Abstract
Introduction
Bacterial sepsis is a life threatening crisis with high mortality and morbidity in neonates. Due to non-specific clinical presentation, diagnosis of sepsis is still a challenge. It can be diagnosed by blood culture but it is time consuming. So, a reliable marker is needed for the diagnosis of neonatal sepsis so that early treatment can be initiated. Various cytokines, chemokines, acute phase reactants, cell surface markers and interferons have been evaluated to find out the effective marker for early diagnosis of neonatal sepsis. In this study, levels of IL-6, CRP and hs-CRP have been analysed which would favour the diagnosis of neonatal sepsis.
Aim
This study aimed to detect the levels of IL-6, CRP and hs-CRP in clinically suspected cases of neonatal sepsis and to evaluate and analyze the above parameters as the early markers of neonatal sepsis in comparison with blood culture.
Materials and Methods
Eighty neonates were included in this study of which 40 were clinically suspected cases of neonatal sepsis who met the inclusion criteria and the other 40 were normal healthy neonates that were taken as controls. After obtaining written informed consent from either parent of all neonates, venous blood samples were collected. Blood culture was performed by conventional method. Estimation of serum IL-6 was done by ELISA method and serum CRP and hs-CRP were done by immunofluorescence assay.
Results
The CRP level >13.49 mg/l showed sensitivity and specificity of 80% and 65.70% respectively. The IL-6 >51.29 pg/ml showed sensitivity of 100% and specificity of 62.86% and hs-CRP showed sensitivity of 90% and specificity of 32.86%. Combination of IL-6 and CRP showed sensitivity and specificity of 100% and 75.71% respectively.
Conclusion
Our study suggests that IL-6 is a highly sensitive marker and CRP is a more specific marker for the diagnosis of neonatal sepsis. hs-CRP is a less reliable marker. So, the combination of IL-6 and CRP are the better predictors of neonatal sepsis.
Keywords: Acute phase reactants, Chemokines, Septicaemia
Introduction
Neonatal sepsis is a clinical syndrome characterized by systemic signs of infection accompanied by bacteraemia in the first month of life [1]. Neonatal sepsis is one of the leading causes of mortality and morbidity worldwide. Early diagnosis of neonatal sepsis is still a challenge because of its non-specific clinical presentation and difficulty in distinguishing from non-infectious conditions like respiratory distress syndrome or maladaptation [1,2]. Neonatal sepsis can be diagnosed by blood culture which is gold standard but it is time consuming and has limited sensitivity [3]. No particular test is available which has diagnostic accuracy [1]. Early detection of neonatal sepsis reduces the inadvertent use of antibiotics, the cost of treatment and also prevents the emergence of drug resistant strains.
An ideal diagnostic biomarker should display excellent sensitivity and negative predictive value as well as excellent specificity and positive predictive value [2]. Now-a-days acute phase proteins, pro-inflammatory cytokines, adhesion molecules, cell surface markers and chemokines are being used to diagnose neonatal sepsis. The commonly used markers are tumour necrosis factor- alpha (TNF-alpha), Interleukin-6 (IL-6), Interleukin-8 (IL-8), C-Reactive protein (CRP), highly sensitive C-reactive protein (hs-CRP) and pro-calcitonin (PCT). The above mentioned markers have been studied extensively for the early diagnosis of neonatal sepsis [4]. These markers complement the clinical and risk factor evaluation in the diagnosis of neonatal sepsis. None of the laboratory sepsis marker has 100% sensitivity and 100% specificity. The reliability of the marker is mainly based on the clinical status of the neonate. If the neonate is critically ill and negative for the sepsis markers or if the neonate is healthy and positive for sepsis markers it does not provide much information on the infectious status of the neonate. Sepsis markers are primarily useful in neonates with clinically unclear infectious status [5]. A rapid diagnostic test is necessary, to differentiate infected from uninfected neonates because, delay in start of antibiotics could lead to early mortality within hours of development of symptoms [6].
C - reactive protein (CRP) is an acute phase reactant, produced in the liver and a frequently used marker. It has a half –life of 24 to 48 hours. But as it takes 10 to 12 hours to respond to an infection, it is unreliable [2]. Procalcitonin concentrations increase within 2 to 4 hours, after an exposure, peaks at 6 to 8 hours and remains elevated for the next 24 hours. However, its reference level changes during the first 48 hours of birth [2]. Interleukin 6 (IL-6), a chemokine produced by the T and B lymphocytes is more sensitive than CRP, but it cannot be used as a sole marker of sepsis, as it has a short half life. IL-8 is a frequently studied pro-inflammatory cytokine used as a marker of neonatal sepsis, with a sensitivity of 90% and specificity between 75%–100% [2]. Highly sensitive CRP (hs-CRP) is more sensitive than the conventional CRP, hs-CRP assays measures the CRP levels lower than that measured by the conventional CRP assays. When measured with a high sensitivity analytic method, CRP can be used as a diagnostic marker of neonatal infection. This is because newborns cannot produce sufficient amounts of acute-phase proteins and so they respond to infection with a smaller increase in CRP compared to adults [7]. It has also been demonstrated that hs CRP measurement below 1 mg/l provides increased sensitivity for neonatal infection [8].
Many studies have evaluated diagnostic markers for neonatal sepsis such as acute phase reactants, cell surface molecules and chemokines, but none of them can consistently diagnose sepsis [1]. Previous studies done by Kocabas et al., Prashanth et al., Abdollahi et al., have employed different inclusion and exclusion criteria, different sampling time and different methods for quantification of different markers [4,9,10]. In this study, levels of Interleukin-6 (IL-6), C- reactive protein (CRP) and highly sensitive C- reactive protein (hs-CRP) have been evaluated in neonates less than 28-day-old by cost-effective methods as potential early neonatal sepsis markers.
Aim
This study aimed to detect the levels of IL-6, CRP and hs-CRP in clinically suspected cases of neonatal sepsis to analyse the above parameters and to evaluate them as the early markers of neonatal sepsis in comparison to blood culture.
Materials and Methods
A prospective study was conducted at Chettinad Hospital and Research Institute, Kelambakkam, Chennai from April 2014 to March 2015. After obtaining written informed consent from either parent, venous blood samples were collected from neonates.
The approval of the Institutional Ethical committee was obtained before the commencement of the study.
A total of 80 neonates were included of which 40 were clinically suspected cases of sepsis admitted in NICU who met the inclusion criteria and were considered as cases and the other 40 were normal healthy neonates that were taken as controls. Both cases and controls were within 28 days of age.
Preterm and term neonates (< 28 days of age) of both sexes showing signs of both early and late onset sepsis were included in the study. Clinical signs and symptoms of sepsis include at least three of the following: Abdominal distension, temperature instability, dyspnoea, tachypnoea (>70/min), feeding intolerance, hepatosplenomegaly, lethargy or irritability, tachycardia (HR>190bpmin), bradycardia (HR<90bpmin). Neonates born with congenital anomalies or those neonates who have received antibiotics or undergone any surgical procedure were excluded from the study.
From each neonate, 3ml of venous blood was collected under strict aseptic precautions. A 1ml of the collected blood was inoculated directly on the brain heart infusion broth for blood culture. The remaining 2ml of blood was transferred to a plain vacutainer and allowed to clot for 30 minutes and then centrifuged at 1000 x g for 15 minutes. The serum was separated and stored at -70°C. Quantification of IL-6 was performed by the sandwich Enzyme Linked Immuno Sorbent Assay (ELISA) using the Booster Immunoleader kit as per the manufacturer’s instructions; Serum CRP and hs-CRP levels were estimated by immunofluorescence assay by using QDx instacheckTM hsCRP analyser manufactured by Piramal Healthcare. QDx Instacheck Reader is a fluorescence scanning instrument used for Immunoassay Test based on antigen-antibody reaction and fluorescence technology.
Blood Culture
A 1ml of blood was collected in 10 ml Brain Heart Infusion (BHI) broth and it was incubated for the duration of 7 days at 370 Celsius and observed for signs of growth. Subcultures were made on blood agar, chocolate agar and MacConkey agar at 24 hours, 72 hours and at the end of 6th day. Organisms isolated by subculture were identified using Gram stain and the appropriate biochemical tests like catalase test, coagulase test, oxidase test, IMViC test, triple sugar ion agar. Antibiotic susceptibility testing was done as per CLSI 2014 guidelines using Muller Hinton agar by Kirby-Bauer disk diffusion method. The antibiotic discs which were used for the Gram negative bacilli were Ampicillin (20μg), Aztreonam (30μg), Gentamycin (10μg), Amikacin (30μg), Cefazolin (30 μg), Cefuroxime (30μg) Ceftazidime (30μg), Cefotaxime (30μg), Ceftriaxone (30μg), Cefepime (30μg), Piperacillin/tazobactam (100/10μg), Imipenem (10μg), Meropenem (10 μg), Polymyxin B (300 units) and Colistin (10μg). Penicillin, Ampicillin, Azithromycin (15μg), Cefoxitin (30μg), Cefotaxime (30μg), Chloramphenicol (30μg), Clindamycin (2μg), Erythromycin (15μg), Vancomycin (30μg), Teicoplanin (30μg)), Ciprofloxacin, Ofloxacin (5μg), Linezolid (30μg) and Tetracycline (30μg) were used to determine the susceptibility patterns of the Gram positive cocci. MRSA, ESBL and carbapenemase production were detected as per the CLSI guidelines 2014 [11].
Statistical Analysis
Statistical analysis was done by using SPSS software version 21. Mean and Standard Deviation (SD) was calculated to assess the level of CRP and IL-6. The difference in the level of IL-6 and CRP among the case groups and control groups were assessed using student t-test. The difference between the two groups was considered to be statistically significant, when the p-value was less than 0.05, while performing student t-test. The sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) for all the parameters were calculated. Receiver Operating Characteristics (ROC) curve was drawn to determine the sensitivity, specificity for all the parameters and Area Under the Curve (AUC) was compared.
Results
Out of the 40 clinically suspected cases of neonatal sepsis, blood culture was positive in only 10 neonates (25%). The commonly isolated bacteria were Klebsiella species (30%), Coagulase negative Staphylococcus (CONS) (30%), Acinetobacter species (10%), Citrobacter (10%), Enterococcus (10%), and Micrococci (10%) [Table/Fig-1]. In our study we also observed that, the rise in the serum level of sepsis markers were high in all the culture positive neonates, but in case of sepsis due to CONS the rise in the level of the markers was not very high as when compared to other organisms.
[Table/Fig-1]:
Organisms isolated in culture positive patients (n=10).
| Organism | Number (n=10) | Percentage (%) |
|---|---|---|
| Klebsiella spp. | 3 | 30% |
| CONS | 3 | 30% |
| Acinetobacter spp. | 1 | 10% |
| Citrobacter spp. | 1 | 10% |
| Enterococcus spp. | 1 | 10% |
| Micrococci | 1 | 10% |
Regarding the antibiotic sensitivity pattern of the organisms isolated, 2 out of the 3 Klebsiella isolates were resistant only to (66%) to cefazolin, cotrimoxazole and ciprofloxacin and one isolate was resistant to cefuroxime, cefotaxime, cefepime, aminoglycosides, piperacillin tazobactam, imipenem and meropenem. None of the isolates were resistant to polymixin B and colistin. The 3 CoNS isolates were all resistant to Penicillin. One isolate (33%) was resistant to Tetracycline, Cloxacillin and Cefazolin. All the isolates (100%) were sensitive to Clindamycin, Vancomycin, Teicoplanin and Linezolid. The single Citrobacter isolate was sensitive to the first and second line of antibiotics whereas the Acinetobacter isolate was multidrug resistant and was sensitive only to Polymyxin B and Colistin.
The serum CRP value was high in clinically suspected cases of neonatal sepsis (33.33±26.35mg/l) when compared to healthy controls (8.55±4.93mg/l) and there is statistically significant difference (p-value <0.0001) between two groups. The serum IL-6 level was significantly higher in cases group (179.64±163.09 pg/ml) when compared to control group (24.25±13.65 pg/ml) it is statistically significant (p-value <0.0001) [Table/Fig-2].
[Table/Fig-2]:
Difference in distribution of markers between cases and controls.
| Marker | Controls 40 (Mean±SD) | Cases 40 (Mean±SD) | t-statistic | p-value |
|---|---|---|---|---|
| CRP mg/l | 8.554±4.930 | 33.332 ± 26.351 | -5.845 | <0.0001 |
| IL-6 pg/ml | 24.257 ± 13.653 | 179.642± 163.097 | -6.004 | <0.0001 |
A 25% of controls and 5% of cases showed hs-CRP values <0.5 mg/l and 57.5% of controls and 82.5% of cases showed the values >3 mg/l indicating increased risk of infection. Chi square test showed statistically significant difference (p-value <0.009) between the 2 groups. [Table/Fig-3] demonstrates the distribution of the CRP values in the cases and controls. [Table/Fig-4] shows the cut-off value, sensitivity, specificity, positive predictive value and negative predictive value of various parameters of sepsis. In our study, based on the cut-off value of CRP >13.495 mg/l showed sensitivity of 80% and specificity of 65.7% and cut-off value of IL-6 >51.29 pg/dl showed sensitivity of 100% and specificity of 62.86%.
[Table/Fig-3]:
Difference in hs-CRP values between cases and controls.
| hs-CRP mg/l | Controls n (%) | Cases n (%) | Chi square | p-value |
|---|---|---|---|---|
| <0.5 | 10 (25) | 2(5) | 6.798 | 0.009 |
| 0.5-1 | 2(5) | 2(5) | ||
| 1-3 | 5(12.5) | 3(7.5) | ||
| >3 | 23 (57.5) | 33 (82.5) |
[Table/Fig-4]:
Characteristics of diagnostic markers.
| S. No | Markers | Cut-off | Sensiti-vity | Specifi-city | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|---|
| 1. | hs-CRP mg/l | >3 | 90% | 32.86% | 16.07 | 95.83 |
| 2. | CRP mg/l | >13.495 | 80% | 65.7% | 25 | 95.83 |
| 3. | IL-6 pg/ml | >51.29 | 100% | 62.86% | 27.78 | 100 |
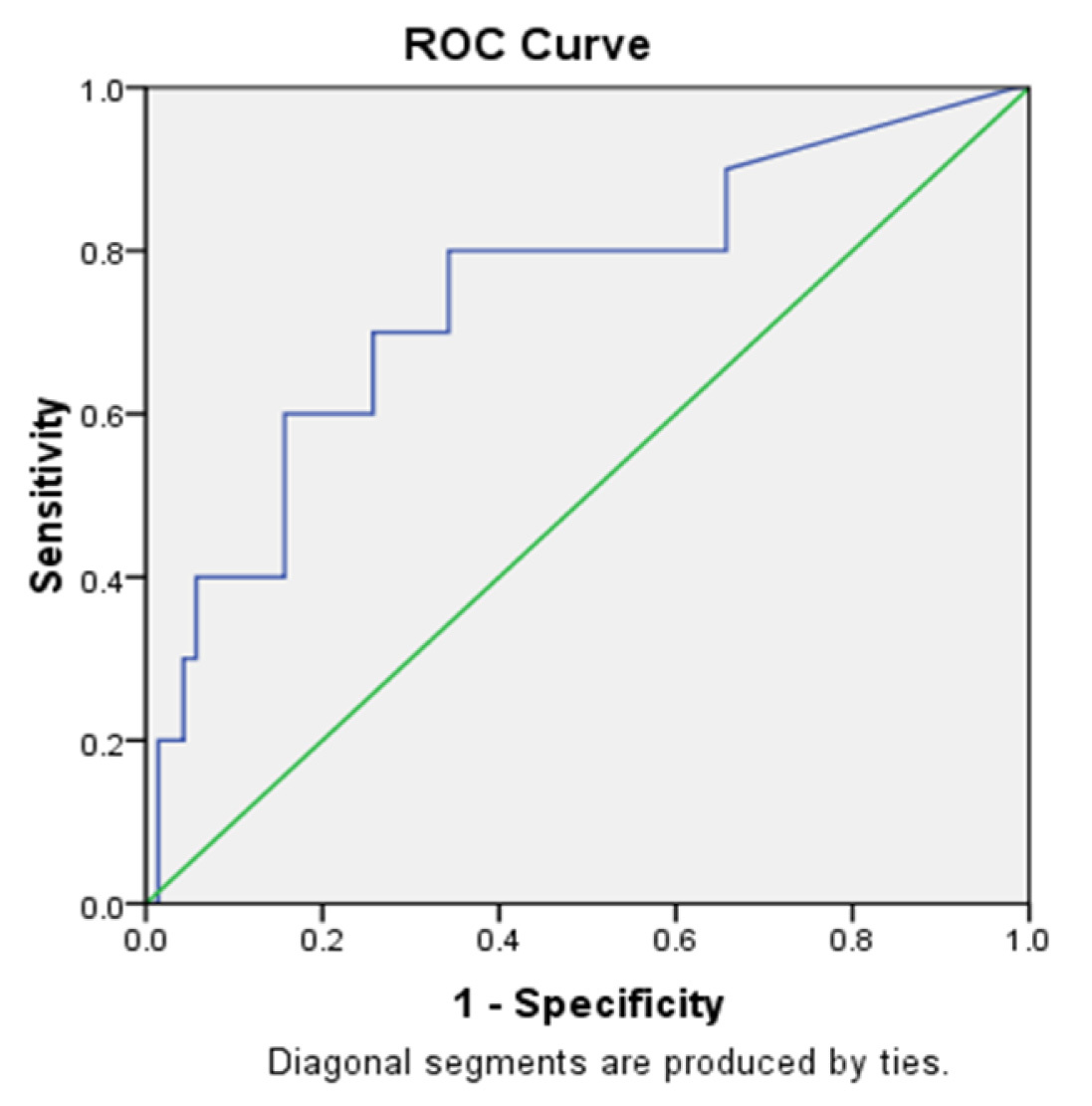
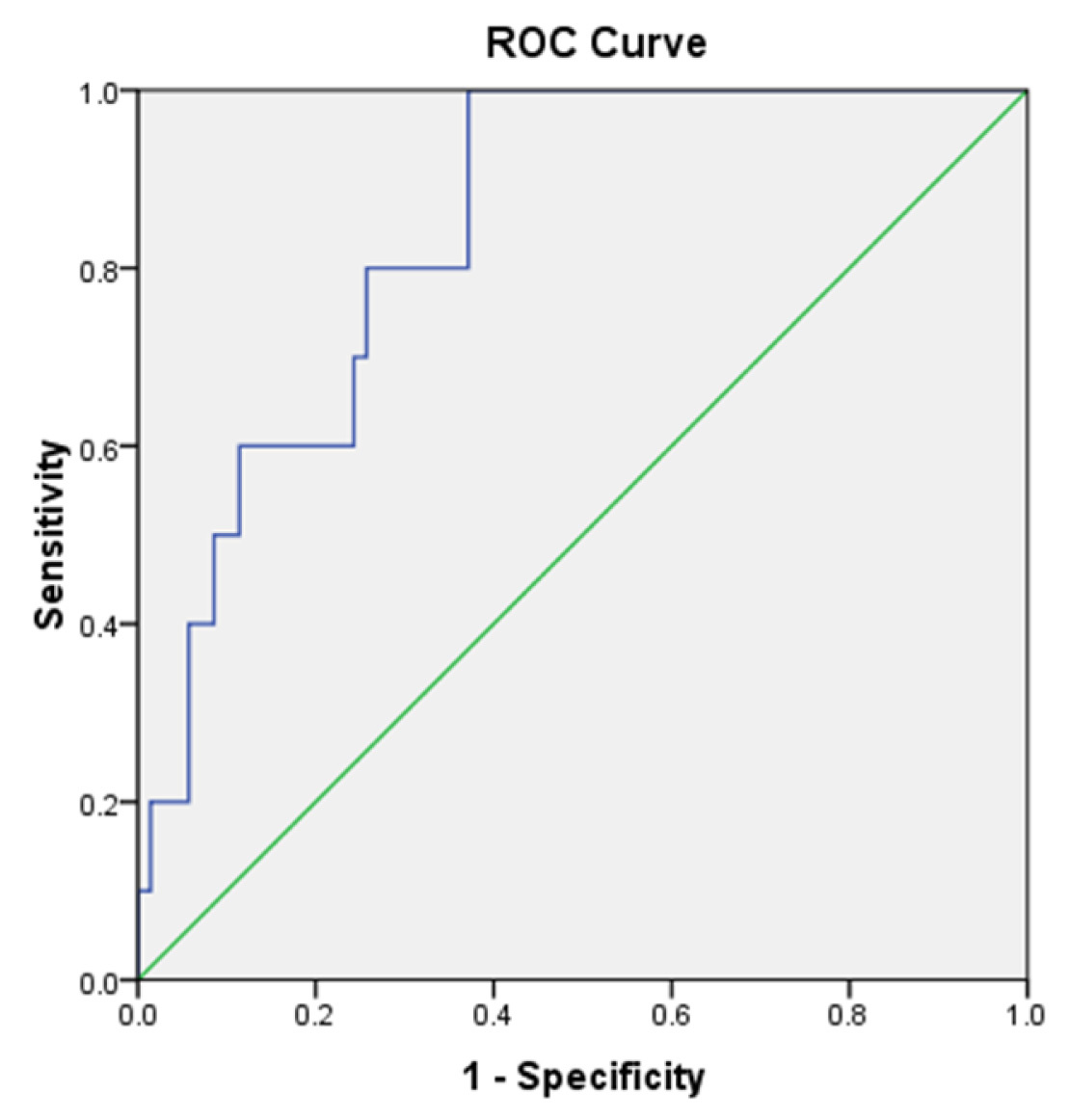
ROC of the CRP [Table/Fig-5] and IL-6 [Table/Fig-6] shows the sensitivity of 80% and 100%, and specificity of 65.7% and 62.86% respectively. In our study, IL-6 showed sensitivity of 100% and negative predictive value of 100% and CRP showed high specificity of 65.7% and negative predictive value of 95.83%. when compared to the other markers hs-CRP has low specificity [Table/Fig-7].
[Table/Fig-5]:
Receiver Operating Characteristics Curve- CRP.
 | ||||
|---|---|---|---|---|
| Area | Std. Errora | Asymptotic Sig.b | Asymptotic 95% Confidence Interval | |
| Lower Bound | Upper Bound | |||
| 0.748 | 0.089 | 0.012 | 0.573 | 0.923 |
Cutoff- 13.495 mg/l (Sensitivity=80%, Specificity= 65.7%)
Note: the above figure shows that the cut off value of CRP - 51.29 pg/ml has sensitivity of 80% and specificity of 65.7%.
[Table/Fig-6]:
Receiver Operating Characteristics Curve- IL-6.
 | ||||
|---|---|---|---|---|
| Area | Std. Errora | Asymptotic Sig.b | Asymptotic 95% Confidence Interval | |
| Lower Bound | Upper Bound | |||
| 0.843 | 0.052 | 0.000 | 0.741 | 0.945 |
Cut off 51.29pg/ml (Sensitivity=100%, Specificity=62.9%)
Area Under the Curve- IL6
a. Under the nonparametric assumption
b. Null hypothesis: true area = 0.5
Note: the above figure shows that the cut off value of IL-6 - 51.29 pg/ml has sensitivity of 100% and specificity of 62.9%.
[Table/Fig-7]:
Characteristics of combination of markers
| S. No | Marker | Sensiti-vity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|
| 1 | hs-CRP and CRP | 70% | 67.14% | 23.33 | 94 |
| 2 | IL6 and hs-CRP | 90% | 71.43% | 31.03 | 98.04 |
| 6 | IL6 and CRP | 100% | 75.71% | 32 | 96.36 |
The culture positive cases among the clinically suspected septic neonate group demonstrated slightly higher levels of serum biomarkers (IL-6, CRP and hs-CRP) as when compared to culture negative suspected septic neonates [Table/Fig-8].
[Table/Fig-8]:
Comparison of sepsis biomarker levels between Culture positive and Culture Negative neonates with sepsis.
| S.N | Sepsis Biomarker |
Measured Ranges in Culture positive neonates (n =10) |
Measured Ranges in Culture Negative neonates (n = 30) |
|---|---|---|---|
| 1 | hs-CRP | 3 to 5 mg/litre | 0.8 to 5 mg/litre |
| 2 | CRP | 5.27 mg/Litre to 91.8 mg/ litre | 5.0 mg/ litre to 66.75 mg/litre |
| 3 | IL- 6 | 52.92 to 655 picogram / ml | 18 to 366 picogram /ml |
Discussion
Neonatal sepsis is still a major cause of death in neonates due to late onset of symptoms and non-specific clinical presentation [9]. The blood culture is the gold standard for the diagnosis but it is time consuming and it is often negative, it may be due to inadequate sample collection or administration of antibiotics intra-partum or intermittent or low density bacteraemia [3]. In this study the cytokine IL-6 and the most commonly used acute phase reactant CRP as well as hs-CRP were evaluated to find out which of the above markers alone or in combination would be reliable and a better predictor of neonatal sepsis. The markers showed significant difference between the cases and the control groups. Blood culture was positive only in 10 out of 40 neonates in clinically suspected cases of neonatal sepsis.
Among the culture positive cases the most common organisms isolated were Klebsiella species (30%) and Coagulase negative staphylococcus (CONS) (30%). A study done by Prasanth et al., showed that Klebsiella species was the most common organism and another study done by Klingenberg et al., suggested that CONS is the most common pathogen in case of late-onset neonatal sepsis its association with the biofilm forming strains inhibits the host immune system in counteracting the infection. Both the studies correlate with our study results [12]. A study done by Jyoti et al., also suggested that the most common pathogen isolated in the culture positive sepsis cases were Gram negative organisms (56%) when compared to Gram positive organisms (44%) and in that Gram negative organism the most common isolate being Klebsiella pneumoniae which is consistent with our study [13].
Antibiotic sensitivity testing of the bacterial isolates showed that one Klebsiella isolate (33%) was multi drug resistant while the other 2(66%) were resistant only to first generation cephalosporins. This correlates with the study findings of Haque et al., who also reported low sensitivity to Ampicillin and cefotaxime for both Gram positive and Gram negative bacilli [14]. Another study by Jyoti et al., also reported increased susceptibility to Imipenem for the Gram negative bacilli Gram positive cocci had 100% susceptibility to Vancomycin, Teicoplanin and Linezolid which was also similar to Haque’s study [13,14].
In our study, the serum C-reactive protein (CRP) level was significantly higher in the clinically suspected neonatal sepsis groups than the control group and it showed higher specificity than other markers. This result is consistent with other studies [2,15,16]. A study done by Prasanth et al., showed that CRP has high specificity which correlates with our study results and it also suggest that the high C-reactive protein (CRP) level is a better indicator of severe bacterial infection in neonates [9]. CRP is the most extensively studied and commonly available laboratory test used for the diagnosis of neonatal sepsis [1]. Hofer et al., in their study, stated that serial CRP measurement along with other markers such as interleukins improves the diagnostic accuracy of neonatal sepsis [1]. There are studies which states that CRP level is a good predictor of severe bacterial infection in neonatal sepsis, but the increase in CRP level is low in case of sepsis due to CONS infection which correlates with our study and it was proposed that it causes less inflammation and tissue damage as it is a low-level pathogen [17].
The hs-CRP value <0.5mg/l indicates no risk of infection, the value 0.5-1mg/l indicates low risk, the value 1-3 mg/l indicates average risk and the value >3 mg/l indicates high risk of infection in neonates. The serum level of highly sensitive C-reactive protein (hs-CRP) was significantly higher in the clinically suspected sepsis group than in the control group in our study. hs-CRP is more sensitive than the conventional CRP as it can measure very low levels of CRP. Similar results were observed in a study done by Abdollahi et al., were the hs-CRP level was significantly higher in the sepsis group than the control group [10]. Another study done by Abdollahi et al., also propose that hs-CRP may also be used in combination with other sepsis markers like interleukin(IL-6) and procalcitonin (PCT) would be a better predictor of neonatal sepsis than using it alone [18]. In our study hs-CRP has very low specificity and positive predictive value than the other markers in the diagnosis of neonatal sepsis. This hs-CRP is used mostly to assess the cardiovascular risk and it can also be used as the prognostic indicator in patients with acute coronary syndrome [19]. In this study, based on the sensitivity, specificity, positive predictive value and negative predictive value obtained it was found that, in [Table/Fig-7] the suspected cases of neonatal sepsis the levels of IL-6 >51.29 pg/dl and CRP >13.49 mg/l showed high sensitivity of 100% and specificity of 75.71% respectively. So the above results suggest that the CRP and IL-6 were better predictors and reliable markers of neonatal sepsis than hs-CRP.
In our study the serum level of interleukin-6(IL-6) is significantly higher in the clinically suspected cases of neonatal sepsis group than the normal healthy controls. This result correlates with other studies [4,9,10,20,21]. A study by Smulian et al., reported that IL-6 level showed a highly significant value even in umbilical cord blood in septic neonates [22]. We observed 100% sensitivity for IL-6 in the clinically suspected neonatal sepsis group. In a study done by Buck et al., also demonstrates similar sensitivity in culture positive and clinical sepsis neonates [23]. A study done by Messer et al., demonstrates 100% sensitivity in the first 12 hours of life [24]. The other studies done by Kocabas et al., and Prasanth et al., showed different sensitivity results as when compared to our study, this may be due to differences in the type of sample collected, that is venous blood or umbilical vein blood, the age of neonate at the onset of infection, mainly due to the difference in the inclusion criteria and absence of standardized cut-off value for IL-6 in these studies could be the factors contributing to the variation in sensitivity [6,9]. In our study the specificity of IL-6 is 62% which correlates with the study done by Dollner et al., [25]. Our study also shows a negative predictive value of 100% which almost correlates with study done by Messer et al., [24].
A study done by van Dissel et al., suggests that, when the serum levels of TNF-α and IL-6 were measured together for the diagnosis of neonatal sepsis it would provide a sensitivity and specificity of 60% and 100% respectively [26]. Our study IL-6 and hs-CRP showed sensitivity and specificity of 100% and 71% respectively [Table/Fig-7].
The measurement of these sepsis markers is of utmast important only in case of neonates with unclear infectious status. These markers can be reliably used only if there is a standardisation of inclusion, exclusion criteria, cut-off values and the methods used for quantification of the markers.
Conclusion
The diagnosis of neonatal sepsis is still a challenge for both the laboratory as well as clinicians due to, absence of standardized cut-off values for sepsis markers and non-specific clinical presentation. So a reliable test is needed to diagnose neonatal sepsis.
From this study, we conclude that the IL-6 is the highly sensitive marker and CRP is the more specific marker for the identification of neonatal sepsis. hs-CRP is a less reliable marker. The combination of IL-6 and CRP has the high sensitivity and negative predictive value when compared to other markers. Therefore a combination of markers i.e. IL-6 and CRP would be the better predictors of neonatal sepsis.
Financial or Other Competing Interests
None.
References
- [1].Hofer N, Zacharias E, Müller W, Resch B. An update on the use of c-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25–36. doi: 10.1159/000336629. [DOI] [PubMed] [Google Scholar]
- [2].Mally P, Xu J, Hendricks-Muñoz KD. Biomarkers for neonatal sepsis: recent developments. Research and Reports in Neonatology. 2014;4:157–68. [Google Scholar]
- [3].Al-Zahrani AK, Ghonaim MM, Hussein YM, Eed EM, Khalifa AS, Dorgham LS. Evaluation of recent methods versus conventional methods for diagnosis of early-onset neonatal sepsis. J Infect Dev Ctries. 2015;9(4):388–93. doi: 10.3855/jidc.5950. [DOI] [PubMed] [Google Scholar]
- [4].Kocabaş E, Sarikçioğlu A, Aksaray N, Seydaoğlu G, Seyhun Y, Yaman A. Role of procalcitonin, C-reactive protein, interleukin-6, interleukin-8 and tumor necrosis factor-alpha in the diagnosis of neonatal sepsis. Turk J Pediatr. 2007;49(1):7–20. [PubMed] [Google Scholar]
- [5].Chiesa C, Panero A, Osborn JF, Simonetti AF, Pacifico L. Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin Chem. 2004;50(2):279–87. doi: 10.1373/clinchem.2003.025171. [DOI] [PubMed] [Google Scholar]
- [6].Ng PC, Li K, Leung TF, Wong RPO, Li G, Chui KM, et al. Early Prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and rantes in preterm infngants. Clin Chem. 2006;52(6):1181–89. doi: 10.1373/clinchem.2005.062075. [DOI] [PubMed] [Google Scholar]
- [7].Ishibashi M, Takemura Y, Ishida H, Watanabe K, Kawai T. (C-Reactive protein kinetics in newborns: application of a high-sensitivity analytic method in its determination. Clinical Chemistry. 2002;48(7):1103–06. [PubMed] [Google Scholar]
- [8].Edgar DM, Gabriel V, Ruth Gallimore J, McMillan SA, Grant J. A prospective study of the sensitivity, specificity and diagnostic performance of soluble intercellular adhesion molecule 1, highly sensitive C-reactive protein, soluble E-selectin and serum amyloid A in the diagnosis of neonatal infection. J. BMC Pediatrics. 2010;10:22.1–16. doi: 10.1186/1471-2431-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Prashant A, Vishwanath P, Kulkarni P, SathyaNarayana P, Gowdara V, Nataraj SM, et al. Comparative assessment of cytokines and other inflammatory markers for the early diagnosis of neonatal sepsis–a case control study. PLoS ONE [Internet] 2013[cited 2015 May 24];8(7) doi: 10.1371/journal.pone.0068426. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3711816/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abdollahi A, Shoar S, Nayyeri F, Shariat M. Diagnostic value of simultaneous measurement of procalcitonin, interleukin-6 and hs-crp in prediction of early-onset neonatal sepsis. Mediterr J Hematol Infect Dis [Internet] 2012[cited 2015 Jul 7];4(1) doi: 10.4084/MJHID.2012.028. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3375671/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Clinical Laboratory Standards Institute. Performance standards fot Antimicrobial Susceptibility testing. Twenty fourht international supplement. M100(S 24) Volume 34. [Google Scholar]
- [12].Klingenberg C, Aarag E, Rønnestad A, Sollid JE, Abrahamsen TG, Kjeldsen G, et al. Coagulase-negative staphylococcal sepsis in neonates. Association between antibiotic resistance, biofilm formation and the host inflammatory response. Pediatr Infect Dis J. 2005;24(9):817–22. doi: 10.1097/01.inf.0000176735.20008.cd. [DOI] [PubMed] [Google Scholar]
- [13].Jyothi P, Basavaraj MC, Basavaraj PV. Bacteriological profile of neonatal septicaemia and antibiotic susceptibility pattern of the isolates. J Nat Sci Biol Med. 2013;4(2):306–09. doi: 10.4103/0976-9668.116981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Haque SM, Jahan N, Mannan MA, Hasan M, Begum M, Rob S, et al. Identification of bacterial isolates in neonatal sepsis and their antimicrobial susceptibility. Mymensingh Med J. 2014;23(4):709–14. [PubMed] [Google Scholar]
- [15].Mathai E, Christopher U, Mathai M, Jana AK, Rose D, Bergstrom S. Is C-reactive protein level useful in differentiating infected from uninfected neonates among those at risk of infection? Indian Pediatr. 2004;41(9):895–900. [PubMed] [Google Scholar]
- [16].Ng PC. Diagnostic markers of infection in neonates. Arch Dis Child – Fetal Neonatal Ed. 2004;89(3):F229–35. doi: 10.1136/adc.2002.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rønnestad A, Abrahamsen TG, Gaustad P, Finne PH. C-reactive protein (CRP) response patterns in neonatal septicaemia. APMIS Acta Pathol Microbiol Immunol Scand. 1999;107(6):593–600. doi: 10.1111/j.1699-0463.1999.tb01597.x. [DOI] [PubMed] [Google Scholar]
- [18].Abdollahi A, Morteza A, Nayyeri F. Procalcitonin, Interleukin-6 and high sensitivity c reactive protein in the early prediction of neonatal sepsis, are they correlated? Pediatr Res. 2011;70(S5):426. [Google Scholar]
- [19]. TB_High Sensitivity C- ReactiveProtein_hsCRP.pdf [Internet]. [cited 2015 Aug 23]. Available from: http://portals.clevelandclinic.org/portals/66/PDF/TechBriefs/TB_HighSensitivityCReactiveProtein_hsCRP.pdf.
- [20].Kurt ANC, Aygun AD, Godekmerdan A, Kurt A, Dogan Y, Yilmaz E. Serum IL-1β, IL-6, IL-8, and TNF-α levels in early diagnosis and management of neonatal sepsis. Mediators Inflamm [Internet] 2007 [cited 2015 Jul 6];2007 doi: 10.1155/2007/31397. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2220039/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chiesa C, Pellegrini G, Panero A, Osborn JF, Signore F, Assumma M, et al. C-Reactive Protein, Interleukin-6, and Procalcitonin in the Immediate Postnatal Period: Influence of Illness Severity, Risk Status, Antenatal and Perinatal Complications, and Infection. Clin Chem. 2003;49(1):60–68. doi: 10.1373/49.1.60. [DOI] [PubMed] [Google Scholar]
- [22].Smulian JC, Vintzileos AM, Lai YL, Santiago J, Shen-Schwarz S, Campbell WA. Maternal chorioamnionitis and umbilical vein interleukin-6 levels for identifying early neonatal sepsis. J Matern Fetal Med. 1999;8(3):88–94. doi: 10.1002/(SICI)1520-6661(199905/06)8:3<88::AID-MFM4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- [23].Buck C, Bundschu J, Gallati H, Bartmann P, Pohlandt F. Interleukin-6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics. 1994;93(1):54–58. [PubMed] [Google Scholar]
- [24].Messer J, Eyer D, Donato L, Gallati H, Matis J, Simeoni U. Evaluation of interleukin-6 and soluble receptors of tumor necrosis factor for early diagnosis of neonatal infection. J Pediatr. 1996;129(4):574–80. doi: 10.1016/s0022-3476(96)70123-3. [DOI] [PubMed] [Google Scholar]
- [25].Døllner H, Vatten L, Austgulen R. Early diagnostic markers for neonatal sepsis: Comparing C-reactive protein, interleukin-6, soluble tumour necrosis factor receptors and soluble adhesion molecules. J Clin Epidemiol. 2001;54(12):1251–57. doi: 10.1016/s0895-4356(01)00400-0. [DOI] [PubMed] [Google Scholar]
- [26].van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Frölich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet Lond Engl. 1998;351(9107):950–53. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]


