Abstract
Introduction
HMG-CoA (3-hydroxy-3- methylglutary lcoenzyme A) reductase inhibitors (statins) have anti-inflammatory properties which may be particularly useful in rheumatoid arthritis to suppress disease activity and inflammatory factors.
Aim
The purpose of this clinical trial was to determine anti-inflammatory properties of statins in rheumatoid arthritis.
Materials and Methods
Eighty Iranian patients with rheumatoid arthritis, aged between 19 to 75 years were recruited to take part in this randomized, double-blind placebo-controlled trial. Subjects were randomly allocated to two groups to take atorvastatin or placebo 40 mg daily as an adjunct to current disease-modifying anti-rheumatic drugs (DMARDs) treatment. Disease Activity Score-28 (DAS28), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), swollen joint count (SJC) & tender joint count (TJC) were assessed before and after three months intervention.
Results
Analysis was based on intention to treat. DAS28 significantly declined in the atorvastatin group in comparison with placebo (p< 0.001). SJC, TJC, CRP and ESR also were significantly dropped in the atorvastatin group in comparison with placebo.
Conclusion
It can be concluded that atorvastatin can suppress RA activity and inflmmatory factors in RA patients for high to moderate grade of inflmmation.
Keywords: HMG-CoA, Anti-inflammatory agents, Erythrocyte sedimentation rate, Swollen joint count, Tender joint count
Introduction
Rheumatoid arthritis (RA) is a chronic illness related to joint inflammation, progressive disability and complications in patients which have adverse impacts on their quality of life [1,2]. It is revealed that statins (3-Hydroxy-3 methyl-glutaryl coenzyme A (HMG-CoA) inhibitors) which are used in hyperlipidemia and prevention of CAD due to cholesterol-lowering effects have anti-inflammatory and immune-modulatory impacts [3].
Statins had shown anti-inflammatory functions in several studies [3]. This potential of having anti-inflammatory properties have raised attention to its probable benefits in routine therapy of rheumatoid arthritis (RA) [4–6]. Furthermore statins have a safety profile, this may decrease demand for the long-term disease modifying antirheumatic drugs (DMARDs) which are currently used for RA treatment [7]. However, the largest surveys applied to evaluate the impacts of statins in RA did not report significant results [8,9], thus the advantages of statin therapy related to disease activity in RA patients remains debatable.
There is contradictory evidence about efficacy of statins in custom treatment of rheumatoid arthritis. However, debatable available data, promotes the need for more research to find the precise dosage and beneficial impact of statin therapy on RA disease activity [10]. The present clinical trial was proposed to determine the impacts of atorvastatin on inflammation and disease activity in rheumatoid arthritis.
Aim
The aim of this randomized, double-blind placebo-controlled trial was to determine anti-inflammatory properties of statins in rheumatoid arthritis (RA) patients.
Materials and Methods
The study was accepted by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (AJUMS), Iran (ajums.REC.1393.31) and was registered in the Iranian Registry of Clinical Trials (IRCT ID:IRCT 2014051817738N1). The trial was carried out from June 2014 to October 2014 with rheumatoid arthritis (RA) patients, referring to the out-patients Rheumatology Clinic of the Golestan Hospital, Ahvaz, Iran.
Sample size was calculated by this formula:
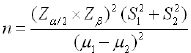 |
(S2=2.6), S1=2.34) with regard to the most possible sample size, mean difference was 2 [4].
A total of 80 patients with rheumatoid arthritis, of whom (14 male, 66 female) were randomised to participate in this study and were randomly assigned to two groups to take either 40 mg atorvastatin or placebo daily for three months [Table/Fig-1]. All patients filled in a written consent. The participants’ age was between 19 to 75-year-old. The subjects were all compared against the 2010 American College of Rheumatology (ACR) and European League with the Rheumatism collaborative (EULAR) criteria [11]. Their disease activity was moderate to severe despite current (DMARDs) therapy. All patients had used only DMARDs including hydroxychloroquine and MTX (methotrexate) jointly with prednisone. None had history of any other injectable drugs such as Anti-TNF agents or other DMARDs. Patients excluded due to instability of DMARD use before study implementation, current usage of statins and NSAIDs, contraindication of statins therapy such as renal disease or impaired liver enzymes, pregnancy, hepatic disease, smoking, disease duration longer than 5 years and any kind of cancer.
[Table/Fig-1]:
![[Table/Fig-1]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/d97f/4948444/1ced72f6663e/jcdr-10-OC32-g001.jpg)
CONSORT flow chart of subjects’ recruitment process for 2-group parallel randomized trial. ITT, intention to treat.
Only patients who met the inclusion criteria and signed consent were invited to participate in this clinical trial. Patients were recruited with active RA in addition with sufficient and stable dose of similar DMARD for at least 3 months and were asked to keep their DMARD dose from study admission. Both groups used hydroxychloroquine 3.5 mg/kg daily, prednisolone7.5 mg daily and 0.2 mg/kg methotrexate weekly. During study, dose of these drugs were fixed until next visit, patients didn’t use any other drug such as NSAID or any other that may effect on their symptoms. Patients were computer randomized by an independent administrator at an off-site location and both patients and researchers were uninformed of the type of allocated drug among two groups. Atorvastatin and placebo tablets were supplied by the Faculty of Pharmacy. Patients followed-up to 3 months.
Disease activity score (DAS28), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), swollen joint count (SJC) & tender joint count (TJC) were measured before and after 3 months of taking atorvastatin & placebo 40 mg daily among two allocated groups. It should be mentioned that 3 weeks after starting trial, we asked patients about myalgia to ensure about a rare probable complications of statins such as myopathy. If there were such symptoms, we had to stop atorvastatin and check Creatine phosphokinase (CPK) and Lactate Dehydrogenase (LDH). No complication in this matter was reported in this trial.
Intention to treat analysis has been done, which means subjects who did not finish 3 months of clinical trial were also considered. The disease activity score (DAS28), is calculated at each visit using the patient’s ESR and also number of tender and swollen joints(out of 28). According to the National Rheumatoid Arthritis Society [12] a DAS28 > 5.1 demonstrates active disease, a DAS28 ≤3.2 demonstrates well controlled disease, and a score < 2.6 qualifies for remission. If the DAS28 score is > 3.2 or trends higher over time, this scoring may lead the provider to adjust current therapies to help slow disease progression.
Statistical Analysis
The analysis of the results was carried out using Statistical Package for Social Sciences (SPSS) version 20. Descriptive statistics such as means, range and standard deviation were used to present subject’s demographic information. Paired t-tests & two-sample independent t-tests were employed to define mean differences of continuous variables within-group and between two groups respectively at baseline and the end of intervention. To find possible baseline differences between the two groups, chi square test was used for categorical socio-demographic and clinical variables. The p-value more than 0.05 means that two groups were similar at baseline. Data analysis was based on intention to treat analysis analysed by Intention-To-Treat (ITT) and for an alteration in laboratory variables, we assumed no change for dropouts by reassigning their baseline value forward to 3months. The associated 95% CI, and p<0.05 was considered as the significant level.
Results
In this randomized, double-blind placebo-controlled trial, 80 RA patients were randomized to take 40 mg atorvastatin or placebo. There were not any significant differences for demographic data between the two groups at baseline. Atorvastatin was well tolerated among subjects. The flow diagram of subject recruitment process for 2 group parallel randomized trial is shown in the [Table/Fig-1].
The age of rheumatoid arthritis patients ranged from 19 to 75-year-old. The mean age was 46.38 ± 12.43 years. There were not any significant differences for clinical variables at baseline between two groups except for SJC and ESR. The comparisons between two groups and within each study arm are described in [Table/Fig-2]. Disease activity score (DAS28), C-reactive protein (CRP), Erythrocyte Sedimentation Rate (ESR), swollen joint count (SJC) & Tender Joint Count (TJC) were significantly declined in patients who were assigned to the atorvastatin group in comparison with placebo (p< 0.001) [Table/Fig-2].
[Table/Fig-2]:
Demographic data, baseline and end values of clinical variables in rheumatoid arthritis patients following 3 months taking of atorvastatin (n=40) or placebo (n=40).
| Variables | Atorvastatin (n=40) | Placebo (n=40) | p* | |
|---|---|---|---|---|
| Demographics | ||||
| Gender | Male | 5(12.5%) | 9(22.5%) | |
| Female | 35(87.5%) | 31(77.5%) | 0.24 | |
| Disease duration (years) | 1 | 33(82.5%) | 28(70%) | |
| 2 | 3(7.5%) | 5(12.5%) | 0.18 | |
| ≥3 | 4(10%) | 7(17.5%) | ||
| Age | 47.8±10.30 | 44.95±14.24 | 0.31 | |
| Clinical outcome measures | ||||
| TJC | ||||
| Baseline | 9.28±4.24 | 10.48±3.90 | 0.191 | |
| End | 2.80±2.25 | 10.90±3.41 | 0.000 * | |
| **P | 0.000** | 0.576 | ||
| SJC | ||||
| Baseline | 7.33±3.34 | 9.32±3.70 | 0.013* | |
| End | 1.68±1.62 | 9.53±3.21 | 0.000* | |
| **P | 0.000** | 0.705 | ||
| ESR | ||||
| Baseline | 51.73±24.47 | 42.23±9.06 | 0.024* | |
| End | 27±12.96 | 41.90±11.21 | 0.000* | |
| **P | 0.000** | 0.787 | ||
| DAS28 | ||||
| Baseline | 5.64±0.67 | 5.78±0.58 | 0.314 | |
| End | 3.76±0.77 | 5.84±0.56 | 0.000* | |
| **P | 0.000** | 0.631 | ||
* Significant at p<0.05
Note: Data were expressed as mean ± SD
P* resulted from independent two sample t-test to compare baseline with end values in each groups;
P** resulted from paired t-test to compare baseline with end values within a group
Comparison of mean DAS28 and its trend in RA patients before and after the intervention among two groups are shown in [Table/Fig-3,4] respectively. Classification of DAS28 (EULAR criteria) in RA patients before and after the intervention were defined in [Table/Fig-5]. Almost 39 of 40 (97.5%) patients in atorvastatin group had moderate or low DAS28 in comparison with 3 of 40 assigned in the placebo group after 3 months [Table/Fig-5]. Classification of C-reactive protein among two groups before and after intervention is presented in [Table/Fig-6].
[Table/Fig-3]:
![[Table/Fig-3]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/d97f/4948444/0b4d9716f044/jcdr-10-OC32-g002.jpg)
Comparison of mean of DAS28 in patients with rheumatoid arthritis before and after 3 months intervention among two groups; statin (n=40), placebo (n=40).
[Table/Fig-4]:
![[Table/Fig-4]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/d97f/4948444/28d523ab6cff/jcdr-10-OC32-g003.jpg)
Trends of mean DAS28 in patients with rheumatoid arthritis before and after 3 months intervention among two groups; statin (n=40), placebo (n=40).
[Table/Fig-5]:
![[Table/Fig-5]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/d97f/4948444/732c0d75cb60/jcdr-10-OC32-g004.jpg)
Classifications of DAS28 in patients with rheumatoid arthritis before and after 3 months intervention among two groups; statin (n=40), placebo (n=40).
[Table/Fig-6]:
![[Table/Fig-6]:](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/d97f/4948444/8ea66f172685/jcdr-10-OC32-g005.jpg)
Classifications of C-reactive protein (CRP) in patients with rheumatoid arthritis before and after 3 months intervention among two groups; statin (n=40) placebo (n=40).
Discussion
The beneficial effects of anti-inflammatory properties of statin have been reported in several studies [4,13–15]. Primary results come from laboratory test in vitro and from animals [16,17]. Recently, the beneficial anti-inflammatory properties of statins have been investigated among humans in diseases with high levels of inflammation such as RA [4], sepsis [13], organ transplantation [14] and multiple sclerosis [15]. The current clinical trial has been done to determinethe anti-inflammatory impact of statin in rheumatoid arthritis because the clinical trials in this matter are not sufficient.
The results of the current study revealed obvious suppression for acute-phase variables (ESR and CRP) and a significant decline in mean DAS28 score, swollen/tender joint counts in RA patients after 3months of atorvastatin treatment with a dose of 40 mg daily. While the level of change is modest, it can provide evidence that statins can be beneficial in inflammatory disease. The impact of atorvastatin in patients with moderate to severe RA revealed that atorvastatin with a dose of 40 mg daily can significantly suppress disease activity. Similarly, the first clinical trial (TARA)(4)aimed to find-out anti-inflammatory role of statins on rheumatoid arthritis was a double-blind, placebo-controlled trial among 116 RA patients with 6 months follow-up. Atorvastatin with a dosage of 40 mg daily had the same results as our trial after 6 months compared with those assigned to the placebo arm.
However, the TARA study had the bigger sample size and longer follow-up time, but the indication stated by the trial was small, more proof was required. Standard RA treatment included DMARDs, NSAIDs, and steroids were allowed. Patients used different DMARDs, Also, the study demonstrated that those in the intervention group using methotrexate had similar results to those in the same group using a different DMARD; while there were no differences between case and control group regarding the type of used DMARDs in our study and taking NSAIDs was in exclusion criteria.
The immunomodulatory properties of statins in RA patients have been reported in three small clinical trials [18–20]. In one study, 30 RA patients were randomly allocated to a group to take methotrexate and prednisone and another group assigned to take those 2 drugs plus atorvastatin (40 mg/day). Statin had significant effect on suppression of RA disease activity after 6 months while sample size of the study was less than our trial and there was no placebo group [18].
The effects of low-dose (10 mg daily) of simvastatin was evaluated in another study [20] for 12 weeks among 24 RA patients. Reduction in ESR and CRP levels was reported. While the other trial [21] with small sample size {atorvastatin (n=11) & placebo (n=9)) and high-dose of statin (80 mg/day} for 12 weeks had no impact on disease activity of rheumatoid arthritis. However, atorvastatin with a dose of 20 mg have been stated to have a positive effect on the reduction of disease activity and inflammatory markers [22] but there was no control group and dose of the statins was different from our trial.
Recently, two meta-analysis [23,24] have been done on randomised controlled trials (RCTs) among RA patients taking either statins or control. The first one [23] included 15 studies with overall 992 RA patients (487 patients allocated to statins therapy). Data revealed that statins down-regulate inflammatory factors and lead to decline on disease activity, tender joint count (TJC) and swollen joint count (SJC), Erythrocyte Sedimentation Rate (ESR) and C-reactive protein (CRP). This meta-analysis explored the anti-inflammatory impacts of statins on suppression of RA activity, which can be prescribed as an effective therapy for RA patients [23]. In the second recent meta-analysis, a total 13 controlled clinical trials, including 737 adult RA patients to compare the effect of statins with placebo were included [24]. Eleven studies were based on DAS28, whereas the other 2 studies were based on ESR or CRP. It was reported that statins therapy significantly decreased swollen/tender joint counts, ESR, and CRP in comparison with placebo and might be effective to suppress RA disease activity in patients with more active disease than others with moderate or low disease activity [24].
A systematic review [25] of five studies [4,18,21,26,27] including four randomized-controlled trials and a prospective cohort observational study [26] revealed that statin medications in only three studies [4,18,28] provide a statistically significant decrease in the DAS28, while all five studies demonstrated that the statins therapy improves one or more individual markers of systemic inflammation. Four studies [4,18,26,27] reported improvement in CRP and three studies showed an improvement in ESR [4,18,27], three studies indicated a decreased incidence of swollen joint counts [4,18,26] and three studies an improvement in tender joint counts [18,26,27]. The result of this systematic review demonstrates a trend of decreased inflammation in rheumatoid arthritis patients related to statin therapy. However, the picture remains unclear for the improvement in rheumatoid arthritis disease progression with adjuvant statin use. A cross-sectional study [29] among 209 RA patients also reported taking statins was correlated with a higher functional status; while, there was not any relationship regarding statins therapy and RA disease activity.
Regardless of all supportive results that mentioned above, in a case-control study among 508 RA patients, after adjustment for cardiovascular risk factors was reported that statins offer significant cardiovascular benefits; while have impact on immune regulation, which can raise autoimmunity and lead to diseases like rheumatoid arthritis [28]. Even though, a large observational cohort study [26] among 4152 RA patients in which 279 patients (6.7%) were statin users with lower C-reactive protein and swollen joint counts indicated that statins have beneficial effects in reduction of RA disease activity in the daily practice of rheumatology.
Limitation
The limitations of this study should be considered as small sample size and single-center design. Longitudinal clinical trials in more populations are necessary to determine the anti-inflammatory properties of statins and its clinical long term impacts in rheumatoid arthritis patients. Future studies should consider the direct impact of DMARDs on systemic inflammatory markers. It is believed that in a large high-powered interventional study, with more rheumatoid arthritis patients, controlled dose and long-term exposure to statins, the effects on RA disease activity should be better explored.
Conclusion
Our findings showed a beneficial effect of atorvastatin on suppression of disease activity, tender & swollen joint counts and plasma markers of inflammation such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in RA patients despite existing DMARD therapy. Although it may not be prescribed for first line use, these properties of atorvastatin might be useful for vascular protection, immune modulatory and anti-inflammatory potential. Statins therapy may be prescribable in patients with RA (or other patients with chronic systemic inflammation).
Acknowledgments
This study is part of thesis for Dr. Mehrdad Dargahi-Malamir. Special thanks to Ahvaz Jundishapur University of Medical Sciences for the financial support (Thesis Grant No.U-93014).
Financial or Other Competing Interests
As Declared above.
References
- [1].Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- [2].Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. The American Journal of Managed Care. 2007;13(Suppl 9):S237–51. [PubMed] [Google Scholar]
- [3].Arnaud C, Burger F, Steffens S, Veillard NR, Nguyen TH, Trono D, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(6):1231–36. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- [4].McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363(9426):2015–21. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- [5].Klareskog L, Hamsten A. Statins in rheumatoid arthritis—two birds with one stone? Lancet. 2004;363(9426):2011–12. doi: 10.1016/S0140-6736(04)16485-4. [DOI] [PubMed] [Google Scholar]
- [6].Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. Journal of Immunology. 2003;170(3):1524–30. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- [7].Paraskevas KI. Statin treatment for rheumatoid arthritis: a promising novel indication. Clinical Rheumatology. 2008;27(3):281–87. doi: 10.1007/s10067-007-0806-8. [DOI] [PubMed] [Google Scholar]
- [8].Lodi S, Evans SJ, Egger P, Carpenter J. Is there an anti-inflammatory effect of statins in rheumatoid arthritis? Analysis of a large routinely collected claims database. British Journal of Clinical Pharmacology. 2010;69(1):85–94. doi: 10.1111/j.1365-2125.2009.03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lodi S, Carpenter J, Egger P, Evans S. Design of cohort studies in chronic diseases using routinely collected databases when a prescription is used as surrogate outcome. BMC Medical Research Methodology. 2011;11:36. doi: 10.1186/1471-2288-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bansback N, Ara R, Ward S, Anis A, Choi HK. Statin therapy in rheumatoid arthritis: a cost-effectiveness and value-of-information analysis. Pharmaco Economics. 2009;27(1):25–37. doi: 10.2165/00019053-200927010-00004. [DOI] [PubMed] [Google Scholar]
- [11].Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Annals of the Rheumatic Diseases. 2010;69(9):1580–88. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- [12].Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA) Arthritis care & research. 2011;63(Suppl 11):S14–36. doi: 10.1002/acr.20621. [DOI] [PubMed] [Google Scholar]
- [13].Zhang S, Luo L, Wang Y, Rahman M, Lepsenyi M, Syk I, et al. Simvastatin protects against T cell immune dysfunction in abdominal sepsis. Shock. 2012;38(5):524–31. doi: 10.1097/SHK.0b013e31826fb073. [DOI] [PubMed] [Google Scholar]
- [14].Kobashigawa JA, Moriguchi JD, Laks H, Wener L, Hage A, Hamilton MA, et al. Ten-year follow-up of a randomized trial of pravastatin in heart transplant patients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24(11):1736–40. doi: 10.1016/j.healun.2005.02.009. [DOI] [PubMed] [Google Scholar]
- [15].Markovic-Plese S, Singh AK, Singh I. Therapeutic potential of statins in multiple sclerosis: immune modulation, neuroprotection and neurorepair. Future Neurology. 2008;3(2):153. doi: 10.2217/14796708.3.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barsante MM, Roffe E, Yokoro CM, Tafuri WL, Souza DG, Pinho V, et al. Anti-inflammatory and analgesic effects of atorvastatin in a rat model of adjuvant-induced arthritis. European Journal of Pharmacology. 2005;516(3):282–89. doi: 10.1016/j.ejphar.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [17].Yamagata T, Kinoshita K, Nozaki Y, Sugiyama M, Ikoma S, Funauchi M. Effects of pravastatin in murine collagen-induced arthritis. Rheumatology International. 2007;27(7):631–39. doi: 10.1007/s00296-006-0270-9. [DOI] [PubMed] [Google Scholar]
- [18].El-Barbary AM, Hussein MS, Rageh EM, Hamouda HE, Wagih AA, Ismail RG. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. The Journal of Rheumatology. 2011;38(2):229–35. doi: 10.3899/jrheum.100582. [DOI] [PubMed] [Google Scholar]
- [19].Abud-Mendoza C, de la Fuente H, Cuevas-Orta E, Baranda L, Cruz-Rizo J, Gonzalez-Amaro R. Therapy with statins in patients with refractory rheumatic diseases: a preliminary study. Lupus. 2003;12(8):607–11. doi: 10.1191/0961203303lu429oa. [DOI] [PubMed] [Google Scholar]
- [20].Kanda H, Yokota K, Kohno C, Sawada T, Sato K, Yamaguchi M, et al. Effects of low-dosage simvastatin on rheumatoid arthritis through reduction of Th1/Th2 and CD4/CD8 ratios. Modern rheumatology / the Japan Rheumatism Association. 2007;17(5):364–68. doi: 10.1007/s10165-007-0589-4. [DOI] [PubMed] [Google Scholar]
- [21].Charles-Schoeman C, Khanna D, Furst DE, McMahon M, Reddy ST, Fogelman AM, et al. Effects of high-dose atorvastatin on antiinflammatory properties of high density lipoprotein in patients with rheumatoid arthritis: a pilot study. The Journal of Rheumatology. 2007;34(7):1459–64. [PubMed] [Google Scholar]
- [22].Van Doornum S, McColl G, Wicks IP. Atorvastatin reduces arterial stiffness in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2004;63(12):1571–5. doi: 10.1136/ard.2003.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lv S, Liu Y, Zou Z, Li F, Zhao S, Shi R, et al. The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: a meta-analysis. Clinical and Experimental Rheumatology. 2015;33(1):69–76. [PubMed] [Google Scholar]
- [24].Xing B, Yin YF, Zhao LD, Wang L, Zheng WJ, Chen H, et al. Effect of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitor on disease activity in patients with rheumatoid arthritis: a meta-analysis. Medicine. 2015;94(8):e572. doi: 10.1097/MD.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Johnson SK. The Effects of HMG-CoA Inhibitors (Statins) on Rheumatoid Arthritis Disease Progression. A Systematic Review. 2012 [Google Scholar]
- [26].Okamoto H, Koizumi K, Kamitsuji S, Inoue E, Hara M, Tomatsu T, et al. Beneficial action of statins in patients with rheumatoid arthritis in a large observational cohort. The Journal of Rheumatology. 2007;34(5):964–68. [PubMed] [Google Scholar]
- [27].Maki-Petaja KM, Booth AD, Hall FC, Wallace SM, Brown J, McEniery CM, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. Journal of the American College of Cardiology. 2007;50(9):852–58. doi: 10.1016/j.jacc.2007.04.076. [DOI] [PubMed] [Google Scholar]
- [28].de Jong HJ, Klungel OH, van Dijk L, Vandebriel RJ, Leufkens HG, van der Laan JW, et al. Use of statins is associated with an increased risk of rheumatoid arthritis. Annals of the Rheumatic Diseases. 2012;71(5):648–54. doi: 10.1136/ard.2011.155622. [DOI] [PubMed] [Google Scholar]
- [29].Villafradez-Diaz M, Santiago-Casas Y, Nieves-Plaza M, Morales M, Rodriguez V, Rios G, et al. Association of the use of statins with disease activity and functional status in Puerto Ricans with rheumatoid arthritis. Puerto Rico Health Sciences Journal. 2014;33(1):3–8. [PMC free article] [PubMed] [Google Scholar]


