Methacholine challenge tests only determine airway hyper-responsiveness to a nonspecific agent, and can be associated with relatively low sensitivity and specificity; therefore, they should not be used in isolation to diagnose asthma. Accordingly, alternative adjunctive measures of pulmonary function have been recommended in cases in which an individual cannot produce high-quality spirometry data. This report describes a case involving a 30-year-old man with a primary complaint of dyspnea with exercise. The ensuing discussion briefly reviews these alternative measures and the inherent limitations of the methacholine challenge test.
Keywords: Airway responsiveness, Bronchoprovocation, Methacholine, Specific conductance
Abstract
A 30-year-old Caucasian man presented to the pulmonary function laboratory for a methacholine challenge test. Following inhalation of the final dose of methacholine, the forced expiratory volume in 1 s (FEV1) was 8% below baseline. However, the patient complained of chest tightness and dyspnea, similar to the symptoms he experienced after running. Repeat specific airway conductance was found to be 73% below baseline, indicating marked airway hyper-responsiveness. Because the reduction in specific airway conductance was accompanied by familiar symptoms, the post-test probability of asthma increases, even in the absence of a 20% reduction in FEV1.
Abstract
Un homme blanc de 30 ans a subi un test de provocation à la méthacholine au laboratoire des fonctions pulmonaires. Après l’inhalation de la dernière dose de méthacholine, son volume expiratoire maximal par seconde (VEMS) avait reculé de 8 % par rapport aux valeurs de départ. Cependant, le patient s’est plaint d’une oppression thoracique et de dyspnée, des symptômes similaires à ceux qu’il ressentait après avoir couru. La répétition de la résistance spécifique des voies respiratoires se situait à 73 % sous les valeurs de départ, démontrant une hyperréactivité marquée des voies respiratoires. Puisque la diminution de la résistance spécifique des voies respiratoires s’accompagnait de symptômes familiers, la probabilité post-test d’asthme augmente, même en l’absence d’une réduction de 20 % du VEMS.
CASE PRESENTATION
A 30-year-old Caucasian man presented to the pulmonary function laboratory for a pulmonary function test (PFT) and methacholine challenge test. The patient’s chief complaint was dyspnea with exercise. He had never smoked but worked as an electrician, often in dusty environments. The patient reported a family history of asthma among two of his siblings. Measured height and weight on the day of testing indicated a body mass index of 28.1 kg/m2. Baseline PFT data are summarized in Table 1, the flow-volume loop and volume-time curve from spirometry are shown in Figure 1. All testing satisfied American Thoracic Society (ATS)/European Respiratory Society acceptability and repeatability quality standards (1). Baseline spirometry suggested a restrictive ventilatory defect, which was confirmed by lung volume measurements via whole body plethysmography.
TABLE 1.
Baseline pulmonary function data
| Parameter | Actual | LLN | Pred | % Pred | z-score |
|---|---|---|---|---|---|
| FVC, L | 3.87 | 4.05 | 5.03 | 77 | −1.95 |
| FEV1, L | 2.93 | 3.33 | 4.17 | 70 | −2.40 |
| FEV1/FVC, % | 76 | 72 | 83 | NR | −1.16 |
| TLCPLETH, L | 5.04 | 5.59 | 6.74 | 75 | NR |
| RV, L | 1.20 | 1.02 | 1.70 | 71 | NR |
| RV/TLC, % | 23 | 21 | 26 | 88 | NR |
| sGaw, L/s/cm H2O/L | 0.26 | 0.11 | 0.26 | 100 | NR |
FEV1 Forced expiratory volume in 1 s; FVC Forced vital capacity; LLN Lower limit of normal; NR Not reported; Pred Predicted value; RV Residual volume; RV/TLC Ratio of RV to total lung capacity (TLC); sGaw Specific airway conductance; TLCPLETH TLC via plethysmography
Figure 1).
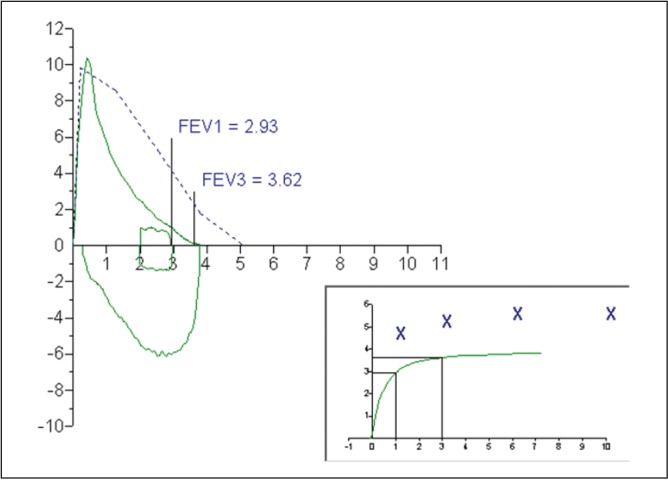
Baseline flow-volume loop and volume-time curve. FEV1 Forced expiratory volume in 1 s (L); FEV3 Forced expiratory volume after 3 s (L)
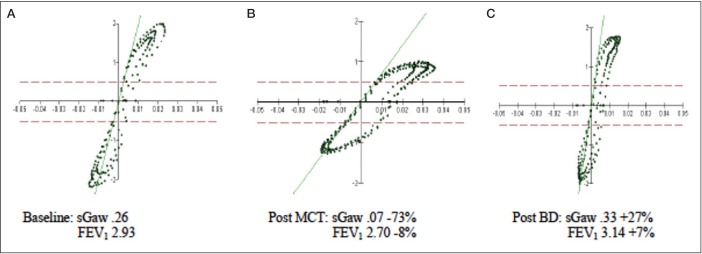
Following baseline testing, a methacholine challenge test was performed using the five-breath dosimeter technique (2). Following inhalation of the final dose of methacholine (20 mg/mL), the forced expiratory volume in 1 s (FEV1) was 8% below baseline. Such a modest reduction in FEV1 would typically be regarded as a ‘negative’ methacholine challenge test; however, the patient complained of chest tightness and dyspnea. He reported that these symptoms were similar to those he experienced after running. There were no stridor or indications of inducible laryngeal obstruction. The patient’s symptoms in the absence of a significant decline in FEV1 prompted repeat measurement of specific airway conductance (sGaw) as an alternative method to assess the airway response to methacholine inhalation. The sGaw was found to be 73% below baseline, indicating significant airway narrowing compatible with marked airway hyper-responsiveness. Following bronchodilator administration (2.5 mg albuterol via small volume nebulizer) both the FEV1 and sGaw values were larger than those recorded during baseline testing, suggesting a degree of pretest bronchoconstriction (Table 2, Figure 2).
TABLE 2.
Forced expiratory volume in 1s (FEV1) and Specific airway conductance (sGaw) before and after methacholine challenge testing (MCT)
| Baseline | Post MCT | Δ, % | Post BD | Δ, % | |
|---|---|---|---|---|---|
| FEV1, L | 2.93 | 2.70 | −8 | 3.14 | +7 |
| sGaw, L/s/cm H2O/L | 0.26 | 0.07 | −73 | 0.33 | +27 |
BD Bronchodilator
Figure 2).
Open-shutter breathing during plethysmography testing. A Baseline testing before methcholine challenge test (MCT). B Post MCT. C Post bronchodilator administration. BD Bronchodilator; FEV1 Forced expiratory volume in 1 s (L); sGaw Specific airway conductance (L/s/cm H2O/L)
DISCUSSION
The 1999 ATS guideline for methacholine challenge testing states that FEV1 should be the primary outcome measure for the discovery of airway hyper-responsiveness (2). The guideline recognizes that alternative measures of pulmonary function (eg, sGaw, impulse oscillometry) may be used during a methacholine challenge test; however, these measures are recommended only when the patient can not produce high-quality spirometry data. A whole-body plethysmograph (often called a ‘body box’) is required to measure sGaw. The patient is asked to breathe small volumes at a rate of 1.5 breaths/s to 2.5 breaths/s. Flow at the mouth is plotted against the pressure inside the plethysmograph (ie, ‘box pressure’). After the collection of several breaths, a shutter or valve is closed so that mouth (alveolar) pressure can be plotted against box pressure. This is accomplished by having the patient continue shallow breathing at a rate of approximately 1 breath/s. Following open and closed shutter breathing, mouth pressure can be divided by flow to calculate airway resistance. The reciprocal of resistance is conductance. Conductance values provide limited information because conductance shares a linear relationship with lung volume. sGaw is considered to be a more useful value because it represents the conductance adjusted to the lung volume where the measurement was recorded. In other words, the influence of lung volume on conductance is removed.
The ATS guideline recommends that a larger reduction in sGaw (eg, 45%) be used as a cut-off point for a ‘positive’ methacholine challenge test. Khalid et al (3) evaluated sGaw and FEV1 in 138 patients undergoing a methacholine challenge test. The researchers found that a 51% to 52% reduction in sGaw was a more appropriate cut-off point for a positive methacholine challenge test than the 45% reduction suggested by the ATS. A remarkable finding was that 32 patients with an FEV1 decline <20% exhibited a reduction in sGaw >50%.
In a similar study, Parker and McCool (4) measured FEV1 and sGaw following methacholine challenge testing in 248 consecutive patients with asthma-like symptoms. Forty patients showed a response to methacholine as assessed by sGaw (≥40% reduction) without a significant decline in FEV1 (<20%). The obvious question that arises from these observations is whether using FEV1 as the sole outcome measure during methacholine challenge testing results in false-negative tests in some patients. In other words, does the positive sGaw/negative FEV1 response indicate asthma or an expected response in some nonasthmatic patients or both? Parker and McCool (4) found that subjects with this response had a higher baseline sGaw and forced expiratory flow between 25% and 75% of the forced vital capacity (FEF25–75) to forced vital capacity ratio, suggesting that dysanapsis (large tracheobronchial tree compared with lung size) may be causal. The authors offered other possible mechanisms for the positive sGaw/negative FEV1 response including disproportionate proximal airway narrowing, differences in airway compliance and airway smooth muscle distribution.
Both studies used the five-breath dosimeter technique with inhalation of aerosol to total lung capacity. Cockcroft and Davis (5) showed that this technique results in false-negative methacholine challenge tests as judged by FEV1 in patients with mild asthma. It is not clear whether sGaw and FEV1 are equally affected by the bronchodilatory and bronchoprotective effects of deep inhalation.
In addition to nonspecific airway challenge testing (eg, methacholine, histamine), Larbanois et al (6) reported that 13% of patients undergoing specific inhalation challenge had a ≥50% decline in sGaw without a ≥20% decline in FEV1. Because sGaw is believed to be more reflective of large airway function (7), the authors speculated that this pattern may, in part, be related to the site of aerosol deposition. Given the complexity of pulmonary structure and function in the realm of airway hyper-responsiveness (8), the etiology of the positive sGaw/negative FEV1 response is likely multifactorial.
Whether one chooses to follow FEV1, sGaw or both, the inherent limitations of bronchial challenge tests must be appreciated to avoid misinterpretation and misdiagnosis. Methacholine challenge tests are not perfect, the sensitivity and specificity can be <60% and 70%, respectively (9). Methacholine challenge tests simply determine the presence of airway hyper-responsiveness to a nonspecific agent. The presence of airway hyper-responsiveness only increases the probability of asthma in patients with an intermediate or high pretest probability of asthma (10). Therefore, methacholine challenge tests should not be used in isolation to diagnose asthma. It would also be incorrect to assume that every patient with a positive sGaw/negative FEV1 response to methacholine challenge testing has asthma. This would be especially suspect in patients with a low pretest probability of asthma. In the present case, however, the patient had an intermediate pretest probability of asthma due to his asthma-like symptoms and family history. The fact that the large reduction in sGaw during methacholine challenge testing was accompanied by familiar symptoms that he had experienced after exercise increases the post-test probability of asthma, even in the absence of a 20% reduction in FEV1.
Footnotes
DISCLOSURES: The author is a consultant for Morgan Scientific Inc, USA. The has no financial disclosures or conflicts of interest to declare relating to this article.
REFERENCES
- 1.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 2.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing – 1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 3.Khalid I, Morris ZQ, DiGiovine B. Specific conductance criteria for a positive methacholine challenge test: Are the American Thoracic Society guidelines rather generous? Respir Care. 2009;54:1168–74. [PubMed] [Google Scholar]
- 4.Parker AL, McCool FD. Pulmonary function characteristics in patients with different patterns of methacholine airway hyperresponsiveness. Chest. 2002;121:1818–23. doi: 10.1378/chest.121.6.1818. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft DW, Davis BE. The bronchoprotective effect of inhaling methacholine by using total lung capacity inspirations has a marked influence on the interpretation of the test result. J Allergy Clin Immunol. 2006;117:1244–48. doi: 10.1016/j.jaci.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Larbanois A, Delwiche JP, Jamart J, Vandenplas O. Comparison of FEV1 and specific airway conductance in assessing airway response to occupational agents. Allergy. 2003;58:1256–60. doi: 10.1046/j.1398-9995.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaminsky DA. What does airway resistance tell us about lung function? Respir Care. 2012;57:85–96. doi: 10.4187/respcare.01411. [DOI] [PubMed] [Google Scholar]
- 8.Winkler T, Venegas JG. Complex airway behavior and paradoxical responses to bronchoprovocation. J Appl Physiol. 2007;103:655–63. doi: 10.1152/japplphysiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 9.Anderson SD, Charlton B, Weiler JM, Nichols S, Spector SL, Pearlman DS, A305 Study Group Comparison of mannitol and methacholine to predict exercise-induced bronchoconstriction and a clinical diagnosis of asthma. Respir Res. 2009;10:4. doi: 10.1186/1465-9921-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busse WW. What is the best diagnostic approach for wheezing patients with normal spirometry? Respir Care. 2012;57:39–46. doi: 10.4187/respcare.01449. [DOI] [PubMed] [Google Scholar]