infections where our strategy allows identification and, eventually, large-scale production of human antibodies against infectious agents such as bacteria, viruses, or parasites. One such example is the anti-Borrelia antibody rOCB-NB1-s13 described here, which indeed reacts to a surface antigen of the pathogenic intruder. Such antibodies, which were selected in vivo for optimal reactivity against the most relevant antigenic epitopes, could be produced as prophylactic or therapeutic agents for “passive immunization.”
Materials and Methods
Clinical Samples.
CSF from patient NB1 with NB and patients MS1, MS2, MS3, and MS4 with clinically defined relapsing–remitting MS (40) were used for analysis of OCBs. Clinical data are listed in Table S1. For testing reactivity to FAM84A by ELISA, we used CSF samples from patients with MS (n = 21), NB (n = 13), other infections of the CNS (n = 7), cranial nerve palsy (n = 9), and noninflammatory neurological diseases (NINDs; n = 17). Informed consent was obtained from all patients. The study was approved by the Institutional Review Boards of the Ludwig-Maximilians-University, Karolinska Hospital, and the University of Ulm.
Table S1.
Clinical data of MS and NB patients
| MS1 | MS2 | MS3 | MS4 | NB1 | |
| Age, y | 33 | 27 | 34 | 31 | 25 |
| Sex | M | F | M | M | M |
| Interval,* mo | 18 | 17 | 108 | 2 | 2 |
| Diagnosis | RRMS | RRMS | RRMS | RRMS | NB |
| Therapy | None | See below† | None | None | Antibiotics (ceftriaxone) |
| CSF cell nos., cells per µL | 40.7 | 3.0 | 3.0 | 7.0 | 11.0 |
| CSF albumin, mg/L | 378 | 121 | 270 | 230 | 788 |
| IgGindex | 1.74 | 0.50 | 0.77 | 1.16 | 0.60 |
Age, sex, time interval between symptom onset and lumbar puncture (LP), diagnosis, and therapy before LP are given. F, female; M, male; RRMS, relapsing–remitting MS.
Time interval between symptom onset and LP (mo).
Plasma exchange for steroid-resistant exacerbation, maintenance therapy with dimethyl fumarate.
Analysis of CSF Ig Transcriptomes.
We generated IgG-H and -L chain transcriptomes from CSF B cells as described (24) with few exceptions detailed in SI Materials and Methods.
Purification, Separation by 2D Gel Electrophoresis, and Mass Spectrometry of CSF IgG Molecules.
IgG antibodies were purified from CSF supernatant by Protein G Dynabeads, deglycosylated by N-glycosidase F, and subjected to IEF gel electrophoresis using 24-cm polyacrylamide gels and SDS/PAGE. All steps were performed under nonreducing conditions. Spots that contained the H2L2 heterodimer were excised, in gel digested by trypsin, and subjected to MALDI-TOF/TOF mass spectrometry. Peptides were aligned to patient-specific transcriptome databases by using MASCOT (24). Matching H and L chains could only be identified in some of the spots, where only one dominant H and one dominant L chain were unambiguously detected. This is not always the case, because a distinctly visible OCB need not be monoclonal, but may contain several comigrating other antibody chains. Details of the procedures are given in SI Materials and Methods.
Cloning, Expression, and Characterization of rOCB, rOCB Fragments, and Target Antigens.
We cloned full-length recombinant antibodies of the H2L2-chain pairs of OCBs MS1-s2, MS1-s8, MS1-s9, NB1-s13, MS3-s1, and MS4. As a control, we expressed the MOG-specific antibody 8-18C5. For the constant regions, we used the human IgG1- and κ-regions. The IgG-H chains were extended with either His6 and V5 tags or with a His6 tag only. In parallel, we generated recombinant Fab fragments, which were extended by a His6 tag. The IgG-H and -L chain pair of MS2-s5 was expressed as scFv in inclusion bodies in E. coli and refolded in vitro. All rOCB or rFabs were expressed in HEK293EBNA cells, purified by immobilized metal affinity chromatography, and characterized by SDS/PAGE, Western blotting, mass spectrometry, circular dichroism spectroscopy, and flow cytometry (r8-18C5 only) (Fig. S3). MKNK1, FAM84A, and AKAP17A were produced in HEK293 cells. In addition, soluble FAM84A was produced in E. coli. Individual amino acids in regions homologous to AKAP17A were exchanged to alanine by site-directed mutagenesis. Recombinant target proteins were characterized as rOCBs. Details are given in SI Materials and Methods.
Detection and Validation of OCB Antigens.
For detection of Borrelia-specific antigens by rOCB-NB1-s13, the Borrelia afzelii + VlsE IgG ELISA Testkit (Virotech) and anti-Borrelia-EUROLINE-RN-AT immunoblot (EUROIMMUN) were used. To identify candidate antigens of the OCB antibodies from MS patients, ProtoArrays (Version 5.0; Human Protein Microarrays, Invitrogen) were used. Each array contained ∼9,400 human proteins produced in insect cells. Data were analyzed by using the ProtoArray Prospector program (Version 5.2.1; Life Technologies). Positive signals were defined as duplicate signals five times higher than the mean signal of the array and three times higher than the signal produced by the secondary antibody and an isotype control antibody (e.g., r8-18C5). Positive signals were sorted by signal intensity. All signals were visually confirmed. All candidate antigens were validated by independent experiments using recombinant proteins produced in either human HEK293 cells (FAM84A, AKAP17A, and MKNK1) or E. coli (FAM84A). FAM84A, AKAP17A, and MKNK1 recognition by rOCB-MS1-s8 and -s2 and rscFv-MS2-s5, respectively, were validated by immunoprecipitation. MKNK1 and FAM84A recognition by rscFv-MS2-s5 and rOCB-MS1-s8, respectively, were validated by ELISA. Antigens were detected by rOCB antibodies in transfected HEK293 cells (Fig. S4). All experimental details and the control antibodies are described in SI Materials and Methods.
Acknowledgments
We thank Eduardo Beltrán, Josef Kellermann, Maria Kalemanov, Naoto Kawakami, Edgar Meinl, Dieter Jenne, and Volker Fingerle for valuable discussion and Joachim Malotka, Monica Zobawa, Ingrid Eiglmeier, and Elisabeth Weyher for expert technical assistance. This work was supported by Deutsche Forschungsgemeinschaft Grants SFB-571-A1, CRC-128-A5, and CRC-128-B8; the University of Ulm (Bausteinprojekt); and the Friedrich-Bauer-Stiftung. T.O. and M.K. have received grants from the Knut and Alice Wallenberg Foundation, the Swedish Research Council, and the AFA Försäkring foundations, Sweden.
Footnotes
Conflict of interest statement: B.O. is now an employee at Biogen, but was affiliated with the Ludwig-Maximilians-University at the time the study was performed; i.e., Biogen was not a sponsor of this study. J.B. is now an employee at Bayer, but was affiliated with the Ludwig-Maximilians-University at the time the study was performed; i.e., Bayer was not a sponsor of this study. M.S. has received honoraria for lecturing and/or travel expenses for attending meetings from Bayer and Biogen Idec. T.O. has received lecture and/or advisory honoraria, as well as unrestricted MS research grants from Biogen, Novartis, Genzyme, Merck, and TEVA, none of which has had any relation to this study. W.K. has received advisory honoraria, speaker's fees, or unrestricted travel grants from Biogen, Novartis, and Merck-Serono, none of which has had any relation to this study.
This article is a PNAS Direct Submission.
Data deposition: The IgG sequences reported in this paper have been deposited in the BioProject database, www.ncbi.nlm.nih.gov/bioproject (BioProject ID PRJNA294639). The ProtoArray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE72789).
See Commentary on page 7696.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522730113/-/DCSupplemental.
References
- 1.Antel J, Bar-Or A. Roles of immunoglobulins and B cells in multiple sclerosis: From pathogenesis to treatment. J Neuroimmunol. 2006;180(1-2):3–8. doi: 10.1016/j.jneuroim.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122(4):1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman L. Immunology of relapse and remission in multiple sclerosis. Annu Rev Immunol. 2014;32:257–281. doi: 10.1146/annurev-immunol-032713-120227. [DOI] [PubMed] [Google Scholar]
- 4.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 5.Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: Migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol. 2006;59(6):880–892. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- 6.Awad A, et al. Analyses of cerebrospinal fluid in the diagnosis and monitoring of multiple sclerosis. J Neuroimmunol. 2010;219(1-2):1–7. doi: 10.1016/j.jneuroim.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Stangel M, et al. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat Rev Neurol. 2013;9(5):267–276. doi: 10.1038/nrneurol.2013.41. [DOI] [PubMed] [Google Scholar]
- 8.Qin Y, et al. Clonal expansion and somatic hypermutation of V(H) genes of B cells from cerebrospinal fluid in multiple sclerosis. J Clin Invest. 1998;102(5):1045–1050. doi: 10.1172/JCI3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baranzini SE, et al. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J Immunol. 1999;163(9):5133–5144. [PubMed] [Google Scholar]
- 10.Owens GP, et al. Restricted use of VH4 germline segments in an acute multiple sclerosis brain. Ann Neurol. 1998;43(2):236–243. doi: 10.1002/ana.410430214. [DOI] [PubMed] [Google Scholar]
- 11.von Büdingen HC, et al. B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest. 2012;122(12):4533–4543. doi: 10.1172/JCI63842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beltrán E, et al. Intrathecal somatic hypermutation of IgM in multiple sclerosis and neuroinflammation. Brain. 2014;137(Pt 10):2703–2714. doi: 10.1093/brain/awu205. [DOI] [PubMed] [Google Scholar]
- 13.Stern JN, et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med. 2014;6(248):248ra107. doi: 10.1126/scitranslmed.3008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palanichamy A, et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci Transl Med. 2014;6(248):248ra106. doi: 10.1126/scitranslmed.3008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hohlfeld R, Dornmair K, Meinl E, Wekerle H. The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol. 2016;15(3):317–331. doi: 10.1016/S1474-4422(15)00313-0. [DOI] [PubMed] [Google Scholar]
- 16.von Büdingen HC, Harrer MD, Kuenzle S, Meier M, Goebels N. Clonally expanded plasma cells in the cerebrospinal fluid of MS patients produce myelin-specific antibodies. Eur J Immunol. 2008;38(7):2014–2023. doi: 10.1002/eji.200737784. [DOI] [PubMed] [Google Scholar]
- 17.Owens GP, et al. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Ann Neurol. 2009;65(6):639–649. doi: 10.1002/ana.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanter JL, et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12(1):138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- 19.Yu X, et al. Intrathecally synthesized IgG in multiple sclerosis cerebrospinal fluid recognizes identical epitopes over time. J Neuroimmunol. 2011;240-241:129–136. doi: 10.1016/j.jneuroim.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cepok S, et al. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. J Clin Invest. 2005;115(5):1352–1360. doi: 10.1172/JCI23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan KM, et al. Lipid arrays identify myelin-derived lipids and lipid complexes as prominent targets for oligoclonal band antibodies in multiple sclerosis. J Neuroimmunol. 2011;238(1-2):87–95. doi: 10.1016/j.jneuroim.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Querol L, et al. Protein array-based profiling of CSF identifies RBPJ as an autoantigen in multiple sclerosis. Neurology. 2013;81(11):956–963. doi: 10.1212/WNL.0b013e3182a43b48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villar LM, et al. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest. 2005;115(1):187–194. doi: 10.1172/JCI22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obermeier B, et al. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med. 2008;14(6):688–693. doi: 10.1038/nm1714. [DOI] [PubMed] [Google Scholar]
- 25.Martin R, Martens U, Sticht-Groh V, Dörries R, Krüger H. Persistent intrathecal secretion of oligoclonal, Borrelia burgdorferi-specific IgG in chronic meningoradiculomyelitis. J Neurol. 1988;235(4):229–233. doi: 10.1007/BF00314352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obermeier B, et al. Related B cell clones that populate the CSF and CNS of patients with multiple sclerosis produce CSF immunoglobulin. J Neuroimmunol. 2011;233(1-2):245–248. doi: 10.1016/j.jneuroim.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130(3):443–454. [PMC free article] [PubMed] [Google Scholar]
- 28.Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30(2):E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbour AG, et al. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun. 2008;76(8):3374–3389. doi: 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 31.Robinson WH, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8(3):295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 32.Vermeulen N, et al. Identification of a novel autoantigen in inflammatory bowel disease by protein microarray. Inflamm Bowel Dis. 2011;17(6):1291–1300. doi: 10.1002/ibd.21508. [DOI] [PubMed] [Google Scholar]
- 33.Karcher D, Van Sande M, Lowenthal A. Micro-electrophoresis in agar gel of proteins of the cerebrospinal fluid and central nervous system. J Neurochem. 1959;4(2):135–140. doi: 10.1111/j.1471-4159.1959.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 34.Holmøy T. The discovery of oligoclonal bands: A 50-year anniversary. Eur Neurol. 2009;62(5):311–315. doi: 10.1159/000235944. [DOI] [PubMed] [Google Scholar]
- 35.Owens GP, Gilden D, Burgoon MP, Yu X, Bennett JL. Viruses and multiple sclerosis. Neuroscientist. 2011;17(6):659–676. doi: 10.1177/1073858411386615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60(1):12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 37.Grabar P. “Self” and “not-self” in immunology. Lancet. 1974;1(7870):1320–1322. doi: 10.1016/s0140-6736(74)90685-0. [DOI] [PubMed] [Google Scholar]
- 38.Ho PP, et al. Identification of naturally occurring fatty acids of the myelin sheath that resolve neuroinflammation. Sci Transl Med. 2012;4(137):137ra73. doi: 10.1126/scitranslmed.3003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson RA, et al. Anti-DNA antibodies are a major component of the intrathecal B cell response in multiple sclerosis. Proc Natl Acad Sci USA. 2001;98(4):1793–1798. doi: 10.1073/pnas.031567598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58(6):840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 41.Nohra R, et al. RGMA and IL21R show association with experimental inflammation and multiple sclerosis. Genes Immun. 2010;11(4):279–293. doi: 10.1038/gene.2009.111. [DOI] [PubMed] [Google Scholar]
- 42.Pröbstel AK, et al. Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology. 2011;77(6):580–588. doi: 10.1212/WNL.0b013e318228c0b1. [DOI] [PubMed] [Google Scholar]