Abstract
The genetic modification and characterization of T-cells with chimeric antigen receptors (CARs) allow functionally distinct T-cell subsets to recognize specific tumor cells. The incorporation of costimulatory molecules or cytokines can enable engineered T-cells to eliminate tumor cells. CARs are generated by fusing the antigen-binding region of a monoclonal antibody (mAb) or other ligand to membrane-spanning and intracellular-signaling domains. They have recently shown clinical benefit in patients treated with CD19-directed autologous T-cells. Recent successes suggest that the modification of T-cells with CARs could be a powerful approach for developing safe and effective cancer therapeutics. Here, we briefly review early studies, consider strategies to improve the therapeutic potential and safety, and discuss the challenges and future prospects for CAR T-cells in cancer therapy.
Adoptive immunotherapy for cancer has a long and somewhat checkered history; the first observations that immune system engagement had antitumor effects are commonly attributed to William Coley, who observed the regression of sarcoma following severe bacterial infections in the 1890s (1). However, the seminal finding that hematopoietic stem cell transplantation (HSCT) using syngeneic donors was less effective at preventing relapse of leukemia compared with sibling donors provided the founding rationale for adoptive T-cell therapy (2). Additionally, the direct isolation and ex vivo activation of the tumor-infiltrating lymphocytes (TILs) was tested in multiple early-phase studies and resulted in durable responses in melanoma (3).
Recently, laboratory studies of chimeric antigen receptor (CAR)–specific T-cells have been viewed with exceptional interest for clinical development at an array of academic institutions. The redirection of T-cells to tumor antigens by expressing transgenic chimeric antigen receptors takes advantage of potent cellular effector mechanisms via human leukocyte antigen (HLA)–independent recognition. The potential of this approach has recently been demonstrated in clinical trials, wherein T-cells expressing CAR were infused into adult and pediatric patients with B-cell malignancies, neuroblastoma, and sarcoma (4–12). We discuss below the important progress that has been made in this young field and the challenges that remain. We also describe recent impressive clinical outcomes using CAR-modified T-cells, which have generated a great deal of excitement.
Chimeric Antigen Receptors
Anatomy of CARs
CARs are recombinant receptors that typically target surface molecules (13). CARs are typically composed of an extracellular antigen-recognition moiety that is linked, via spacer/hinge and transmembrane domains, to an intracellular signaling domain that can include costimulatory domains and T-cell activation moieties. CARs recognize unprocessed antigens independently of their expression of major histocompatibility antigens, which is unlike the physiologic T-cell receptors (TCRs). Hence, CAR T-cells can circumvent some of the major mechanisms by which tumors avoid major histocompatibility class (MHC)–restricted T-cell recognition such as the downregulation of HLA expression or proteasomal antigen processing, two mechanisms that contribute to tumor escape from TCR-mediated immunity (14–16). Another feature of CARs is their ability to bind not only to proteins but also to carbohydrate (17,18), ganglioside (19,20), proteoglycan (21), and heavily glycosylated protein (22,23), thereby expanding the range of potential targets. CARs typically engage the target via a single-chain variable fragment (scFv) derived from antibodies, although natural ligands (known as first-generation CARs) and Fabs fragment (Fab) selected from libraries have also been used (24). Individual scFvs derived from murine immunoglobulins are normally used. However, human antimouse antibody responses can occur and block antigen recognition by CARs when CAR-modified T-cells are transferred into patients. In addition to antigen-specific approaches, two “universal” CAR systems have recently been reported. These CARs house avidin (25) or antifluorescein isothiocyanate (FITC)–specific scFvs (26) that confer the recognition of tumors with biotinylated or bound FITC–conjugated monoclonal antibodies. Recently, some studies (27) have described the design of a dual-specific CAR designated a “TanCAR,” which recognizes each target antigen individually and provides full T-cell activation upon encountering both antigens by incorporating two antigen recognition moieties in tandem separated by a flexible linker.
The second element within a CAR molecule is the structure of the spacer/hinge domain between the targeting moiety and the T-cell plasma membrane (28). Commonly used sequences are derived from IgG subclasses such as IgG1, IgG4, and IgD and CD8 domains (22,29), of which IgG1 has been the most extensively used (30). The extracellular domain spacer/hinge profoundly affects CAR function and scFv flexibility. Notably, although some CARs require hinge regions for optimal function, others do not (31–33). Indeed, the distance between the T-cell and the tumor cell is influenced by the position of the epitope and the length of the spacer regions, and this affects the tumor recognition and signaling of T-cell cytokine production and proliferation and can also affect synapse formation between the T-cell and target cell (34). Similar to the spacer/hinge domain, the CAR transmembrane (TM) domain also impacts the CARs’ expression on the cell surface. Accordingly a variety of TM domains are derived from T-cell molecules such as CD3ζ (35), CD4 (36, 37), CD8 (38, 39), or CD28 (40). Fusion molecules that incorporate a CD28 TM domain lead to high expression of CAR compared with CD3ζ TM domains (40). Although little is known about the definitive principles of the spacer/hinge regions and the TM regions, the design of CARs for targeting novel antigens must take these aspects into account. Studies suggest that for many target molecules, spatial constraints are able to affect antigen binding and that the nonsignaling extracellular spacer and the TM domain can be critical determinants in optimizing CAR design.
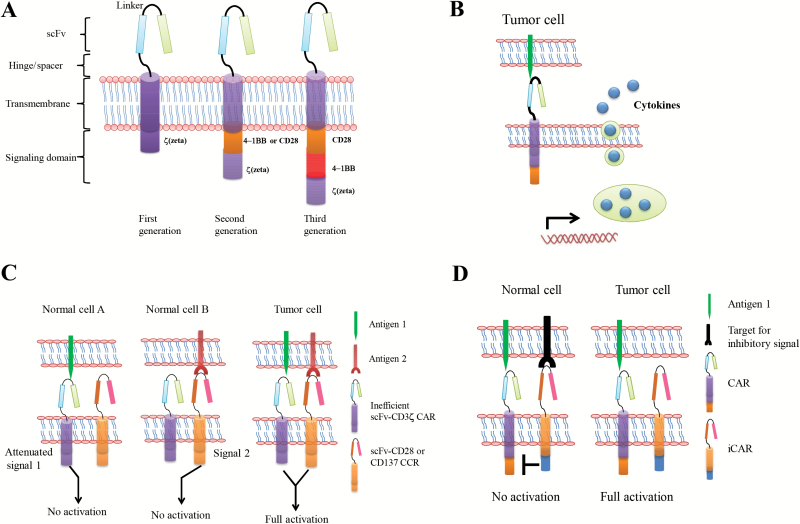
Of crucial importance for CAR design is the intracellular signaling modules, which are derived from lymphocyte signal-initiating molecules. These so-called first-generation CAR designs include the ζ-chain of the TCR/CD3 complex and the γ-chain of the high-affinity receptor for immunoglobulin E (FcεRI), which were shown to initiate the phosphatidylinositol and tyrosine kinase cascade, thus leading to gene transcription, cell activation, and cellular responses to diseased cells (41–43). Tumor cells can induce antigen-specific tolerance or anergy based on MHC class I–restricted antigen presentation and a simultaneous lack of costimulatory ligands. CAR design therefore aims to provide the appropriate costimulatory signals to activate effector T-cells, and improved responses can be achieved through the incorporation of the costimulatory signal (known as second-generation CARs), which may include ICOS (inducible costimulatory), OX40 (CD134), CD28, 4-1BB (CD137), CD27, DAP10, or other costimulatory domains alone and in tandem with CD3ζ (44–49) (Figure 1A). Some studies have demonstrated that CARs that provide costimulatory signaling enhance cytokine production and enable sequential rounds of T-cell proliferation in response to tumor-associated antigen (TAA) in vitro and in vivo compared with CARs that contain CD3ζ in isolation (44–48). Similarly, some studies have shown that 4-1BB signaling ameliorates CAR T-cell exhaustion induced by persistent CAR signaling (50). Although second-generation CARs enable increased T-cell antitumor activity, even this may not initiate the full signaling capabilities of T-cells. Consequently, third-generation CARs have been developed that include two costimulatory signals, such as CD28, 4-1BB, and CD3ζ, in the CAR gene constructs. These CARs have been shown to enhance cytokine production and tumor growth inhibition in mice (51–53). More recently, some studies focused on optimizing CAR design, showing that “armored CAR T-cells,” which include cytokine-secreting CAR T-cells (54–56) and ligand-modified (4-1BBL [57] and CD40L [58]) CAR T-cells have substantial antitumor activity and can reprogram the immunosuppressive tumor microenvironment. Fourth-generation CAR T-cells redirected for universal cytokine killing (TRUCKs) have been described, where the vector encoding the CAR construct also possesses a cytokine expression cassette (Figure 1B). The cytokine, usually a pro-inflammatory cytokine, may be constitutively produced or induced once the T-cell is activated by the CAR in the target tissue. The basic mechanism is that CAR T-cells, when activated by their CAR, deposit pro-inflammatory cytokine in the targeted tumor lesion, which in turn attracts an innate immune cell response toward those cancer cells that are invisible to CAR T-cells (54,55). In an alternate strategy, CAR T-cells that were modified to constitutively express CD40L or 4-1BBL demonstrated enhanced tumor efficacy and had a profound effect on the tumor microenvironment. These properties are likely to be useful to translate this promising immunotherapy to solid tumors.
Figure 1.
Schematic representation of the chimeric antigen receptor (CAR) structure. A) CARs target surface antigens in a major histocompatibility class–independent manner and are comprised of an extracellular portion typically derived from an antibody and intracellular signaling modules derived from T-cell signaling proteins. First-generation CARs contain a single cytoplasmic domain. Second- and third-generation CARs contain combinations of signaling domains. B) CAR T-cells redirected for universal cytokine killing (TRUCKs) employ a vector encoding the CAR construct that also possesses a cytokine expression cassette. These cytokines such as IL-12 can effectively recruit other components of the immune system to enhance the antitumor immune response toward those cancer cells that are invisible to CAR T-cells. C) To increase the specificity of the CAR T-cells, T-cell signal 1 is separated from signal 2. Both target antigens that are expressed on tumor cells must be engaged to deliver signals 1 and 2 and fully activate CAR T-cells. Normal cells that express only one of two antigens do not signal T-cells sufficiently to accomplish full activation. D) A CAR that delivers a dominant inhibitory signal such as PD-1 and CTLA-4 is coexpressed with a CAR capable of full T-cell activation. Engaging both antigens on normal cells could inhibit T-cell function, whereas encountering only the activating ligand on tumor cells generates a sustained T-cell response. CAR = chimeric antigen receptor; CCR = chimeric costimulatory receptor; iCAR = inhibitory CAR.
Gene Transfer of CARs
For CAR therapeutic approaches, gene transfer technology has also rapidly developed, with an expanding series of gene transfer platforms now available that can efficiently introduce the CAR transgene cassettes into primary T-cells. Approaches for the introduction of the CAR transgene use either nonviral gene transfer of DNA plasmids, in vitro-transcribed mRNA species, or viral-mediated transduction. Nonviral-based DNA transfection was initially used because of its low immunogenicity and low risk of insertional mutagenesis. Although this approach was safe, the cells were short-lived after the transfer, likely owing to the long-term culture and the antibiotic selection for T-cells bearing the stable integrants (59,60). Transposon-based systems, which can integrate transgenes more efficiently than plasmids that do not contain an integrating element (61,62), are starting to be evaluated in the context of CAR therapy (63). The relative advantages and disadvantages of these different integrating systems have not been elucidated but will depend on safety, CAR expression levels, ease of manufacturing usage, and cost. Transposon/transposase systems such as Sleeping Beauty (SB) (64,65) and piggyback (66,67) can lead to the stable integration of a transgene; however, these systems may be less effective overall. Alternative approaches that do not depend on transgene integration and that use RNA electroporation (68,69) lead to transient CAR expression. Clinical trials in which solid tumors are treated with RNA-electroporated CAR T-cells have been reported (70,71), and the safety and efficacy results will provide valuable information for future cancer therapies using genetically modified T-cells. Currently, virtually all CAR studies have depended on retroviral vectors, including gammaretroviral and lentiviral vectors (72). Most retroviral vectors are derived from murine leukemia virus or human immunodeficiency virus-1. Gammaretroviruses that efficiently and permanently transduce T-cells have preliminarily been proven safe for use to transfer CARs into primary human T-cells with a proven ability to exert a therapeutic effect (4). Lentiviral vectors also efficiently and permanently transduce T-cells and hold particular appeal in light of their potential to transduce nondividing cells (73). Unlike hematopoietic stem cells (74,75), T-cells seem highly resistant to retroviral vector-induced transformation (76–79).
Approaches to T-Cell Culture Production
There are important differences in the T-cell expansion processes employed at different centers (Table 1 and 2). CAR T-cells are generally produced within 10 days to three weeks of the ex vivo culture (5,80,81) although some studies required a longer culture time (82). T-cells or virus-specific T-cells in recent clinical trials are commonly activated and expanded using anti-CD3 antibody in combination with either anti-CD28 (83,84) antibody costimulation or coculture with peripheral blood mononuclear cells derived from antilogous patients (7,80) or donors (85,86). While the dose of CAR T-cells that is the most effective for treating a patient with advanced cancer remains unknown, clinical adoptive T-cell therapy studies have shown that a greater number of cells is preferable, with up to 1011 T-cells infused into the patient (87). To date, IL-2 has been the cytokine of choice to drive this ex vivo T-cell expansion. However, the in vitro culture process can also be adapted to modify T-cells for the desired effector function by selectively adding other common γ-chain cytokines, for example, IL-7 and IL-15, to the culture media during the ex vivo culture (88,89). Some studies showed that IL-7 and IL-15 both resulted in cultured T-cells and successfully expanded CAR T-cells with a more favorable T-memory stem cell phenotype (90). These studies are likely to influence the manufacturing of CAR T-cells as investigators seek to encode CARs into T-cells that preserve the functional capacity of T memory stem cells.
Table 1.
Published results from clinical trials of CAR T-cells targeting hematologic malignancies
| Reference | Antigen | Gene-transfer vector used | Endomains | Cell culture | Cell dose | Conditioning regimen | Cytokine support | No. of patients | Responses to CAR T-cells | Persistence |
|---|---|---|---|---|---|---|---|---|---|---|
| Till 2008 (161) | CD20 | Electroporation | CD3ζ | 30ng/mL OKT3+50U/mL IL-2; 2–4 mo | 108/m2 to 3.3×109/ m2 (3 infusions 2–5 d apart | CVP, FND, none, 131I-tositumomab | IL-2 twice daily for 14 d in last 4 patients | 9 enrolled, 7 treated (7 FL) | 2 NED, 1 PR, 4 SD | 5–21 d alone, 5–7wk with IL-2 |
| Jesen 2010 (82) | CD19 or CD20 | Electroporation | CD3ζ | 30ng/mL OKT3+ 25U/mL IL-2 + irradiated LCL feeders; approximately 106 d | 108/m2 to 2×109/ m2 (3–5 infusions) | 25mg/m2 fludarabine× 5 for FL | 5x105 IU/ m2 BID IL-2 for 5 d in FL | 4 (2 FL, 2 DLBCL) | Two patients continues to be in remission after autologous HSCT | 24h to 7 d |
| Savoldo 2011 (81) | CD19 | Gammaretrovirus | CD3ζ vs CD28- CD3zeta | OKT3+100U/mL IL-2; 6–18 d | 2×107/m2-2×108/ m2, 1–2 infusions | None | None | 6 (NHL) | 2 SD, 4 PD | CD28-CD3zeta persistented 4wk to 6 mo |
| Brentjens 2011 (80) | CD19 | Gammaretrovirus | CD28-CD3ζ | CD3/CD28 beads (3:1) + 100U/mL IL-2; 11–19 d | 0.4×107 CART- cells/kg-3×107 CART-cells/kg, 2–3 infusions | None or 1.5 and 3g/m2 cyclophosphamide | None | 9 (8 CLL and 1 ALL) | 1 death, 1 reduction in lymphadenopathy, 1 B-cell aplasia, 1 PD, 2 SD, 3 no objective response | 1–8 wk |
| Poter 2011 (5); Kalos 2011* (6) | CD19 | Lentiviral | CD137- CD3ζ | CD3/CD28 beads + 100U/mL IL-2; 10±2 d | 1.46×105 CART- cells/kg; 1×107 CART-cells/kg; 1.6×107 CART- cells/kg | Bendamustine or 4mg/m2 pentostatin/ 600mg/ m2 cyclophosphamide | None | 3 CLL | 2 CR, 1 PR | Up to 180 d |
| Kochenderfer 2010 (162); Kochenderfer 2012 (97) | CD19 | Gammaretrovirus | CD28- CD3ζ | 50ng/mL OKT3+ 300 IU/mL IL-2; 24 d | 0.3–3×107 CART-cells/kg (3 infusions) | 60mg/kg cyclophosphamide×2 (day-7 and day-6) and fludarabine 25mg/ m2×5 (day - 5 to day -1) | 720000 IU/ kg every 8h as toletated | 8 (3 FL, 4 CLL, 1 SMZL) | 1 death with influenza, 1 PD, 5 PR, 1CR | Up to 3–6 mo |
| Till 2012 (101) | CD20 | Electroporation | CD137- CD28- CD3ζ | 30ng/mL OKT3+50U/mL IL-2; >69 d | 1x108-3.3x109/ m2 (3 infusions) | 1000mg/m2 cyclophosphamide for 2 d | 250000 IU/ m2 IL-2 for 14 d | 3 (2 MCl, 1 FL) | 2 patients had no evidence of disease progression, 1 delayed PR | Up to 9–12 mo |
| Brentjens 2013 (7); Davila 2014 (98) | CD19 | Gammaretrovirus | CD28- CD3ζ | CD3/CD28 beads (3:1) + 100U/mL IL-2; 11–19 d | 1.5–3 x108 CAR T-cells/kg (2 infusions) | 1.5 - 3g/m2 cyclophosphamide | None | 16 adults with ALL | 12 MRD negative; 7 went to allo-HSCT | Lasted 21 d - 56 d |
| Groop 2013 (8) | CD19 | Lentiviral | CD137- CD3ζ | CD3/CD28 beads + 100U/mL IL-2; 10±2 d | 1.4 x106 and 1.2 x107 CAR T-cells/kg (a single dose) | One with etoposide- cyclophosphamide and one with none | None | 2 children with ALL | 2 CR; one is ongoing at 11 mo after treatment, one relapsed with CD19 negative after 2 mo | Approximately persisted 2 mo and 6 mo |
| Ritchie 2013 (102) | Lewis-Y | Gammaretrovirus | CD28- CD3ζ | 30ng/mL OKT3 and 600 IU/mL IL-2; 12 d | 1.48–9.2 x106 CAR T- cells/kg | Fludarabine 30mg/m2×5 and cytarabine 2g/m2×5 | None | 5 enrolled (4 treated), AML | 2 SD, 1 transient reduction in blasts, 1 transient cytogenetic remission | Persisted up to 10 mo |
| Cruz 2013 (86) | CD19 | Gammaretrovirus | CD28- CD3ζ | Ad5f35pp65- transduced EBV- LCLs + 100U/mL IL-2; 5–6 wk | 1.9 x107 - 1.13 x108 T-cells (allogeneic donor derived) (3 infusion) | None | None | 8 (4 ALL and 4 CLL) | 2 CCR, 1 CR, 1 PR, 1 SD, 3 PD | Persisted for a median of 8wk in blood and up to 9wk at disease sites |
| Kochenderfer 2013 (85) | CD19 | Gammaretrovirus | CD28- CD3ζ | 50ng/mL OKT3+ 300 IU/mL IL-2; 8 d | 0.4–7.8 x106 CAR T- cells/kg (allogeneic donor derived) | None | None | 10 (4 CLL, 2 DLBCL, 4 MCL) | 2 PD, 6 SD,1 PR, 1 CR | Minimal numbers of CAR-expressing T-cells were detectable beyond 1 mo after infusion |
| Kochenderfer 2014* (84) | CD19 | Gammaretrovirus | CD28- CD3ζ | 50ng/mL OKT3+ 300 IU/mL IL-2; 10 d | 1–5 x106 CAR T-cells/kg | 120 or 60mg/kg cyclophospha and fludarabine 25mg/ m2×5 | None | 15 (9 DLBCL, 2 indolent lymphomas, 4 CLL) | 8 CR, 4 PR, 1 SD, 2 NE | Peaked between 7 and 17 d after infusion; persisted up to approximately 75 d |
| Lee 2014* (10) | CD19 | Gammaretrovirus | CD28- CD3ζ | 50ng/mL OKT3+ 300 IU/mL IL-2; 11 d | 1 x106/kg and 3 x106/kg | Fludarabine 25mg/ m2×3 and 900mg/m2 | None | 21 children and yong adults (1 DLBCL, 20 ALL) | 12 MRD negative (9 went to allo and 2 relapsed with CD19- negative leukaemia), 1 CRi, 3 SD, 4 PD | Peak expansion occurring around day 14; no CAR T-cells were detected after day 68 in any patient |
| Maude 2014* (9) | CD19 | Lentiviral | CD137- CD3ζ | CD3/CD28 beads + 100U/mL IL-2; 10±2 d | 0.76×106 - 20.6×106 CAR T-cells/kg | Main cyclophospha / fludarabine | None | 30 children and adults with relapsed and refractory ALL | 27 CR (2 patients with blinatumomab- refractory disease and 15 who had undergone allo) | The probability of persistence of CTL019 at 6 mo was 68% |
| Wang 2014 (100) | CD20 | Lentiviral | CD137- CD3ζ | 5 ug/ul OKT3+1000U/ mL IL-2; 10–12 d | 3.6×106 - 23.5×106 CAR T-cells/kg (3–5 infusions) | None, COED, COD, CHODE, ESHAP | None | 7 DLBCL | 1 CR, 3 PR, 1 SD, 1 PD, 1 NE | Up to 220 d |
| Wang 2014 (103) | CD33 | Lentiviral | CD137- CD3ζ | 5 ug/ul OKT3+1000U/ mL IL-2; 13 d | 4.25×108 CAR T-cells (4 infusion) | None | None | 1 AML | Transient reduction in blasts 2wk after infusion; this patient died 13 wk | Approximately 60 d |
| Dai 2015* (99) | CD19 | Lentiviral | CD137- CD3ζ | 500ng/ul OKT3+500U/ mL IL-2; 10–12 d | 0.33×107 - 1.26×107 CAR T-cells/kg (3–5 infusions) | C-MOAD, none | None | 9 adults with relapsed and refractory ALL (6 with extramedullary leukemia involvement) | 3 PD, 2 MRD-, 2 CR in BM and PB with PR of extramedullary lesions, 1 CNS1, 1 hematological improvement and reduction of blast counts of bone marrow | Maintained a high level for more than 6wk, maitained for at least 6–12 wk |
| Poter 2015 (11) | CD19 | Lentiviral | CD137- CD3ζ | CD3/CD28 beads + 100U/mL IL-2; 10±2 d | 0.14×108 - 11×108 CAR T (1–3 infusions) | Fludarabine/ cyclophosphamide, pentostatin/ cyclophosphamide, and bendamustine | None | 18 enrolled, 14 treated (relapsed and refractory CLL) | 4 CR, 4 PR | Up to 14–49 mo in patients with CR |
| Garfall 2015 (104) | CD19 | Lentiviral | CD137- CD3ζ | CD3/CD28 beads + 100U/mL IL-2; 10±2 d | 5×107 CAR T (1 infusions) | Day 12 after ASCT and 140mg/m2 melphalan for cell infusion | None | 1 MM | 1 CR | 47 d |
* Response assessment was done on week 4 (within 4 days) after CAR T-cell infusion. ALL = acute lymphoblastic leukemia; Allo-HSCT = allogeneic hematopoietic stem cell transplantation; AML = acute myeloid leukemia; ASCT = autologous stem cell transplantation; C-MOAD = cyclophosphamide, mitoxantrone, vindesine, cytarabine, and dexamethasone; CCR = continuous complete response; CHODE = cyclophosphamide, doxorubicin, vincristine, dexamethasone, and etoposide; CLL = chronic lymphocytic leukemia; CNS = central nervous system; COED = cyclophosphamide, vincristine, etoposide, and dexamethasone; COD = cyclophosphamide, vincristine, and dexamethasone; CR = complete response; CVP = cyclophosphamide, vincristine, and prednisone; DLBCL = diffuse large B-cell lymphoma; EMV = Epstein-Barr virus; ESHAP = etoposide, carboplatin, high-dose cytosine, and methylprednisolone; FL = follicular lymphoma; FND = fludarabine, mitoxantrone, and dexamethasone; LCL = lymphoblastoid cell line; MCL = mantle cell lymphoma; MM = multiple myeloma; MRD = minimal residual disease; NE = not evaluable; NHL = non-Hodgkin’s lymphoma; PD = progressive disease; PR = partial response; SD = stable disease; SMZL = Splenic marginal zone lymphoma.
Table 2.
Published results from clinical trials of CAR T-cells targeting solid tumors
| Reference | Antigen | Gene-transfer vector used | Endomains | Cell culture | Cell dose | Conditioning regimen | Cytokine support | No. of patients | Responses to CAR T-cells | Persistence |
|---|---|---|---|---|---|---|---|---|---|---|
| Kershaw 2006 (111) | α-folate receptor | Gammaretrovirus | FcRγ | 10ng/mL OKT3+600 IU/mL IL-2; 21–56 d | 3×109-1.69×1011 T-cells (1–3 infusions) | None | IL-2 9(720000 IU/ kg) was given i.v. every 12h in cohort 1 | 14 patients with ovarian cancer | 14 PD | 14–21 d |
| Park 2007 (71) | CD171 | Electroporation | CD3ζ | 30ng/mL OKT3+50U/mL IL-2 + irradiated PBMC/ lymphoblastoid cell line feeders; 14 d (1–3 infusions) | 1×108/m2 -1.1×109/m2 | Salvage chemotherapy | None | 6 children with neuroblastoma | 1 PR, 5 PD | Short (1–7 d) in patients with bulky disease, but significantly longer (42 d) in a patient with a limited disease burden |
| Lamers 2013 (108) | CAIX | Gammaretrovirus | FcRγ | 10ng/mL OKT3+100 IU/mL IL-2; approximately 21 d | 0.2×109-2.1×109 CAR T-cells (5 infusions) | None | 5×105 U/m2 twice daily administered for 20 d | 12 patients with metastatic renal cell carcinoma | 12 NR | Up to 3–5 wk |
| Louis 2011 (20) | GD2 | Gammaretrovirus | CD3ζ | OKT3+100 or 50U/mL IL-2 + irradiated PBMC/ lymphoblastoid or PBMC; 12–18 d and 36–54 d | 2×107/m2 -1×108 CAR T-cells/m2 | None | None | 19 patients with neuroblastoma | 8 NED, 3 CR, 1 PR, 1 SD, 4 PD, 2 tumor necrosis | ≥6 wk |
| Morgan 2010 (107) | HER2 | Gammaretrovirus | CD137- CD28-CD3ζ | 50ng/mL OKT3+300 IU/mL IL-2 (a rapid expansion) procedure: 6000 IU/mL + 50ng/mL OKT3 + irradiated PBMC feeders; 24 d | 1010 T-cells | 60mg/kg cyclophos phamide ×2 and flurodarabine 25mg/m2 ×5 | None | 1 patients with colorectal cancer | Died of cytokine release syndrome | Died 5 d after treatment |
| Brown 2015* (70) | IL13Rα2 | Electroporation | CD3ζ | 30ng/mL OKT3+50U/mL IL-2; approximately 63 d | 9.6×108 - 15.35×108 CD8+ T (11–17 infusions) | None | None | 13 enrolled, 3 treated (glioblastoma) | 3 PD | Up to 184 d |
| Katz 2015† (106) | CEA | Gammaretrovirus | CD28-CD3ζ | 50ng/mL OKT3+3000U/ mL IL-2; 17–25 d | Cohort 1: 10.1×109 CAR T; Cohort 2:30×109 CAR T (3 infusion) | None | Cohort 1: none; Cohort 2: 75 000U/ kg/day | 6 patients with denocarcinoma liver metastases | 5 PD, 1 SD | Approximately 2 wk |
| Ahmed 2015 (12) |
HER2 | Gammaretrovirus | CD28-CD3ζ | OKT3 or CD3/CD28 beads + 100U/mL IL-2; 12–21 d | 1×104/m2 -1×108 CAR T-cells/m2 (1–9 infusions) | None | None | 19 patients with sarcoma | 4 SD | Up to 18 mo |
* Imaging to assess response was performed during the week 3 rest cycle and after week 5. CR = complete response; NED = no evidence of disease; PBMC=peripheral blood mononuclear cell; PD = progressive disease; PR = partial response; SD = stable disease.
† Liver MRI and PET examinations were performed within one month prior to the first infusion and then within one month following the third CART cell infusion.
Use in the Clinic
Hematologic Malignancies
There are 20 publications reporting clinical trials of the use of CAR-modified T-cells in hematological malignancies in acute lymphoblastic leukemia (ALL) by targeting CD19, in chronic lymphoblastic leukemia (CLL) by targeting CD19, in multiple myeloma by targeting CD19, in lymphoma by targeting CD19 or CD20, and in acute myeloid leukemia (AML) by targeting Lewis Y antigen or CD33. The CAR designs, manufacturing processes, and clinical outcomes are summarized in Table 1. These clinical trials followed the same basic steps, including a patient T-cell apheresis, retroviral or lentiviral CAR transduction, T-cell expansion, and conditioning chemotherapy prior to the T-cell infusion. However, each group follows a slightly different protocol, which varies by the following factors: vector design (the same CD28/CD3ζ dual-signaling domain was used at the NCI and Memorial Sloan-Kettering Cancer Center; and 4-1BB/CD3ζ was used at the University of Pennsylvania and PLA General Hospital), infused T-cell product, T-cell manufacturing, the conditioning chemotherapy strategy, cytokine support for the infused T-cells, the tumor targeted, the age of the treated patient population, the timing of the CAR T-cell infusion, the degree of tumor burden at the time of therapy, and the T-cell dosage and derivation (91,92). The effects of the differences in protocols are unclear.
The most investigated target to date is CD19, an appealing target for immunotherapy, as it is uniformly expressed by most of B-cell malignancies but not in normal tissues other than those originating from the B-cell lineage (93,94). Human T-cells expressing different CD19 CARs eradicated systemic B-cell tumor xenografts established in immunodeficient mice (36,46,59,95), effectively paving the way for several ongoing clinical trials. Anti-CD19 CAR T-cells have thus ushered in a new paradigm for evaluating CAR technology. The reported clinical outcomes of CD19-specific CAR T-cells were recently reviewed elsewhere (92,96) and are briefly summarized here.
CLL
CD19-specific CAR T-cells induced a clinically significant response in 40 patients with advanced chemotherapy-refractory and high-risk CLL by groups at the National Cancer Institute (84,85,97), Memorial Sloan-Kettering Cancer Center (80), Baylor College of Medicine (86), and the Abramson Family Cancer Research Institute at the University of Pennsylvania (5,6,11); of the 40 patients, 10 achieved complete remission (CR), 10 achieved partial remission (PR), and five achieved stable disease (SD). Notably, of these 40 patients, eight patients received infusion of allogeneic, rather than autologous, T-lymphocytes engineered to express a CD19-specific CAR without preconditioning at two different medical centers (National Cancer Institute [NCI] and Baylor College of Medicine). Some studies have shown that CD19-specific CAR T-cells containing the CD3ζ activation domain and the 4-1BB (CD137) costimulatory domain proliferated in vivo, eliminated high tumor burdens, and persisted with ongoing functional activity beyond three years (5,6). In one of these studies, 14 patients with relapse and refractory CLL received T-cells expressing a CD19 CAR; the overall response rate in these patients was eight of 14 (57%), with four CR and four PR (11). Other studies have also reported clinically significant responses in CLL patients to T-cells directed with slightly different CAR designs also targeting CD19 but containing a CD28 costimulatory domain (11–15). The reasons for the different outcomes across the 40 patients with CLL treated at four different centers include important differences in the CAR design, lymphodepleting strategy, derivation of T-cells, and selection of chemosensitive patients. These variables are among many (91) that may affect outcome and need further study.
ALL
We and three other groups successively published follow-up studies in adult and pediatric ALL, which are summarized in Table 1. The results obtained at the four different centers all showed a dramatic complete remission rate, a rare occurrence for phase I studies in oncology, particularly considering the poor prognosis of patients with relapsed ALL. Maude et al. reported a 90% CR rate in 30 pediatric and adult patients with ALL treated at Children’s Hospital of Philadelphia (CHOP) and UPenn phase I trials (9). Davila et al. reported an 88% CR rate in 16 adult patients proceeding to allogeneic stem cell transplantation shortly after CAR T-cell treatment with relapsed ALL treated at MSSKC (7,98). Lee et al. reported a 66.7% CR rate in an NCI intent-to-treat analysis of 20 children and young adults with ALL (10). Finally, Dai recently reported a 56% overall survival rate at 18 weeks in nine adult B-ALL patients with extramedullary leukemia and observed that donor-derived anti-CD19 CAR T-cells can cause graft-vs-host disease (GVHD) and the regression of extramedullary B-ALL (99). Forty-six percent of patients in the four studies had a prior history of allogeneic SCT.
Durable remissions were also observed in all four studies but were reported in only approximately half of the patients who ultimately transitioned to allo-SCT in the NCI and MSKCC groups. In the CHOP/Penn medical center, sustained remission was achieved with a six-month event-free survival rate of 67% and a 78% overall survival rate. Finally, in the PLA General Hospital (PLAGH), the rate of overall survival at 18 weeks was 56%. Across the trials and the CAR designs, the ALL patients in our group tended to have lower response rates to CAR T-cell treatment compared with the other groups. The reason for this low response rate may be related to the patient characteristics. The patients in our group had confirmed CNS leukemia and isolated extramedullary leukemia, both of which have been considered high-risk for ALL with a poor prognosis. These results suggest that CD19-directed CAR T-cells can potentially produce durable remission, but a more extensive follow-up is required across trials as differences are likely.
B-ALL remains a challenge because of relapse after CD19-directed T-cell therapy. CD19-positive and CD19-negative blasts have been observed in relapsed patients post-CD19-directed T-cell therapy. A lack of CAR-directed T-cell persistence or a decreased efficiency of CAR T-cells leads to relapse of ALL that retains a CD19-positive blast. Optimized CAR designs (optimal costimulatory domains), gene transfer technologies, optimal formations of the final cell product, and second CAR-19 T-cell infusions may prevent relapse of ALL by boosting T-cell persistence. However, CD19-negative blasts are not prevented by enhanced T-cell persistence. Single-target therapy may select for and spur CD19 escape variants or CD19 antigen loss. The group at the NCI observed that CD19-negative blasts emerged in the two patients treated with CD19-directed therapy who retained the expression of CD22, and based on this retention they developed CAR T-cells targeting the B-cell antigen CD22 that can be used for treating CD19-negative relapse and can be combined with a CD19-modified CAR in the future (32). Combination or tandem CARs targeting both CD19 and CD22 may prevent escape because of antigen loss, but further studies are needed (24).
Lymphoma
Modest clinical responses have been achieved in studies wherein first-generation CARs have been transferred to adoptively transferred lymphocytes for treating lymphoma as summarized in Table 1. However, second- and third-generation CARs showed encouraging clinical outcomes in lymphoma by groups at the Fred Hutchinson Cancer Research Center (101), NCI (84), and PLAGH (100). Our group reported targeting CD20 containing a 4-1BB costimulatory domain in patients with diffuse large B-cell lymphoma and showed one CR, three PR, and one SD in six evaluable patients (100). Till et al. reported using a CD20-directed CD28.4-1BBζ CAR in which two of the three patients with relapsed indolent B-cell and mantle cell lymphomas who received anti-CD20 CAR T-cells remained progression-free for 12 and 24 months, respectively, while the third patient experienced an objective partial response (101). Finally, Kochenderfer et al. (84) reported the clinical activity of autologous T-cells modified to express a CD19-directed CAR in nine patients with diffuse large B-cell lymphoma (DLBCL) and two patients with indolent lymphomas. They showed that seven with DLBCL were evaluable, with four patients achieving complete response and the other two patients with indolent lymphomas achieving one CR and one PR (84). Taken together, these clinical studies are highly encouraging, with many reports of objective clinical responses in patients with advanced refractory lymphoma.
AML
Two groups reported on the feasibility of CAR T-cell therapy in AML. Richie et al. conducted a study of T-cells redirected against the Lewis Y antigen in four AML patients and showed that two patients achieved SD, one patient achieved a transient reduction in blasts, and a fourth patient showed transient cytogenetic remission (102). The other study, conducted by our group, employed an anti-CD33 CAR and reported a transient reduction in blasts in one patient with advanced AML (103). These two studies demonstrate the safety and potential utility of CAR T-cells for treating AML.
Multiple Myeloma
More recently, Garfall et al. tested the therapeutic potential of autologous T-cells modified to express a CD19-specific CAR (104) in one patient with refractory multiple myeloma previously subjected to myeloablative chemotherapy (melphalan, 140mg/m2) and autologous transplantation (Table 1). The patient experienced ongoing complete clinical and molecular remissions despite the absence of CD19 expression in most neoplastic cells. Although this is only a case report and further information and validation are necessary, the use of anti-CD19 CAR T-cells in conjunction with autologous transplantation is regarded as a promising strategy for treating multiple myeloma.
Solid Tumors
Despite several case series having reported clinically significant responses in patients with CD19-positive malignancies, clinical experience targeting solid tumor antigens with CAR T-cells is considerably more limited (Table 2)(4,12,20,70,71,105–111). Most trials using first-generation CAR T-cells that are specific to solid tumors have failed to achieve effective antitumor responses (70–71, 111) although one trial with 19 neuroblastoma patients treated with CAR T-cells specific for the GD2 ganglioside showed 3 CRs (20). Encouraging clinical reports (12) are also emerging from other centers using HER2.CD28.ζ-CAR in 19 patients with metastatic or recurrent HER2-positive sarcoma, with four patients experiencing disease stabilization for 12 weeks to 14 months. However, CAR T-cells face a unique set of challenges in the case of solid tumors. Some of the key issues appear to be the absence of unique tumor-associated antigens, the inefficient homing of T-cells to tumor sites, and the limited persistence of CAR T-cells. Moreover, the immunosuppressive microenvironment within the tumor tends to strongly inhibit CAR T-cell function. Despite a large number of investigated targets, few are truly tumor specific or ubiquitously expressed on tumor cells but not expressed on normal cells. Targets with merely higher levels of expression on tumor cells than on normal tissues have been selected as potential alternatives; and, while this approach raises safety concerns (112,113), it is worth pursuing. While a seemingly herculean task, fulfilling all of the above requirements can be accomplished effectively through both intrinsic and/or extrinsic modifications of CAR T-cells. The specific issues that help CAR T-cells to achieve their full therapeutic potential in clinical studies and developing solutions are discussed below.
CAR Safety
On-Target, Off-Tumor Toxicity
A major concern is the risk of “on-target, off-tumor” toxicity, resulting in the immune-mediated destruction of normal tissues that express the targeted antigen. B-cell aplasia is an expected on-target result of successful CD19-specific CAR T-cell therapy (5,80,97) and has served as a useful pharmacodynamic marker of CAR T-cell functional persistence. Fortunately, B-cell aplasia can be effectively managed by infusion with gamma globulin as replacement therapy. One recent case report of acute toxicities associated with CAR T-cell infusion described a patient who received a third-generation HER2-specific CAR (107), which is expressed at a low level in several normal tissues, including the heart and pulmonary vasculature. Similarly, there have been clinical trials of “on-target, off-tumor” toxicities following CAR T-cell treatment, the most informative example being the cholestatic effect of T-cells specific for carbonic anhydrase IX (110). Other examples, for which no toxicities have been reported to date, include CD171-, GD2-, CEA- and IL13Rα2-redircted CAR T-cell therapy (4,70,71,106). Surprisingly, in our study (99) grade 2–3 GVHD was observed in patients with mixed chimerism who received substantial donor-derived anti-CD19 CAR T-cells three to four weeks after cell infusions. These results indicate that the toxicity management of GVHD for enrolled patients with mixed chimerism treated with donor-derived CAR-modified T-cells must be conducted with extreme caution (99). To avoid untoward outcomes, it is imperative to choose highly tumor-specific antigen molecules to target and to refine the affinity and specificity of the CAR, the cell dose, and the conditioning regimens used prior to cell infusion.
Cytokine Release Syndrome
The second major concern is that of “cytokine release syndrome” associated with CAR T-cell therapy. Large numbers of activated T-cells can produce cytokine release syndrome (CRS), which manifests in high fever, hypotension, and hypoxia, potentially resulting in organ failure, and is related to production of several proinflammatory cytokines, including IL-6, TNFα, and IFNγ, secondary to CAR T-cell activation. Some studies have proposed C-reactive protein as an indicator of severe CRS (98); however, although high C-reactive protein (CRP) is associated with the severity of CRS in several studies, the assessment of its use as a predictive biomarker is ongoing (9,114). The clinical and laboratory manifestations overlap with those of the macrophage activation syndrome (MAS) (8). Fortunately, IL-6 blockade using the IL-6 receptor antagonist tocilizumab is now used off-label to control severe CRS/MAS without compromising T-cell efficacy (8). In addition to using cytokine-blocking drugs, some studies have used steroids to treat CRS (8,10,98). Another important observation is that the severity of CRS may be directly associated with the tumor burden at the time of infusion of the CAR-directed T-cells (9,10). This association indicates that either infusing CAR T-cells into patients earlier in the course of their disease or using pre-infusion conditioning chemotherapy combined with intensive chemotherapy to reduce the tumor burden may substantially reduce the risk of severe CRS.
Neurologic Toxicities
Neurologic symptoms including delirium, dysphasia, akinetic mutism, and seizures have been reported in a handful of ALL patients treated with anti-CD19 CAR T-cells in some trials (9,10,98,99). These symptoms are self-limited, resolving over several days without intervention or apparent long-term sequelae. Although certainly associated with the presence of CAR T-cells in the spinal fluid of most patients, these symptoms do not appear to be prevented by IL-6 blockade. The mechanism of these symptoms is unclear and warrants careful and greater investigation.
Strategies to Improve CAR Safety
Because CAR-modified T-cells can elicit a remarkable antitumor response as well as severe toxicities, strategies to improve CAR safety may become necessary (Figure 2). Transfecting T-cells with mRNA encoding the CAR to provide transient expression of the CAR in transferred T-cells may be useful for alleviating immediate toxicity (115). Additionally, suicide genes encoded into the transduced cells as a countermeasure have been evaluated in two caspase-based systems: herpes simplex virus–derived enzyme thymidine kinase (HSV-tk) and inducible caspase-9. Both systems selectively and efficiently eliminated the vast majority of transferred T-cells (116,117). However, these two approaches have two drawbacks, namely that the transient expression of the CAR may sensitize the patient to the CAR (118) and that the induced transgene may also be potentially immunogenic (119–121). A novel fully human CAR may overcome the issues of transgene immunogenicity and has been tested in an animal model (122), but the animal model development remains in its early stages. Some groups have employed an alternate approach based on the human epidermal growth factor receptor (EGFR) to generate a small, truncated derivative (tEGFR). tEGFR can function as a cell marker for elimination by antibody-dependent, cell-mediated cytotoxicity upon cetuximab treatment (123). Alternatively, some lessons learned from nature may be applied to the synthetic biology of the CAR. One strategy to increase the specificity of the CAR is to split the signals required to activate the transduced T-cells between two separate CAR molecules (Figure 1C). This strategy has proven to be successful in vitro and in mouse models, wherein the primary antigen receptor is shared by the tumor and some normal cells but is designed to have low affinity and delivers an attenuated signal. A second CAR target was specified for an antigen on the tumor, but not the normal cells, with the target for delivering costimulatory signals (124). However, the transferred T-cells only eliminated the target cells that expressed a combination of antigens. An alternative strategy for achieving tumor specificity is to employ both activating and inhibitory CAR (iCAR) (Figure 1D). In a preclinical model, the co-expression of two CARs that operate as logic gates was demonstrated, such that signaling through the activating receptor is inhibited when the CAR T-cells encounter a normal cell (125), thereby improving tumor selectivity. More recently, some studies using a strategy to adjust the affinities of the scFv component of CAR to discriminate malignant from normal cells based on the density of target antigen expression have demonstrated a CAR with reduced affinity, enabling T-cells to distinguish tumor overexpressing the target from normal cells that express it at physiologic levels (126,127). Moreover, reducing tissue trafficking via the incorporation of a chemokine receptor (128) into effector cells or the administration of a drug blocking CCR5 (129) may alleviate toxicity. Overall, alongside the development of scientific technology to control CRS and equip CAR T-cells to prevent on-target off-tumor toxicities or eliminate activated T-cells, the safety profile of CAR T-cell therapy has improved greatly.
Figure 2.
Strategies to improve chimeric antigen receptor (CAR) T-cell therapy. There are various potential strategies to genetically modify T-cells for adoptive therapy to improve CAR T-cell efficacy and safety. Ultimately, combination therapy can be used to enhance the therapeutic potential of CAR T-cells. CAR = chimeric antigen receptor; CCR = chimeric costimulatory receptor; iCAR = inhibitory CAR; EGFR = epidermal growth factor receptor; HSV = herpes simplex virus; IDO = indoleamine 2, 3-dioxygenase; Treg = regulatory T-cell; TRUCKs = T-cells redirected for universal cytokine killing; VEGFR = vascular endothelial growth factor receptor.
Homing
The trafficking of targeted effector T-cells to tumor sites is a prerequisite for exerting their functions against tumors. Indeed, data accumulated primarily in the context of adverse events demonstrated that infused T-cells do, in fact, traffic throughout the body and home to sites where a target antigen is expressed (130,131). Chemokines play a major role in defining lymphocyte migration; hence, genetically modifying T-cells to express appropriate chemokine receptors can change the migration patterns of T-cells so that they can move towards the relevant tumor chemokine receptors. This approach was initially demonstrated by expressing CCR4 on T-cells that are specific to the Hodgkin’s lymphoma marker CD30, thereby helping to increase their localization to tumor sites (128). Similar approaches have been used to express CCR2b to target T-cells toward neuroblastoma (132) and to express a CAR that recognizes VEGFR2, which is consequently expressed in tumor blood vessels and serves as a target to increase T-cell homing to tumor tissues (133). Alternatively, recent data showed that inducing the expression of the enzyme heparanase in T-cells co-expressing a tumor-specific CAR improves their capacity to degrade the extracellular matrix without compromising their viability, expansion, or effector function and consequently promoting increased antitumor activity (134). However, strategies involving genetically redirecting the migration of T-cells to enhance tumor-specific homing remain in the preclinical phase. They also offer an approach to focus on blocking inhibitor of migration (135) or using radiotherapy to normalize the often chaotic structure of tumor blood vessels (136) (Figure 2).
Persistence
CAR T-cells must survive and also possibly proliferate to achieve effective disease clearance and protection from recurrence. T and B-cell numbers fixed within an individual are reduced to baselines by homeostatic mechanisms after infection. Similarly, conditioning chemical or radiological depletion of lymphocytes can lead to enhanced engraftment and to the persistence of the infused cells through a homeostatic mechanism mediated by the removal of inhibiting regulatory T-cells (137) and the availability of homeostatic cytokines to facilitate proliferation of the transferred T-cells (138). However, conditioning chemotherapy is associated with considerable complications, principally susceptibility to infection.
Efforts to improve the persistence of CAR T-cells focus on the phenotypically defined populations that may proliferate and survive for longer periods, preferably naive or central memory T-cells (139,140). CD19-specific CD8+ central memory T-enriched cells expressing naive markers, such as CD62L, may improve engraftment in the patient as compared with more effector/differentiated T-cells (141). Recent data have identified and characterized stem cell memory T-cells and have shown that Wnt signaling promotes the propagation of these cells (142,143). Some studies have used T-cells specific to EBV and directed them to the GD2 antigen expressed by neuroblastoma tumor cells. This approach appears to increase numbers of CAR T-cells, at least in the short term (20).
An indirect strategy to supply cytokines to enhance the proliferation and persistence of CAR T-cells involving the introduction of the IL-12 gene into CD19-specific CAR T-cells has shown that T-cells retained a central memory effector phenotype and exhibited increased antitumor activity (144). Similarly, CD19-specific redirected T-cells maintained antitumor effects and persisted longer following transduction with a vector encoding an IL-15 gene (56). However, an advantage of this approach lies in the absence of the systemic cytokine IL-2, which could impinge upon T-cell function, such as the stimulation and expansion of regulatory T-cells (145). Initial studies using T-cells that were engineered to express cytokines have been successful in vitro although the feasibility of this approach in vivo is unclear.
Alternative or complementary approaches for enhancing CAR T-cells include multiple cell infusion cycles. A limitation of this strategy is that autologous CAR T-cells must be custom made. Some studies used transcription activator-like effector nuclease (TALEN) gene-editing technology to permanently knock out TCR αand βexpression and to abrogate the third-party T-cell’s potential for GVHD from the CD19-directed T-cells to overcome the key barriers of the adoptive transfer of third-party CAR T-cells, thus facilitating “off-the-shelf” CAR T-cell immunotherapy (146). Recent studies have also used this TALEN-mediated editing approach to develop and to aid in the large-scale manufacturing of T-cells that do not express TCRαβ and CD52, a protein targeted by alemtuzumab, which can be administered with or following alemtuzumab treatment, mediating lymphodeletion/immunosuppression and thereby enhancing engraftment (147). The manufacturing platform is highly reproducible irrespective of the donor source, and the multiplex genome-edited T-cell manufacturing platform shows potential efficacy as an “off-the-shelf” immunopharmaceutical for investigating tumor treatment.
Overcoming Immunosuppression
Despite the fact that adoptive immunotherapy using gene-modified T-cells expressing antigen-specific CAR is a promising approach for treating cancer, the CAR T-cell must face a barrage of immunosuppressive factors within the tumor microenvironment of solid tumors. Therefore, in solid tumors CAR T-cells are further genetically engineered to incorporate countermeasures against immunosuppressive factors in the tumor microenvironment (Figure 2). For example, CAR T-cells can be potentially engineered to include the expression of a dominant-negative form of the TGFβ receptor to overcome the inhibitory effects of TGFβ (148); to target NKG2D to recognize NKG2D ligands expressed on immunosuppressive cells, such as MDSCs, and to regulate T-cells and endothelial cells within the tumor microenvironment (149–151); to mute inhibitory immune receptors such as CTLA-4 (152); to reduce sensitivity to Fas-induced apoptosis (153) and to facilitate the expression of survival genes such as Bcl-X(L) (154). Remarkable and sustained upregulation of CD25 expression (155) and the co-expression of 4-1BBL (57) or CD40L (58) may protect the CAR T-cells against the immunosuppressive tumor microenvironment. Alternately, recent studies observed that CAR T-cell therapy, in combination with anti-PD1 antibody (156,157) or GM-CSF neutralization (105), can resist tumor immunosupression and thereby improve antitumor activity in preclinical models. Moreover, a PD1-CD28 chimeric receptor can convert the tumor PD-L1 to a ligand that transmits a CD28 costimulatory signal to CD8+ T-cells, which thereby enhances its antitumor activity (158). Similarly, resistance to other immunosuppressive factors, such as indoleamine 2, 3-dioxygenase, can be achieved using conditioning chemotherapy, including fludarabine and cyclophosphamide, prior to CAR T-cell infusion (159).
In an alternate strategy, CAR T-cells engineered with a CAR-inducible IL-12 cassette secrete IL-12 (TRUCKs) upon CAR engagement of the cognate antigen (54) and therefore can enhance antitumor function and are better able to impact local suppressive cells, such as Treg cells within the tumor stroma that are aimed at recruiting a second wave of immune cells in a locally restricted fashion to initiate the recognition of cancer cells that have lost the expression of the CAR target antigen (160). Moreover, CAR T-cells with IL-12 release enable more effective antitumor destruction compared with CAR T-cells alone and may not require conditioning chemotherapy (55). Thus, TRUCKs exhibited antitumor activity in solid tumors with different cancer cell phenotypes and actually changed the tumor microenvironment.
Thus, a marvelous array of strategies has been conceived to resist tumor immune evasion mechanisms and thereby enhance the antitumor function of CAR T-cell therapy. However, these approaches have been used in isolation as proof-of-principle demonstrations for each strategy. Further improvements will undoubtedly follow in future clinical trials.
Conclusions
Although CAR T-cell therapy is emerging as a powerful therapy and is likely to be incorporated into mainstream oncologic treatment soon, some important biologic tasks require additional investigation, including optimizing CAR signaling, defining the optimal target antigen, optimizing cell manufacturing methods, enhancing CAR T-cell therapy safety, the optimal conditioning for CAR T-cell therapy to delineate optimal combinatorial strategies to improve the therapeutic potential of CAR T-cells, and identifying the active “ingredient” of the CAR T-cell products (the optimal CAR T-cell substrates). Several obstacles to the effectiveness of this therapy remain, particularly in treating solid tumors. Addressing issues specific to the treatment of solid tumors, such as tumor heterogeneity, antigen loss, an immunosuppressive microenvironment, and accessibility challenges, will be imperative. Genetic modifications can meet some of the requirements for an effective immune response against solid tumors, including making T-cells respond against tumors and resistant to the tumor immunosuppression microenvironment, as well as improving homing to tumors and persistence for the long term. Such genetic modifications can be envisioned as the future approaches in this rapidly evolving field. This is a very positive development that shows that CAR T therapy undoubtedly marks a new era in cancer therapy and the beginning of personalized cell therapy with targeted specifications.
Funding
This work is supported by the Science and Technology Planning Project of Beijing City (number Z151100003915076 to WDH) and the National Natural Science Foundation of China (number 31270820 and number 81230061 to WDH).
The Science and Technology Planning Project of Beijing and the National Natural Science Foundation of China (the sponsor of the study) were not involved in the design of the study, the writing of the article, or the decision to submit the article for publication.
References
- 1. Soderqvist T, Stillwell C. A commotion in the blood: Life, death, and the immune system. J Hist Biol. 1999;32(1):205–215. [DOI] [PubMed] [Google Scholar]
- 2. Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pule MA, Savoldo B, Myers GD, et al. Virus-specific T-cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalos M, Levine BL, Porter DL, et al. T-cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia. Science Transl Med. 2011;3(95):95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T-cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T-cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T-cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T-cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T-cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T-cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmed N, Brawley VS, Hegde M, et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T-cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol. 2015;33(15):1688–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eshhar Z, Waks T, Bendavid A, et al. Functional expression of chimeric receptor genes in human T-cells. J Immunol Methods. 2001;248(1–2):67–76. [DOI] [PubMed] [Google Scholar]
- 14. Zhou G, Levitsky H. Towards curative cancer immunotherapy: overcoming posttherapy tumor escape. Clin Dev Immunol. 2012;2012:124187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh R, Paterson Y. Immunoediting sculpts tumor epitopes during immunotherapy. Cancer Res. 2007;67(5):1887–1892. [DOI] [PubMed] [Google Scholar]
- 16. Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–488. [DOI] [PubMed] [Google Scholar]
- 17. Hombach A, Heuser C, Sircar R, et al. T-cell targeting of TAG72+ tumor cells by a chimeric receptor with antibody-like specificity for a carbohydrate epitope. Gastroenterology. 1997;113(4):1163–1170. [DOI] [PubMed] [Google Scholar]
- 18. Westwood JA, Smyth MJ, Teng MW, et al. Adoptive transfer of T-cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc Natl Acad Sci U S A. 2005;102(52):19051–19056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rossig C, Bollard CM, Nuchtern JG, et al. Targeting of G(D2)-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int J Cancer. 2001;94(2):228–236. [DOI] [PubMed] [Google Scholar]
- 20. Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T-cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abken H, Hombach A, Heuser C, et al. A novel strategy in the elimination of disseminated melanoma cells: chimeric receptors endow T-cells with tumor specificity. Recent Results Cancer Res. 2001;158:249–264. [DOI] [PubMed] [Google Scholar]
- 22. Wilkie S, Picco G, Foster J, et al. Retargeting of human T-cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180(7):4901–4909. [DOI] [PubMed] [Google Scholar]
- 23. Chekmasova AA, Rao TD, Nikhamin Y, et al. Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T-cells genetically targeted to the MUC16 antigen. Clin Cancer Res. 2010;16(14):3594–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hegde M, Corder A, Chow KK, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T-cells in glioblastoma. Mol Ther. 2013;21(11):2087–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Urbanska K, Lanitis E, Poussin M, et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72(7):1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamada K, Geng D, Sakoda Y, et al. Redirecting gene-modified T-cells toward various cancer types using tagged antibodies. Clin Cancer Res. 2012;18(23):6436–6445. [DOI] [PubMed] [Google Scholar]
- 27. Grada Z, Hegde M, Byrd T, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moritz D, Groner B. A spacer region between the single chain antibody- and the CD3 zeta-chain domain of chimeric T-cell receptor components is required for efficient ligand binding and signaling activity. Gene Ther. 1995;2(8):539–546. [PubMed] [Google Scholar]
- 29. Hombach A, Hombach AA, Abken H. Adoptive immunotherapy with genetically engineered T-cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther. 2010;17(10):1206–1213. [DOI] [PubMed] [Google Scholar]
- 30. Kradin R, Kurnick J, Gifford J, et al. Adoptive immunotherapy with interleukin-2 (IL-2) results in diminished IL-2 production by stimulated peripheral blood lymphocytes. J Clin Immunol. 1989;9(5):378–385. [DOI] [PubMed] [Google Scholar]
- 31. James SE, Greenberg PD, Jensen MC, et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J Immunol. 2008;180(10):7028–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121(7):1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guest RD, Hawkins RE, Kirillova N, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005;28(3):203–211. [DOI] [PubMed] [Google Scholar]
- 34. Dustin ML, Depoil D. New insights into the T-cell synapse from single molecule techniques. Nat Rev Immunol. 2011;11(10):672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kahlon KS, Brown C, Cooper LJ, et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T-cells. Cancer Res. 2004;64(24):9160–9166. [DOI] [PubMed] [Google Scholar]
- 36. Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T-cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hombach AA, Heiders J, Foppe M, et al. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T-cells. Oncoimmunology. 2012;1(4):458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song DG, Ye Q, Carpenito C, et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T-cells is enhanced by costimulatory signaling through CD137 (4-1BB). Cancer Res. 2011;71(13):4617–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tammana S, Huang X, Wong M, et al. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T-cells against B-cell malignancies. Hum Gene Ther. 2010;21(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pule MA, Straathof KC, Dotti G, et al. A chimeric T-cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T-cells. Mol Ther. 2005;12(5):933–941. [DOI] [PubMed] [Google Scholar]
- 41. Irving BA, Weiss A. The cytoplasmic domain of the T-cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64(5):891–901. [DOI] [PubMed] [Google Scholar]
- 42. Letourneur F, Klausner RD. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins. Proc Natl Acad Sci U S A. 1991;88(20):8905–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Romeo C, Amiot M, Seed B. Sequence requirements for induction of cytolysis by the T-cell antigen/Fc receptor zeta chain. Cell. 1992;68(5):889–897. [DOI] [PubMed] [Google Scholar]
- 44. Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–684. [DOI] [PubMed] [Google Scholar]
- 45. Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. [DOI] [PubMed] [Google Scholar]
- 46. Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T-cells. Cancer Res. 2006;66(22):10995–11004. [DOI] [PubMed] [Google Scholar]
- 47. Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T-cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172(1):104–113. [DOI] [PubMed] [Google Scholar]
- 48. Song DG, Ye Q, Poussin M, et al. CD27 costimulation augments the survival and antitumor activity of redirected human T-cells in vivo. Blood. 2012;119(3):696–706. [DOI] [PubMed] [Google Scholar]
- 49. Altvater B, Landmeier S, Pscherer S, et al. 2B4 (CD244) signaling via chimeric receptors costimulates tumor-antigen specific proliferation and in vitro expansion of human T-cells. Cancer Immunol Immunother. 2009;58(12):1991–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Long AH, Haso WM, Shern JF, et al. 4-1BB costimulation ameliorates T-cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhong XS, Matsushita M, Plotkin J, et al. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T-cell-mediated tumor eradication. Mol Ther. 2010;18(2):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang LX, Westwood JA, Moeller M, et al. Tumor ablation by gene-modified T-cells in the absence of autoimmunity. Cancer Res. 2010;70(23):9591–9598. [DOI] [PubMed] [Google Scholar]
- 53. Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T-cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106(9):3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chmielewski M, Kopecky C, Hombach AA, et al. IL-12 release by engineered T-cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71(17):5697–5706. [DOI] [PubMed] [Google Scholar]
- 55. Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T-cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24(6):1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhao Z, Condomines M, van der Stegen SJ, et al. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T-cells. Cancer Cell. 2015;28(4):415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Curran KJ, Seinstra BA, Nikhamin Y, et al. Enhancing antitumor efficacy of chimeric antigen receptor T-cells through constitutive CD40L expression. Mol Ther. 2015;23(4):769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cooper LJ, Topp MS, Serrano LM, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101(4):1637–1644. [DOI] [PubMed] [Google Scholar]
- 60. Jensen MC, Clarke P, Tan G, et al. Human T lymphocyte genetic modification with naked DNA. Mol Ther. 2000;1(1):49–55. [DOI] [PubMed] [Google Scholar]
- 61. Dupuy AJ, Akagi K, Largaespada DA, et al. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature. 2005;436(7048):221–226. [DOI] [PubMed] [Google Scholar]
- 62. Huang X, Wilber AC, Bao L, et al. Stable gene transfer and expression in human primary T-cells by the Sleeping Beauty transposon system. Blood. 2006;107(2):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jin Z, Maiti S, Huls H, et al. The hyperactive Sleeping Beauty transposase SB100X improves the genetic modification of T-cells to express a chimeric antigen receptor. Gene Ther. 2011;18(9):849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maiti SN, Huls H, Singh H, et al. Sleeping beauty system to redirect T-cell specificity for human applications. J Immunother. 2013;36(2):112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Singh H, Manuri PR, Olivares S, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68(8):2961–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Manuri PV, Wilson MH, Maiti SN, et al. piggyBac transposon/transposase system to generate CD19-specific T-cells for the treatment of B-lineage malignancies. Hum Gene Ther. 2010;21(4):427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nakazawa Y, Huye LE, Dotti G, et al. Optimization of the PiggyBac transposon system for the sustained genetic modification of human T lymphocytes. J Immunother. 2009;32(8):826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao Y, Zheng Z, Cohen CJ, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13(1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Birkholz K, Hombach A, Krug C, et al. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T-cells for use in the adoptive immunotherapy of cancer. Gene Ther. 2009;16(5):596–604. [DOI] [PubMed] [Google Scholar]
- 70. Brown CE, Badie B, Barish ME, et al. Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T-cells in Patients with Recurrent Glioblastoma. Clin Cancer Res. 2015; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15(4):825–833. [DOI] [PubMed] [Google Scholar]
- 72. Suerth JD, Schambach A, Baum C. Genetic modification of lymphocytes by retrovirus-based vectors. Curr Opin Immunol. 2012;24(5):598–608. [DOI] [PubMed] [Google Scholar]
- 73. June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9(10):704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kustikova OS, Schiedlmeier B, Brugman MH, et al. Cell-intrinsic and vector-related properties cooperate to determine the incidence and consequences of insertional mutagenesis. Mol Ther. 2009;17(9):1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Riviere I, Dunbar CE, Sadelain M. Hematopoietic stem cell engineering at a crossroads. Blood. 2012;119(5):1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Newrzela S, Cornils K, Li Z, et al. Resistance of mature T-cells to oncogene transformation. Blood. 2008;112(6):2278–2286. [DOI] [PubMed] [Google Scholar]
- 77. Recchia A, Bonini C, Magnani Z, et al. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T-cells. Proc Natl Acad Sci U S A. 2006;103(5):1457–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scholler J, Brady TL, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T-cells. Sci Transl Med. 2012;4(132):132ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T-cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118(18):4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T-cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T-cells in humans. Biol Blood Marrow Transplant. 2010;16(9):1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32(7):689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T-cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T-cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cruz CR, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T-cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu S, Riley J, Rosenberg S, et al. Comparison of common gamma-chain cytokines, interleukin-2, interleukin-7, and interleukin-15 for the in vitro generation of human tumor-reactive T lymphocytes for adoptive cell transfer therapy. J Immunother. 2006;29(3):284–293. [DOI] [PubMed] [Google Scholar]
- 89. Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T-cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9(7):480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Xu Y, Zhang M, Ramos CA, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T-cells and are preserved by IL-7 and IL-15. Blood. 2014;123(24):3750–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Davila ML, Brentjens R, Wang X, et al. How do CARs work?: Early insights from recent clinical studies targeting CD19. Oncoimmunology. 2012;1(9):1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Maus MV, Grupp SA, Porter DL, et al. Antibody-modified T-cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123(17):2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li YS, Wasserman R, Hayakawa K, et al. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5(6):527–535. [DOI] [PubMed] [Google Scholar]
- 94. Li YS, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178(3):951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T-cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13(18 Pt 1):5426–5435. [DOI] [PubMed] [Google Scholar]
- 96. Maude SL, Teachey DT, Porter DL, et al. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125(26):4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T-cells. Blood. 2012;119(12):2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T-cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dai H, Zhang W, Li X, et al. Tolerance and efficacy of autologous or donor-derived T-cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. OncoImmunology. 2015;4(11):e1027469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang Y, Zhang WY, Han QW, et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T-cells. Clin Immunol. 2014;155(2):160–175. [DOI] [PubMed] [Google Scholar]
- 101. Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ritchie DS, Neeson PJ, Khot A, et al. Persistence and efficacy of second generation CAR T-cell against the LeY antigen in acute myeloid leukemia. Mol Ther. 2013;21(11):2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang QS, Wang Y, Lv HY, et al. Treatment of CD33-directed chimeric antigen receptor-modified T-cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther. 2015;23(1):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Garfall AL, Maus MV, Hwang WT, et al. Chimeric Antigen Receptor T-cells against CD19 for Multiple Myeloma. N Engl J Med. 2015;373(11):1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Burga RA, Thorn M, Point GR, et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother. 2015;64(7):817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Katz SC, Burga RA, McCormack E, et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin Cancer Res. 2015;21(14):3149–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T-cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T-cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21(4):904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lamers CH, Langeveld SC, Groot-van Ruijven CM, et al. Gene-modified T-cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol Immunother. 2007;56(12):1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24(13):e20–e22. [DOI] [PubMed] [Google Scholar]
- 111. Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T-cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hosing C, Kebriaei P, Wierda W, et al. CARs in chronic lymphocytic leukemia -- ready to drive. Curr Hematol Malig Rep. 2013;8(1):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Brentjens RJ, Curran KJ. Novel cellular therapies for leukemia: CAR-modified T-cells targeted to the CD19 antigen. Hematology Am Soc Hematol Educ Program. 2012;2012:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Maude SL, Barrett D, Teachey DT, et al. Managing cytokine release syndrome associated with novel T-cell-engaging therapies. Cancer J. 2014;20(2):119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhao Y, Moon E, Carpenito C, et al. Multiple injections of electroporated autologous T-cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70(22):9053–9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ciceri F, Bonini C, Stanghellini MT, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10(5):489–500. [DOI] [PubMed] [Google Scholar]
- 117. Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Maus MV, Haas AR, Beatty GL, et al. T-cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Marktel S, Magnani Z, Ciceri F, et al. Immunologic potential of donor lymphocytes expressing a suicide gene for early immune reconstitution after hematopoietic T-cell-depleted stem cell transplantation. Blood. 2003;101(4):1290–1298. [DOI] [PubMed] [Google Scholar]
- 120. Berger C, Flowers ME, Warren EH, et al. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T-cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107(6):2294–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Traversari C, Marktel S, Magnani Z, et al. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109(11):4708–4715. [DOI] [PubMed] [Google Scholar]
- 122. Song DG, Ye Q, Poussin M, et al. A fully human chimeric antigen receptor with potent activity against cancer cells but reduced risk for off-tumor toxicity. Oncotarget. 2015;6(25):21533–21546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang X, Chang WC, Wong CW, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118(5):1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kloss CC, Condomines M, Cartellieri M, et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T-cells. Nat Biotechnol. 2013;31(1):71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215):215ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Caruso HG, Hurton LV, Najjar A, et al. Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity. Cancer Res. 2015;75(17):3505–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Liu X, Jiang S, Fang C, et al. Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T-cells Exhibit an Increased Therapeutic Index against Tumors in Mice. Cancer Res. 2015;75(17):3596–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Di Stasi A, De Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113(25):6392–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367(2):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T-cells in myeloma and melanoma. Blood. 2013;122(6):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cameron BJ, Gerry AB, Dukes J, et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T-cells. Sci Transl Med. 2013;5(197):197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Craddock JA, Lu A, Bear A, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T-cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33(8):780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chinnasamy D, Yu Z, Kerkar SP, et al. Local delivery of interleukin-12 using T-cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res. 2012;18(6):1672–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Caruana I, Savoldo B, Hoyos V, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21(5):524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Buckanovich RJ, Facciabene A, Kim S, et al. Endothelin B receptor mediates the endothelial barrier to T-cell homing to tumors and disables immune therapy. Nat Med. 2008;14(1):28–36. [DOI] [PubMed] [Google Scholar]
- 136. Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62(5):1462–1470. [PubMed] [Google Scholar]
- 137. Yao X, Ahmadzadeh M, Lu YC, et al. Levels of peripheral CD4(+)FoxP3(+) regulatory T-cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119(24):5688–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T-cells. J Exp Med. 2005;202(7):907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8+ T-cells derived from central memory cells establishes persistent T-cell memory in primates. J Clin Invest. 2008;118(1):294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hinrichs CS, Borman ZA, Cassard L, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T-cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106(41):17469–17474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang X, Naranjo A, Brown CE, et al. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T-cells manufactured at clinical scale. J Immunother. 2012;35(9):689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Graef P, Buchholz VR, Stemberger C, et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T-cells. Immunity. 2014;41(1):116–126. [DOI] [PubMed] [Google Scholar]
- 143. Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T-cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pegram HJ, Purdon TJ, van Leeuwen DG, et al. IL-12-secreting CD19-targeted cord blood-derived T-cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia. 2015;29(2):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38(1):13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Torikai H, Reik A, Liu PQ, et al. A foundation for universal T-cell based immunotherapy: T-cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119(24):5697–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Poirot L, Philip B, Schiffer-Mannioui C, et al. Multiplex Genome-Edited T-cell Manufacturing Platform for “Off-the-Shelf” Adoptive T-cell Immunotherapies. Cancer Res. 2015;75(18):3853–3864. [DOI] [PubMed] [Google Scholar]
- 148. Foster AE, Dotti G, Lu A, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother. 2008;31(5):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang T, Sentman CL. Cancer immunotherapy using a bispecific NK receptor fusion protein that engages both T-cells and tumor cells. Cancer Res. 2011;71(6):2066–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhang T, Sentman CL. Mouse tumor vasculature expresses NKG2D ligands and can be targeted by chimeric NKG2D-modified T-cells. J Immunol. 2013;190(5):2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T-cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183(11):6939–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Condomines M, Arnason J, Benjamin R, et al. Tumor-Targeted Human T-cells Expressing CD28-Based Chimeric Antigen Receptors Circumvent CTLA-4 Inhibition. PLoS One. 2015;10(6):e0130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Dotti G, Savoldo B, Pule M, et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105(12):4677–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Eaton D, Gilham DE, O’Neill A, et al. Retroviral transduction of human peripheral blood lymphocytes with Bcl-X(L) promotes in vitro lymphocyte survival in pro-apoptotic conditions. Gene Ther. 2002;9(8):527–535. [DOI] [PubMed] [Google Scholar]
- 155. Chang ZL, Silver PA, Chen YY. Identification and selective expansion of functionally superior T-cells expressing chimeric antigen receptors. J Transl Med. 2015;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology. 2013;2(10):e26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. John LB, Devaud C, Duong CP, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T-cells. Clin Cancer Res. 2013;19(20):5636–5646. [DOI] [PubMed] [Google Scholar]
- 158. Prosser ME, Brown CE, Shami AF, et al. Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T-cells modified to express a PD1:CD28 chimeric receptor. Mol Immunol. 2012;51(3–4):263–272. [DOI] [PubMed] [Google Scholar]
- 159. Ninomiya S, Narala N, Huye L, et al. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T-cells and is downregulated by lymphodepleting drugs. Blood. 2015;125(25):3905–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cheadle EJ, Gornall H, Baldan V, et al. CAR T-cells: driving the road from the laboratory to the clinic. Immunol Rev. 2014;257(1):91–106. [DOI] [PubMed] [Google Scholar]
- 161. Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T-cells. Blood. 2008;112(6):2261–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T-cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]