Although currently major contributors to morbidity and mortality, cardiovascular disease (CVD) and chronic respiratory diseases, such as chronic obstructive pulmonary disease, are projected to rank among the top three in global disease burden by 2020. Multimorbidity is a common feature in CVD and chronic respiratory disease, and patients often share the same pathological characteristics including anatomical location of disease, dyspnea and fatigue, among several others. However, patients entering rehabilitation programs for either condition are functionally diverse; therefore, individuals with respiratory comorbidities may not fare as well in cardiac rehabilitation programs. This retrospective cohort study investigated several relevant functional parameters and outcomes.
Keywords: Aerobic execise, Cardiac rehabilitation, Comorbidities, COPD, Smokers
Abstract
OBJECTIVE:
To describe the prevalence and impact of respiratory comorbidities on patients undergoing cardiac rehabilitation (CR).
METHODS:
A retrospective review of a CR database (1999 to 2004) of patients with ischemic heart disease with ≥10 pack per year (ppy) smoking history and respiratory comorbidities (RC), non-respiratory comorbidities (NRC) and no comorbidities (NC) was performed. Primary outcomes at zero, six and 12 months included peak oxygen uptake (VO2peak), maximum workload, resting heart rate, ventilatory anaerobic threshold and anthropometrics. Analyses were performed on individuals who completed the program, adjusting for age, sex and baseline VO2peak.
RESULTS:
Of 5922 patients, 1247 had ≥10 ppy smoking history: 77 (6.2%) had RC; 957 (76.7%) had NRC; and 213 (17.1%) had NC. The program completion rate for each group was similar for the RC (46.8%), NRC (55.8%) and NC groups (57.3%) (P=0.26). The RC group had the lowest baseline fitness levels (P<0.002). For VO2peak, there were significant differences among groups (P=0.02) and improvements over program duration (P<0.0001). There were no significant differences in other outcomes.
CONCLUSIONS:
There was a low prevalence of patients with comorbid chronic obstructive pulmonary disease in CR when based on physician referral documentation. This is likely underestimated and/or reflects a referral bias. Diagnostic testing at CR entry would provide a more accurate measure of the prevalence and severity of disease. CR participation resulted in significant and similar improvements in most key CR outcomes in all groups including similar completion rate. A CR model was effective for patients with coexisting RCs. Strategies to improve access and diagnosis should be explored.
Abstract
OBJECTIF :
Décrire la prévalence et les effets des comorbidités respiratoires sur les patients en réadaptation cardiaque (RC).
MÉTHODOLOGIE :
Les chercheurs ont réalisé l’analyse rétrospective d’une base de données de RC (de 1999 à 2004) dont ils ont extrait les patients atteints d’une maladie cardiaque ischémique ayant des antécédents de tabagisme d’au moins dix paquets par année (ppa) et des comorbidités respiratoires (CR), des comorbidités non respiratoires (CNR) ou aucune comorbidité (AC). Les résultats primaires en début d’étude, au sixième et au douzième mois incluaient la consommation maximale d’oxygène (VO2max), la charge de travail maximale, la fréquence cardiaque au repos, le seuil anaérobie ventilatoire et les données anthropométriques. Les chercheurs ont effectué des analyses chez les personnes qui ont terminé le programme, après rajustement selon l’âge, le sexe et la VO2max en début d’étude.
RÉSULTATS :
Des 5 922 patients, 1 247 avaient des antécédents de tabagisme d’au moins 10 ppa : 77 (62 %) avaient des CR, 957 (76,7 %), des CNR, et 213 (17,1 %), AC. Le taux d’achèvement du programme était similaire dans les groupes ayant des CR (46,8 %), des CNR (55,8 %) et AC (57,3 %) (P=0,26). Le groupe ayant des CR présentait le taux de forme physique le plus faible en début d’étude (P<0,002). Les différences entre les groupes étaient significatives pour ce qui est de la VO2max, (P=0,02) et de l’amélioration pendant la durée du programme (P<0,0001). Les autres résultats ne présentaient aucune différence significative.
CONCLUSIONS :
Peu de patients atteints d’une maladie pulmonaire obstructive chronique comorbide allaient en RC selon les documents d’orientation des médecins. Ce nombre est probablement sous-estimé ou reflète un biais d’orientation. Les tests diagnostiques à l’arrivée en RC fourniraient une mesure plus précise de la prévalence et de la gravité de la maladie. Dans tous les groupes, la participation à la RC s’associait à des améliorations significatives et similaires à l’égard de la plupart des principaux résultats liés aux RC au sein des groupes, y compris un taux d’achèvement similaire. Un modèle de RC était efficace pour les patients présentant des CR. Il faudra chercher des stratégies pour améliorer l’accès et le diagnostic.
Cardiovascular and chronic respiratory disease are leading causes of morbidity and mortality in the United States and worldwide (1). In 2008, cardiovascular disease (CVD) caused 17 million deaths worldwide (48% of all noncommunicable diseases), and chronic obstructive pulmonary disease (COPD) and asthma caused 4.2 million deaths. These noncommunicable diseases, according to the World Bank/WHO, will rank in the top three of burdened diseases worldwide by 2020 (1,2).
CVD and respiratory disease share many comorbid characteristics including multimorbidity (≥2 conditions) as a common feature (3). The prevalence of multimorbidity increases with age and results in poor health outcomes (4,5), stressing the importance of effective health care interventions for this population (1). The specific prevalence of respiratory comorbidity (eg, COPD, asthma) in cardiac disease has varied in the literature: 9% to 39% in patients with acute or nonacute cardiac morbidities (eg, acute myocardial infarction, cardiac surgery) (6–9). The method by which respiratory disease was defined in these studies and the cohort investigated may have contributed to this high level of variability in prevalence.
Cardiac rehabilitation and pulmonary rehabilitation are common interventions for CVD and COPD (10,11). The population of patients who enter these programs share many characteristics: a common intrathoracic location of the pathology; the frequent coexistence of cardiac and pulmonary disease; and shared symptoms, such as dyspnea, fatigue, psychological disturbances, deconditioning and exercise intolerance (12–14). In addition, they share common rehabilitation goals and outcomes including improvement in exercise tolerance, which can reduce future morbidity and disability, as well as enhance quality of life (10,13,15). Although the principles of both pulmonary and cardiac rehabilitation are similar, the patients who enter these programs are functionally diverse (16). In many cardiac rehabilitation patients (without heart failure), the chief functional limitation and cause of exercise intolerance is deconditioning and, in some patients, CVD rate-limiting angina or ischemia (13–17). In many pulmonary rehabilitation patients, functional limitations are more extensive: work inefficiency due to impairment in lung mechanics; inspiratory muscle fatigue; ineffective gas exchange; right ventricular dysfunction; alterations in peripheral muscle metabolism; acute exacerbations; and malnutrition (13,18,19). These greater limitations may place patients with respiratory comorbidities enrolled in a cardiac rehabilitation program at a disadvantage compared with those without airflow limitations.
There is currently little information regarding the prevalence and impact of respiratory comorbidities on patients who have completed a cardiac rehabilitation program. In their retrospective review, King et al (8) noted a decreased likelihood of cardiac rehabilitation attendance in patients with a history of COPD or asthma. In a cohort study, Savage et al (20) found that chronic lung disease was one of the comorbidities that significantly predicted no improvement in peak oxygen uptake that occurred in 20% of 385 cardiac rehabilitation patients. Identifying the characteristics of cardiac rehabilitation patients with respiratory comorbidities, and the impact of this comorbidity on key outcomes, is the first step toward adapting rehabilitation needs and improving care.
The present article describes the characteristics and effects of exercise training for individuals with respiratory comorbidities and enrolled in a traditional cardiac rehabilitation program. The specific objective was to compare the prevalence, demographic, aerobic and functional characteristics, and risk factor profile of individuals with: respiratory comorbidities (RCs); nonrespiratory comorbidities (NRCs); and no comorbidities (NCs) using a retrospective database review. All individuals included in the present study participated in the Cardiovascular Prevention and Rehabilitation Program at the Toronto Rehabilitation Institute/University Health Network (Toronto, Ontario) and had a ≥10 pack per year (ppy) smoking history. We hypothesized that the prevalence of respiratory comorbidities would be low in the cardiac rehabilitation program. This subgroup would also have lower cardiovascular fitness compared with the subgroups without RCs, but exhibit similar improvements after completing the program; providing support for the enrollment of these patients into standard cardiac rehabilitation programs. This is important considering the poor availability of pulmonary rehabilitation programs for individuals with chronic respiratory disease (21,22).
METHODS
Study design
The present study was a retrospective review of a database found in the Cardiac Rehabilitation and Secondary Prevention Program at Toronto Rehabilitation Institute. The program admits 1800 patients annually, with the majority of admissions for percutaneous coronary intervention, coronary artery bypass graft (CABG) surgery and myocardial infarction. Data were extracted from all available cases from January 1999 to May 2004. The present study was approved by the Toronto Rehabilitation Research Ethics Board.
Patients
The entire cohort of cardiac rehabilitation patients from January 1999 to May 2004 was initially reviewed. Only patients with a primary cardiac diagnosis and a ≥10 pack per year (ppy) smoking history were analyzed comparing the following three groups: RC, NRC and NC. Identifying comorbidities was based on physician referral information; formal pulmonary function testing was not conducted. Although there is no consensus or standardized ppy smoking history associated with COPD to increase the likelihood of identifying true cases, both the label of ‘COPD’ (or other chronic respiratory disease such as interstitial lung disease or asthma) based on physician referral and a smoking history of at least 10 ppy (23,24), was required. Physician referrals were used to identify COPD because other standard measures (25) of diagnoses, such as spirometry and clinical assessment (including dyspnea), were not collected as part of the program.
Cardiac rehabilitation intervention
Participants were referred to the cardiac rehabilitation program by their family physicians, surgeon or other health care provider. The program was led by an interprofessional team of physicians, physiotherapists, nurses, kinesiologists, psychologists and dietitians. Each participant was assigned to a case manager. Participants attended 90 min classes once per week for six to 12 months, and monthly classes for four to 12 months. Cardiopulmonary exercise tests (CPETs) were conducted in all participants at baseline, six and 12 months, except in those who prematurely discontinued the program. Classes included aerobic training, resistance training, education sessions, as well as psychosocial and dietary counselling. One exercise session was conducted in the facility each week, with the balance of the exercise being completed in the home/community. Exercise sessions, both at the facility and home/community, were tracked via diaries. The initial walking prescription was set at a distance of approximately 1.6 km per day and an intensity equivalent to the ventilatory anaerobic threshold (VAT) and/or 60% to 80% oxygen uptake (VO2peak). Prescriptions were progressed every two weeks, increasing distance to a maximum of 6.4 km and then increasing intensity to a maximum of 80% of VO2peak (maximum duration of 60 min). Thereafter, training intensity was adjusted to maintain a pace equivalent to 80% of VO2peak. Resistance training exercises were initiated eight weeks after aerobic training and included lower body, upper body and trunk-stabilizing exercises. Participants were advised to gradually progress from 10 to 15 repetitions and then to increase resistance by 5 kg, or one exercise band level and reduce the number of repetitions to 10.
Outcomes
Baseline variables were compared among the three groups and included age, sex, smoking history, presence of angina, occupational status, whether the patient completed the program (completed all diagnostic testing at baseline, six and 12 months, and attended classes over 12 months), anthropometrics (body mass index [BMI], waist circumference, body fat percentage) and cardiorespiratory status at rest and during CPET (heart rate [HR], blood pressure [BP], workload, oxygen uptake [VO2peak], VAT and presence of symptoms).
The following variables were compared among groups and over three different time points (baseline, immediately after discharge [six months] and 12 months after the program): anthropometrics (BMI, waist circumference, body fat percentage), cardiorespiratory status at rest and during cardiopulmonary exercise testing (HR, BP, workload, VO2peak, VAT and presence of symptoms).
Measurements
Body fat percentage was assessed using bioelectrical impedance for patients referred after 1999 (Tanita TBF-300A, Japan) and, before this, by skin fold measurements (26). Waist circumference was measured at the narrowest part of the torso between the iliac crest and xiphoid process, or at the level of the iliac crest after normal exhalation (27). CPET was performed on either an upright cycle ergometer (Ergoselect 200P, Germany) or a treadmill (same modality pre- and post-training) depending on patient balance and comfort. On the cycle, workload was increased by either 8.3 Watts or 16.7 Watts every minute, maintaining a pedalling rate of 60 rpm. On the treadmill, the Bruce or Modified Bruce protocol was selected (27). Gas samples were collected via calibrated metabolic cart (SensorMedics Vmax Encore, USA) with continuous monitoring of 12-lead electrocardiography (ECG) (Marquette Case 80, GE Healthcare, USA) and BP. The test was terminated at peak volitional effort (unable to maintain treadmill speed or pedalling rate) if a physiological maximum was achieved, or if the patient exhibited adverse clinical signs or symptoms. VAT was determined using a combination of the V-slope and ventilatory equivalents methods (28,29) by agreement between the supervising physician and technologist.
Data analysis
Descriptive statistics (mean ±SD, frequencies and counts) were used to describe all groups. A one-way ANOVA (continuous) and χ2 test (categorical) were used to compare baseline characteristics among groups. To evaluate the effects of the cardiac rehabilitation intervention, mixed factorial ANOVA (continuous variables) and logistic regression (categorical variables) was used for the between-subject, between-group and within-subject effects at each time point (zero, six and 12 months), adjusted for age, sex and baseline VO2peak. Pairwise post hoc tests were performed for the cardiac rehabilitation intervention outcomes if there were significant findings (with Bonferroni adjustment). An alpha level ≤0.05 was considered to be statistically significant (SAS version 9.3 [SAS, USA] and SPSS version 20 [IBM Corporation, USA]).
RESULTS
There were a total of 5922 patients in the database from January 1999 to May 2004, in whom 266 (4.5%) had respiratory comorbidities (only COPD, no other chronic respiratory diseases were identified). The number of patients with ischemic heart disease and a smoking history of >10 ppy was 1247 (and the focus for the present analysis). Of this smoking cohort, 77 (6.2%) had an RC; 957 (76.7%) had at least one NRC and; 213 (17.1%) had no NC. NRCs included diabetes, cardiac conduction deficits (eg, atrial fibrillation), cancer, cardiomyopathy, congestive heart failure, cerebrovascular disease, hypertension, peripheral vascular disease, pericarditis and thyroid abnormalities. The proportion of patients from this smoking cohort who completed the program did not significantly differ among groups: RC (n=36 [46.8%]); NRC (n=534 [55.8%]); and NC (n=122 [57.3%]) (P=0.26) (Figure 1).
Figure 1).
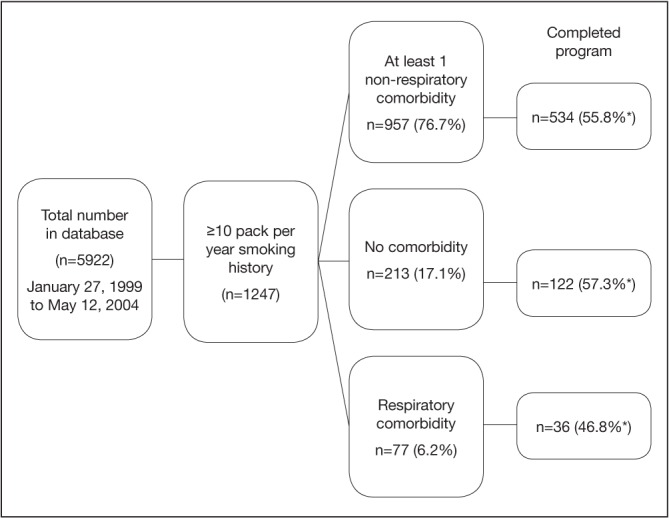
Flow diagram of 5922 patient records from the Toronto Rehabilitation Institute Cardiac Rehabilitation and Secondary Prevention Program (Toronto, Ontario) database from January 27, 1999 to May 12, 2004. *Percentage based on sample size of non-respiratory comorbidity (n=957), no comorbidity (n=213) and respiratory comorbidity (n=77) subgroups
Baseline characteristics of comparative groups
The RC group was older compared with the other two groups (mean ± SD) of 67.2±10.1 years of age versus 61.2±10.1 NRC and 60.5±8.3 NC (P=0.001), and had more female patients (eight of 36 [22%] versus 58 of 534 [11%] NRC and five of 122 [4%] NC; P=0.004). The RC group had the lowest fitness levels at baseline compared with the other two groups: VO2peak 16.0±3.8 mL/kg/min versus 18.3±4.9 NRC and 19.2±5.2 mL/kg/min NC (P=0.002). Table 1 summarizes details regarding other significantly different outcomes among the three subgroups at baseline.
TABLE 1.
Baseline characteristics of subgroups that completed the Toronto Rehabilitation Institute Cardiac Rehabilitation and Secondary Prevention Program (Toronto, Ontario), January 1999 to May 2004
| Characteristic | Comorbidities | P | ||
|---|---|---|---|---|
|
| ||||
| Respiratory (n=36) | Non-respiratory (n=534) | None (n=122) | ||
| Female sex | 8 (22.2) | 58 (10.9) | 5 (4.1) | 0.004 |
| Age, years | 67.2±10.1 | 61.1±10.1 | 60.5±8.3 | 0.001 |
| Smoking status | 0.78 | |||
| Quit | 33 (91.7) | 484 (90.6) | 113 (92.6) | |
| Current | 3 (8.3) | 50 (9.4) | 9 (7.4) | |
| Pack per year smoked | 48.3 (32.4) | 37.0 (27.1) | 36.3 (29.9) | 0.06 |
| Occupational status | 0.0002 | |||
| Employed | 5 (14.3) | 211 (39.5) | 52 (42.6) | |
| Retired | 22 (62.9) | 198 (37.1) | 39 (32.0) | |
| Disability pension | 4 (11.4) | 9 (1.7) | 2 (1.6) | |
| Sick leave | 2 (5.7) | 96 (18.0) | 24 (19.7) | |
| Unemployed | 2 (5.7) | 17 (3.2) | 3 (2.5) | |
| Body mass index, kg/m2 | 27.3±3.6 | 28.4±4.2 | 28.2±3.9 | 0.48 |
| Waist circumference, cm | 95.2±10.9 | 98.4±11.7 | 99.3±10.8 | 0.18 |
| Body fat percentage | 24.6±8.0 | 23.7±5.8 | 23.3±8.5 | 0.72 |
| Resting heart rate, beats/min | 70.5±13.1 | 67.2±12.6 | 66.7±12.2 | 0.28 |
| Resting systolic blood presssure, mmHg | 145.0±18.7 | 139.8±21.9 | 138.8±20.7 | 0.31 |
| Resting diastolic blood pressure, mmHg | 77.5±11.6 | 75.7±12.2 | 75.5±11.3 | 0.68 |
| Maximum heart rate, beats/min | 115.9±22 | 119.0±21.7 | 121.4±22.0 | 0.36 |
| Maximum systolic blood presssure, mmHg | 190.2±27.3 | 187.1±27.5 | 186.8±26.0 | 0.80 |
| Maximum diastolic blood pressure, mmHg | 83.6±12.7 | 83.7±12.9 | 83.0±12.1 | 0.83 |
| Maximum workload, W | 99.9±38.7 | 124.6±39.6 | 133.8±37.8 | 0.00004 |
| Peak oxygen uptake (VO2peak), mL/kg/min | 16.0±3.8 | 18.3±4.9 | 19.2±5.2 | 0.002 |
| Ventilatory anaerobic threshold, mL/kg/min | 11.4±2.3 | 12.3±2.7 | 12.6±2.8 | 0.08 |
| Angina | 1 (2.9) | 19 (3.6) | 1 (0.8) | 0.56 |
| Symptoms during cardiopulmonary exercise test | 8 (23.5) | 160 (30.0) | 38 (31.1) | 0.85 |
| Exercise at home | 31 (91.2) | 384 (72.0) | 89 (73.0) | 0.29 |
| Primary diagnosis | 0.0002 | |||
| Coronary artery bypass graft surgery | 15 (41.7) | 213 (39.9) | 73 (59.8) | |
| Percutansoue coronary intervention | 2 (5.6) | 97 (18.2) | 9 (7.4) | |
| Myocardial infarction | 11 (30.6) | 158 (29.6) | 23 (18.9) | |
| Ischemic heart disease (no intervention) | 8 (22.2) | 66 (12.4) | 17 (13.9) | |
| Medications | ||||
| Respiratory | 18 (50.0) | 279 (52.2) | 69 (56.6) | 0.65 |
| β-blockers | 14 (38.9) | 424 (79.4) | 96 (78.7) | <0.0001 |
| Lipid-lowering agent | 26 (72.2) | 407 (76.2) | 98 (80.3) | 0.17 |
| Platelet inhibitor | 34 (94.4) | 488 (91.4) | 109 (89.3) | 0.60 |
| Anti-anxiolytics | 6 (16.7) | 67 (12.5) | 20 (16.4) | 0.45 |
| Wait time to program entry, days | 51.4±40.6 | 42.2±21.6 | 41.7±22.2 | 0.06 |
Data presented as n (%) or mean ± SD unless otherwise indicated. Bolded values indicate statistical significance (ie, P<0.05)
Cardiac rehabilitation outcomes for comparative groups
For VO2peak, there were significant differences among groups, adjusted for age and sex (P=0.02). The NC group (over all three time points) had significantly greater VO2peak (21.3±6.0 mL/kg/min) compared with the RC group (16.7±3.8 mL/kg/min). There were also significant improvements over time (P=0.0002), adjusted for age and sex. Overall (ie, all groups together) VO2peak was significantly greater at six (20.6±5.9 mL/kg/min) and 12 months (21.0±6.2 mL/kg/min) versus baseline (18.3±4.9 mL/kg/min) (both P<0.0001). There was no significant group × time interaction (Table 2, Figure 2).
TABLE 2.
Comparison of body composition, resting and maximal exercise test outcomes for three groups at baseline, six and 12 months (respiratory comorbidity [RC, n=36]; non-respiratory comorbidity [NRC, n=534]; no comorbidity [NC, n=122])
| Characteristic | Time, months | Mean (all groups) | P | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0 | 6 | 12 | Group | Time | Group × time | ||
| Peak oxygen uptake (VO2peak), mL/kg/min | |||||||
| RC | 15.9±3.9 | 17.0±3.9 | 17.1±3.9 | 16.7±3.8 | 0.02* | <0.0001** | 0.20 |
| NRC | 18.2±4.9 | 20.5±5.9 | 20.8±6.2 | 19.9±5.8 | |||
| NC | 19.4±5.1 | 22.1±5.9 | 22.6±6.2 | 21.3±6.0 | |||
| Mean (all time periods) | 18.3±4.9 | 20.6±5.9 | 21.0±6.2 | ||||
| Maximum workload, W | |||||||
| RC | 100.5±39.2 | 113.0±38.7 | 111.6±37.4 | 107.9±38.0 | 0.11 | <0.0001** | 0.78 |
| NRC | 121.6±36.2 | 137.8±41.2 | 140.5±45.4 | 134.1±43.2 | |||
| NC | 131.4±32.5 | 147.8±35.8 | 151.1±39.7 | 143.5±38.8 | |||
| Mean (all time groups) | 122.2±36.2 | 138.2±40.8 | 140.8±44.7 | ||||
| Ventilatory anaerobic threshold, mL/kg/min | |||||||
| RC | 11.8±2.0 | 12.0±2.3 | 12.2±1.8 | 11.8±2.1 | 0.18 | 0.12 | 0.26 |
| NRC | 12.3±2.6 | 13.8±3.4 | 14.1±3.6 | 13.3±3.5 | |||
| NC | 12.6±2.8 | 14.3±3.7 | 14.8±4.6 | 13.9±3.9 | |||
| Mean (all time groups) | 12.4±2.6 | 13.8±3.5 | 14.2±3.8 | ||||
| Resting heart rate, beats/min | |||||||
| RC | 70.2±13.2 | 65.2±9.4 | 65.7±10.3 | 67.3±11.2 | 0.36 | 0.68 | 0.80 |
| NRC | 67.3±12.6 | 64.2±11.2 | 65.0±11.8 | 65.4±11.9 | |||
| NC | 66.6±12.2 | 63.3±10.3 | 63.7±11.2 | 64.6±11.3 | |||
| Mean (all time groups) | 67.3±12.6 | 64.1±11.0 | 64.8±11.6 | ||||
| Resting systolic blood pressure, mmHg | |||||||
| RC | 144.9±19.0 | 140.9±16.9 | 145.1±15.2 | 143.6±16.9 | 0.84 | 0.81 | 0.28 |
| NRC | 139.7±21.9 | 140.5±20.6 | 141.6±20.0 | 140.6±20.9 | |||
| NC | 138.9±20.8 | 140.2±19.0 | 138.6±17.4 | 139.1±19.2 | |||
| Mean (all time groups) | 139.8±21.6 | 140.5±20.1 | 141.2±19.3 | ||||
| Resting diastolic blood pressure, mmHg | |||||||
| RC | 77.2±11.6 | 72.1±10.8 | 76.0±9.6 | 75.0±10.8 | 0.96 | 0.85 | 0.32 |
| NRC | 75.7±12.2 | 74.4±11.8 | 76.2±11.1 | 75.5±11.9 | |||
| NC | 75.6±11.3 | 75.5±12.1 | 76.1±10.3 | 75.7±11.2 | |||
| Mean (all time groups) | 75.8±12.0 | 74.5±11.8 | 76.1±10.9 | ||||
| Body mass index, kg/m2 | |||||||
| RC | 27.0±3.3 | 27.5±3.1 | 27.6±3.2 | 27.5±3.4 | 0.49 | 0.12 | 0.54 |
| NRC | 28.2±4.1 | 28.3±4.2 | 28.6±4.6 | 28.3±4.4 | |||
| NC | 28.3±3.9 | 28.3±4.2 | 28.5±4.2 | 28.3±4.1 | |||
| Mean (all time groups) | 28.1±4.0 | 28.2±4.2 | 28.6±4.5 | ||||
| Waist circumference, cm | |||||||
| RC | 94.2±9.8 | 95.3±9.8 | 95.7±9.5 | 95.8±10.4 | 0.53 | 0.15 | 0.50 |
| NRC | 98.4±11.6 | 98.1±11.3 | 98.6±11.6 | 98.4±11.6 | |||
| NC | 99.5±10.8 | 98.9±10.7 | 99.5±10.6 | 99.2±10.7 | |||
| Mean (all time groups) | 98.4±11.4 | 98.1±11.1 | 98.7±11.3 | ||||
| Body fat percentage | |||||||
| RC | 25.6±7.3 | 25.2±4.8 | 27.2±7.0 | 25.3±7.7 | 0.53 | 0.36 | 0.36 |
| NRC | 27.4±7.9 | 26.9±7.2 | 27.9±8.2 | 24.4±6.2 | |||
| NC | 33.7±19.8 | 28.7±2.8 | 28.9±4.0 | 24.4±7.2 | |||
| Mean (all time groups) | 28.2±10.4 | 27.1±6.6 | 28.0±7.6 | ||||
| Angina | |||||||
| RC | 1 (2.9) | 0 (0) | 0 (0) | 1 (2.8) | 0.41 | 0.99 | 0.63 |
| NRC | 19 (3.6) | 10 (1.9) | 9 (1.7) | 36 (6.7) | |||
| NC | 1 (0.8) | 1 (0.8) | 3 (2.5) | 5 (4.1) | |||
| Mean (all time groups) | 21 (3.0) | 11 (1.6) | 12 (1.7) | ||||
| Symptoms*** during cardiopulmonary exercise test | |||||||
| RC | 8 (22.2) | 5 (13.9) | 4 (11.1) | 17 (47.2) | 0.36 | 0.52 | 0.07 |
| NRC | 160 (30.0) | 80 (15.0) | 13 (2.4) | 253 (47.4) | |||
| NC | 38 (31.1) | 22 (18.0) | 4 (3.3) | 64 (52.4) | |||
| Mean (all time groups) | 206 (29.8) | 107 (15.5) | 21 (3.0) | ||||
Data presented as mean ± SD or n (%) unless otherwise indicated. All analyses adjusted for age, sex and baseline VO2peak (except VO2peak, which was adjusted only for age and sex).
Statistically significant (bold) pairwise post-hoc tests (Bonferroni adjustment) at P=0.05 level: NC>RC;
Statistically significant (bold) pairwise post-hoc tests (Bonferroni adjustment) at P=0.05 level: six-month > baseline; 12 month > baseline;
Leg fatigue or shortness of breath
Figure 2).
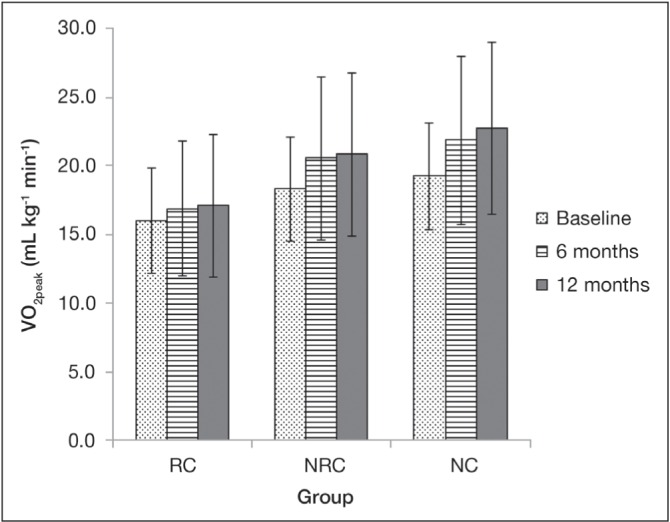
Peak oxygen uptake (VO2peak) in the three comparison groups over time. Error bars represent standard deviation. There were significant differences among groups (P=0.02) (adjusted for age and sex): Non-respiratory comorbidity (NRC) VO2peak significantly greater than the respiratory comorbidity (RC) group (P=0.05). There were significant improvements over time (P<0.0001) (adjusted for age and sex): VO2peak significantly greater at six and 12 months versus baseline (both P<0.0001). There was no significant group × time interaction. NC No comorbidity
There were no significant differences among the three groups or over time (adjusted for age, sex and baseline VO2peak) for: VAT, resting HR, resting systolic or diastolic BP, BMI, waist circumference, body fat percentage, angina or symptoms during exercise (leg fatigue or shortness of breath) (Table 2).
DISCUSSION
In the present retrospective review of a cardiac rehabilitation program database, there was an overall 4.5% prevalence of respiratory comorbidities (presumed COPD). For all groups, there were significant improvements in VO2peak over the 12-month program. The respiratory group had significantly worse VO2peak compared with the NC group. There were no differences among the groups in the other outcomes. Patients enrolled in a cardiac rehabilitation program significantly improved their fitness level over time, despite the presence or absence of comorbidities (when considering age, sex and baseline VO2peak).
The prevalence of COPD was low. This was likely underestimated because diagnoses were based on physician referrals. Systematic spirometry and clinical assessment (that includes assessment of dyspnea) at entry to cardiac rehabilitation, according to standard criteria, such as Global Initiative for Obstructive Lung Disease (GOLD) (25), would have yielded a more accurate number and provided a breakdown of different severity levels (7,9,30). Studies using spirometry and clinical symptoms have shown the highest levels of prevalence. Fuster et al (7) and Soriano et al (9) used clinical assessment and the GOLD (1) spirometric criteria for COPD. Fuster et al (7) found 39% of patients undergoing CABG (n=1412) had COPD. Soriano et al (9) compared the prevalence of airflow limitation in three nonsurgical groups: patients with CVD (n=52), no CVD (n=450) and hospitalized CVD (n=119). Prevalence was estimated at 17.5%, 19.2% and 33.6%, respectively (P<0.05). Soriano et al (9) found airflow limitation was underdiagnosed and, therefore, highly undertreated. In contrast, King et al (8) and Cooper et al (6) used documented history in retrospective chart reviews. King et al (3) estimated that 9% of patients following acute myocardial infarction, percutaneous transluminal coronary angioplasty and/or CABG (n=1254) possessed respiratory comorbidities; Cooper et al (4) estimated 18% in patients receiving isolated CABG (n=104,880). Pulmonary function testing is rarely performed at baseline or at the end of cardiac rehabilitation programs (31–34). Incorporating lung screening may definitively identify airflow limitation and help individualize program needs accordingly. We cannot rule out the possibility that fewer patients with a complex medical history are less likely to be referred to cardiac rehabilitation.
Patients with RCs had the poorest fitness level compared with those with NRCs or NCs. In addition, patients with NRCs often had poorer outcomes compared with those with NCs. COPD, among other comorbidities (peripheral vascular disease, renal disease and diabetes mellitus), has been shown to have the greatest impact on short-term and long-term mortality of patients undergoing CABG surgery (7,35,36). Pathogenic mechanisms explaining why respiratory comorbidities have the greatest burden on outcomes is likely multifactorial: systemic and lung inflammation; hypoxia (alveolar and tissue); hypercapnic acidosis; endothelial dysfunction/vessel wall abnormalities; and polycythemia (14,37).
Despite the burden of respiratory disease, we found the relative improvements in aerobic capacity (ie, VO2peak) after cardiac rehabilitation and the number of patients who completed the program similar for all three groups despite the lowest fitness level at baseline (VO2peak). Similar results have been shown in other studies investigating the efficacy of cardiac rehabilitation in patients with a differing number of medical comorbidities (38); diabetes versus no diabetes (39); with and without stroke (40,41); and with and without chronic kidney disease (42). Rehabilitation benefited the RC and other comorbid groups by increasing their functional and fitness levels, and potentially reducing their risk for death (43).
There is increasing comorbid disease burden among cardiac patients, and an aging population and improved operative survival will expand the number of patients living with prognostically significant comorbidities. A cardiac rehabilitation model may be effective for patients with COPD or a pulmonary rehabilitation model for patients with CVD. Currently, the accessibility of cardiac and pulmonary rehabilitation programs fall below need (21,22,44,45). Pack et al (45) surveyed cardiac rehabilitation program directors in the American Association of Cardiovascular and Pulmonary Rehabilitation database and found only 28% of eligible patients utilized cardiac rehabilitation programs. They suggested modest expansion of all programs operating at capacity would meet, at most, 47% of eligible patients in the United States. Although cardiac rehabilitation utilization is low compared with the number of individuals who need it, pulmonary rehabilitation fares significantly worse. In a systematic review comparing pulmonary rehabilitation programs internationally, Desveaux et al (22) found that the availability of pulmonary rehabilitation services accommodated ≤1.2% of individuals with COPD. In a recent survey of Canadian pulmonary rehabilitation programs, Camp et al (21) found that only 0.4% of all Canadians with COPD (0.8% with moderate to severe) have access to these programs. Having both cardiac and respiratory programs available may increase rehabilitation accessibility for these patients. One potential solution may be to have cardiac and pulmonary rehabilitation programs in one institution or location, such as at Duke Regional Hospital (Durham, North Carolina, USA <www.dukeregional.org/services/cardiac-and-pulmonary-rehabilitation/cardiac-and-pulmonary-rehabilitation/servicepage_view>). Although the two programs at this hospital operate separately, they are likely to be more cost effective and efficient because they share common infrastructure and, presumably, have cross-trained health care professionals. Similar programs can be found in Canada. An alternative rehabilitation model is one that combines COPD and heart failure patients in one program. Evans et al (46,47) completed a study of an exercise rehabilitation program for patients with congestive heart failure and COPD. This combined training program was not only feasible but also significantly improved functional and health status for both groups. Future research may include evaluating combination or adapted rehabilitation training programs for cardiac and respiratory patients and determining the type of COPD patient (eg, severity level) who may benefit.
Limitations
There were a few limitations to our study, the first of which was those common to retrospective cohort designs (48). Identification of respiratory and other comorbidities were based on physician referrals, and may have underestimated specific diagnoses. In addition, we could not delineate the severity of the individuals with COPD. Significant differences in baseline characteristics (ppy, occupational status, respiratory medication, β-blockers, BMI and waist circumference) and external factors beyond the database may have contributed to the results. Second, the present study described outcomes from one cardiac rehabilitation and secondary prevention program in Toronto, Ontario; therefore, the results may not be generalizable to areas with different cultures, practices and policies. Finally, significant results from the numerous post hoc analyses performed may have occurred due to random chance.
CONCLUSIONS
The prevalence of patients with COPD in a cardiac rehabilitation program was low, and likely due to the method for which diagnosis was made (physician referrals). It is recommended that spirometry and clinical assessment according to standard criteria be used to yield more accurate diagnoses and enable individualized programming. Patients with RCs had the poorest fitness level compared with those with NRCs or NCs. However, all groups improved after cardiac rehabilitation and the number of patients who completed the program were similar. Cardiac rehabilitation and pulmonary rehabilitation program accessibility is limited for the CVD and COPD populations. Individuals with COPD may benefit from cardiac rehabilitation programs; individuals with CVD may benefit from pulmonary rehabilitation programs. Adaptation and/or combinations may optimize their accessibility.
Acknowledgments
The authors thank the patients and staff at The Toronto Rehabilitation Institute.
Footnotes
FUNDING: MLN was supported by Toronto Rehabilitation Institute post-doctoral fellowship.
DISCLOSURES: The authors have no additional financial disclosures or conflicts of intetest to declare.
REFERENCES
- 1.Smith Susan M, Soubhi H, Fortin M, Hudon C, O’Dowd T.Interventions for improving outcomes in patients with multimorbidity in primary care and community settings Cochrane Database Syst Rev 20124http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD006560.pub2/abstract. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Global Status Report on Noncommunicable Diseases 2010. 2011. ISBN 978 92 4 068645 8. [Google Scholar]
- 3.Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3:223–8. doi: 10.1370/afm.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith SM, O’Dowd T. Chronic diseases: What happens when they come in multiples? Br J Gen Pract. 2007;57:268–270. [PMC free article] [PubMed] [Google Scholar]
- 5.Fortin M, Soubhi H, Hudon C, Bayliss EA, van den Akker M. Multimorbidity’s many challenges. BMJ. 2007;334:1016–7. doi: 10.1136/bmj.39201.463819.2C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper WA, O’Brien SM, Thourani VH, et al. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: Results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation. 2006;113:1063–70. doi: 10.1161/CIRCULATIONAHA.105.580084. [DOI] [PubMed] [Google Scholar]
- 7.Fuster RG, Argudo JA, Albarova OG, et al. Prognostic value of chronic obstructive pulmonary disease in coronary artery bypass grafting. EurJ Cardiothorac Surg. 2006;29:202–9. doi: 10.1016/j.ejcts.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 8.King KM, Humen DP, Teo KK. Cardiac rehabilitation: The forgotten intervention. Can J Cardiol. 1999;15:979–85. [PubMed] [Google Scholar]
- 9.Soriano JB, Rigo F, Guerrero D, et al. High prevalence of undiagnosed airflow limitation in patients with cardiovascular disease. Chest. 2010;137:333–40. doi: 10.1378/chest.09-1264. [DOI] [PubMed] [Google Scholar]
- 10.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:4. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800. doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reardon JZ, Levine S, Peske G, et al. A comparison of outpatient cardiac and pulmonary rehabilitation patients. J Cardiopulm Rehabil. 1995;15:277–82. doi: 10.1097/00008483-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Milani RV, Lavie CJ. Disparate effects of out-patient cardiac and pulmonary rehabilitation programs on work efficiency and peak aerobic capacity in patients with coronary disease or severe obstructive pulmonary disease. J Cardiopulm Rehabil. 1998;18:17–22. doi: 10.1097/00008483-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Topsakal R, Kalay N, Ozdogru I, et al. Effects of chronic obstructive pulmonary disease on coronary atherosclerosis. Heart Vessels. 2009;24:164–8. doi: 10.1007/s00380-008-1103-4. [DOI] [PubMed] [Google Scholar]
- 15.Leon AS, Franklin BA, Costa F, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: An American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111:369–376. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 16.Ries A. The evolving role of rehabilitation in COPD. Adv Stud Med. 2008;8:457–62. [Google Scholar]
- 17.Anonymous American College of Sports Medicine position stand. Exercise for patients with coronary artery disease. Med Sci Sports Exercise. 1994;26(3) [PubMed] [Google Scholar]
- 18.Antonucci R, Berton E, Huertas A, Laveneziana P, Palange P. Exercise physiology in COPD. Monaldi Arch Chest Dis. 2003;59:134–9. [PubMed] [Google Scholar]
- 19.MacIntyre N. Pulmonary Issues Related to Cardiac Rehabilitation. In: Kraus W, Keteyian S, editors. Cardiac Rehabilitation. Totowa: Human Press Inc; 2007. [Google Scholar]
- 20.Savage PD, Antkowiak M, Ades PA. Failure to improve cardiopulmonary fitness in cardiac rehabilitation. J Cardiopulm Rehabil Prevent. 2009;29:284–91. doi: 10.1097/HCR.0b013e3181b4c8bd. [DOI] [PubMed] [Google Scholar]
- 21.Camp PG, Hernandez P, Bourbeau J, et al. Pulmonary rehabilitation in Canada: A report from the Canadian Thoracic Society COPD Clinical Assembly. CAn Respir J. 2015;22:147–52. doi: 10.1155/2015/369851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desveaux L, Janaudis-Ferreira T, Goldstein R, Brooks D. An international comparison of pulmonary rehabilitation: A systematic review. COPD. 2015;12:144–53. doi: 10.3109/15412555.2014.922066. [DOI] [PubMed] [Google Scholar]
- 23.Rennard SI. In: Chronic Obstructive Pulmonary Disease: Definition, clinical manifestations, diagnosis, and staging. Stoller JK, Hollingsworth H, editors. Waltham, MA: UpToDate; 2014. < www.uptodate.com/contents/chronic-obstructive-pulmonary-disease-definition-clinical-manifestations-diagnosis-and-staging> (Accessed October 16, 2014) [Google Scholar]
- 24.O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2003. Can Respir J. 2003;(10 Suppl A):11a–65a. doi: 10.1155/2003/567598. [DOI] [PubMed] [Google Scholar]
- 25.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 26.Durnin JVGA, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 Years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 27.ACSM . American College of Sports Medicine’s guidelines for exercise testing and prescription. 8th edn. Philadelphia: Lippincott Williams and Wilkins; 2009. [Google Scholar]
- 28.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting an anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–7. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 29.ATS American Thoracic Society/American College of Chest Physicians: ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 30.Gershon AS, Hwee J, Croxford R, Aaron SD, To T. Patient and physician factors associated with pulmonary function testing for COPD: A population study. Chest. 2014;145:272–81. doi: 10.1378/chest.13-0790. [DOI] [PubMed] [Google Scholar]
- 31.Goble A, Worcester M. Best Practice Guidelines for Cardiac Rehabilitation and Secondary Prevention. 1999. < www.health.vic.gov.au/nhpa/downloads/bestpracticecardiacrehab.pdf> (Accessed August 31, 2014.
- 32.Seron P, Lanas F, Rios E, Bonfill X, Alonso-Coello P. Evaluation of the Quality of Clinical Guidelines for Cardiac Rehabilitation: A critical review. J Cardiopulm Rehabil Prev. 2014 doi: 10.1097/HCR.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 33.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: Endorsed by the National Heart, Lung, and Blood Institute. Circulatiom. 2006;113:2363–72. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 34.Stone JA, Arthur HM. Canadian guidelines for cardiac rehabilitation and cardiovascular disease prevention, second edition, 2004: Executive summary. Can J Cardiol. 2005;(21 Suppl D):3d–19d. [PubMed] [Google Scholar]
- 35.Leavitt BJ, Ross CS, Spence B, et al. Long-term survival of patients with chronic obstructive pulmonary disease undergoing coronary artery bypass surgery. Circulation. 2006;114(1 Suppl):4. doi: 10.1161/CIRCULATIONAHA.105.000943. [DOI] [PubMed] [Google Scholar]
- 36.Scrutinio D, Giannuzzi P. Comorbidity in patients undergoing coronary artery bypass graft surgery: Impact on outcome and implications for cardiac rehabilitation. Eur J Cardiovasc PrevRehabil. 2008;15:379–85. doi: 10.1097/HJR.0b013e3282fd5c6f. [DOI] [PubMed] [Google Scholar]
- 37.Choudhury G, Rabinovich R, MacNee W. Comorbidities and systemic effects of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:101–30. doi: 10.1016/j.ccm.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Listerman J, Bittner V, Sanderson BK, Brown TM. Cardiac rehabilitation outcomes: impact of comorbidities and age. J Cardiopulm Rehabil Prev. 2011;31:342–8. doi: 10.1097/HCR.0b013e31822f189c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banzer JA, Maguire TE, Kennedy CM, O’Malley CJ, Balady GJ. Results of cardiac rehabilitation in patients with diabetes mellitus. Am J Cardiol. 2004;93:81–4. doi: 10.1016/j.amjcard.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 40.Tang A, Closson V, Marzolini S, Oh P, McIlroy W, Brooks D. Cardiac rehabilitation after stroke-need and opportunity. J Cardiopulm Rehabil Prev. 2009;29:97–104. doi: 10.1097/HCR.0b013e31819a00d4. [DOI] [PubMed] [Google Scholar]
- 41.Tang A, Marzolini S, Oh P, McIlroy WE, Brooks D. Feasibility and effects of adapted cardiac rehabilitation after stroke: A prospective trial. BMC Neurology. 2010;10:40. doi: 10.1186/1471-2377-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkataraman R, Sanderson B, Bittner V. Outcomes in patients with chronic kidney disease undergoing cardiac rehabilitation. Am Heart J. 2005;150:1140–6. doi: 10.1016/j.ahj.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 43.Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long-term prognosis in 12,169 men referred for cardiac rehabilitation. Circulation. 2002;106:666–71. doi: 10.1161/01.cir.0000024413.15949.ed. [DOI] [PubMed] [Google Scholar]
- 44.Grace SL, Chessex C, Arthur H, et al. Canadian Association of Cardiac Rehabilitation and Canadian Cardiovascular Society joint position paper. J Cardiopulm Rehabil Prev. 2010;2011;31:E1–8. doi: 10.1097/HCR.0b013e318219721f. [DOI] [PubMed] [Google Scholar]
- 45.Pack QR, Squires RW, Lopez-Jimenez F, et al. The current and potential capacity for cardiac rehabilitation utilization in the United States. J Cardiopulm Rehabil Prev. 2014;34:318–26. doi: 10.1097/HCR.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 46.Evans RA, Singh SJ, Collier R, Loke I, Steiner MC, Morgan MD. Generic, symptom based, exercise rehabilitation: Integrating patients with COPD and heart failure. Respir Med. 2010;10:1473–81. doi: 10.1016/j.rmed.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 47.Evans RA. Developing the model of pulmonary rehabilitation for chronic heart failure. Chron Respir Dis. 2011;8:259–69. doi: 10.1177/1479972311423111. [DOI] [PubMed] [Google Scholar]
- 48.Cummings SR, Kohn MA, Hulley SB. Designing cross-sectional and cohort studies Designing Clinical Research. 4th edn. Alphen aan den Rijn: Wolters Kluwer; 2013. pp. 85–96. [Google Scholar]


