Abstract
Background:
Positron emission tomography (PET) scans are often used in cancer patients for staging, restaging, and monitoring for treatment response. These scans are also often used to detect recurrence in asymptomatic patients, despite a lack of evidence demonstrating improved survival. We sought to evaluate utilization of PET for this purpose and relationships with survival for patients with lung and esophageal cancers.
Methods:
Using national Surveillance, Epidemiology, and End Results (SEER) and Medicare-linked data, we identified incident patient cases from 2005 to 2009, with follow-up through 2011. We identified cohorts with primary lung (n = 97 152) and esophageal (n = 4446) cancers. Patient and tumor characteristics were used to calculate risk-adjusted two-year overall survival. Using Medicare claims, we examined PET utilization in person-years (to account for variable time in cohorts), excluding scans for staging and for follow-up of CT findings. We then stratified hospitals by quintiles of PET utilization for adjusted two-year survival analysis. All statistical tests were two-sided.
Results:
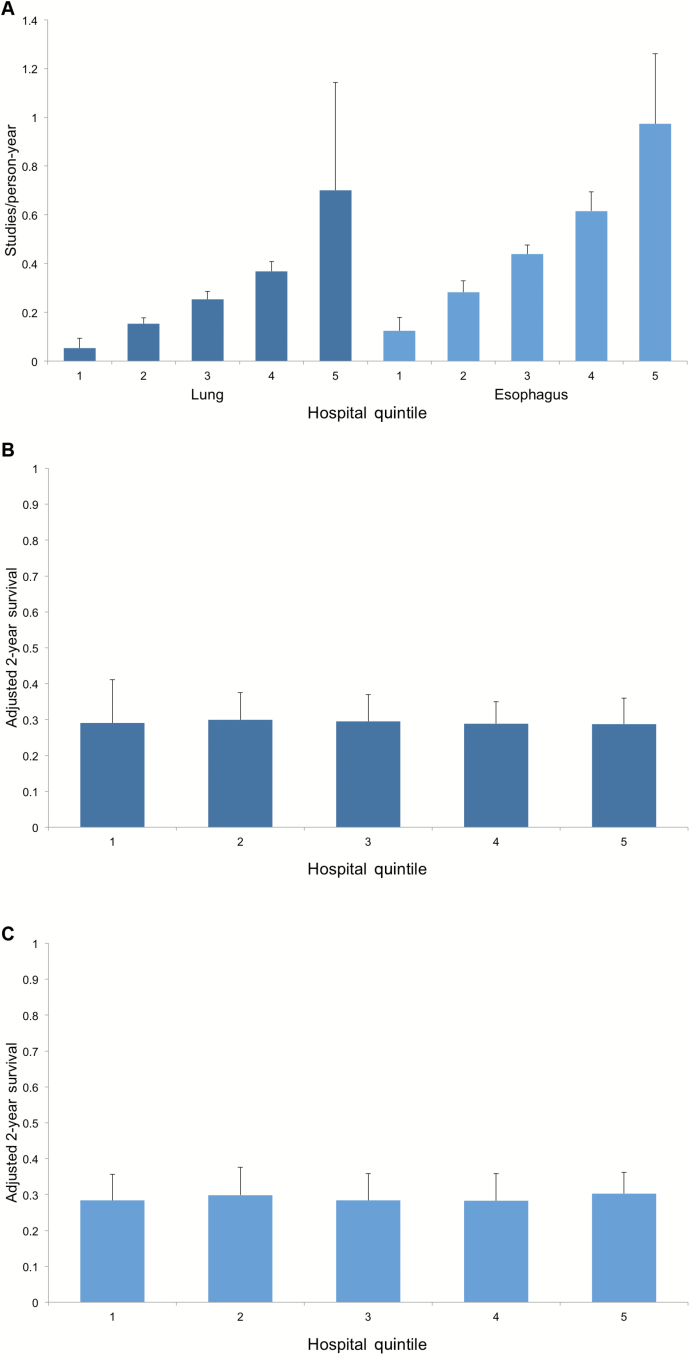
There was statistically significant variation in utilization of PET. Lowest vs highest utilizing hospitals performed .05 (SD = 0.04) vs 0.70 (SD = 0.44) scans per person-year for lung cancer and 0.12 (SD = 0.06) vs 0.97 (SD = 0.29) scans per person-year for esophageal cancer. Despite this, for those undergoing PET, lowest vs highest utilizing hospitals had an adjusted two-year survival of 29.0% (SD = 12.1%) vs 28.8% (SD = 7.2%) for lung cancer (P = .66) and 28.4% (SD = 7.2%) vs 30.3% (SD = 5.9%) for esophageal cancer (P = .55).
Conclusions:
Despite statistically significant variation in use of PET to detect tumor recurrence, there was no association with improved two-year survival. These findings suggest possible overuse of PET for recurrence detection, which current Medicare policy would not appear to substantially affect.
Use of positron emission tomography (PET) has consistently increased since the introduction of these scans in the 1970s (1). While extremely useful in appropriate situations, PET is an expensive technology. Medicare has gradually increased coverage of PET, from initial reimbursement in 1998 for the evaluation of solitary pulmonary nodules and initial staging of non–small cell lung cancers to many more indications (2). To gather evidence regarding the utility of PET for various indications around cancer, Medicare established the National Oncologic PET Registry (NOPR) in 2005 with opening for accrual in 2006. In this registry, reimbursement for PET could be received in exchange for providing data from providers about utility of PET in management decisions, which were ultimately used to guide coverage decisions. This concept is known as Coverage with Evidence Development (CED). Using information gained from this national registry, PET reimbursements were expanded over time to include use in many more cancers for the purposes of staging, restaging, and even treatment monitoring. As a result, the proportion of cancer patients undergoing PET has increased substantially over time, with a number of scans being performed for detection of recurrence in asymptomatic patients who are not currently receiving therapy (3). Despite this, there has been a persistent lack of evidence regarding the use of PET for these purposes in asymptomatic patients (4).
However, it is unclear to what extent PET is actually used for recurrence detection in asymptomatic patients despite the lack of evidence or formal coverage in this setting. There is suspicion that this type of use is actually quite prevalent, leading to the American Society of Clinical Oncology (ASCO) recommendation in their 2013 contribution to the Choosing Wisely campaign that providers “avoid using positron emission tomography or positron emission tomography–computed tomography scanning as part of routine follow-up care to monitor for cancer recurrence in asymptomatic patients who have finished initial treatment to eliminate the cancer unless there is high-level evidence that such imaging will change the outcome” (5). Moreover, in 2013, the Centers for Medicare & Medicaid Services (CMS) issued Decision Memorandum CAG-00181R4, which effectively ended the NOPR for 18F-FDG PET scans and limited routine reimbursement to three scans following initial treatment. While PET for recurrence detection in asymptomatic patients is technically not covered under Medicare, many believe that this three scan limit is aimed at preventing excessive use of PET for this very purpose (1). Despite this, some have advocated for PET in these circumstances because of the potential that earlier detection of recurrence could lead to better outcomes (6,7).
The purpose of this study was to identify how often PETs are actually used to detect recurrence in patients with lung and esophageal cancers despite limited evidence to support their utility. We sought to evaluate hospital-based variation in the use of PET for this purpose and the relationships between use of PET for recurrence detection with survival. Our hypothesis was that there would be statistically significant variation across hospitals in the use of PET with little association with survival in this population of patients with poor prognosis cancers. Further, we hypothesized that the utilization of more than three PET scans after initial treatment is relatively low overall and that current CMS policy limiting PET coverage to three scans is not likely to substantially curb overuse.
Methods
We chose to study lung and esophageal cancers because of their similar poor prognoses and general anatomic location, yet with one being a relatively common malignancy and one being much more rare. We felt that studying both would help to prevent detecting only disease-specific phenomena and establish if patterns of use are present at a hospital level. Because of differences in cohort size, we chose to perform all of our analyses separately for these two diseases. We divided hospitals into quintiles of utilization, allowing us to group and compare hospitals by utilization of PET.
Data Source and Study Population
We identified cohorts of lung and esophageal cancer patients using the national Surveillance, Epidemiology, and End Results (SEER)–Medicare linked dataset. This set includes population-based cancer sociodemographic and tumor characteristic data for patients from the National Cancer Institute’s SEER Program, encompassing approximately 28% of the US population (8). A subset of patients in the SEER registry is linked with Medicare claims data, which allows for examination of the details of diagnostic and therapeutic interventions of inpatient and outpatient medical care. Using this data, we identified incident patient cases of primary lung and esophageal cancers in the period from 2005 through 2009, with follow-up data including Medicare claims through 2011 using relevant ICD-9 diagnosis codes (lung: 162.x, esophageal: 150.x) for primary malignancies. Our exclusion criteria included patients first diagnosed prior to 2005 or with an unknown diagnosis date, patients with a history of previous primary tumor, patients diagnosed on autopsy, patients younger than age 65 years or older than 99 years at diagnosis, patients with HMO or incomplete Medicare part A & B coverage 12 months prior to and after diagnosis, and patients at hospitals treating fewer than 10 patients with the given diagnosis in the study time period (Supplementary Figure 1, available online). In order to perform a hospital-level analysis, we attributed patients to hospitals at which they received the plurality of their care based on magnitude of Medicare payments. We excluded patients for which such a hospital could not be assigned. We identified both PET and CT Medicare claims using relevant Current Procedural Terminology (CPT) codes (Supplementary Table 1, available online). Use of this data for this study was approved by the University of Michigan Institutional Review Board.
Statistical Analysis
For our survival analysis, we included all patients undergoing at least one PET scan. We calculated patient-level two-year survival. Patient tumor and sociodemographic characteristics were analyzed for risk-adjustment and to compare case mix across hospitals. Covariates included age, race, sex, marital status, and American Joint Committee on Cancer (AJCC) 6th edition cancer stage (9). Using multivariable logistic regression, risk-adjusted two-year survival was calculated. In testing model discrimination, area under the receiver operating characteristic curve (AUROC) was 0.81 and 0.77 for lung and esophageal cancers, respectively (10).
In our utilization analysis, we assessed the total number of PET scans billed for each patient using Current Procedural Terminology (CPT) codes, accounting for these studies in inpatient and outpatient settings. For each patient, we excluded all duplicate studies billed on the same date of service, such that only one scan could be counted toward a patient’s total for a given date, even with a different CPT code. Using Medicare claims, it is not possible to know whether or not a scan was performed in a symptomatic patient. Because of this, we took several measures to ensure that we eliminated scans that were performed for staging and follow-up of other imaging. An initial “staging” scan performed within three months following diagnosis was not considered a recurrence detection scan. We wanted to avoid including scans that were used to potentially follow-up on a CT finding (ie, diagnostic or restaging PET). Thus, we excluded any PET beyond three months of diagnosis that was performed within 30 days following a CT scan. Finally, we sought to account for and exclude PET scans for treatment response monitoring. To do this, we performed a sensitivity analysis, repeating initial analyses using only patients with early-stage disease (stage I for lung cancer, stage I and II for esophageal cancer).
Patient time in cohort was calculated, and PET utilization was reported in person-years (ie, scans per patient per year), a measure to account for and standardize the amount of time each patient spends in the cohort (11). We analyzed utilization and survival within hospital-level quintiles, divided evenly based on mean PET utilization. Following this, analysis of variance was used to compare differences in mean PET utilization and survival across quintiles (12). All tests of statistical significance were two-sided, with P values of .05 considered statistically significant. We performed all analyses using Stata release 13 (StataCorp, College Station, TX).
Results
Cohort Characteristics
Using our exclusion criteria, we identified 97 152 patients in 859 hospitals with lung cancer and 4446 patients in 215 hospitals with esophageal cancer in our cohort. The median age of patients for both lung and esophageal cancers was 76 years. The majority of lung and esophageal cancer patients were men (52% and 73%, respectively). In both cohorts, over 80% of patients were white. Over 40% of lung cancer patients (38 761) and 26% (1167) of esophageal cancer patients in the cohort were AJCC stage IV.
PET Utilization
Basic characteristics of PET utilization are shown in Table 1. For lung and esophageal cancers, respectively, the total numbers of PETs were 100 479 and 6162, mean PETs per patient were 1.03 and 1.39, percentages of scans in stage IV patients were 28.0% and 21.8%, and percentages of patients undergoing more than three scans were 7.4% and 11.1%.
Table 1.
Overall and adjusted PET utilization in cohorts
| All scans | Lung | Esophageal |
|---|---|---|
| Total PET studies | 100 479 | 6162 |
| Mean scans/patient | 1.03 | 1.39 |
| Patients receiving, No. (%) | ||
| 0 scans | 50 699 (52.2) | 1805 (40.5) |
| 1 scan | 24 784 (25.5) | 1225 (27.6) |
| 2 scans | 9873 (10.2) | 597 (13.4) |
| 3 scans | 4652 (4.8) | 325 (7.3) |
| >3 scans | 7144 (7.4) | 494 (11.1) |
| Total scans by AJCC stage, No. (%) | ||
| I | 27 810 (27.7) | 1108 (17.9) |
| II | 7032 (7.0) | 1569 (25.5) |
| III | 28 779 (28.6) | 1416 (23.0) |
| IV | 28 161 (28.0) | 1345 (21.8) |
| Mean scans/person-year by AJCC stage, No. (SD) | ||
| I | 0.86 (1.60) | 1.14 (0.54) |
| II | 1.28 (1.93) | 1.57 (2.33) |
| III | 1.33 (2.43) | 1.85 (2.05) |
| IV | 1.36 (3.43) | 1.81 (2.96) |
| Adjusted results following exclusion of “appropriate use” scans* | ||
| Total PET studies | 49 471 | 3160 |
| Mean scans/patient | 0.51 | 0.71 |
| Patients receiving, No. (%) | ||
| 0 scans | 75 598 (77.8) | 3060 (68.8) |
| 1 scan | 11 044 (11.4) | 666 (15.0) |
| 2 scans | 4350 (4.5) | 297 (6.7) |
| 3 scans | 2416 (2.5) | 187 (4.2) |
| >3 scans | 3744 (3.9) | 236 (5.3) |
| Total scans by AJCC stage, No. (%) | ||
| I | 13 982 (28.3) | 560 (17.7) |
| II | 3672 (7.4) | 842 (26.6) |
| III | 14 612 (29.5) | 752 (23.8) |
| IV | 12 751 (25.8) | 639 (20.2) |
| Mean scans/person-year by AJCC stage, No. (SD) | ||
| I | 0.32 (0.72) | 0.36 (0.73) |
| II | 0.47 (0.99) | 0.58 (0.98) |
| III | 0.43 (1.04) | 0.68 (1.07) |
| IV | 0.30 (1.05) | 0.52 (1.25) |
* Excluded first scan within three months of diagnosis (staging) and scans performed within 30 days after computed tomography (CT) scan (follow-up of CT finding). AJCC = American Joint Committee on Cancer; PET = positron emission tomography.
Table 1 also shows results with exclusion of staging PET and follow-up or restaging PET. For lung and esophageal cancers, respectively, total PETs were 49 471 and 3160, the numbers of patients receiving 0 PET studies were 75 598 (77.8%) and 3060 (68.8%), the numbers of patients receiving one PET study were 11 044 (11.4%) and 666 (15.0%), the numbers of patients receiving two PET studies were 4350 (4.5%) and 297 (6.7%), and numbers of patients receiving three PET studies were 2416 (2.5%) and 187 (4.2%). Notably, the percentages of patients of all stages in the cohorts undergoing more than three scans were 3744 (3.9%) and 236 (5.3%), respectively. In other words, the proportions of patients completely unaffected by the 2013 Medicare policy change would have likely been 96.1% and 94.7% for lung and esophageal cancers, respectively.
Finally, as was studied further in our sensitivity analysis, the percentages of all scans occurring in stage I (lung) and stage I and II (esophageal) cancer patients were 28.3% and 44.3%, respectively.
Survival
Using multivariable logistic regression, we calculated risk-adjusted two-year survival. Risk factor statistics are shown in Supplementary Table 2 (available online). For lung cancer, risk factors included advancing age, male sex, black race, single marital status and advancing AJCC stage. For esophageal cancer, risk factors included all of these with the exception of male sex. Using this information, we calculated risk-adjusted two-year survival.
Utilization of PET was analyzed at a hospital level, with hospitals divided into quintiles based on PET utilization (Table 2). For both cancers, higher utilizing hospitals treated higher percentages of white patients, while lower utilizing hospitals treated higher percentages of black patients. Higher utilization occurred at higher volume hospitals in lung cancer. For all cancers, the case mix by AJCC stage was similar across quintiles.
Table 2.
Patient and tumor characteristics across hospital quintiles of PET utilization for lung and esophageal cancers*
| Characteristics | Quintile | ||||
|---|---|---|---|---|---|
| 1 (low) | 2 | 3 | 4 | 5 (high) | |
| Lung | |||||
| Patients, No. | 7842 | 21 922 | 20 827 | 24 306 | 22 255 |
| Age (median), y | 76 | 76 | 76 | 76 | 76 |
| Race, No. (%) | |||||
| White | 6361 (81.1) | 19 015 (86.7) | 18 253 (87.6) | 21 048 (86.6) | 19 620 (88.2) |
| Black | 884 (11.3) | 1645 (7.5) | 1790 (8.6) | 1925 (7.9) | 1515 (6.8) |
| Other | 597 (7.6) | 1262 (5.8) | 784 (3.8) | 1333 (5.5) | 1120 (5.0) |
| AJCC stage, No. (%) | |||||
| I | 1368 (17.4) | 4410 (20.1) | 4279 (20.5) | 4983 (20.5) | 4779 (21.5) |
| II | 299 (3.8) | 893 (4.1) | 876 (4.2) | 1,074 (4.4) | 1019 (4.6) |
| III | 1770 (22.6) | 5349 (24.4) | 4820 (23.1) | 5622 (23.1) | 5375 (24.2) |
| IV | 3326 (42.4) | 8916 (40.6) | 8315 (39.9) | 9677 (39.8) | 8527 (38.3) |
| Esophageal | |||||
| Patients, No. | 771 | 940 | 915 | 953 | 867 |
| Age (median), y | 76 | 77 | 75 | 76 | 74 |
| Race, No. (%) | |||||
| White | 634 (82.2) | 842 (89.6) | 793 (86.7) | 829 (87.0) | 786 (90.7) |
| Black | 77 (10.0) | 68 (7.2) | 93 (10.2) | 80 (8.4) | 42 (4.8) |
| Other | 60 (7.8) | 30 (3.2) | 29 (3.2) | 44 (4.6) | 39 (4.5) |
| AJCC stage, No. (%) | |||||
| I | 135 (17.5) | 191 (20.3) | 192 (21.0) | 162 (17.0) | 177 (20.4) |
| II | 141 (18.3) | 175 (18.6) | 153 (16.7) | 183 (19.2) | 168 (19.4) |
| III | 128 (16.6) | 147 (15.6) | 163 (17.8) | 170 (17.8) | 143 (16.5) |
| IV | 194 (25.2) | 278 (29.6) | 225 (24.6) | 249 (26.1) | 221 (25.5) |
* AJCC = American Joint Committee on Cancer; PET = positron emission tomography.
Hospital-Based Variation in PET Utilization Without Improved Survival
The results of our analysis of utilization and survival across hospitals is shown in Figure 1. For each cancer, there is statistically significant variation in mean PET utilization across quintiles. Lowest vs highest utilizing hospitals performed 0.05 (SD = 0.04) vs 0.70 (SD = 0.44) scans per person-year for lung cancer and 0.12 (SD = 0.06) vs 0.97 (SD = 0.29) scans per person-year for esophageal cancer (P < .001 for both). Despite this variation, for those patients undergoing PET two-year survival was not significantly improved across hospitals. Lowest vs highest utilizing hospitals had a mean adjusted two-year survival of 29.0% (SD = 12.1%) vs 28.8% (SD = 7.2%) for lung cancer (P = .66) and 28.4% (SD = 7.2%) vs 30.3% (SD = 5.9%) for esophageal cancer (P = .55).
Figure 1.
Mean hospital-level positron emission tomography (PET) utilization and survival. A) Shows mean PET utilization across hospital quintiles for lung and esophageal cancers. B) Demonstrates mean adjusted two-year survival in these same quintiles for lung cancer. C) Represents mean adjusted two-year survival in these same quintiles for esophageal cancer. All error bars represent standard deviations.
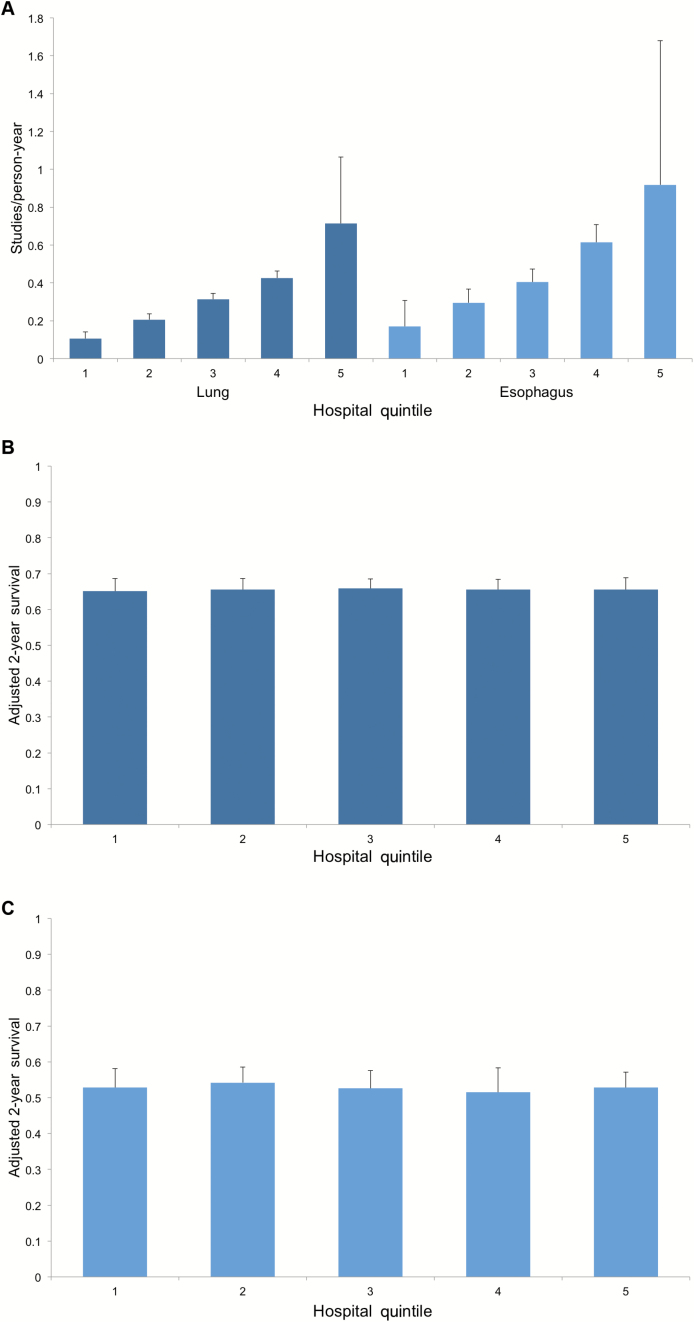
The results of our sensitivity analysis are shown in Figure 2. In this subgroup of patients with early-stage disease, the results are similar. For each cancer, there is statistically significant variation in mean PET utilization across quintiles. Lowest vs highest utilizing hospitals performed 0.11 (SD = 0.04) vs 0.71 (SD = 0.35) scans per person-year for lung cancer and 0.17 (SD = 0.14) vs 0.92 (SD = 0.76) scans per person-year for esophageal cancer (P < .001 for both). Despite this variation, for those patients undergoing PET, two-year survival was not statistically significantly improved across quintiles. Lowest vs highest utilizing hospitals had an adjusted two-year survival of 65.1% (SD = 3.5%) vs 65.5% (SD = 3.3%) for lung cancer and 52.8% (SD = 5.3%) vs 52.8% (SD = 4.4%) for esophageal cancer.
Figure 2.
Sensitivity analysis of mean hospital-level positron emission tomography (PET) utilization and survival in early-stage disease. A) Shows mean PET utilization across hospital quintiles for lung and esophageal cancers. B) Demonstrates mean adjusted two-year survival in these same quintiles for lung cancer. C) Represents mean adjusted two-year survival in these same quintiles for esophageal cancer. All error bars represent standard deviations.
As an additional sensitivity analysis, we compared hospitals across disease groups. We found that 75% of the highest utilizing (4th and 5th quintile) hospitals for esophageal cancer were also in these same quintiles for lung cancer. This suggests that many high-utilizing hospitals are high users across both disease groups.
Discussion
The results of this study show that there is high overall use of PET in lung and esophageal cancers, with statistically significant hospital-based variation in use of PET for recurrence detection. Despite this, there is no association with improved two-year survival in higher utilizing hospitals. This combination of hospital-based variation without survival benefit suggests potential overuse and that efforts to decrease such overuse are warranted. Another striking and perhaps unexpected finding was that more scans are performed in stage IV patients than in any other group in both cancers. Moreover, a major strength of this study is the nationally representative nature of the cohort. SEER-Medicare provides the patient sociodemographic and tumor characteristics necessary for this analysis in addition to the claims-based imaging utilization data that Medicare provides. This allows for analysis of national patterns of PET utilization.
A potential implication of this study is that use of PET for recurrence detection appears to be common in some hospitals. The Decision Memo released by CMS in 2013 limiting patients to three scans following initial anticancer therapy was based on several years of data collection by the NOPR. Interestingly however, over 90% of patients in the NOPR 2006 and 2009 cohorts had fewer than three scans and the mean number per patient was 1.6, with a range of 1 to 29 scans. Even this data would suggest that while this policy may curb extreme overuse it is unlikely to have any effect on over 90% of patients undergoing recurrence detection PET, and our results are consistent with this.
Given this, we believe targets to reduce variation are two-fold. First, CMS should reconsider its policy limiting patients to three scans following initial anticancer therapy. If this is intended to curb overuse, it appears unlikely to do this. However, this should be further studied once more data following this policy becomes available. Second, we believe that this information should be fed back to hospitals to improve practices. This type of activity can possibly be done in a statewide or regional collaborative setting, in which hospital representatives meet regularly to discuss and disseminate best practices, as well as consider collecting data on imaging practices.
Arguments against Medicare’s decision have implied that using PET for recurrence detection in asymptomatic patients might be more sensitive for earlier detection of recurrence or metastatic disease in a more treatable state, thus implying a survival benefit for the use of this expensive technology (1,6,7). The results of our study suggest that this is not likely to be the case for this group of patients with poor prognosis cancers. Some have previously contended that the impact of PET on survival is not likely to be statistically significant in most cases, as PET is a diagnostic test and part of a chain of tests and treatments for these cancer patients (1). But in patients with poor prognosis cancers, does the added information that PET provides yield sufficient value to justify the high cost of such scans, especially for recurrence detection in asymptomatic patients? While NOPR was able to detect changes in management, investigators were not able to identify whether these management changes improved long-term outcomes. Our results support ASCO’s contribution to the Choosing Wisely campaign recommending avoidance of use of PET to detect recurrence in the absence of high-level evidence that such imaging will change outcomes.
Detection of PET for recurrence in asymptomatic patients has been studied in other cancer types. One well-done systematic review covering a diversity of tumor types (lymphoma, colorectal cancer, and head and neck cancer) is especially informative (4). This review looked at 12 studies in total and concluded insufficient evidence to draw conclusions on the clinical impact of PET or PET/CT for surveillance. Our study provides data on PET use in two cancers that were not studied in this review. Lung and esophageal cancers, both poor prognosis cancers, are especially susceptible to variation in treatment patterns and intensity, and our study demonstrates that this is true in the use of PET. Many other previous studies of PET have noted changes in management rather than impact on patient outcomes (13–16). One study described PET findings leading to a change from nontreatment to treatment (30%) more often than a change from treatment to nontreatment (8%) (15). The overwhelming majority of these patients received some change in their therapy, as opposed to a transition to observation or supportive care. It is impossible to know in these datasets how appropriate these decisions are, but what is clear is that there is a lower chance of receiving less aggressive therapy or stopping therapy following PET for staging or treatment monitoring. Our study adds to this, with no evidence of improvement in long-term outcomes with PET to detect tumor recurrence.
Another topic of recent studies is geographic variation in the use of cancer imaging and subsequent implications (11,17,18). One study compared VA and Medicare cohorts of patients to determine differences in imaging utilization and geographic variation (11). Investigators identified similar statistically significant geographic variation in both cohorts but statistically significantly less spending in the VA system. This implies that geographic variation alone is not sufficient to demonstrate overutilization. Yet another recent study demonstrated within-region and not just inter-region variation in use of diagnostic imaging across two distinct types of cancer (17). Some have expressed caution in light of these results, specifically regarding policies sought to curb overuse at the regional level as variation has been shown to exist within regions and simply attempting to make utilization uniform where there is high spending, without regard for appropriateness of use, could have unintended consequences (18). Our main outcome measure of PET utilization is based on a more local measure—hospital-based variation—with evidence of overuse demonstrated not simply by the presence of variation alone, but with a lack of association with improved survival.
There are likely myriad causes for the variation we observed. First, providers are likely to have specific imaging-related practice patterns. Second, hospitals may have different degrees of communication between these varying providers. Perhaps in a hospital where radiologists regularly communicate with oncologists, fewer tests are ordered after discussions of indication and utility.
Perhaps the most substantial limitation to this work is that we cannot directly detect asymptomatic patients using Medicare claims data. We specifically did not include a first scan within three months of diagnosis (which we attributed to staging) and scans performed within 30 days following a CT scan (which would likely be performed to follow-up on a CT finding). While this helps to identify some clearly appropriate scans, it is not possible to say whether an individual patient in our study was asymptomatic. Because of the importance of this point, we also performed a sensitivity analysis using patients with early-stage disease to assess for any differences in our main outcome measures and found no clear differences in patterns of hospital-based variation in PET utilization.
Other limitations to our study include the fact that we used SEER-Medicare, a retrospective linked registry and claims-based dataset. SEER represents 28% of all cancer patients in the United States and is considered an authoritative source for national cancer survival data (8). Importantly, due to the potential for confounding, it is not possible in an observational study such as this, to retrospectively determine cause-and-effect relationships, as to whether PET scans intended to detect tumor recurrence improve survival. Thus we can only say from our study that there does not appear to be a clear association between increased use of PET to detect tumor recurrence as we defined it and improved overall survival.
We were interested in hospital-level patterns in PET utilization, but it is possible that if individual patients within hospitals experienced benefit with increased PET utilization this may not have been detected, even after accounting for clustering. This hospital-level analysis allows us to standardize for patient time in the cohort by reporting scans per person-year. In a patient-level analysis, the only way to achieve similar standardization is to perform a cross-sectional analysis at a given point in time. This type of analysis showed a similar amount of broad variation in PET utilization among patients alive at two years in both cohorts (data not shown). Future studies to address these topics should be prospectively performed to mitigate potential immortality time bias, ecological fallacy, or bias by indication, and focus on multiple levels of evaluation and potential confounders at patient, provider, hospital, and perhaps regional levels. Our study only examines patients in the Medicare population, and thus these results may not be generalizable to younger patients. However, the median ages at diagnosis of lung and esophageal cancer are 70 and 67 years, respectively (19). Our study results come from patients diagnosed between 2005 and 2009, before the current CMS policy that began the three PET scan limit. Because of this, our data can only be used to anticipate how much this policy could be expected to curb overuse. However, our results show that a relatively small proportion of patients is likely to be affected by this policy, and thus it will be interesting, when more recent data becomes available, to see if this policy is associated with changed practice patterns.
Overuse of tests, specifically PET for detection of tumor recurrence after initial treatment, has been raised as a concern, especially in patients with poor prognosis cancers. Our results support this. Providers must take note of available data when making clinical imaging decisions to avoid unnecessary overuse. Medicare’s current policy limits routine reimbursement to three scans, meant to guide subsequent management after completion of initial cancer therapy. Our findings highlight patterns of variation in the use of PET to detect tumor recurrence without clear benefit in long-term patient outcomes, which would not appear to be affected substantially by current Medicare policy.
Funding
Dr. Healy is supported by the National Institutes of Health (grant T32CA009672-24), and Dr. Wong is supported by the Association for Healthcare Research and Quality (grant AHRQ1K08 HS20937-01) and the American Cancer Society (grant RSG-12-269-01-CPHPS). Dr. Reddy has received research funding from GlaxoSmithKline and speaking fees from Covidien Ltd.
Supplementary Material
Notes
Part of this work was presented at the 10th Annual Academic Surgical Congress, Las Vegas, NV, February 3–5, 2015.
The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication or presentation.
Dr. Reddy has received travel sponsorship from Cancer Treatment Centers of America and Novadaq Technologies, Inc. All other authors have no financial conflicts of interest to disclose.
References
- 1. Siegel BA. 2014 Cassen Lecture-What Have We Learned from the National Oncologic PET Registry? J Nucl Med. 2014;55(12):9N–15N. [PubMed] [Google Scholar]
- 2. CMS Manual System: pub 100–03 Medicare national coverage determinations: transmittal 31. Centers for Medicare & Medicaid Servicves. [Google Scholar]
- 3. Dinan MA, Curtis LH, Hammill BG, et al. Changes in the Use and Costs of Diagnostic Imaging Among Medicare Beneficiaries With Cancer, 1999–2006. JAMA. 2010;303(16):1625–1631. [DOI] [PubMed] [Google Scholar]
- 4. Patel K, Hadar N, Lee J, Siegel BA, Hillner BE, Lau J. The lack of evidence for PET or PET/CT surveillance of patients with treated lymphoma, colorectal cancer, and head and neck cancer: a systematic review. J Nucl Med. 2013;54(9):1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schnipper LE, Lyman GH, Blayney DW, et al. American Society of Clinical Oncology 2013 top five list in oncology. J Clin Oncol. 2013;31(34):4362–4370. [DOI] [PubMed] [Google Scholar]
- 6. Rohren EM, Dillehay GL, Jadvar H. SNMMI comment on ASCO 2013 “Choosing wisely” recommendation on use of PET/CT in recurrent cancer surveillance. J Nucl Med. 2014;55(5):699–700. [DOI] [PubMed] [Google Scholar]
- 7. Sobhani I, Tiret E, Lebtahi R, et al. Early detection of recurrence by 18FDG-PET in the follow-up of patients with colorectal cancer. Br J Cancer. 2008;98(5):875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Overview of the Surveillance, Epidemiology, and End Results database: evolution, data variables, and quality assurance. Curr Probl Cancer. 2012;36(4):183–190. [DOI] [PubMed] [Google Scholar]
- 9. Greene FL. AJCC cancer staging manual: Springer Science & Business Media; 2002.
- 10. Hanley J, McNeil BJ. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 11. McWilliams JM, Dalton JB, Landrum MB, Frakt AB, Pizer SD, Keating NL. Geographic variation in cancer-related imaging: Veterans Affairs health care system versus Medicare. Ann Intern Med. 2014;161(11):794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dmitrienko A, D’Agostino R., Sr Traditional multiplicity adjustment methods in clinical trials. Stat Med. 2013;32(29):5172–5218. [DOI] [PubMed] [Google Scholar]
- 13. Hillner BE, Tunuguntla R, Fratkin M. Clinical decisions associated with positron emission tomography in a prospective cohort of patients with suspected or known cancer at one United States center. J Clin Oncol. 2004;22(20):4147–4156. [DOI] [PubMed] [Google Scholar]
- 14. Hillner BE, Siegel BA, Liu D, et al. Impact of Positron Emission Tomography/Computed Tomography and Positron Emission Tomography (PET) Alone on Expected Management of Patients With Cancer: Initial Results From the National Oncologic PET Registry. J Clin Oncol. 2008;26:2155–2161. [DOI] [PubMed] [Google Scholar]
- 15. Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the national oncologic PET registry. J Nucl Med. 2008;49(12):1928–1935. [DOI] [PubMed] [Google Scholar]
- 16. Hillner BE, Siegel BA, Shields AF, et al. The impact of positron emission tomography (PET) on expected management during cancer treatment: findings of the National Oncologic PET Registry. Cancer. 2009;115(2):410–418. [DOI] [PubMed] [Google Scholar]
- 17. Makarov DV, Soulos PR, Gold HT, et al. Regional-Level Correlations in Inappropriate Imaging Rates for Prostate and Breast Cancers: Potential Implications for the Choosing Wisely Campaign. JAMA Oncol. 2015;1(2):186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swisher-McClure S, Bekelman J. Diagnostic Imaging Use for Patients With Cancer Opportunities to Enhance Value. JAMA Oncol. 2015;1(2):194–195. [DOI] [PubMed] [Google Scholar]
- 19. SEER Stat Fact Sheets. Surveillance Research Program, National Cancer Institute http://seercancergov/statfacts Accessed February 22, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.