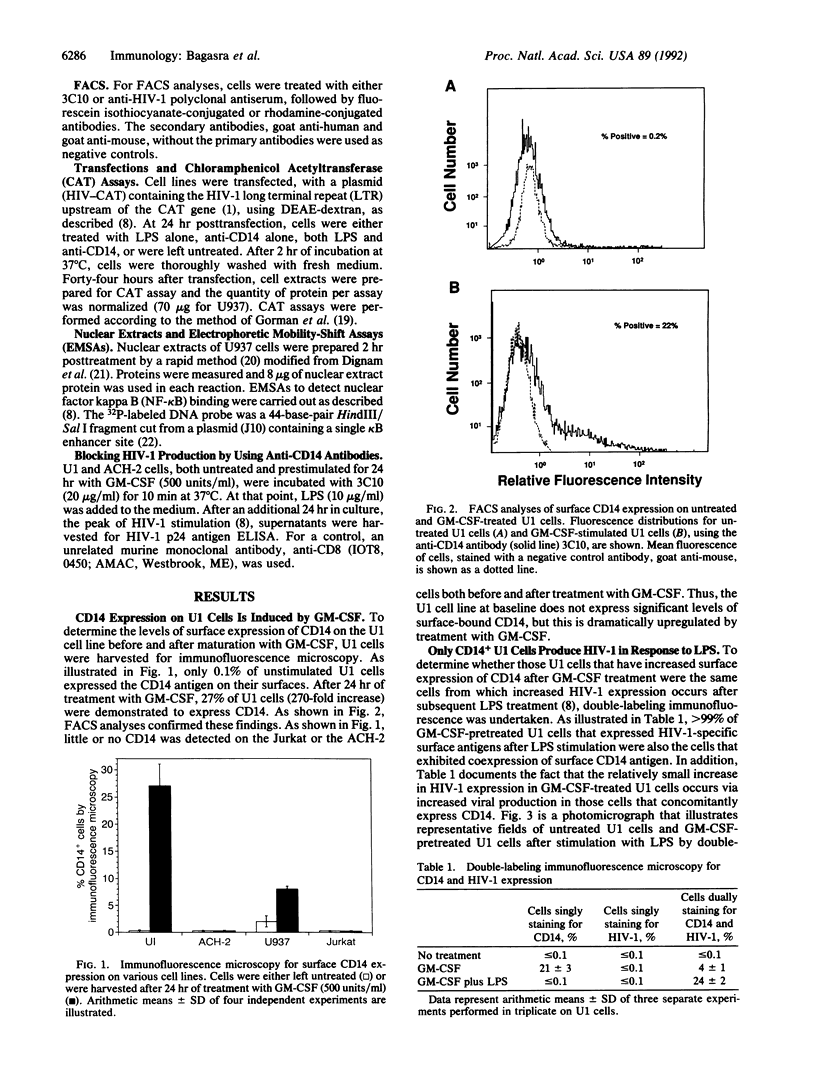
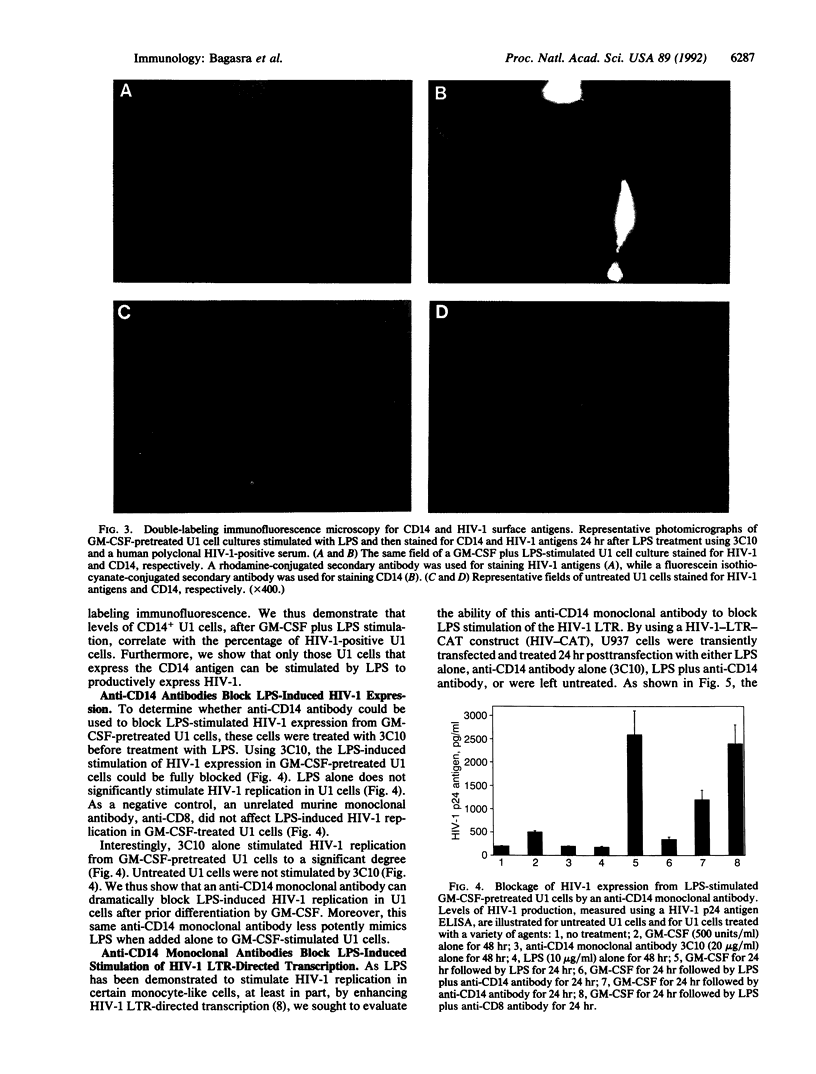
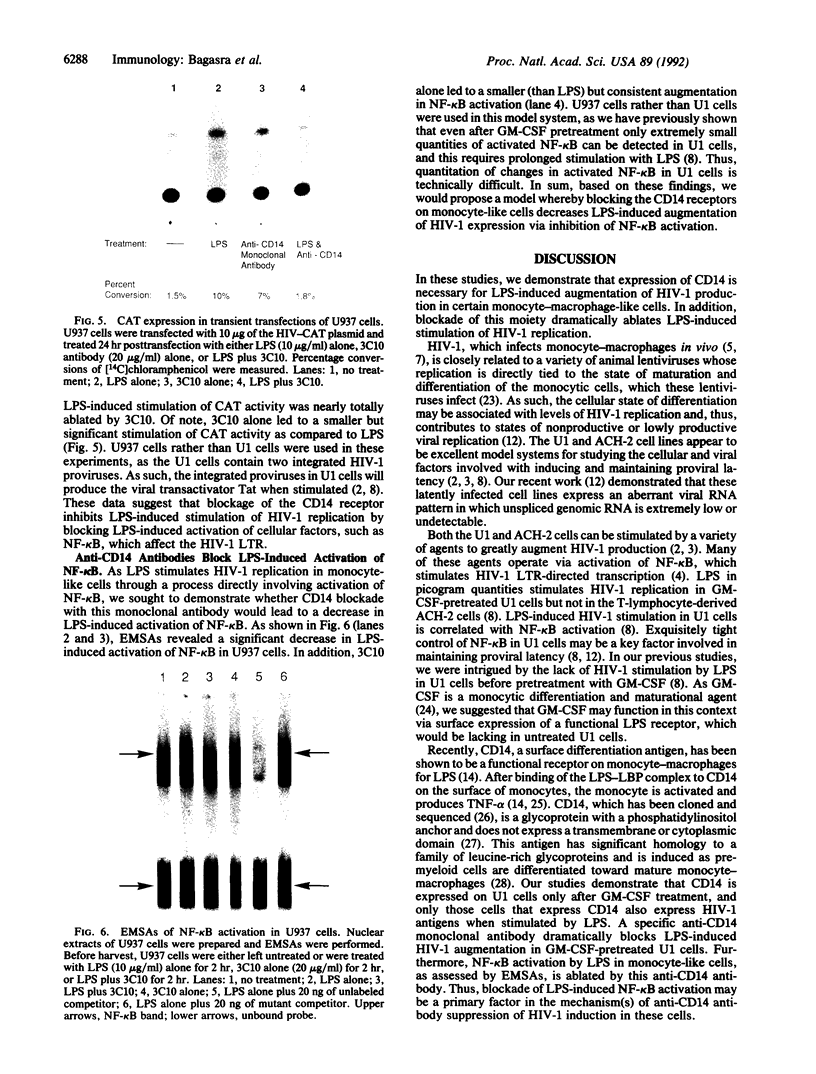
Abstract
Lipopolysaccharide (LPS) potently stimulates human immunodeficiency virus type 1 (HIV-1) long terminal repeat-directed transcription in transfected monocyte-macrophage cell lines and dramatically increases HIV-1 production in the latently infected monocyte-macrophage-like cell line U1. This response to LPS, however, can only be observed after pretreatment of the U1 cells with granulocyte-macrophage colony-stimulating factor (GM-CSF). CD14, the differentiation antigen that acts as a receptor for complexes of LPS and LPS-binding protein, is now demonstrated to be involved in LPS-induced stimulation of HIV-1 replication. CD14 is shown to be expressed on a subpopulation of U1 cells only after treatment with GM-CSF and correlates with HIV-1 production stimulated by LPS. Importantly, only those U1 cells that express CD14 can be induced by LPS to upregulate HIV-1 production. In addition, a monoclonal antibody directed against CD14 can block LPS-induced stimulation of HIV-1 production from these latently infected cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida Y., Pabst M. J. Priming of neutrophils by lipopolysaccharide for enhanced release of superoxide. Requirement for plasma but not for tumor necrosis factor-alpha. J Immunol. 1990 Nov 1;145(9):3017–3025. [PubMed] [Google Scholar]
- Clouse K. A., Powell D., Washington I., Poli G., Strebel K., Farrar W., Barstad P., Kovacs J., Fauci A. S., Folks T. M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989 Jan 15;142(2):431–438. [PubMed] [Google Scholar]
- Cullen B. R., Greene W. C. Regulatory pathways governing HIV-1 replication. Cell. 1989 Aug 11;58(3):423–426. doi: 10.1016/0092-8674(89)90420-0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E., Jr, Corwin L. M. The essential role of the liver in detoxification of endotoxin. Ann N Y Acad Sci. 1966 Jun 30;133(2):668–684. doi: 10.1111/j.1749-6632.1966.tb52397.x. [DOI] [PubMed] [Google Scholar]
- Ferrero E., Goyert S. M. Nucleotide sequence of the gene encoding the monocyte differentiation antigen, CD14. Nucleic Acids Res. 1988 May 11;16(9):4173–4173. doi: 10.1093/nar/16.9.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero E., Hsieh C. L., Francke U., Goyert S. M. CD14 is a member of the family of leucine-rich proteins and is encoded by a gene syntenic with multiple receptor genes. J Immunol. 1990 Jul 1;145(1):331–336. [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Haziot A., Chen S., Ferrero E., Low M. G., Silber R., Goyert S. M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988 Jul 15;141(2):547–552. [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Pomerantz R. J., Kaplan J. C. Pathogenesis of infection with human immunodeficiency virus. N Engl J Med. 1987 Jul 30;317(5):278–286. doi: 10.1056/NEJM198707303170505. [DOI] [PubMed] [Google Scholar]
- Kornbluth R. S., Oh P. S., Munis J. R., Cleveland P. H., Richman D. D. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989 Mar 1;169(3):1137–1151. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Vadas M. A., Lopez A. F., Williamson D. J., Wong G. G., Clark S. C., Wang E. A. Biologic properties in vitro of a recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1986 Jan;67(1):37–45. [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., Lenardo M., Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz R. J., Feinberg M. B., Trono D., Baltimore D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J Exp Med. 1990 Jul 1;172(1):253–261. doi: 10.1084/jem.172.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz R. J., Kuritzkes D. R., de la Monte S. M., Rota T. R., Baker A. S., Albert D., Bor D. H., Feldman E. L., Schooley R. T., Hirsch M. S. Infection of the retina by human immunodeficiency virus type I. N Engl J Med. 1987 Dec 24;317(26):1643–1647. doi: 10.1056/NEJM198712243172607. [DOI] [PubMed] [Google Scholar]
- Pomerantz R. J., Trono D., Feinberg M. B., Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990 Jun 29;61(7):1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- Pomerantz R. J., de la Monte S. M., Donegan S. P., Rota T. R., Vogt M. W., Craven D. E., Hirsch M. S. Human immunodeficiency virus (HIV) infection of the uterine cervix. Ann Intern Med. 1988 Mar;108(3):321–327. doi: 10.7326/0003-4819-108-3-321. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Schütt C., Ringel B., Nausch M., Bazil V., Horejsí V., Neels P., Walzel H., Jonas L., Siegl E., Friemel H. Human monocyte activation induced by an anti-CD14 monoclonal antibody. Immunol Lett. 1988 Dec;19(4):321–327. doi: 10.1016/0165-2478(88)90162-9. [DOI] [PubMed] [Google Scholar]
- Skolnik P. R., Pomerantz R. J., de la Monte S. M., Lee S. F., Hsiung G. D., Foos R. Y., Cowan G. M., Kosloff B. R., Hirsch M. S., Pepose J. S. Dual infection of retina with human immunodeficiency virus type 1 and cytomegalovirus. Am J Ophthalmol. 1989 Apr 15;107(4):361–372. doi: 10.1016/0002-9394(89)90659-4. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Tobias P. S., Ulevitch R. J., Ramos R. A. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989 Oct 1;170(4):1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]