Abstract
Background:
We analyzed the cost-effectiveness of treating incident chronic myeloid leukemia in chronic phase (CML-CP) with generic imatinib when it becomes available in United States in 2016. In the year following generic entry, imatinib’s price is expected to drop 70% to 90%. We hypothesized that initiating treatment with generic imatinib in these patients and then switching to the other tyrosine-kinase inhibitors (TKIs), dasatinib or nilotinib, because of intolerance or lack of effectiveness (“imatinib-first”) would be cost-effective compared with the current standard of care: “physicians’ choice” of initiating treatment with any one of the three TKIs.
Methods:
We constructed Markov models to compare the five-year cost-effectiveness of imatinib-first vs physician’s choice from a US commercial payer perspective, assuming 3% annual discounting ($US 2013). The models’ clinical endpoint was five-year overall survival taken from a systematic review of clinical trial results. Per-person spending on incident CML-CP treatment overall care components was estimated using Truven’s MarketScan claims data. The main outcome of the models was cost per quality-adjusted life-year (QALY). We interpreted outcomes based on a willingness-to-pay threshold of $100 000/QALY. A panel of European LeukemiaNet experts oversaw the study’s conduct.
Results:
Both strategies met the threshold. Imatinib-first ($277 401, 3.87 QALYs) offered patients a 0.10 decrement in QALYs at a savings of $88 343 over five years to payers compared with physician’s choice ($365 744, 3.97 QALYs). The imatinib-first incremental cost-effectiveness ratio was approximately $883 730/QALY. The results were robust to multiple sensitivity analyses.
Conclusion:
When imatinib loses patent protection and its price declines, its use will be the cost-effective initial treatment strategy for CML-CP.
The BCR-ABL1 tyrosine kinase inhibitor (TKI) imatinib (Gleevec, Glivec, Novartis International AG) was approved by the US Food and Drug Administration (FDA) in 2001 to treat incident Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP) and has been shown to produce a high cumulative incidence of complete cytogenetic responses (CCyR) (1–3). Imatinib is also associated with improved survival. After eight years, the overall survival (OS) on the International Randomized Study of Interferon vs STI571 (imatinib) (the IRIS trial) was 85% for patients treated with imatinib, and their freedom from progression to accelerated phase or blast crisis (AP/BC) was 92% (4).
In the past decade, additional TKIs have demonstrated efficacy for treating incident CML-CP (5). Dasatinib (Sprycel, Bristol-Myers Squibb) and nilotinib (Tasigna, Novartis Oncology) were granted first-line approval for the treatment of CML-CP by the FDA. These “second-generation” TKIs have been compared prospectively with imatinib individually but not with each other in incident CML-CP patients (6–9). The second-generation TKIs produce more rapid molecular responses than imatinib at standard doses of 400mg daily, but five-year OS does not differ between the three TKIs (5–9). Most incident CML-CP patients will require life-long, daily TKI-based care (5–10).
In the United States, Novartis’ composition-of-matter patent on imatinib was scheduled to expire in the first quarter of 2015. An agreement between Novartis and Sun Pharmaceutical Industries, Ltd., has deferred generic entry to the first quarter of 2016. Generic imatinib is already available in Canada. For most European Union member countries, Novartis’ patent will also expire in 2016.
Health system spending on incident CML-CP after generic imatinib becomes available is the subject of great interest among patients, physicians, and payers (11,12). Loss of patent exclusivity opens the market to potential competition from multiple manufacturers. The extent of payers’ savings gained from a drug’s generic entry largely depends on whether and to what extent prices decline (13). In Canada, the price of generic imatinib is now 18% to 26% of the branded drug price, and mandatory generic imatinib-first and brand-to-generic substitution policies have resulted in cost savings (14,15).
Physicians’ willingness to prescribe generic drugs is related to patient benefit, including differences in OS and quality-adjusted life-years (QALYs). Generic drug quality may also be a concern, which is in part determined by the strength of individual country drug safety regulations and permeability to drug importation from countries with weaker drug quality standards (16). Anecdotal concerns have been raised that the bioavailability and potency of generic imatinib is not equivalent to the branded drug, based on individual case reports and small case series; however, a recent meta-analysis concluded that these concerns in non-Western countries were unfounded in Canada (17).
The objective of this study was to estimate the five-year cost-effectiveness of treating all incident CML-CP patients with generic imatinib as first-line therapy when it becomes available in 2016 in the United States from a commercial payer’s perspective compared with the current standard of care.
Methods
We hypothesized that initiating treatment among incident CML-CP patients in 2016 with generic imatinib and then switching as needed clinically to dasatinib or nilotinib would be cost-effective over a five-year time horizon in comparison with the current standard of care, a physician’s choice of starting any one of the three approved TKIs and then switching to another TKI if the initial selection lacked efficacy or was not tolerated. Our study took the US commercial payer perspective because these insurers are the largest domestic payers of TKI-based therapy among CML-CP patients, including those insured through the fee-for-service Medicare pharmacy benefit (the “Part D”) program, those who are insured through employer-sponsored plans, and those who are self-insured. The five-year time horizon was chosen to reflect available published data on relevant clinical outcomes associated with CML-CP treatment with the three TKIs. The methods used were based on guidelines set by the US Panel on Cost-Effectiveness in Health and Medicine (18). A panel of CML experts, all members of the European LeukemiaNet (ELN) CML committee, designed this study, identified the study’s main outcomes from a systematic review of published randomized clinical trials, reviewed preliminary results, and made edits to the manuscript to improve interpretation for a clinical audience. The University of Chicago Institutional Review Board approved this study as exempt human-subject research.
Effectiveness
The main outcome measure of the analysis was predicted OS in 2021 among CML-CP patients initiating TKI treatment in 2016. For each TKI, data on actual OS were extracted from the published clinical trials (IRIS, ENESTnd, DASISION, and second-line phase II/III and transplant studies) that formed the basis for regulatory approval of the TKIs for these indications (1–4,6–9,17–21).
The ELN guidelines recommend that CML-CP patients switch initial TKI choice if they do not experience sufficient treatment efficacy or tolerance (22,23). In clinical practice, treatment efficacy among CML-CP patients initiating TKI treatment is monitored using two distinct measures and associated time points: 1) the achievement of a 12-month CCyR or 2) the achievement of a three-month early molecular response (EMR) defined as a reduction in BCR-ABL1 transcript levels to less than 10% compared with a standardized baseline (using the International Scale [IS]) (5,24,25). For each TKI, predicted achievement of CCyR and EMR as well as tolerance data were extracted from the published clinical trials cited above and overseen by the ELN expert panel (1–4,6–9,17–21).
OS, CCyR, and EMR have been associated with increased treatment effectiveness among CML-CP patients. Effectiveness was measured in units of quality-adjusted life-years based on US societal CML-specific preferences reported by Szabo et al. (Table 1) (26). All QALYs were discounted at 3% per year.
Table 1.
US societal health utilities for patients with chronic myeloid leukemia derived from the published literature
| Disease status | QALYs* (range for sensitivity analyses) | Description |
|---|---|---|
| CP, responding to treatment | 0.89 (0.78–0.94) | CCyR |
| CP, not responding to treatment | 0.75 (0.57–0.85) | At diagnosis; or lack of CCyR leading to switch |
| AP, responding to treatment | 0.79 (0.62–0.88) | Szabo 2010 |
| BP, responding to treatment | 0.59 (0.4–0.72) | Szabo 2010 |
| BP, not responding to treatment | 0.22 (0.07–0.34) | Szabo 2010 |
| Treatment changed because of serious adverse events | 0.58 (0.38–0.76) | Switch from first TKI to another |
| Allogeneic transplantation (within 1 y) | 0.6 (0.51–0.69) | Szabo 2010 |
| Allogeneic transplantation (after 1 y) | 0.85 (0.723–0.978) | Szabo 2010 |
| MMR | 0.9 (0.765–0.99) | Szabo 2010 |
| Death | 0 | Anchor |
* Quality-adjusted life-years (QALYs) are extracted from Szabo et al. (2010). They relate to this analysis as the health utility weights derived from the EQ5D index from the US societal perspective and represent measures of effectiveness in this analysis. In general, QALYs range from 0.0 to 1.0, where 0.0 represents death and 1.0 represents full health over one year. This range should be incremental, such that 0.5 QALY is exactly ½ of full health. AP = accelerated phase; BP = blast phase; CP = chronic phase; MMR = major molecular response; QALYs = quality-adjusted life-years; TKI = tyrosine kinase inhibitor.
In rare cases, CML-CP progresses to AP/BC despite patient treatment with a TKI. Allogeneic hematopoietic cell transplantation is indicated for the majority of these patients. The probabilities of patient disease progression after initial treatment with a TKI and the outcome of subsequent transplantation were extracted from the available clinical trial literature (Table 1) (29). Societal QALYs associated with transplantation were extracted from Szabo et al. (26).
Costs
Direct medical costs per patient, per month of overall care components were estimated using 2011 and 2012 Truven Health Analytics MarketScan (Truven Health, Ann Arbor, MI) commercial claims and encounters data (Table 2) (14,30). These data represent the medical care experienced by employees and their dependents enrolled in commercial health insurance plans sponsored by approximately 100 payers, representing more than 50 million covered lives annually. Marketscan data are well studied, and we used the claims that were the basis of previously published reports of the direct costs of domestic CML treatment (29).
Table 2.
Per-patient, per-month costs of treatment by TKI estimated from a cohort of US patients with chronic myeloid leukemia observed in Truven’s MarketScan claims database (2011–2012; 2013 $USD)
| Monthly treatment costs* | Median cost, $ | Mean cost (95% CI), $ |
|---|---|---|
| Imatinib (n = 2616) | ||
| Direct outpatient costs | 11.22 | 696.25 (565.65 to 826.86) |
| Direct inpatient costs | 0 | 1963.48 (440.88 to 3486.08) |
| Direct drug payments | 5032.50 | 4652.59 (4456.98 to 4848.20) |
| Dasatinib (n = 1284) | ||
| Direct outpatient costs | 103.57 | 700.97 (575.02 to 826.92) |
| Direct inpatient costs | 0 | 647.21 (155.45 to 1138.97) |
| Direct drug payments | 7944.80 | 6328.27 (5981.25 to 6675.29) |
| Nilotinib (n = 864) | ||
| Direct outpatient costs | 111.65 | 634.81 (477.65 to 791.97) |
| Direct inpatient costs | 0 | 365.26 (40.92 to 689.60) |
| Direct drug payments | 7636.96 | 6287.03 (5866.54 to 6707.52) |
* All data were extracted from Truven MarketScan using a sample of 397 patients (4764 patient-months). CI = confidence interval; TKI = tyrosine kinase inhibitor.
Direct medical costs were estimated among patients who closely reflected the inclusion criteria of the IRIS, ENESTnd, and DASISION trials using variables reported in the MarketScan data (age 18–65 years), primary diagnosis of CML (International Classification and Diseases, 9th revision [ICD-9], codes 205.1, 205.8, 205.9, 207.8, 208.1, 208.8, and 208.9) with incident disease (newly diagnosed CML between January 1, 2011, and December 31, 2012) and no comorbidities. Costs were estimated among these patients who were treated with imatinib, dasatinib, or nilotinib. For identifying incident cases, the first observed TKI dispensing date was considered the index drug date. Patients were excluded if diagnosed with acute leukemia (lympoid or myeloid; ICD-9 codes 204 and 205.0) or were not continuously enrolled between three months before through 12 months after the index date of drug dispensing.
Costs included in the models were mean monthly CML drug costs for imatinib, dasatinib, and nilotinib and mean monthly costs for other prescription drugs, diagnostic tests and laboratory monitoring, outpatient visits, and hospitalizations (Table 2). Allogeneic transplantation was not observed in the Marketscan cohort. Therefore, the costs of allogeneic transplantation were extracted from Preussler et al. (Table 3) (30). The frequency of use of specific care components and the contribution of these component costs to total per-person, per-month spending were reviewed.
Table 3.
Per-patient, per-year of treatment by TKI estimated from a cohort of patients with chronic myeloid leukemia observed in Truven’s MarketScan claims database (2011–2012; 2013 $USD) for model health states
| Total annual costs after generic entry of imatinib* | Base case (range for sensitivity analyses) |
|---|---|
| 12-month complete cytogenetic response | |
| Imatinib 1 y | 79 373 (65 415–87 748) |
| Imatinib 2–4 y | 45 875 (37 500–54 249) |
| Dasatinib | 92 117 (78 300–105 935) |
| Nilotinib | 87 445 (74 328–100 562) |
| 3-month BCR/ABL1 early molecular response | |
| Imatinib 1–3 mo | 21 937 (18 646–25 228) |
| Dasatinib 1–3 mo | 23 029 (19 575–26 484) |
| Nilotinib 1-3mo | 21 861 (18 582–25 141) |
| Imatinib 4–12 mo | 57 436 (48 821–66 052) |
| Dasatinib 4–12 mo | 69 088 (58 725–79 451) |
| Nilotinib 4–12 mo | 65 584 (55 746–75 422) |
| Imatinib 2–4 y | 45 875 (38 993–52 756) |
| Dasatinib 2–4 y | 92 117 (78 300–105 935) |
| Nilotinib 2–4 y | 87 445 (74 328–100 562) |
| Allogeneic transplant | 245 000 (125 000–300 000) |
* These cost data represent the mean payer prices of drugs, rather than the list price, to accurately represent the US commercial payer perspective. All predicted cost data are based on trends according to Conti and Berndt (2014) and crosschecked with other published estimates of generic prices of oral drugs. Cost of allogeneic transplantation was drawn from Preussler (2012). All other costs are based on cases billed according to ICD-9; see text for a list of ICD-9 codes. Total costs are the sum of direct expenditures for chronic myeloid leukemia. TKI = tyrosine kinase inhibitor.
The model evaluated the per-patient, per-month cost trajectory of imatinib before and after patent expiration based on cancer drug–specific cost trajectories after generic entry recently published by Conti and Berndt (14,33). These trajectories were consistent with previously published studies, as well as contemporaneous Canadian trends in the per-patient, per-month costs following generic entry among oral drugs (14,34–37). The per-patient, per-month cost trajectory of imatinib-based therapy utilized in this study is as follows: 1) 100% of the branded treatment cost prior to generic entry for the first six months; 2) 60% to 80% for the second six months following generic entry; and 3) 10% to 30% thereafter. The midpoint of these ranges was taken for each time period. Only drug-specific costs were assumed to change after the generic entry of imatinib; the frequency of use and the costs of other medical care components were assumed to be stable throughout the study period. This assumption was made based on an examination of MarketScan data and the opinion of the ELN panel. The models assumed that the average dose of imatinib for a given CML-CP patient would not differ before and after generic entry based on the opinion of the ELN panel.
All costs were expressed in 2013 US dollars using the all-urban Consumer Price Index inflation calculator (US Bureau of Labor and Statistics, www.bls.gov/cpi) and discounted at 3% annually. Results reported per-patient, per-month costs aggregated and expressed as total annual per-patient costs.
Analytic Plan
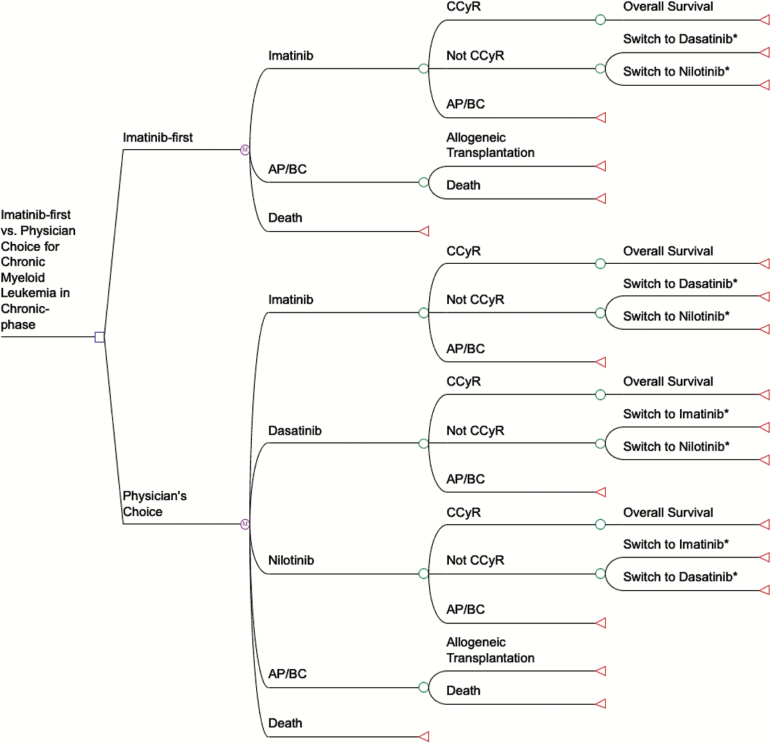
Markov models were constructed to compare the cost-effectiveness of two treatment strategies for a cohort of incident patients with CML-CP enrolled over five years beginning in January 2016 with follow-up through December 2020 (Figure 1) using TreeAge Pro Suite (TreeAge Software Inc, Williamstown, MA). The models compared two strategies: 1) standard care by physician’s choice, initiating newly diagnosed CML-CP treatment with any one of the 3 TKIs, and 2) initially using generic imatinib in all patients and then altering therapy as clinically required in a stepwise approach.
Figure 1.
A simplified Markov diagram of generic imatinib-first vs physician’s choice (the current standard of care) treatment strategies for treating chronic myeloid leukemia in chronic phase. Under physician’s choice, patients have equal probability of beginning on imatinib, dasatinib, or nilotinib and remain on a drug until they reach a chance node. The chance nodes depicted in the figure represent CCyR at 12 months (or, as noted in the Methods, EMR at 3 months). Patients have three possible outcomes prior to reaching the first chance node, whether at 12 months in the first CCyR model or three months in the second EMR model: remaining on TKI therapy, progressing to AP/BC, or death. Patients who respond positively to a TKI stay on it the remainder of time, and overall survival since progression to AP/BC or death is uncommon after that point. Patients who fail their initial TKI because of intolerance or lack of efficacy can switch once (*) at 12 months (or 3 months in the EMR model) to another TKI. Under generic imatinib-first, 100% of patients begin on imatinib and only switch to nilotinib or dasatinib because of intolerance or if a CCyR (or EMR) was not reached. Some patients who fail under TKI therapy transition to the AP/BC state, which includes the risk of death. *Indicates where one switch at 12 months (or 3 months) can take place. AP/BC = accelerated phase/blast crisis; CCyR = complete cytogenetic response; EMR = early molecular response; M = Markov node; TKI = tyrosine kinase inhibitor.
In the physician’s choice strategy, it was assumed that each TKI would command equal probability, or one-third of front-line CML treatment based on the observed distribution of TKI use in the MarketScan data. In both strategies, patients were assumed to switch once to a second-generation TKI in equal proportion if they failed to meet efficacy measures by the appropriate time points defined by ELN guidelines and based on the opinion of the ELN panel. The model included an additive probability that 15% of patients who were intolerant to one second-generation TKI would switch to generic imatinib, which is a less potent ABL1 kinase inhibitor based on observed switches in the MarketScan data and the opinion of the ELN panel (Table 4).
Table 4.
Base case probabilities and range for sensitivity analysis employed in Markov models distinguished by use of the efficacy endpoints of complete cytogenetic response at 12 months or early molecular response at 3 months for newly diagnosed patients with chronic phase chronic myeloid leukemia enrolled on prospective clinical trials
| 12-mo CCyR | 3-mo EMR | |
|---|---|---|
| Probabilities | Base case (range for sensitivity analyses) | Base case |
| Imatinib | ||
| CCyR | 0.7 (0.60–0.81) | 0.65 |
| Not CCyR or intolerant | 0.23 (0.20–0.26) | Remainder |
| Switch to dasatinib | 0.5 | |
| Overall survival | 0.74 (0.63–0.85) | |
| Death* | 0.26 | |
| Switch to nilotinib | 0.5 | |
| Overall survival | 0.74 (0.63–0.85) | |
| Death* | 0.26 | |
| AP/BC | 0.07 (0.060–0.081) | 0.07 |
| Survival | 0.5 (0.43–0.58) | |
| Death* | 0.5 (0.43–0.58) | |
| Dasatinib | ||
| CCyR | 0.85 (0.72–0.98) | 0.84 |
| Not CCyR or intolerant | 0.1 (0.085–0.12) | Remainder |
| Switch to imatinib | 0.15 (0.10–0.50) | |
| Overall survival | 0.3 (0.26–0.35) | |
| Death* | 0.7 | |
| Switch to nilotinib* | 0.85 | |
| Overall survival | 0.3 (0.26–0.35) | |
| Death* | 0.7 | |
| AP/BC | 0.05 (0.043–0.058) | 0.05 |
| Survival | 0.5 (0.43–0.58) | |
| Death* | 0.5 (0.43–0.58) | |
| Nilotinib | ||
| CCyR | 0.8 (0.68–0.92) | 0.9 |
| Not CCyR or intolerant | 0.16 | Remainder |
| Switch to imatinib | 0.15 (0.10–0.50) | |
| Overall survival | 0.75 (0.64–0.86) | |
| Death* | 0.25 | |
| Switch to dasatinib* | 0.85 | |
| Overall survival | 0.75 (0.64–0.86) | |
| Death* | 0.25 | |
| AP/BC | 0.04 (0.034–0.046) | 0.04 |
| Survival | 0.5 (0.43–0.58) | |
| Death* | 0.5 (0.43–0.58) |
* Indicates remainder probability, adding up to 1.0 for all subnodes. Indentations in the listing of model parameters above are representative of the Markov model subnodes in Figure 1. Full description of related model probabilities is available in the Supplementary Materials (available online). Data were extracted from a meta-analysis of published clinical trial results (1–9,19–25). AP/BC = accelerated phase/blast crisis; CCyR = complete cytogenetic response; CML = chronic myeloid leukemia; EMR = early molecular response.
The cost-effectiveness of the alternative treatment strategies was estimated using two distinct Markov models, one for each efficacy measure described above. Details of each Markov model are described in the Supplementary Methods (available online). Results were interpreted as incremental cost-effectiveness ratios (ICERs) at a willingness-to-pay (WTP) threshold of $100 000 per QALY.
Sensitivity Analyses
Univariate and multivariable sensitivity analyses tested parameters with the greatest impact on results, including treatment heterogeneity based on the patient Sokal score for risk, probabilities of reaching a positive CCyR or EMR, and ranges in the expected price drop of imatinib (see the Supplementary Methods, available online) (5). A Bayesian multivariable probabilistic sensitivity analysis (PSA) was performed using 10 000 Monte Carlo simulations; gamma distributions were applied to costs, and beta distributions for probabilities and utilities. The PSA was used to randomly select parameter values from their assumed distributions in order to provide more realistic results as the outcomes may be portrayed for a population with CML-CP.
Results
MarketScan Estimates of Per-Person Costs
MarketScan cost data were from 397 patients diagnosed with CML-CP (Table 2). The average per-person total cost of treatment with branded imatinib ($79 000/year) was similar to that of dasatinib or nilotinib ($87 000-$92 000/year), although the mean annual cost of the drug itself was lower (approximately $59 000 for imatinib compared with $76 000 for dasatinib and $75 000 for nilotinib). Once imatinib loses patent exclusivity, the total costs of imatinib-based treatment (drug plus usual medical care) are expected to drop to about $46 000 per year (Table 3). Per-person cost of CML-CP treatment is largely comprised of drug costs; spending on other routine care components (eg, outpatient office visits and diagnostic tests) is limited, and the use of more expensive care components (eg, hospitalizations, transplants) among CML-CP patients responding to TKI-based treatment is observed to be rare in the MarketScan cohort.
Model Estimates of Effectiveness and Cost
For both models, the clinical effectiveness derived from these two treatment strategies fell in a narrow range, approximately 3.8 to 4.0 QALYs over five years. Physician’s choice was estimated to provide slightly higher effectiveness to patients. However, the cost of imatinib-first therapy was lower than the cost of physician’s choice by about $80 000 to $90 000 over five years.
Base Case Analysis
Both strategies compared with a no-alternative-treatment option met the willingness-to-pay threshold of $100 000 per QALY (CCyR: imatinib-first = $277 401, QALYs = 3.87, $/QALY = $71 679, and physician’s choice = $365 774, QALYs = 3.97, $/QALY = $92 135; EMR: imatinib-first = $281 107, QALYs = 3.82, $/QALY = $73 588, and physician’s choice = $366 819, QALYs = 3.98, $/QALY = $92 166). However, the imatinib-first strategy was cost-effective compared with the current standard of care based on the incremental cost-effectiveness ratio. Based on 12-month CCyR, imatinib-first cost less over five years and offered CML patients only slightly fewer QALYs compared with physician’s choice (ICER = 883 730) (Table 5). Similar results were found using the three-month EMR model (ICER = 535 700).
Table 5.
Base-case results of cost utility analysis based on two Markov models distinguished by use of the efficacy endpoints of complete cytogenetic response at 12 months or early molecular response at 3 months; the ICERs are largely driven by cost differences because there are no clinically significant differences between the measures of effectiveness*
| Treatment | Cost, $ | Effectiveness, QALYs | ICER, $/ QALY |
|---|---|---|---|
| 12-month complete cytogenetic response | |||
| Imatinib-first | 277 401 | 3.87 | |
| Physician’s choice | 365 774 | 3.97 | 883 730 |
| 3-month BCR/ABL1 early molecular response | |||
| Imatinib-first | 281 107 | 3.82 | |
| Physician’s choice | 366 819 | 3.98 | 535 700 |
* ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year.
Sensitivity Analysis
Imatinib-first therapy was cost-effective compared with current standard of care based on risk stratification by low/intermediate or high Sokal risk scores (Table 2; Supplementary Methods, available online). The results were robust to changes based on univariate analyses of all parameters. The most sensitive parameters in the model were the imatinib-associated probabilities of reaching a positive CCyR or EMR. The model was also sensitive to changes in the costs of imatinib in the first year and remaining years.
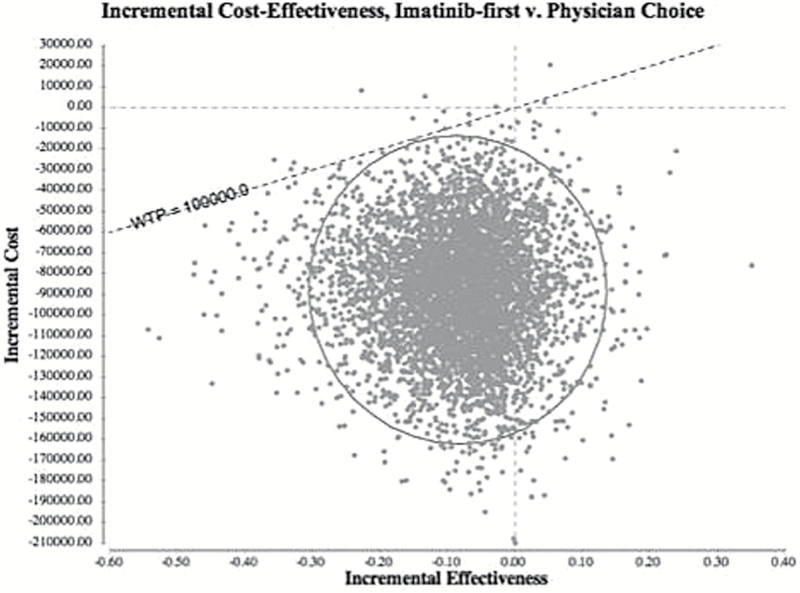
The PSA suggested that imatinib-first therapy was overwhelmingly cost-effective compared with physician’s choice. Overall, 99.7% of simulations determined that imatinib-first therapy had improved net monetary benefit. Approximately 85.1% of iterations identified imatinib-first therapy as cost-effective, that is, less costly at a slightly lower effectiveness compared with standard of care and below a WTP threshold of $100 000 per QALY (Figure 2). In an additional 14.6% of simulations, imatinib-first therapy dominated physician’s choice because it offered both greater utility (higher QALYs) and lower overall cost.
Figure 2.
A Bayesian multivariable probabilistic sensitivity analysis found imatinib-first to be cost-effective compared with physician’s choice in 99.7% of 10 000 Monte Carlo simulations. The circular line indicates the 95% confidence interval of incremental cost-effectiveness ratios (ICERs) among simulations, and the dotted diagonal line indicates the willingness-to-pay threshold, which has a slope of $100 000/QALY. Simulations appearing below this line favor the imatinib-first strategy as cost-effective. Incremental effectiveness along the x-axis is measured in units of quality-adjusted life-years (QALYs). Incremental cost along the y-axis is measured in units of US dollars ($), adjusted for inflation to 2013. The ICER of each simulation is calculated as a ratio of incremental cost over incremental effectiveness ($/QALY).
Discussion
From a US commercial payer perspective, when imatinib loses patent protection in 2016 and lower-cost generic drugs equivalent to branded imatinib become available, it will be the most cost-effective initial treatment strategy for incident CML-CP compared with the current standard of care—physician’s choice of imatinib, dasatinib, or nilotinib over five years. While important to note that imatinib-first offers slightly lower effectiveness, estimated QALYs for both treatment strategies fell in a narrow range for which differences do not appear clinically meaningful (26). Furthermore, imatinib costs much less, holding greater value per QALY. No other alternative variations of the models changed the cost-effectiveness of the imatinib-first treatment strategy over physician’s choice. Interestingly, sensitivity results of both models suggested that if imatinib maintained its current branded price for five more years, imatinib-first would still remain the cost-effective strategy compared with standard of care. These results suggest that the adoption of an imatinib-first strategy to treat newly diagnosed CML-CP patients over the current standard of care when generic imatinib becomes available could translate into substantial savings for US payers. For a commercial insurer covering 100 incident CML-CP patients in 2016, a generic imatinib-first strategy compared with standard of care would save the plan USD $9.12 million (an average of $91 163 per patient) in direct medical costs over five years.
Our results are likely lower bounds on commercial insurer cost savings when generic imatinib becomes available in the United States. This analysis assumes the imatinib-first treatment strategy would be adopted for newly diagnosed CML-CP patients only. Thus, there are two types of patients with prevalent CML-CP excluded from the analysis: 1) those currently treated with nilotinib or dasatinib and 2) those who have been treated with branded imatinib for one year or longer and have responded well to treatment. The selection of generic imatinib for the treatment of both patient types might be affected by generic substitution policies implemented by pharmacies without insurer or patient involvement and/or motivated by patient and/or insurer cost consciousness (38–40). Indeed, depending on the province, Canadian pharmacies are authorized (or even required) to switch all incident and prevalent CML patients using branded imatinib to either of two generic formulations of imatinib (40). In the United States, generic substitution by pharmacists of oral drugs covered under a patient’s pharmacy benefits is allowed or sometimes even mandated although in some states pharmacists must contact the prescribing physician for permission to substitute (40). Alternatively, physicians might choose to adopt this strategy based on their patients’ expressed cost consciousness (38), which might be related to the common use of formularies by insurers with differential patient out-of-pocket copayment requirements between generic and branded drugs (42).
The analysis has a number of limitations. The models assume perfect patient adherence to TKI-based treatment across all treatment modalities studied and no change in TKI usage patterns before and after imatinib’s generic entry. These assumptions are subject to several caveats (44–46). For example, Dusetzina et al. found that among a commercially insured CML patient population in the United States patients with higher copayments were more likely to be nonadherent to imatinib-based therapy (47). Patients with higher copayments were 42% more likely to be nonadherent to the recommended daily dosing. Poor adherence to TKI therapy leads to unfavorable clinical outcomes (44–46). To the extent patient nonadherence to TKI-based treatment is related to out-of-pocket pharmacotherapy costs, generic entry of imatinib may lead to better treatment adherence among selected patients. This limitation would bias results in favor of the imatinib-first strategy.
This study did not employ a lifetime time horizon. While this horizon is appealing from a societal perspective, such an analysis requires modeling assumptions regarding OS, generic entry dates, associated future pricing and use pattern changes among the competing, currently available TKIs, and CML-CP treatment innovation in the future. None of these parameters are known at this time, and the ELN expert panel was unable to come to consensus regarding reasonable ranges of values for each of these parameters for additional sensitivity analyses. This is an important topic for future study. Similarly, an important unknown is the fraction of newly diagnosed CML-CP patients who might eventually be able to discontinue TKI therapy because their disease has been suppressed to an undetectable level for a long time period, or perhaps even been cured (48–52). This treatment strategy holds great potential for health system cost savings, even if a more expensive TKI were required for several years to achieve this stopping point.
The study is also limited by lacking data about additional switches among first-, second-, and third-generation TKIs following three- or 12-month efficacy/tolerability endpoints. The study derived outcomes and the achievement of efficacy and tolerance milestones based on a systematic review of randomized controlled clinical trial results that only followed patients through one possible switch related to initial TKI-associated lack of efficacy or intolerance. This omission does not substantially bias these results because switching between one currently available second-generation TKI to another or a third-generation TKI would have little impact on treatment costs given the largely equivalent current market prices of these branded drugs. Patients who switch to a third TKI because of lack of efficacy or intolerance are often candidates for allogeneic transplantation, which was included in the models. More generally, results may not apply to patients with comorbidities, older age, or lack of access to appropriate specialty care who would have been excluded from the clinical trials upon which this analysis is based.
In 2016, we anticipate that the per-patient, per-month cost of imatinib will drop 60% to 90% once it loses patent protection while second generation TKIs dasatinib and nilotinib will hold their high costs steady. From a US payer perspective, imatinib will be the cost-effective initial treatment strategy for CML-CP compared with dasatinib and nilotinib.
Funding
The following sources of unrestricted funding are to be noted: A F32-HS023710 National Research Service Award from the Agency for Healthcare Research and Quality (AHRQ) supported William V. Padula’s participation in this research. The National Institutes of Health Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) K12 Program and the North Carolina Translational and Clinical Sciences Institute (UL1TR001111) supported S. Dusetzina’s participation in this research. A K07-CA138906 award from the National Cancer Institute funded R. Conti’s participation in this research.
Supplementary Material
Notes
No organization, including the AHRQ, the National Cancer Institute, and the University of Chicago, is responsible for the design and conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
All authors have followed guidelines set by the International Committee of Medical Journal Editors.
Author contributions: design and conduct of the study: Conti, Padula, Larson; collection, management, analysis, and interpretation of the data: Conti, Padula, Larson, Dusetzina, Apperley, Baccarani, Eigendorff, F. Guilhot, J. Guilhot, Mahon, Martinelli, Mayer, Müller, Niederwieser, Saussele, Schiffer, Silver, Simonsson, and Hehlmann; preparation, review, or approval of the manuscript: Conti, Padula, Larson, Dusetzina, Apperley, Baccarani, Eigendorff, F. Guilhot, J. Guilhot, Mahon, Martinelli, Mayer, Müller, Niederwieser, Saussele, Schiffer, Silver, Simonsson, and Hehlmann; decision to submit the manuscript for publication: Conti, Padula, Larson, Dusetzina, Apperley, Baccarani, Eigendorff, F. Guilhot, J. Guilhot, Mahon, Martinelli, Mayer, Müller, Niederwieser, Saussele, Schiffer, Silver, Simonsson, and Hehlmann. Conti and Padula had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The information reported in this manuscript was previously presented at: 1) Larson RA, Conti RM, Padula WV, et al. What Is the Most Cost-Effective Strategy for Treating Newly Diagnosed Chronic Phase Chronic Myeloid Leukemia (CML) after Imatinib Loses Patent Exclusivity? In: American Society of Hematology annual meeting; December 8, 2014; San Francisco, CA; Blood. 2014;124:Abstract 738; and 2) Padula WV, Larson RA, Conti RM. Is it cost-effective to start all new chronic phase CML patients on imatinib when it loses patent exclusivity? In: Society of Medical Decision Making; October 2014; Miami, FL.
W. Padula, S. Dusetzina, R. Conti, J. Apperley, B. Simonsson, F. Guilhot, and J. Guilhot have no conflicts of interest to disclose.
The following colleagues have professional relationships with named companies that have vested interest in the CML market, which did not influence this study in any way: R. Larson: Novartis, Bristol-Myers-Squibb, Pfizer, and Ariad; R. Hehlmann: Novartis and Bristol-Myers Squibb; M. Baccarani: Ariad, Bristol-Myers Squibb, Novartis, and Pfizer; E. Eigendorff: Bristol-Myers Squibb and Novartis; F. X. Mahon: Ariad, Bristol-Myers Squibb, Novartis, and Pfizer; J. Mayer: Ariad, Bristol-Myers Squibb, and Novartis; G. Martinelli: Novartis, Bristol Myers, Pfizer, and Ariad and is supported by “Next Generation Sequencing platform for targeted Personalized Therapy of Leukemia - NGS-PTL,” FP7-HEALTH-2012-INNOVATION-1 Seventh framework programme 2011, and by Universita’-Regione ER 2012 projects; M. Müller: Novartis, Bristol-Myers Squibb, and Ariad; C. Schiffer: Ariad, Novartis, Bristol-Myers Squibb, Pfizer, and Teva; R. Silver: Novartis and Bristol-Myers Squibb; D. Niederwieser: Novartis, Bristol-Myers Squibb, Gentium; S. Saussele: Novartis, Bristol-Myers Squibb, and Ariad.
References
- 1. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. [DOI] [PubMed] [Google Scholar]
- 2. Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. [DOI] [PubMed] [Google Scholar]
- 3. Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349(15):1423–1432. [DOI] [PubMed] [Google Scholar]
- 4. Deininger M, O’Brien SG, Guilhot F, et al. International Randomized Study of Interferon Vs STI571 (IRIS) 8-Year Follow up: Sustained Survival and Low Risk for Progression or Events in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Treated with Imatinib. ASH Annual Meeting Abstracts. 2009;114(22):1126. [Google Scholar]
- 5. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cortes J, Saglio G, Baccarani M, et al. Final Study Results of the Phase 3 Dasatinib Versus Imatinib in Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Trial (Dasision, CA180-056). ASH Annual Meeting Abstracts. 2014;124(21). [Google Scholar]
- 7. Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. [DOI] [PubMed] [Google Scholar]
- 8. Larson RA, Kim DW, Issaragrisil S, et al. Efficacy and Safety of Nilotinib vs. Imatinib in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP): Long-Term Follow-Up of ENESTnd. ASH Annual Meeting Abstracts. 2014;124(21). [Google Scholar]
- 9. Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. [DOI] [PubMed] [Google Scholar]
- 10. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conti RM. Why are cancer drugs commonly the target of numerous schemes to extend patent exclusivity? Health Affairs Blog. December 4, 2013. [Google Scholar]
- 12. Kantarjian H, Steensma D, Rius Sanjuan J, et al. High cancer drug prices in the United States: reasons and proposed solutions. J Oncol Pract. 2014;10(4):e208-e211. [DOI] [PubMed] [Google Scholar]
- 13. Walensky RP, Sax PE, Nakamura YM, et al. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med. 2013;158(2):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conti RM, Berndt ER. Specialty drug prices and utilization after loss of US patent exclusivity, 2001–2007. In. NBER Working Paper Series 20016: National Bureau of Economic Research (NBER); 2014. [Google Scholar]
- 15. Generic Imatinib. http://cmlsociety.org/generic-tyrosine-kinase-inhibitors-tkis-arrive-in-canada/. Accessed January 30, 2016.
- 16. Dunne S, Shannon B, Dunne C, et al. A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacol Toxicol. 2013;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Lemos ML, Kyritsis V. Clinical efficacy of generic imatinib. J Oncol Pharm Pract. 2015;21(1):76–79. [DOI] [PubMed] [Google Scholar]
- 18. Gold M, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 19. Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22(6):1200–1206. [DOI] [PubMed] [Google Scholar]
- 20. Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123(9):1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2014;123(4):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jabbour E, le Coutre PD, Cortes J, et al. Prediction of outcomes in patients with Ph+ chronic myeloid leukemia in chronic phase treated with nilotinib after imatinib resistance/intolerance. Leukemia. 2013;27(4):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah NP, Guilhot F, Cortes JE, et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood. 2014;123(15):2317–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marin D, Milojkovic D, Olavarria E, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112(12):4437–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010;116(19):3758–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Efficace F, Baccarani M, Breccia M, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood. 2011;118(17):4554–4560. [DOI] [PubMed] [Google Scholar]
- 27. Williams LA, Garcia Gonzalez AG, Ault P, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood. 2013;122(5):641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szabo SM, Levy AR, Davis C, et al. A multinational study of health state preference values associated with chronic myelogenous leukemia. Value Health. 2010;13(1):103–111. [DOI] [PubMed] [Google Scholar]
- 29. Innes AJ, Apperley JF. Chronic Myeloid Leukemia-Transplantation in the Tyrosine Kinase Era. Hematol Oncol Clin North Am. 2014;28(6):1037–1053. [DOI] [PubMed] [Google Scholar]
- 30. Padula W, Allen R, Nair K. Determining the cost of obesity and its common comorbidities from a commercial claims database. Clin Obesity. 2014;4(1):53–58. [DOI] [PubMed] [Google Scholar]
- 31. Guerin A, Chen L, Dea K, et al. Economic benefits of adequate molecular monitoring in patients with chronic myelogenous leukemia. J Med Econ. 2014;17(2):89–98. [DOI] [PubMed] [Google Scholar]
- 32. Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conti RM, Padula WV, Larson RA. Changing the cost of care for chronic myeloid leukemia: the availability of generic imatinib in the USA and the EU. Ann Hematol. 2015; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grabowski H, Vernon J. Longer patents for increased generic competition in the US. The Waxman-Hatch Act after one decade. Pharmacoeconomics. 1996;10(Suppl 2):110–123. [DOI] [PubMed] [Google Scholar]
- 35. Grabowski H, Vernon J. Brand loyalty, entry and price competition in pharmaceuticals after the 1984 Drug Act. J Law Econ. 1992;35:331–350. [Google Scholar]
- 36. Reiffen D, Ward MR. Generic drug industry dynamics. Rev Econ Statistics. 2005;87(1):37–49. [Google Scholar]
- 37. Wiggins SN, Maness R. Price Competition in Pharmaceuticals: The Case of Anti‐infectives. Econ Inquiry. 2004;42(2):247–263. [Google Scholar]
- 38. Motheral BR. Pharmaceutical step-therapy interventions: a critical review of the literature. J Manag Care Pharm. 2011;17(2):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nayak RK, Pearson SD. The Ethics Of ‘Fail First’: Guidelines And Practical Scenarios For Step Therapy Coverage Policies. Health Affairs. 2014;33(10):1779–1785. [DOI] [PubMed] [Google Scholar]
- 40. Pearson CF. Consumers face more hurdles to accessing drugs in exchange plans compared to employer coverage. Washington, DC: Avalere Health; March 2014. [Google Scholar]
- 41. Paradis PE, Latremouille-Viau D, Moore Y, et al. Projected economic impact of clinical findings of generic entry of topiramate on G4 European countries. Curr Med Res Opin. 2009;25(7):1793–1805. [DOI] [PubMed] [Google Scholar]
- 42. 2013–2014 Prescription Drug Benefit Costs and Plan Design Report. In: Pharmacy Benefit Management Institute; August 2013; Plano, TX. [Google Scholar]
- 43. Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117(14):3733–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–5411. [DOI] [PubMed] [Google Scholar]
- 46. Dusetzina SB, Winn AN, Abel GA, et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306–311. [DOI] [PubMed] [Google Scholar]
- 47. Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035. [DOI] [PubMed] [Google Scholar]
- 48. Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424–430. [DOI] [PubMed] [Google Scholar]
- 49. Delphine R, Nicolini F, Tulliez M, et al. Dasatinib or nilotinib discontinuation in chronic phase-chronic myeloid leukemia patients with durably undetectable BCR-ABL transcripts: Interim analysis of the STOP 2G-TKI study with a minimum follow-up of 12 months – on Behalf of the French CML Group FILMC. ASH Annual Meeting Abstracts. 2014;124(21). [Google Scholar]
- 50. Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515–522. [DOI] [PubMed] [Google Scholar]
- 51. Mahon FX, Etienne G. Deep molecular response in chronic myeloid leukemia: the new goal of therapy? Clin Cancer Res. 2014;20(2):310–322. [DOI] [PubMed] [Google Scholar]
- 52. Mahon FX. Is going for cure in chronic myeloid leukemia possible and justifiable? Hematology Am Soc Hematol Educ Program. 2012;2012:122–128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.