Abstract
In the official joint policy document of the American Thoracic Society and European Respiratory Society (Hsia et al., 2010), the need for proper stereologic assessment of lungs was emphasized. In this document it was emphasized that for the quantitative analysis of lung histologic sections, one of the most robust and reliable methods is point and intercept counting (Knudsen et al., 2010). One of the practical aspects of this method is how many points or intercepts are needed. The answer to this question has been considered from a theoretical perspective, and it depends on the relative magnitudes of the methodological and biologic variabilities. Although it is generally accepted that in a normal lung, one needs only 100–200 points to sufficiently lower the methodological variability, given the increased variability often seen in experimental emphysematous lung injury, the requisite number of points of intercepts has not been evaluated. In this study, we examined this question by focusing on some of the relevant sampling levels in mice with extensive elastase-induced emphysema. Using fixed samples of tissue blocks, we varied the number of sampling points or intercepts from about 25 to 1000 in control and emphysematous lungs. Our results show that, at the sampling levels investigated, even with the increased heterogeneity in the lung tissue damage caused by elastase, the number of sampling points needed to detect changes is similar to what is needed for control mice.
Keywords: stereology, mean linear intercept, internal surface area, COPD
INTRODUCTION
In the quantitative analysis of lung histologic sections, one of the most robust and reliable methods is point and intercept counting. This traditional method has been used for many years and is one of the methods that have been highlighted in the recent official joint policy document of the American Thoracic Society and European Respiratory Society (Hsia et al., 2010). In addition, the relative merits of this approach versus a computerized approach were compared with specific experimental lung sections (Knudsen et al., 2010). Although there are a number of sources of variability when doing this kind of quantitative histologic analysis, one of the important practical issues in the use of the point counting approach is the number of points or intercepts needed. The answer to this question depends on the relative magnitudes of the methodological and biologic variabilities, and it has been theoretically shown that one only needs to count sufficient numbers such that the biologic variability is more than twice the methodological variability (Gundersen and Osterby, 1981; Ochs and Muhlfeld, 2013). This is often referred to as the “do more less well” principle (Gundersen and Osterby, 1981; Mouton, 2011), and it means that it is generally wise to spend extra time and effort to do more animals, since in most cases, especially with experimental lung injury models, there is considerable interanimal variability.
One of the most common applications for quantitative morphology is in the analysis of experimental emphysema. Emphysema is defined as the irreversible loss of alveolar surface area (Snider et al., 1985), and simply observing an increase in the mean airspace chord length (Lm) does not by itself show this. Because Lm is a function of lung volume, if lung volume and Lm increase, the alveolar surface area could remain unchanged (Mitzner and Weibel, 2009; Hsia et al., 2010). In the quantitative analysis of lung histologic sections to assess alveolar surface area (or Lm), the number of sampling points or intercepts utilized by different investigators varies widely and seldom even reported. Although the number of sampling points in normal lungs is generally agreed upon (Mitzner and Weibel, 2009; Hsia et al., 2010), this may not be the same in emphysematous lungs, which have increased heterogeneity in the lung tissue damage and increased variability among animal. Thus, there remains some uncertainty whether additional counting events might be needed in experimental emphysema.
In this study, we compared the biologic and a key aspect of the methodological variability in control and elastase treated C57BL/6J mice, under conditions where we changed the number of parenchymal sampling points between approximately 25 and 1000 on different groups of histologic images from each mouse. Our results show that even with increased heterogeneity from lung tissue damage caused by elastase, the number of sampling points needed to detect changes is not different from that needed for control mice.
METHODS
All experiments were performed using 8–10 week-old, male C57BL/6J mice (Jackson Laboratories) under the approval of the Johns Hopkins University Animal Care and Use Committee. Mice were housed in a pathogen free facility, continually provided filtered air and water, maintained on a 12-hour light/dark cycle, and fed autoclaved food ad libitum. 5 mice were used as controls (PBS) and 8 were treated with 6U of elastase suspended solubilized in PBS and delivered to the lung by aspiration (Foster et al., 2001). To deliver the PBS with or without elastase with this procedure, the mice were anesthetized by placing them in a chamber ventilated with 3% isofluorane for 5 minutes. Upon removal the tongue was gently extracted, and either the 6 U dose of elastase or PBS was placed on the rear of the tongue, and the nares were then briefly blocked to force the liquid on the tongue to be aspirated. Mice were returned to the animal housing and allowed to recover for 3 weeks.
After 3 weeks, mice were sacrificed with an anesthetic overdose, and death was confirmed by the absence of heartbeat seen after opening the thorax. The trachea was cannulated and lungs were fixed with formalin instillation at 25 cmH2O and embedded in paraffin. Prior to embedding, fixed lung volumes were measured by water immersion (Scherle, 1970). For this analysis only the left lung was used for quantitative stereology. With a sharp razor, 3 cuts were made transverse to the long axis of the left lung at 3–5 mm intervals working from the apex to the base to create 4 tissue blocks. The first 3 of these tissue blocks (3–5mm thick) were embedded in paraffin, and the caudal surfaces of each block of were used for the subsequent quantitative analysis. From each block we cut an initial set of five micron sections after first cutting to a depth where we could see lung surrounded by pleura, as shown in Figure 1. A systematic uniform random sampling procedure (Hsia et al., 2010) was used to take ≈thirty to forty 20X digital images (2560×1920 pixels at 0.17 μm/px) from these sections. For each lung section, a field of view was selected with no lung visible. The microscope stage was moved laterally in 1 or 2 mm increments. When there was no longer lung in the field, the stage moved vertically and the process repeated with other sections until the desired number of images was acquired. To get different sets of unique sections to analyze, this procedure was repeated twice more, cutting additional 5 μm sections at greater than ≈100 μm depths into the blocks, and taking a new random set of images from each new set of slides. This process thus gave us 3 unique sets of images from each lung.
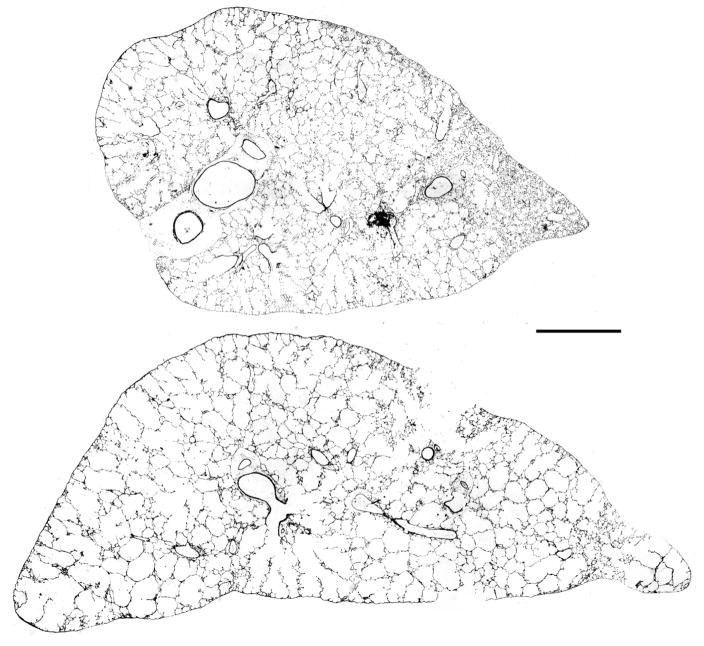
Figure 1.
Low power (2.5x) images of sections of the left lung from two of the elastase treated mice that had very different Lm. In the mouse lung shown in the upper section, there is considerable heterogeneity of the damage, whereas in the lung from another mouse shown in the lower section, the damage seems more uniform with a more severe degree. The mean Lm averaged from all the sampling densities done in each mouse shown was 68 μm in the upper lung and 134 μm in the lower lung. Bar = 1000 μm.
Each of these image sets were then analyzed by overlaying them with a grid consisting of 15 short line segments with shorter vertical lines to mark the endpoints as described (Knudsen et al., 2010). The histology was quantified by counting the number of intercepts with alveolar walls, and identifying where the end points of each line fell. With this traditional system of quantification (Knudsen et al., 2010) we then calculated the following:
where d = length of a single test line, Vp = parenchymal volume, Ialv = number intercepts with alveolar septal walls, and Pair = number of points hitting air spaces in alveoli and ducts.
Each different set of images from each animal was also analyzed 3 or 4 times by varying the number of points counted. This was done by systematically ignoring line segments, thus counting only every other or every third or fourth line segment. Thus for each set of images, at least three different numbers of points were counted. For the control and emphysematous lungs this procedure provided us with 10 random data sets in each mouse, where the number of points counted varied from approximately 25 to 1000.
For each of the data sets within a mouse, we used the mean and standard deviation (SD) of Lm and Surface Area to calculate the coefficient of variation (CV) as CV = SD/mean. We refer to the as the “within mouse” CV, and it measures the methodological variation. We also obtained an average Lm and S in each mouse taken from all of the measurements, and used these means to calculate the CV for the between mouse variability. We refer to this as the “between mouse” CV, and it reflects the biologic variability of our limited sample. We note here that there is a sound theoretical basis for expressing the observed overall variance (CV2) as the sum of the biologic variance and the measurement variance. However, since we did not use a rigorous random sampling from all lobes of the mouse lung and have only focused on one aspect of the measurement variance, the application of this formula here would be incomplete. However, we were able to provide a prediction of the CE of the intersection density based on data using the formula for the coefficient of error of a ratio estimate presented by Howard and Reed (Howard and Reed, 2005). For each of the control mice these estimates are 0.045, 0.036, 0.038, 0.058, and 0.038, and for the emphysematous mice these estimates are 0.037, 0.081, 0.035, and 0.105.
RESULTS
The 6U of elastase creates a rather severe stable emphysematous lung. As is often the case, there is a considerable variation among mice in how they respond to intratracheal elastase. The extent of this variability is illustrated in Figure 1 by representative low magnification sections from 2 mice in our study with the most extreme differences. The mean Lm in all control and elastase treated mice was 41 μm and 85 μm, respectively, consistent with a substantial degree of damage caused by the elastase. The respective alveolar surface areas in these left lungs were 225 cm2 and 118 cm2.
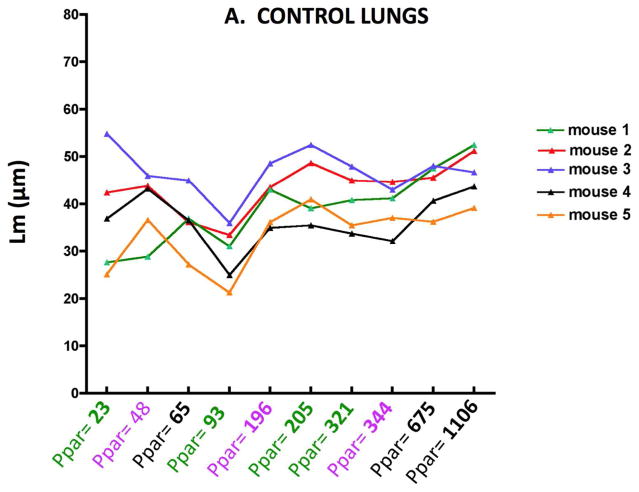
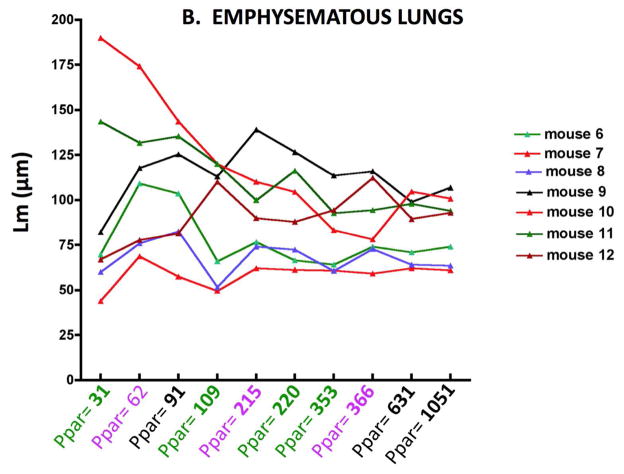
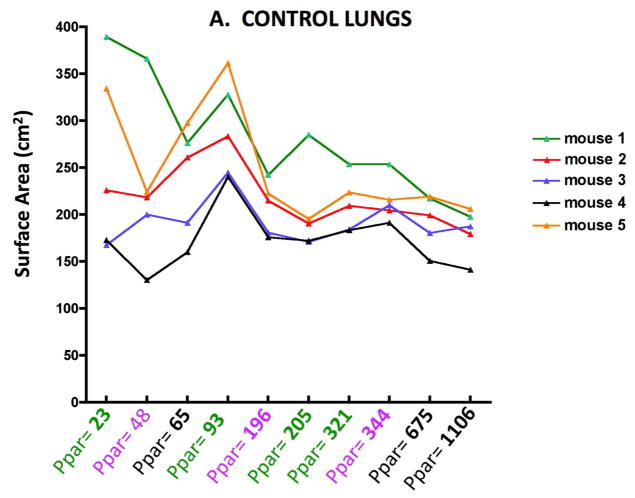
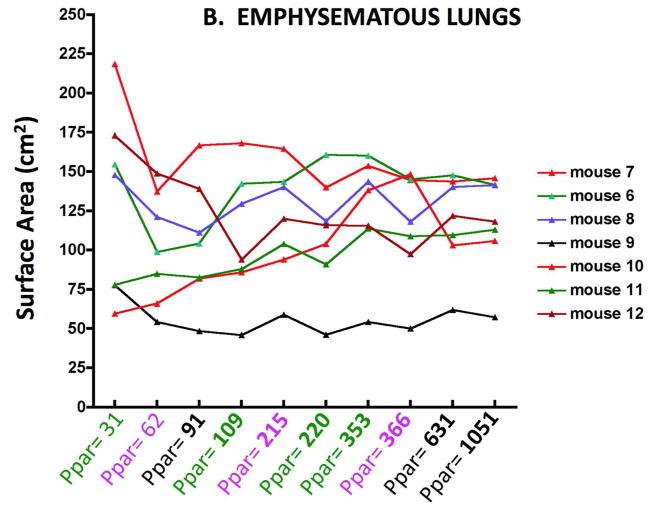
Figure 2 shows the Lm results for individual control (2A) and elastase treated (2B) mice obtaining by varying the number of sampling points. The abscissa shows the number of points counted, and the different colors indicate each independent set of images. As might be expected, there is greater variability between mice with the elastase treatment, but with any of the sampling densities, the Lm in emphysematous lungs was significantly different from the control lungs (p<0.01). A similar variability is mirrored in the surface area (SA) as shown in Figure 3A and B. With either Lm or SA, below a sampling density of about 100 points the variability clearly increases.
Figure 2.
Figure 2A. Lm variability with increasing numbers of parenchymal sampling points (Ppar) in normal control lungs of individual mice.
Figure 2B. Lm variability with increasing numbers of parenchymal sampling points (Ppar) in emphysematous lungs of individual mice. Note change of ordinate scale from Figure 1A.
Figure 3.
Figure 3A. Surface area variability with increasing numbers of parenchymal sampling points (Ppar) in normal control lungs of individual mice.
Figure 3B. Surface area variability with increasing numbers of parenchymal sampling points (Ppar) in emphysematous lungs of individual mice. Note change of ordinate scale from Figure 2A.
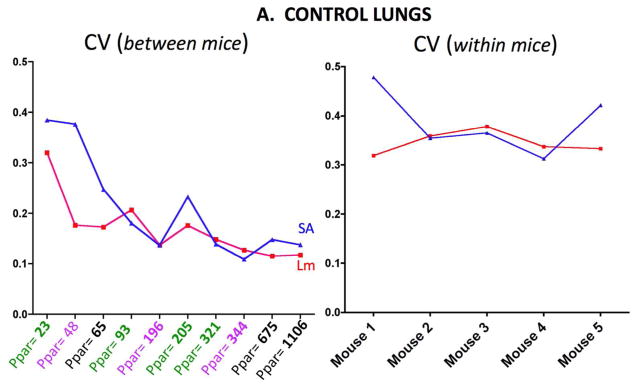
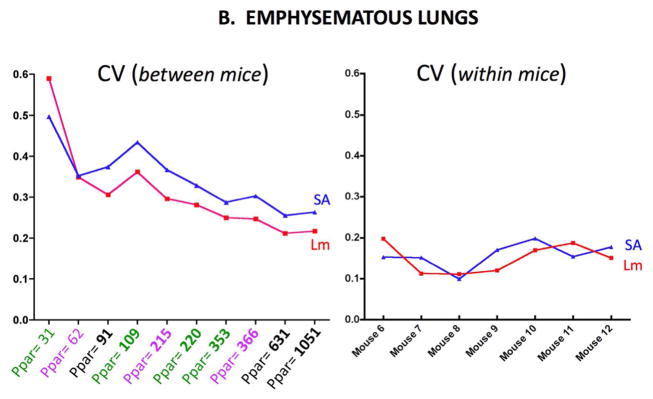
Figure 4A shows the between mice CV (left) and within mice CV (right) for the emphysematous lungs. The Lm and SA results are shown in each graph. Figure 4B shows the same measurements for the control lungs. The between mice variability increases in both control and emphysematous mice as the number of sampling points decreases.
Figure 4.
Figure 4A. CV (SD/mean) in control lungs for Lm and Surface Area (SA) measurements between mice (left panel) and for measurements within mice (right panel). The between mice abscissa shows the total number of parenchymal points (Ppar) counted.
Figure 4B. CV (SD/mean) in emphysematous lungs for Lm and Surface Area (SA) measurements between mice (left panel) and for measurements within mice (right panel). The between mice abscissa shows the average number of parenchymal points (Ppar) counted.
DISCUSSION
The results presented in this report indicate that higher density sampling is not needed even with increased variability associated with elastase-induced emphysema. While there is variability associated with varying the number of points counted, this variability is not very sensitive over the range counted in either control or emphysematous lungs. In addition this sampling variability is comparable in magnitude to the biologic (between animal) variability in control mice, but is considerably less than the biologic variability in emphysematous mice. This issue of relative variability has been theoretically analyzed (Gundersen and Jensen, 1987; Gundersen et al., 1999), and this theory leads to practical predictions that approximately 200 sampling events should be sufficient (Mühlfeld et al., 2012). Above ≈200 parenchymal points, there is no advantage of sampling with higher density in either control or emphysematous lungs. Our observations shown in Figures 2 and 3 support this recommendation. Indeed, at any of the sampling levels, a simple T-test would find a significant difference in Lm (p<0.01) or SA (p<0.03) if the number of sampling points exceeds 100. This observation in the emphysematous lungs was somewhat surprising given the increased variation in surface area and Lm between animals and the heterogeneous distribution of the elastase injury. Although we could detect differences in Lm and SA with the level of emphysematous injury using a relatively small sample size (5 control and 7 emphysema nice), if the emphysematous injury were less severe, it is likely that the number of mice in might have to be increased.
We should emphasize that there are several limitations to this study, that may preclude a more general application of the results. Of major importance is that this study did not consider all the different levels of sampling variability that could be present. For example, there is a variability that would occur by sampling different tissue blocks throughout the whole lung, as well as variability in how the sections could be cut from such blocks. Considering all these possible levels of variability would have made this a very extensive complex study, and we chose to focus on one particular practical and useful issue concerned with the requisite number of counting events. Due to the fixed sampling of tissue blocks, the important contribution of block sampling variability could not be studied. Thus, the analysis in the present study was restricted to within-block sampling variability, and if these other variances are greater than the within block variability that we have restricted our study to, the number of points/intercepts counted may need to be independently determined.
We also did not attempt to sample the right lung, but rather chose to duplicate what is commonly done by many investigators to reduce experimental costs. Investigators will often use one lung for lavage or tissue processing and the other for quantitative morphology. The underlying assumption for this procedure is that whatever the pathology is, there are no systematic differences between left and right lungs. We think this is a very reasonable assumption, and we are unaware of any generalized lung injury models where this has been shown to be untrue. Furthermore, it is likely the elastase delivered by our method follows a similar widespread dispersion pattern as was shown radiograpically for a 99mtechnetium labeled colloid solution (Foster et al., 2001). The other bias in our sampling procedure is that we did not slice sections from the left lung in a randomly manner. One reason not to do this is technical, since it is more difficult to cut the left lung along its long axis. However, there is again an underlying assumption that the lung parenchyma is relatively isotropic, that is that it matters little from which direction one views the parenchyma. If true, then it won’t matter how we cut the tissue blocks. While there is some indication that the lung is not completely isotropic (Mitzner et al., 2008), at the level of injury we are looking for in an emphysema model, the anisotropy would be expected to be relatively small. Thus we do not think that the biased transverse slicing of the left lung will introduce a systematic error, and the fact that we used the same procedure for both control and emphysematous lungs makes it even less so. Lastly, we chose to fix the lungs with formaldehyde instillation and embed the lungs in paraffin, processes known to cause substantial lung shrinkage (Hsia et al., 2010). One can use glutaraldehyde and embed in glycol methacrylate, and this approach will cause minimal shrinkage. Although such an approach brings the lung closer to the in vivo state, we chose not to use this approach simply because nearly all published studies employ the simpler procedure as done here. We did not attempt to correct for such shrinkage, nor the likelihood that the shrinkage might be less in emphysematous lungs with less elastic recoil, even with the same processing. However, this differential shrinkage between control any emphysematous lungs is likely to be small, and it would likely have only secondary effects on the variability. And it is the variability that was the focus of this present work.
In our analysis, we measured both surface area and Lm, variables that depend not only on the number of parenchymal points counted, but also on the number of intercepts of the line segments with alveolar walls. So an obvious corollary of our analysis is the question of how many intercepts should be counted as well. It turns out, however, that the number of intercepts is normally fairly close to the number of parenchymal points. On average, in control lungs the number of intercepts was 11% greater than the number of parenchymal points, so if the results were plotted as a function of intercepts, the curves would look very similar. With the extensive degree of emphysema we induced, however, the loss of alveolar walls leads to the average number of intercepts being about 30% less than the number of parenchymal points. Thus if we had used the abscissa as intercepts instead of parenchymal points the range would run from about 70 to 700 intercepts. This difference, however, would have little effect on the conclusions we draw from these observations.
With regard to practical recommendations based on our present work, we can conclude that even with a severe level of emphysema, the recommendation, that in any given animal, one need not count more than about 200 points is valid. Of course this assumes that the block to block variability mentioned above was not extensive and that acquisition of histologic sections and images was done in a random manner, but without such unbiased sample preparation even a higher density sampling would be inadequate. Although this manual point counting takes slightly longer than having the microscope software determine intercepts and surface area, with the need to only count 100–200 points, it only takes 5–10 min for a trained investigator or technician to quantify each mouse lung. The major time involved in the whole stereological process becomes the sectioning and capturing of the random images for analysis. Since this is required no matter what analysis is done, the advantages of the manual counting procedure (Knudsen et al., 2010) would seem to justify the slight additional time cost. An additional advantage of this method is that in addition to the measurement of Lm and SA, one can also obtains tissue density and septal thickness.
Contributor Information
Nathachit Limjunyawong, Email: nlimjuny@jhsph.edu.
Alexandra Kearson, Email: akearso1@jhu.edu.
Sandhya Das, Email: s_thulasidas@yahoo.co.in.
Wayne Mitzner, Email: wmitzner@jhsph.edu.
References
- Foster WM, Walters DM, Longphre M, Macri K, Miller LM. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J Appl Physiol. 2001;90:1111–1117. doi: 10.1152/jappl.2001.90.3.1111. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Osterby R. Optimizing sampling efficiency of stereological studies in biology: or ‘do more less well!’. J Microsc. 1981;121:65–73. doi: 10.1111/j.1365-2818.1981.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Three-dimensional measurement in microscopy. 2. Oxford: Bios; 2005. Unbiased stereology. [Google Scholar]
- Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen L, Weibel ER, Gundersen HJ, Weinstein FV, Ochs M. Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. J Appl Physiol. 2010;108:412–421. doi: 10.1152/japplphysiol.01100.2009. [DOI] [PubMed] [Google Scholar]
- Mitzner W, Fallica J, Bishai J. Anisotropic Nature of Mouse Lung Parenchyma. Ann Biomed Eng. 2008 doi: 10.1007/s10439-008-9538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzner W, Weibel ER. Comment on Kang, et al. “Cigarette Smoke selectively enhances viral PAMP. J Clin Invest. 2009;118 doi: 10.1172/JCI32709. http://www.jci.org/eletters/view/32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR. Unbiased stereology : a concise guide. Baltimore: Johns Hopkins University Press; 2011. [Google Scholar]
- Mühlfeld C, Knudsen L, Ochs M. Stereology and morphometry of lung tissue. In: Taatjes DJ, Roth J, editors. Cell Imaging Techniques: Methods and Protocols. New York: Humana Press; 2012. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- Ochs M, Muhlfeld C. Quantitative microscopy of the lung: a problem-based approach. Part 1: basic principles of lung stereology. Am J Physiol Lung Cell Mol Physiol. 2013;305:L15–22. doi: 10.1152/ajplung.00429.2012. [DOI] [PubMed] [Google Scholar]
- Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57–60. [PubMed] [Google Scholar]
- Snider GL, Kleinerman J, Thurlbeck WM, Bengali ZH. The definition of emphysema: Report of a National Heart, Lung, and Blood Institute workshop. Am Rev Respir Dis. 1985;132:182–185. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]