Abstract
As with other mitochondrial respiratory chain components, marked clinical and genetic heterogeneity is observed in patients with a cytochrome c oxidase deficiency. This constitutes a considerable diagnostic challenge and raises a number of puzzling questions. So far, pathological mutations have been reported in more than 30 genes, in both mitochondrial and nuclear DNA, affecting either structural subunits of the enzyme or proteins involved in its biogenesis. In this review, we discuss the possible causes of the discrepancy between the spectacular advances made in the identification of the molecular bases of cytochrome oxidase deficiency and the lack of any efficient treatment in diseases resulting from such deficiencies. This brings back many unsolved questions related to the frequent delay of clinical manifestation, variable course and severity, and tissue-involvement often associated with these diseases. In this context, we stress the importance to study different models of these diseases, but also discuss the limitations encountered in most available disease models. In the future, with the possible exception of replacement therapy using genes, cells or organs, a better understanding of underlying mechanism(s) of these mitochondrial diseases is presumably required to develop efficient therapy.
Keywords: Mitochondrial diseases, Cytochrome c oxidase, genetic diseases, mtDNA
1. Cytochrome oxidase, an overview of structure, function, and regulation
Cytochrome oxidase (COX; EC 1.9.3.1) is the unique terminal oxidase of the mitochondrial respiratory chain (RC) in mammals (Fig. 1). COX also referred to as complex IV is made up of thirteen subunits that catalyze the transfer of electrons from ferro-cytochrome c to molecular oxygen. This exergonic reaction is coupled to proton transfer across the inner membrane, which contributes to the electrochemical gradient used for ATP synthesis (Fig. 1A). The electrochemical gradient is also crucial for preserving the capacity of mitochondria to exchange metabolites and ions with the surrounding cytosol and other organelles.
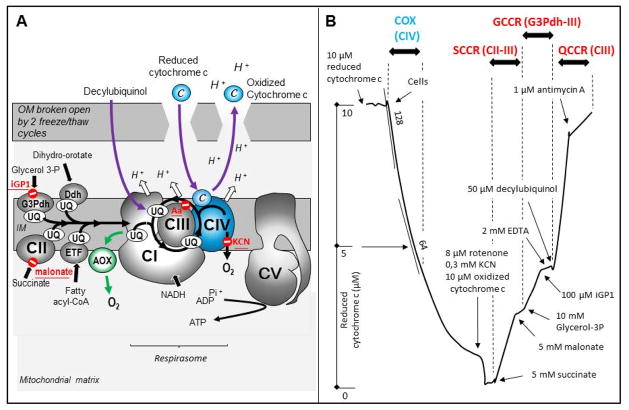
Figure 1. Cytochrome oxidase location in the respiratory chain and activity assay in human skin fibroblasts.
A. Schematic representation of the respiratory chain in the inner mitochondrial membrane showing the interaction of cytochrome oxidase (complex IV) with complexes I and III in a super-complex (respirasome). The site of action of specific inhibitors is indicated in red. The green arrow shows the alternative oxidase (AOX) by-pass, which when expressed in COX-defective human mitochondria or flies rescues their various phenotypes. The assay of COX with externally added cytochrome c requires the permeabilization of the outer membrane. B. Cytochrome oxidase is assayed spectrophotometrically by measuring using a double-wavelength spectrophotometer (550–540 nm) the oxidation of reduced cytochrome c in skin fibroblasts permeabilized by 2 successive freeze/thaw cycles. The reaction is first order with respect to substrate concentration and is thus diminished by half when half of the reduced cytochrome c is consumed. Subsequent sequential addition of rotenone, cyanide, oxidized cytochrome c and succinate measures reduction of cytochrome c, first by the succinate-cytochrome c reductase (CII plus CIII). The activity is essentially rate controlled by CII and can be inhibited by malonate, a competitive inhibitor of CII. Further addition of glycerol-3 phosphate measures the activity from the glycerol-3 phosphate dehydrogenase (G3Pdh) to CIII. This activity can be selectively inhibited by iGP1(143). Finally, addition of decylubiquinol in the presence of EDTA is used to measure antimycin-sensitive CIII activity. Abbreviations: The RC complexes are abbreviated as, CI, CII, CIII, CIV, and the ATP synthase as CV; c, cytochrome c; COX, cytochrome oxidase; Ddh, the dihydroorotate dehydrogenase which catalyze the production of uridine, an essential step for the synthesis of nucleic acids; EDTA, ethylenediamine tetraacetic acid; ETF, the electron transfer flavoprotein involved in the oxidation of fatty acids; G3Pdh, the glycerol 3-phosophate dehydrogenase; GCCR, iGP1-sensitive glycerol 3-phosphate; IM, inner membrane; KCN, potassium cyanide; OM, outer membrane; QCCR, antimycin-sensitive decylubiquinol-cytochrome c reductase; SCCR, malonate-sensitive cytochrome c reductase; UQ, ubiquinone 50, or coenzyme Q10.
The three largest subunits of COX are encoded in mitochondrial DNA (mtDNA). These core subunits contain all the heme and metal prosthetic groups needed for catalysis. The remaining ten subunits are products of nuclear genes that are translated as pre-proteins on cytosolic ribosomes, imported to different compartments of mitochondria by the TIM and TOM transport machineries and possibly modified before entering the COX assembly pathway (1, 2). A large number of factors, sometimes specific to COX assembly and in other cases with broader specificity, are known to facilitate the various steps of COX assembly and its incorporation into the RC super-complexes, also referred to as the respirasome (Fig. 2 and 3).
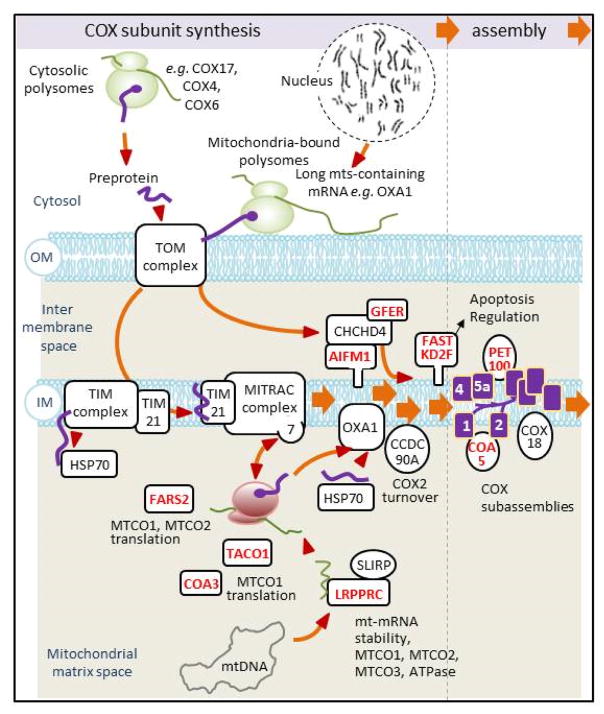
Figure 2. Synthesis and assembly of COX subunits.
A scheme summarizing what is presently known about the pathways for the integrated synthesis and assembly of COX subunits expressed from the nuclear and mitochondrial genomes. Protein subunits translated on cytosolic ribosomes with N-terminal presequences are first transported by the outer membrane TOM complex and are subsequently matured and sorted by the TIM and MITRAC machineries to the intermembrane space, inner membrane, or matrix where they interact with partner proteins to form assembly intermediates (144–146). Subunits encoded by mtDNA genes are translated on mitochondrial ribosomes attached to the matrix side of the inner membrane. Following insertion into the inner membrane by Oxa1 they interact with their nucleo-cytoplasmic partners to form subcomplexes that subsequently assemble into COX. The overall process is assisted by numerous proteins acting in transport, translation, chaperoning of different assembly steps. Oxa1 is also involved in the biogenesis of other respiratory chain complexes. Some of the genes coding for ancillary factors (indicated in red) have been found to be mutated in COX deficient patients. IM, inner membrane; OM, outer membrane; 1, 2, 4, 5a, COX subunits (purple)
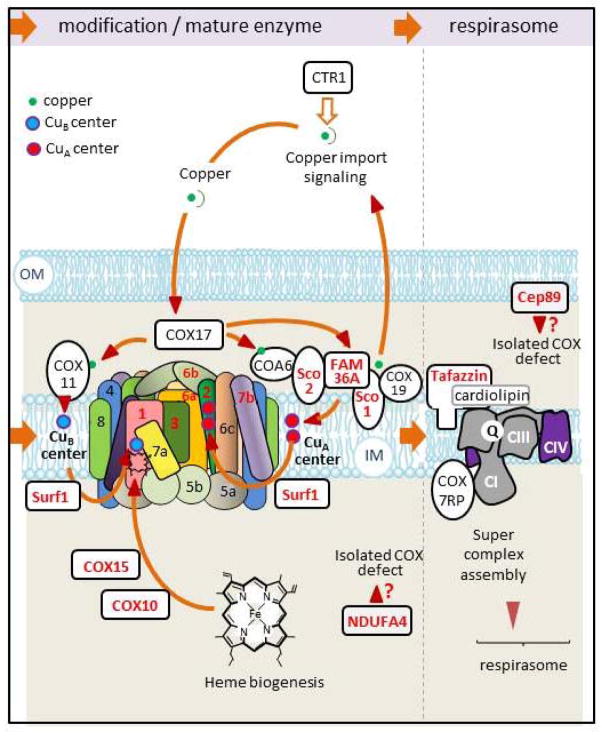
Figure 3. Maturation and insertion of COX into the respiratory chain.
Mammalian COX exists as a dimer. Each monomer consists of 13 different subunits. At present human mutations leading to a COX deficiency have been identified in six structural subunits including the three mtDNA-encoded core proteins (in red) and in 9 ancillary proteins (also indicated in red). The catalytic activity of COX depends on heme a, a3 and two copper centers (CuA and CuB) linking COX biosynthesis to both copper and heme metabolism. Maturation of COX active centers involves a number of factors, some of which have also been found mutated in COX deficient patients (denoted in red). IM, inner membrane; OM, outer membrane; 1, 2, 4, 5a, 5c, 6a, 6b, 6c, 7a, 7b, 8, the different COX subunits; CI, CII, CIII, CIV, the various complexes of the respiratory chain
Complex IV of the mammalian RC has been shown to interact with complexes I and III with variable stoichiometry to form the respirasome (3, 4). This organization of the respiratory chain into a respirasome, de facto, isolates kinetic pools of electron carriers, including mobile carriers such as ubiquinone(5), to efficiently channel electrons supplied by various dehydrogenases of mitochondria to the appropriate segments of the RC (6). In the context of mitochondrial diseases, a primary loss of COX may have secondary effects on the organization of the respirasome thereby eliciting more complex biochemical phenotypes (7, 8).
In mammals, the composition of tightly-bound subunits of the COX core is constant. However, the expression of several nuclear-encoded isoforms of one or more imported subunits may vary depending on the developmental stage and the tissue (Table I; Fig. 4). Indeed, extensive SDS-PAGE analyses together with immunological and/or sequencing data by the Kadenbach and Grossman groups have demonstrated the occurrence of several isoforms of the COX4, COX6A, COX6B, COX7A and COX8 (1, 2) subunits that are variably expressed during fetal development in mammals (9) (Table I; Fig. 4). COX activity is regulated by the ATP/ADP ratio, which affects its phosphorylation status (10), and association of COX into dimers, as well as its interactions with cardiolipin (11) and other proteins (2). Finally, beside the thirteen tightly bound subunits observed by the Kadenbach group and later confirmed by the Yoshikawa group by X-ray crystallography (12), a more loosely bound 14th subunit product of the NDUFA4 gene was detected in stoichiometric amounts when analyzed by BN-PAGE (13). This idea gained support from the finding that a mutation in NDUFA4 results in a specific COX deficiency (14).
Table I.
The human cytochrome c oxidase subunits and isoforms
| Subunit name and symbol | Alternative names | OMIM n° |
|---|---|---|
| CYTOCHROME c OXIDASE SUBUNIT I; MTCO1 | COI; COX1 | 516030 |
| CYTOCHROME c OXIDASE SUBUNIT II; MTCO2 | COII; COX2 | 516040 |
| CYTOCHROME c OXIDASE SUBUNIT III; MTCO3 | COIII; COX3 | 516050 |
| CYTOCHROME c OXIDASE, SUBUNIT IV, ISOFORM 1; COX4I1 | COX4 | 123864 |
| CYTOCHROME c OXIDASE, SUBUNIT IV, ISOFORM 2; COX4I2 | COX IV-2; COX4-2 | 607976 |
| CYTOCHROME c OXIDASE, SUBUNIT Va; COX5A | 603773 | |
| CYTOCHROME c OXIDASE, SUBUNIT Vb; COX5B | 123866 | |
| CYTOCHROME c OXIDASE, SUBUNIT VIa, POLYPEPTIDE 1; COX6A1 | LIVER ISOFORM; COX6AL | 602072 |
| CYTOCHROME c OXIDASE, SUBUNIT VIa, POLYPEPTIDE 2; COX6A2 | MUSCLE ISOFORM; COX6AH; COX6AM | 602009 |
| CYTOCHROME c OXIDASE, SUBUNIT VIb POLYPEPTIDE 1; COX6B1 | 124089 | |
| CYTOCHROME c OXIDASE, SUBUNIT VIb POLYPEPTIDE 2; COX6B2 | Cancer/Testis antigen 59 CT59 | Not available |
| CYTOCHROME c OXIDASE, SUBUNIT VIc; COX6C | 124090 | |
| CYTOCHROME c OXIDASE, SUBUNIT VIIa, POLYPEPTIDE 1; COX7A1 | MUSCLE ISOFORM; COX7AM | 123995 |
| CYTOCHROME c OXIDASE, SUBUNIT VIIa, POLYPEPTIDE 2; COX7A2 | LIVER ISOFORM 1; COX7AL; COX7AL1 | 123996 |
| CYTOCHROME c OXIDASE, SUBUNIT VIIb; COX7B | 300885 | |
| CYTOCHROME c OXIDASE, SUBUNIT VIIc; COX7C | 603774 | |
| CYTOCHROME c OXIDASE, SUBUNIT VIII; COX8 | 123870 |
Figure 4. The non-overlapping clinical symptoms of COX deficiency and expression territories of COX subunits.
On the left are shown ubiquitously expressed subunits which when mutated result in a constellation of symptom but sparing numerous organs. The representations in the middle and on the right show that mutation in genes encoding subunits with more specific organ/tissue expression does not necessarily result in symptoms affecting the predicted organs. Subunits encoded in mtDNA are depicted in green. Genes with identified mutations in patients with COX deficiency are shown in yellow (or green).
2. Genetic diseases characterized by a COX deficiency
Patients with a COX deficiency can present surprisingly variable clinical phenotypes. As of now mutations in more than 20 mitochondrial and nuclear genes associated with COX deficiency are known to lead to a constellation of phenotypes. Parameters such as lactic acidosis in the blood or cerebro-spinal fluid, ragged-red fibers in adult skeletal muscle biopsies are hallmarks of mitochondrial diseases but they are not specific to COX deficiencies. The following circumstances may modulate the severity and clinical manifestations of a COX and more generally an RC mutation; a) the organs being affected (e.g. a heart specific COX defect is unlikely to change the general metabolic equilibrium of the organism), b) the severity of the deficiency (e.g. partial COX defect can have deleterious consequence, yet not significantly changing metabolic equilibrium under basal conditions), and c) the capacity to cope with a COX defect (e.g. course of disease resulting from a given mutation often differ between patients). As a result, even the absence of typical manifestation of COX (or RC) deficiency should not exclude a clinical anamnesis by advertised clinician, which may justify proceeding to further investigation.
3. New methods, old questions
The large scale screening with micro-chips identifying both mtDNA and nuclear gene mutations has been partially responsible for the steady increase in the number of identified mutations in patients suspected to have dysfunctional mitochondria (see MITOMAP for an update)(15). Unfortunately, except for known or non-ambiguous mutations affecting COX-related genes, there currently is no simple way to ascribe a clinical phenotype to the reported base change even when the latter is in a gene of known function. Examples abound showing that the nature of the amino acid change, its location in the protein, or its evolutionary conservation are not sufficient to establish pathogenicity. Consequently, a biochemical screen of the RC complexes function is usually required. COX activity can be conveniently assayed in virtually all human tissues and cells (16) (Fig. 1B). It should be kept in mind, however, that the results obtained with tissue samples do not always lead to a correct identification of the underlying lesion. Indeed, errors can occur when the mutation shows a high degree of tissue specific expression and therefore is not necessarily detectable in the particular sample analyzed. Another source of ambiguity may arise with mutations causing an accumulation of mitochondria, thus compensating for the decrease of the enzyme and giving the impression of a normal activity. This is not uncommon, especially in the skeletal muscle but can be avoided by normalizing the activity of the deficient enzyme to another RC component or to a marker for mitochondrial mass (Fig. 1B) (17).
4. Human diseases caused by genetically-determined COX deficiency
A sensible and useful way of classifying COX deficiencies takes into consideration their genetic origin. Indeed mutations in the same gene are expected to affect a common step or activity in the same tissue and consequently have a similar outcome. However, what is observed in patients is an impressive diversity of phenotypes independently of whether the mutation affects the biosynthesis/maturation of a mitochondrial or nucleus-encoded COX subunit or of its assembly into the mature complex and ultimately super-complex. A number of excellent reviews have been written on various aspects of COX deficiencies (18–20) and databases are regularly updated incorporating the rapidly growing body of new information (15).
4a. Mutations in mitochondrial COX genes
Numerous diseases resulting from mutations in the mitochondrial genes encoding MTCO1, MTCO2 and MTCO3 have been reported. The mutations in each of these three COX subunits have been associated with a variety of more or less severe phenotypes (Table II). In addition, COX3 mutations have also been connected to Alzheimer disease (21) but this has been disputed as the underlying cause may be an overproduction of superoxides by the mitochondrial RC rather than by the COX deficiency (22).
Table II.
Mutations in COX assembly and structural genes and their phenotypic consequences in human and animals (As in the text, for all cited species, human nomenclature has been used (HGNC) (92)
| Mutant gene | Known clinical phenotype in human | Phenotype in animal |
|---|---|---|
| MTCO1 | MELAS syndrome (93), myopathy (94), rhabdomyolysis(95), prostate cancer (96), myoglobinuria (97), motoneurone disease (98), exercise intolerance (99), epilepsy (100), acquired idiopathic sideroblastic anemia (101), multisystem disorders (102), deafness, LHON, or mitochondrial sensorineural hearing loss (103) | Mus musculus no overt phenotype (62) |
| MTCO2 | Encephalomyopathy (104) LHON (105) myopathy (106), hypertrophic cardiomyopathy (107), Alpers-Huttenlocher like disease (108), encephalomyopathy (109), pseudoexfoliation glaucoma (110), asthenozoospermy (111), rhabdomyolysis (112) | |
| MTCO3 | MIDD (113), LHON (99), myopathy (114), asthenozoospermia (115), Leigh disease (116), myoglobinuria (117), sporadic bilateral optic neuropathy (118), rhabdomyoloysis (119), encephalopathy (120), progressive encephalopathy, MELAS or non-arteritic ischemic optic neuropathy (121), hypertensive end-stage renal disease (122) | |
| COX6B1 | Severe infantile encephalomyopathy (23) | |
| COX6A1 | Recessive axonal or mixed form of Charcot-Marie-Tooth disease (24) | Drosophila melanogaster, COX deficiency and premature death (58). |
| COX7B | Microphthalmia with linear skin lesions (25) | |
| COX4I2, (COX6A1 COX6A2, COX7A1, COX7A2 | Failure to thrive, psychomotor delay, progressive leucodystrophy, encephalomyopathy, epilepsy, hypotony, hepatopathy, anemia, lactic acidosis, and visual impairment (123) |
Caenorhabditis elegans, knock-down of COX4 and COX5a homologs using RNA interference shortened lifespans (60). Mus musculus COX4I2 KO lung pathology (63) |
| COX6A2, | Mus musculus KO led to cardiac dysfunction (65) | |
| COX7A1, | Mus musculus; KO of the heart/skeletal muscle-specific COX7A1 exercise intolerance reminiscent of a mild myopathy (66) or dilated cardiomyopathy (67) | |
| COX7A2 | ||
| AIFM1 | Encephalomyopathy (124), prenatal ventriculomegaly (125), hearing loss, external ophthalmoplegia, ataxia and muscle wasting (126), infantile motor neuron disease (127) | |
| COA3 | Neuropathy, exercise intolerance, obesity, and short stature (128) | |
| COA5 | Cardiomyopathy (129) | |
| COX10 | Neonatal tubulopathy and encephalopathy, Leigh syndrome, or cardiomyopathy (130, 131) | Mus musculus transient liver KO caused increase of hepatocytes apoptosis (68), muscle-specific KO led to myopathy (69); forebrain-specific Cox10 deletion resulted in astroglyosis and inflammation (70) |
| COX15 | Early-onset cardiomyopathy, or Leigh syndrome (132, 133) | |
| FAM36A | Ataxia and muscle hypotonia (134) | |
| FARS2 | Infantile-onset epilepsy (69) | |
| FASTKD2 | Encephalomyopathy in (135) | |
| PET100 | Infantile lactic acidosis (136); Leigh syndrome (137) | |
| SCO1 | Neonatal-onset hepatic failure and encephalopathy (138) | |
| SCO2 | Neonatal cardioencephalomyopathy; Myopia (36, 139) |
Mus musculus A heterozygous KI/KO for SCO2 developed muscle weakness(71, 72) podocyte-specific KO of KLF6 (Krüppel-like factor 6) acting on SCO2-transcription increased focal segmental glomerulosclerosis induced by adryamicin (73) |
| SURF1 | Leigh syndrome (140); villous atrophy and hypertrichosis, without central nervous system pathology(141) |
D. melanogaster. Ubiquitous post-transcriptional silencing mostly larvae death some reaching pupal stage dying as early imagos (59). Silencing in the central nervous system, cephalic low COX activity associated with behavioral and electrophysiological abnormality (59). Danio rerio. COX reduced expression induced by using morpholinos, tissue-specific consequences with increased apoptosis in neural tissues but not in the heart that however showed time-increased poor performance (61) Mus musculus SURF1 KO did not manifest disease phenotype despite 30–40% decreased COX activity |
| TACO1 | Leigh syndrome (142) |
Altogether, mutations in the 3 mtDNA encoded COX subunits can result in more than twenty different phenotypes. The degree of heteroplasmy (co-existence of variable levels of mutant and non-mutant mtDNA) in different tissues has been invoked to account for the large number of phenotypes associated with mutations in these mitochondrial genes. Although heteroplasmy may be a contributing factor, it cannot be the entire explanation as a similar clinical variability is observed in patients harboring mutations in nuclear genes encoding RC components and assembly factors.
4b. Mutations in nuclear COX genes
Relatively few mutations have been reported in nuclear genes encoding COX subunits and prior to the discovery of the first mutation in COX6B1 (23), it was suggested that such mutations might be incompatible with life. A mutation in COX6B1 was associated with severe infantile encephalomyopathy, a quite typical presentation for a mitochondrial disease (23). In a more recent study a mutation in COX6A1 was shown to cause a neurological disorder characterized by a recessive axonal or mixed form of Charcot-Marie-Tooth disease (24). In addition, a mutation in COX7B was identified in a patient presenting microphthalmia with linear skin lesions, an unusual phenotype for a mitochondrial disease (25). Loss-of-function mutations in nuclear-encoded subunits, although based on still limited data, appear to result in a loss of COX activity and accumulation of COX partially assembled complexes (26), however, the differences in clinical phenotype cannot be related to the function of a particular COX subunit or to its level of expression. A possible factor contributing to this phenomenon is the existence in human mitochondria of isoforms of some COX subunits with differential tissue-specific expression (Table 1 and Fig. 4). COX6B1 like COX6A1 is ubiquitously expressed, while COX6B2 and COX6A2 are expressed mainly in testes and muscle tissue, respectively (Fig 4). Thus, isoform expression by itself may sometimes explains the phenotypic variability associated with mutations in these genes.
4c. Mutant COX assembly genes
Much more frequent mutations affecting COX occur in genes encoding protein factors involved in the biosynthesis and assembly of this enzyme (Fig. 2 and 3, in red). Mutations in 15–20 genes, depending on whether they elicit a singular COX or a predominantly COX deficiency in patients, have been identified. Some of these genes products are factors known to act in pathways other than COX assembly (e.g. AIF1M, or LRPPRC)(27–33).
The temporally and perhaps spatially coordinated biogenesis of different RC complexes and their assembly into supercomplexes is likely to rely on factors that are not specific to a single RC complex. For example the product of OXA1, which was initially thought to be a specific COX factor (34), turned out to function as a more general inner membrane insertase of mitochondrial gene products. However, most COX factors that have been described, mainly from studies of Saccharomyces cerevisiae, and for which there are human homologues, have functions confined to COX biogenesis. With a few exceptions COX assembly factors are ubiquitously expressed in humans and when mutated affect multiple organs. The SCO1 and SCO2 proteins are such exceptions as they display a tissue-specific pattern of expression that has been claimed to fit with the phenotype observed in patients with mutations in these proteins (35). Even in this case, however, it is not always easy to correlate the presentation of the disease with tissue distribution of the protein. A case in point is the highly specific presentation as an autosomal-dominant high-grade myopia in patients with mutations in SCO2. This phenotype is difficult to reconcile with the restricted expression territory of the gene (36, 37).
Mutations in COX assembly factors, similar to those in the structural genes exhibit a multiplicity of phenotypes (see Table II). A number of studies have concluded that COX activity is also critical for cell proliferation in lung, breast cancers, nasopharyngeal carcinomas and gliomas, perhaps by favoring metabolic reprograming required for cell proliferation (38–42).
More than twenty phenotypes observed in patients with mutations in nuclear genes encoding COX subunits and assembly factors are not unlike those described for mutations in the mitochondrial genes. As already mentioned, the fact that these genes have a nuclear origin and are inherited by Mendelian laws excludes heteroplasmy as a contributing factor to the large diversity of phenotypes. Tissue-specific expression of some isoforms can also be discounted as there are numerous examples of gene products that have no isoforms yet also showing a range of different phenotypes.
A number of factors may contribute to the phenotypic variability. The function(s) of at least some proteins encoded by these nuclear genes is far from being completely understood and might be more diversified than presently recognized. Indeed, some of these genes products attributed to play a role in the assembly of COX could also functions in the biogenesis of other RC complexes. Mitochondria, depending on the organ and its energy needs (43, 44), could be differently affected despite expressing a similar biochemical defect. Additionally, because mitochondria are intimately connected with and strongly influence other cellular activities (45), the capacity of organs and the whole organism to cope with mitochondrial dysfunction could depend on the mobilization of genetic resources, expected to be unique to each individual. Evidence is only beginning to emerge now showing that genetic background plays a crucial role in determining the consequence of mitochondrial dysfunctions (46, 47).
5. Studies of COX deficiency in cultured cells, and micro-organisms
Because of their low invasiveness, biopsies of skin fibroblasts or lymphocytes from patient blood samples have been (and still are) extensively used in studies of mitochondrial diseases(16). They have been instrumental in clarifying questions related to the biochemistry of mitochondrial dysfunction and the levels of mtDNA heteroplasmy as a function of cell divisions (48, 49). Additionally, primary cell cultures have proved to be useful to establish the deleterious character of a number of COX gene mutations, especially exploiting their requirement for glucose in culture media. These cells have been in particular useful in devising rational pharmacological (50) and genetic (51) approaches to fight the consequences of COX deficiencies. Exciting recent studies offer the promise that COX deficiencies in patients might one day be ameliorated by an AOX (alternative oxidase) by-pass. Human COX-defective fibroblasts unable to grow in the absence of glucose as a result of COX15 silencing were rescued by ectopic expression of AOX from Ciona intestinalis (52). This non-proton motive oxidase restores NADH and succinate oxidation in mitochondria without increasing their ATP generating capacity (Fig. 1A). The rescue of COX-defective fibroblasts by AOX suggests that the deleterious effect of the COX deficiency in these cells and under the culture conditions used is not the result of lowered ATP production. This is also supported by recent data showing AOX rescue of the different phenotypes seen in COX deficient fruit flies (53).
A number of important questions about COX deficiency remains unanswered, and, for obvious reasons they cannot be solved by studies of cultured cells. Foremost are tissue specificity and development-related phenotypes. In view of this shortcoming several COX defective models have been created to unravel COX subunit/isoform function, hopefully to model human mitochondrial diseases, and ideally to find ways to counteract disease-causing mutations.
Most of our basic understanding of COX and its assembly comes from studies of microorganisms, particularly of the unicellular yeast, Saccharomyces cerevisiae. In addition to the large arsenal of genetic tools for manipulating the mitochondrial and nuclear genes of this organism, it is also extremely useful in validating the pathogenic impact of a particular mutation identified in a constituent of the RC (54). A requirement for such heterologous complementation tests is the presence of a yeast homolog for a mutated human gene. Although this is usually the case with genes coding for RC complexes II, III, and IV, importantly human complex I has not homolog in S. cerevisiae, which uses instead two different dehydrogenases completely unrelated to human complex I. The absence of CI in S. cerevisiae mitochondria implies a different organization of the respirasome that could also influence the deficit related phenotype. There are also important differences in the genetic systems of mammalian and yeast mitochondria. The absence of introns in mammalian mtDNA, and the existence of different sets of translational activators and regulatory proteins than those present in yeast implies that the balanced output of nuclear and mitochondrial gene products of COX and other complexes of the RC must be attained by regulatory mechanisms different from those described in yeast (55, 56),(57). Obviously, yeast is also not the best system to study questions related to tissue specificity and the effects on developmental parameters of COX and RC deficiencies.
6. Higher organisms to study COX deficiency
Several groups have exploited the fruit fly (Drosophila melanogaster), the worm (Caenorhabditis elegans), the Zebrafish (Danio rerio) or the mouse as models for genetic modification of COX-related genes to evaluate their consequent phenotypes in a higher organism. Interestingly, the phenotypes observed in these model organisms have not always been consistent with those of human patients. A mutation in COX6A of Drosophila caused a COX deficiency and premature death, while different mutations in the human gene, similarly causing COX deficiency, result in encephalomyopathy (58). Body-wide post-transcriptional silencing of the fly homolog of the COX-assembly gene SURF1 caused extensive larvae death as early as the imago stage of pupal development (59). Flies with SURF1 silenced in the central nervous system only, reached adulthood with low cephalic COX activity and with behavioral and electrophysiological abnormality (59). Knock-down of the COX4 and COX5A homologs using RNA interference shortened the lifespan of worms. Surprisingly, the authors reported that the COX defect was accompanied by a loss of complex I function (60). Reduced expression of either COX5A or SURF1 induced with morpholinos in Zebra fish elicited a panoply of tissue-specific abnormalities, including increased apoptosis in neural tissues but not in the heart. Heart function, however, decreased with time (61).
Several genes for COX subunits have also been targeted in the mouse. No obvious phenotype could be linked to a mild COX1 point mutant, a missense mutation at nucleotide 6589 (T6589C) converting a highly conserved valine at codon 421 to alanine (V421A) (62). A KO of the nuclear COX4I2, however, caused a lung pathology stressing the importance of the gene product for normal lung function (63). In human, a mutation in COX4I2 causes exocrine pancreatic insufficiency, dyserythropoeitic anemia, and calvarial hyperostosis (64) affecting organs/tissues supposedly not expressing this protein, which might suggest an indirect effect of the mutation. A KO of COX6A2 led to cardiac dysfunction (65) and a KO of the heart/skeletal muscle-specific COX7A1 to exercise intolerance reminiscent of a mild myopathy (66) or dilated cardiomyopathy (67). Known assembly factors for COX have also been inactivated in the mouse. A transient liver KO of COX10 induced hepatocytes apoptosis (68) and a muscle-specific KO of COX10 led to myopathy (69). A forebrain-specific COX10 deletion resulted in astrogliosis and inflammation (70). A SURF1 KO did not manifest a disease phenotype despite 30–40% decreased in COX activity, suggesting that the mitochondrial concentration of this enzyme is higher than required to sustain cell growth and survival. Heterozygous mice with SCO2 KI/KO developed muscle weakness (71, 72), while podocyte-specific KO of KLF6 (Krüppel-like factor 6) acting on SCO2 transcription, increased focal segmental glomerulosclerosis induced by adryamicin (73). A heterozygous KO of CHCHD4 exhibited a COX deficiency and reduced weight gain (28).
Genetic manipulation of the model organisms produce in general rather well defined phenotypes.. This contrasts sharply with the large variation in phenotypes frequently observed in patients with mutations in the homologous genes. This discrepancy can be explained to some extent by the use of clonally-selected animals and by organ-specific targeting (46). The major impact of genetic background in the phenotypic expression of an RC dysfunction can also account for the variability (74). This is clearly demonstrated by the large number of phenotypes observed in a genetically heterogeneous Harlequin mouse population harboring a proviral insertion that reduces expression of the AIF1M gene by 80% (75), resulting only in a significant complex I deficiency (27, 74).
7. Therapeutic hopes
Since our last review on cytochrome oxidase, more than 10 years ago (76), very few approaches have demonstrated efficacy in counteracting COX deficiency in patients. Yet in a small number of cases (77, 78), without human intervention and mostly for unknown reason, a partial reversal of the disease has been seen. This gives hope that reversing disease phenotype owing to an efficient therapy is not out of reach. This particularly holds true when considering mtDNA mutations where partial change of mutant load might be sufficient to counteract at least disease progression. This has been shown possibly reachable in cultured human cells by using mitochondria-targeted nuclases (mitoTALEN) specifically identifying mutant mtDNA(79). Furthermore, thanks to the rapid progress being made in gene vectorization, gene therapy for a number of human diseases including COX deficiency has become an attainable goal. Accordingly, gene therapy has shown some promise in alleviating Leber Hereditary Optic Neuropathy (LHON), which was established to be in part related to a COX deficiency (80). In a mouse model of LHON stemming from a mutation in the mitochondrial ND4 gene, reversal of the disease was observed by optimizing the allotopic expression in the nucleus of an adeno-associated virus-harboring a version of ND4 modified for mitochondrial import and translation (81–83). LHON disease is particularly suitable for this approach as the retinal ganglion cell layer, which as a consequence of its degeneration is instrumental for the loss of vision, can be easily accessed. Gene therapy as a means of treating patients with LHON is still in the early stage with trials being done in France and the US. Unfortunately, this approach is hampered by problems of accessibility for most COX-related disorders affecting other organs or tissues.
Approaches alternative to gene therapy have also been explored to fight COX deficiency and other RC defects (84). A ketogenic diet has been claimed to increase energy metabolism in the brain by enhancing mitochondrial biogenesis, which in turn raises the cellular concentrations of adenosine and ATP, enhances neuron–glia interactions and may even shift the level of heteroplasmy (84). Numerous studies in which mixture(s) of non-toxic dietary supplements (creatine, lipoic acid, CoQ10, etc.) have been tested in patients, claimed to ameliorate some symptoms. Definitive evidence of their efficacy, however, is lacking. On the other hand, treating the symptoms such as strokes with arginine in MELAS patients with a COX-defect may help (85). Some drugs should however be avoided. For example, valproate, but possibly other antiepileptic drugs as well, have been shown to trigger hepatic failure in COX-defective patients (86, 87). Because of a lack of evidences for increased oxidative stress in COX impaired cells (88, 89), the use of antioxidants is also unlikely to be effective for COX deficient patients.
The pan-PPAR agonist bezafibrate has been shown to rescue RC deficiency in COX-defective human cells (50) (90) and in COX-defective mouse (69). However, this effect of bezafibrate could not be reproduced in a SURF KO, a SCO2 KO/KI, and in a muscle-restricted mouse with a COX15 KO. On the other hand, treatment of these three COX-defective mice with the AMPK agonist AICAR led to a partial recovery of COX (91). At this time it is important that these observations be corroborated by additional experiments and hopefully confirmed in patients.
8. Conclusion
Much progress has been made in our understanding of the molecular basis for COX deficiencies in patients, thereby reducing diagnostic wavering and allowing the clinician to better inform family members. The numerous mutations in structural and assembly genes identified in COX deficient patients has also served as an incentive to better understand their functions. Research along this line has revealed that in some cases genes products involved in COX biogenesis also play a more general role in maintaining the respiratory integrity of mitochondria by participating in the assembly pathways for other RC complexes. These studies have contributed to the significant progress made in recent years in deciphering mechanisms responsible for the biogenesis of the respiratory chain complexes and the role of the factors involved in this process.
The same cannot be said of the efforts to find ways of slowing down the course of these often devastating diseases. The emergence of gene therapy gives hope that development of vectors allowing targeting of a specific organ will be paralleled by equal strides in the treatment of diseases including those stemming from mitochondrial disorders.
Acknowledgments
PB and PR thanks the Association Française contre les Maladies Mitochondriales (AMMI), the Association Française contre l’Ataxie de Friedreich (AFAF), and the Association Française contre les Myopathies (AFM), MCD thanks l’association Ouvrir Les Yeux (OLY) and AFM for their support.
Funding information
PR and MCD were recipients of grants from ANR, and AT from NIH Grant GM1118640.
Abbreviation list
- COX
cytochrome c oxidase
- mtDNA
mitochondrial DNA
- RC
respiratory chain
Footnotes
Declaration of interest
The authors declare no conflict of interest.
References
- 1.Pierron D, Wildman DE, Huttemann M, Markondapatnaikuni GC, Aras S, Grossman LI. Cytochrome c oxidase: evolution of control via nuclear subunit addition. Biochim Biophys Acta. 2012 Apr;1817(4):590–7. doi: 10.1016/j.bbabio.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadenbach B, Huttemann M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion. 2015 Jul 17;24:64–76. doi: 10.1016/j.mito.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Schagger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000 Apr 17;19(8):1777–83. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schagger H, de Coo R, Bauer MF, Hofmann S, Godinot C, Brandt U. Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J Biol Chem. 2004 Aug 27;279(35):36349–53. doi: 10.1074/jbc.M404033200. [DOI] [PubMed] [Google Scholar]
- 5.Gutman M. Electron flux through the mitochondrial ubiquinone. Biochim Biophys Acta. 1980 Dec 22;594(1):53–84. doi: 10.1016/0304-4173(80)90013-0. [DOI] [PubMed] [Google Scholar]
- 6.Rustin P, Moreau F, Lance C. Malate Oxidation in Plant Mitochondria via Malic Enzyme and the Cyanide-insensitive Electron Transport Pathway. Plant Physiol. 1980 Sep;66(3):457–62. doi: 10.1104/pp.66.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz F, Fukui H, Garcia S, Moraes CT. Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Mol Cell Biol. 2006 Jul;26(13):4872–81. doi: 10.1128/MCB.01767-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saada A, Edvardson S, Shaag A, Chung WK, Segel R, Miller C, Jalas C, Elpeleg O. Combined OXPHOS complex I and IV defect, due to mutated complex I assembly factor C20ORF7. J Inherit Metab Dis. 2011 May 24; doi: 10.1007/s10545-011-9348-y. [DOI] [PubMed] [Google Scholar]
- 9.Bonne G, Seibel P, Possekel S, Marsac C, Kadenbach B. Expression of human cytochrome c oxidase subunits during fetal development. Eur J Biochem. 1993 Nov 1;217(3):1099–107. doi: 10.1111/j.1432-1033.1993.tb18342.x. [DOI] [PubMed] [Google Scholar]
- 10.Huttemann M, Lee I, Grossman LI, Doan JW, Sanderson TH. Phosphorylation of mammalian cytochrome c and cytochrome c oxidase in the regulation of cell destiny: respiration, apoptosis, and human disease. Adv Exp Med Biol. 2012;748:237–64. doi: 10.1007/978-1-4614-3573-0_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musatov A, Robinson NC. Bound cardiolipin is essential for cytochrome c oxidase proton translocation. Biochimie. 2014 Oct;105:159–64. doi: 10.1016/j.biochi.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshikawa S, Muramoto K, Shinzawa-Itoh K, Mochizuki M. Structural studies on bovine heart cytochrome c oxidase. Biochim Biophys Acta. 2012 Apr;1817(4):579–89. doi: 10.1016/j.bbabio.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Balsa E, Marco R, Perales-Clemente E, Szklarczyk R, Calvo E, Landazuri MO, Enriquez JA. NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab. 2012 Sep 5;16(3):378–86. doi: 10.1016/j.cmet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Pitceathly RD, Rahman S, Wedatilake Y, Polke JM, Cirak S, Foley AR, Sailer A, Hurles ME, Stalker J, Hargreaves I, et al. NDUFA4 mutations underlie dysfunction of a cytochrome c oxidase subunit linked to human neurological disease. Cell Rep. 2013 Jun 27;3(6):1795–805. doi: 10.1016/j.celrep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MITOMAP. A Human Mitochondrial Genome Database. 2007 doi: 10.1093/nar/24.1.177. http://wwwmitomaporg. [DOI] [PMC free article] [PubMed]
- 16.Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM, Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta. 1994 Jul;228(1):35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 17.Rustin P, Chretien D, Bourgeron T, Wucher A, Saudubray JM, Rotig A, Munnich A. Assessment of the mitochondrial respiratory chain. Lancet. 1991 Jul 6;338(8758):60. doi: 10.1016/0140-6736(91)90057-v. [DOI] [PubMed] [Google Scholar]
- 18.DiMauro S, Tanji K, Schon EA. The many clinical faces of cytochrome c oxidase deficiency. Adv Exp Med Biol. 2012;748:341–57. doi: 10.1007/978-1-4614-3573-0_14. [DOI] [PubMed] [Google Scholar]
- 19.Alfadhel M, Lillquist YP, Waters PJ, Sinclair G, Struys E, McFadden D, Hendson G, Hyams L, Shoffner J, Vallance HD. Infantile cardioencephalopathy due to a COX15 gene defect: report and review. Am J Med Genet A. 2011 Apr;155A(4):840–4. doi: 10.1002/ajmg.a.33881. [DOI] [PubMed] [Google Scholar]
- 20.Chadha R, Shah R, Mani S. Analysis of reported SCO2 gene mutations affecting cytochrome c oxidase activity in various diseases. Bioinformation. 2014;10(6):329–33. doi: 10.6026/97320630010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamblet NS, Ragland B, Ali M, Conyers B, Castora FJ. Mutations in mitochondrial-encoded cytochrome c oxidase subunits I, II, and III genes detected in Alzheimer’s disease using single-strand conformation polymorphism. Electrophoresis. 2006 Feb;27(2):398–408. doi: 10.1002/elps.200500420. [DOI] [PubMed] [Google Scholar]
- 22.Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic Biol Med. 2014 Jun;71:390–401. doi: 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Massa V, Fernandez-Vizarra E, Alshahwan S, Bakhsh E, Goffrini P, Ferrero I, Mereghetti P, D’Adamo P, Gasparini P, Zeviani M. Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am J Hum Genet. 2008 Jun;82(6):1281–9. doi: 10.1016/j.ajhg.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamiya G, Makino S, Hayashi M, Abe A, Numakura C, Ueki M, Tanaka A, Ito C, Toshimori K, Ogawa N, et al. A mutation of COX6A1 causes a recessive axonal or mixed form of Charcot-Marie-Tooth disease. Am J Hum Genet. 2014 Sep 4;95(3):294–300. doi: 10.1016/j.ajhg.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Indrieri A, van Rahden VA, Tiranti V, Morleo M, Iaconis D, Tammaro R, D’Amato I, Conte I, Maystadt I, Demuth S, et al. Mutations in COX7B cause microphthalmia with linear skin lesions, an unconventional mitochondrial disease. Am J Hum Genet. 2012 Nov 2;91(5):942–9. doi: 10.1016/j.ajhg.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fornuskova D, Stiburek L, Wenchich L, Vinsova K, Hansikova H, Zeman J. Novel insights into the assembly and function of human nuclear-encoded cytochrome c oxidase subunits 4, 5a, 6a, 7a and 7b. Biochem J. 2010 Jun 15;428(3):363–74. doi: 10.1042/BJ20091714. [DOI] [PubMed] [Google Scholar]
- 27.Vahsen N, Cande C, Briere JJ, Benit P, Joza N, Larochette N, Mastroberardino PG, Pequignot MO, Casares N, Lazar V, et al. AIF deficiency compromises oxidative phosphorylation. Embo J. 2004 Nov 24;23(23):4679–89. doi: 10.1038/sj.emboj.7600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hangen E, Feraud O, Lachkar S, Mou H, Doti N, Fimia GM, Lam NV, Zhu C, Godin I, Muller K, et al. Interaction between AIF and CHCHD4 Regulates Respiratory Chain Biogenesis. Mol Cell. 2015 Jun 18;58(6):1001–14. doi: 10.1016/j.molcel.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Ruzzenente B, Metodiev MD, Wredenberg A, Bratic A, Park CB, Camara Y, Milenkovic D, Zickermann V, Wibom R, Hultenby K, et al. LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J. 2012 Jan 18;31(2):443–56. doi: 10.1038/emboj.2011.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer K, Buettner S, Ghezzi D, Zeviani M, Bano D, Nicotera P. Loss of apoptosis-inducing factor critically affects MIA40 function. Cell Death Dis. 2015;6:e1814. doi: 10.1038/cddis.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu F, Morin C, Mitchell G, Ackerley C, Robinson BH. The role of the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III mRNA. Biochem J. 2004 Aug 15;382(Pt 1):331–6. doi: 10.1042/BJ20040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasarman F, Nishimura T, Antonicka H, Weraarpachai W, Shoubridge EA. Tissue-specific responses to the LRPPRC founder mutation in French Canadian Leigh Syndrome. Hum Mol Genet. 2015 Jan 15;24(2):480–91. doi: 10.1093/hmg/ddu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mourier A, Ruzzenente B, Brandt T, Kuhlbrandt W, Larsson NG. Loss of LRPPRC causes ATP synthase deficiency. Hum Mol Genet. 2014 May 15;23(10):2580–92. doi: 10.1093/hmg/ddt652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonnefoy N, Chalvet F, Hamel P, Slonimski PP, Dujardin G. OXA1, a Saccharomyces cerevisiae nuclear gene whose sequence is conserved from prokaryotes to eukaryotes controls cytochrome oxidase biogenesis. J Mol Biol. 1994 Jun 3;239(2):201–12. doi: 10.1006/jmbi.1994.1363. [DOI] [PubMed] [Google Scholar]
- 35.Brosel S, Yang H, Tanji K, Bonilla E, Schon EA. Unexpected vascular enrichment of SCO1 over SCO2 in mammalian tissues: implications for human mitochondrial disease. Am J Pathol. 2010 Nov;177(5):2541–8. doi: 10.2353/ajpath.2010.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran-Viet KN, Powell C, Barathi VA, Klemm T, Maurer-Stroh S, Limviphuvadh V, Soler V, Ho C, Yanovitch T, Schneider G, et al. Mutations in SCO2 are associated with autosomal-dominant high-grade myopia. Am J Hum Genet. 2013 May 2;92(5):820–6. doi: 10.1016/j.ajhg.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang D, Li J, Xiao X, Li S, Jia X, Sun W, Guo X, Zhang Q. Detection of mutations in LRPAP1, CTSH, LEPREL1, ZNF644, SLC39A5, and SCO2 in 298 families with early-onset high myopia by exome sequencing. Invest Ophthalmol Vis Sci. 2015 Jan;56(1):339–45. doi: 10.1167/iovs.14-14850. [DOI] [PubMed] [Google Scholar]
- 38.Guo Q, Zhang H, Zhang L, He Y, Weng S, Dong Z, Wang J, Zhang P, Nao R. MicroRNA-21 regulates non-small cell lung cancer cell proliferation by affecting cell apoptosis via COX-19. Int J Clin Exp Med. 2015;8(6):8835–41. [PMC free article] [PubMed] [Google Scholar]
- 39.Liu F, Zhang W, You X, Liu Y, Li Y, Wang Z, Wang Y, Zhang X, Ye L. The oncoprotein HBXIP promotes glucose metabolism reprogramming via downregulating SCO2 and PDHA1 in breast cancer. Oncotarget. 2015 Jul 30; doi: 10.18632/oncotarget.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasan S, Guha M, Dong DW, Whelan KA, Ruthel G, Uchikado Y, Natsugoe S, Nakagawa H, Avadhani NG. Disruption of cytochrome c oxidase function induces the Warburg effect and metabolic reprogramming. Oncogene. 2015 Jul 6; doi: 10.1038/onc.2015.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliva CR, Markert T, Gillespie GY, Griguer CE. Nuclear-encoded cytochrome c oxidase subunit 4 regulates BMI1 expression and determines proliferative capacity of high-grade gliomas. Oncotarget. 2015 Feb 28;6(6):4330–44. doi: 10.18632/oncotarget.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen YA, Wang CY, Hsieh YT, Chen YJ, Wei YH. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle. 2015;14(1):86–98. doi: 10.4161/15384101.2014.974419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazat JP, Rossignol R, Malgat M, Rocher C, Faustin B, Letellier T. What do mitochondrial diseases teach us about normal mitochondrial functions…that we already knew: threshold expression of mitochondrial defects. Biochim Biophys Acta. 2001 Mar 1;1504(1):20–30. doi: 10.1016/s0005-2728(00)00236-x. [DOI] [PubMed] [Google Scholar]
- 44.Gusdon AM, Fernandez-Bueno GA, Wohlgemuth S, Fernandez J, Chen J, Mathews CE. Respiration and substrate transport rates as well as reactive oxygen species production distinguish mitochondria from brain and liver. BMC Biochem. 2015;16(1):22. doi: 10.1186/s12858-015-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turnbull DM, Rustin P. Genetic and biochemical intricacy shapes mitochondrial cytopathies. Neurobiol Dis. 2015 Feb 12; doi: 10.1016/j.nbd.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Benit P, El-Khoury R, Schiff M, Sainsard-Chanet A, Rustin P. Genetic background influences mitochondrial function: modeling mitochondrial disease for therapeutic development. Trends Mol Med. 2010 May;16(5):210–7. doi: 10.1016/j.molmed.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Gil Borlado MC, Moreno Lastres D, Gonzalez Hoyuela M, Moran M, Blazquez A, Pello R, Marin Buera L, Gabaldon T, Garcia Penas JJ, Martin MA, et al. Impact of the mitochondrial genetic background in complex III deficiency. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–3. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 49.Bourgeron T, Chretien D, Rotig A, Munnich A, Rustin P. Fate and expression of the deleted mitochondrial DNA differ between human heteroplasmic skin fibroblast and Epstein-Barr virus-transformed lymphocyte cultures. J Biol Chem. 1993 Sep 15;268(26):19369–76. [PubMed] [Google Scholar]
- 50.Bastin J, Aubey F, Rotig A, Munnich A, Djouadi F. Activation of peroxisome proliferator-activated receptor pathway stimulates the mitochondrial respiratory chain and can correct deficiencies in patients’ cells lacking its components. J Clin Endocrinol Metab. 2008 Apr;93(4):1433–41. doi: 10.1210/jc.2007-1701. [DOI] [PubMed] [Google Scholar]
- 51.Rustin P, Jacobs HT. Respiratory chain alternative enzymes as tools to better understand and counteract respiratory chain deficiencies in human cells and animals. Physiol Plant. 2009 Dec;137(4):362–70. doi: 10.1111/j.1399-3054.2009.01249.x. [DOI] [PubMed] [Google Scholar]
- 52.Dassa EP, Dufour E, Goncalves S, Paupe V, Hakkaart GA, Jacobs HT, Rustin P. Expression of the alternative oxidase complements cytochrome c oxidase deficiency in human cells. EMBO Mol Med. 2009 Apr;1(1):30–6. doi: 10.1002/emmm.200900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kemppainen KK, Rinne J, Sriram A, Lakanmaa M, Zeb A, Tuomela T, Popplestone A, Singh S, Sanz A, Rustin P, et al. Expression of alternative oxidase in Drosophila ameliorates diverse phenotypes due to cytochrome oxidase deficiency. Hum Mol Genet. 2014 Apr 15;23(8):2078–93. doi: 10.1093/hmg/ddt601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaignard P, Menezes M, Schiff M, Bayot A, Rak M, Ogier de Baulny H, Su CH, Gilleron M, Lombes A, Abida H, et al. Mutations in CYC1, encoding cytochrome c1 subunit of respiratory chain complex III, cause insulin-responsive hyperglycemia. Am J Hum Genet. 2013 Aug 8;93(2):384–9. doi: 10.1016/j.ajhg.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrientos A, Zambrano A, Tzagoloff A. Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J. 2004 Sep 1;23(17):3472–82. doi: 10.1038/sj.emboj.7600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pierrel F, Khalimonchuk O, Cobine PA, Bestwick M, Winge DR. Coa2 is an assembly factor for yeast cytochrome c oxidase biogenesis that facilitates the maturation of Cox1. Mol Cell Biol. 2008 Aug;28(16):4927–39. doi: 10.1128/MCB.00057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mick DU, Fox TD, Rehling P. Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation. Nat Rev Mol Cell Biol. 2011 Jan;12(1):14–20. doi: 10.1038/nrm3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W, Gnanasambandam R, Benjamin J, Kaur G, Getman PB, Siegel AJ, Shortridge RD, Singh S. Mutations in cytochrome c oxidase subunit VIa cause neurodegeneration and motor dysfunction in Drosophila. Genetics. 2007 Jun;176(2):937–46. doi: 10.1534/genetics.107.071688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zordan MA, Cisotto P, Benna C, Agostino A, Rizzo G, Piccin A, Pegoraro M, Sandrelli F, Perini G, Tognon G, et al. Post-transcriptional silencing and functional characterization of the Drosophila melanogaster homolog of human Surf1. Genetics. 2006 Jan;172(1):229–41. doi: 10.1534/genetics.105.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suthammarak W, Yang YY, Morgan PG, Sedensky MM. Complex I function is defective in complex IV-deficient Caenorhabditis elegans. J Biol Chem. 2009 Mar 6;284(10):6425–35. doi: 10.1074/jbc.M805733200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baden KN, Murray J, Capaldi RA, Guillemin K. Early developmental pathology due to cytochrome c oxidase deficiency is revealed by a new zebrafish model. J Biol Chem. 2007 Nov 30;282(48):34839–49. doi: 10.1074/jbc.M703528200. [DOI] [PubMed] [Google Scholar]
- 62.Fan W, Waymire KG, Narula N, Li P, Rocher C, Coskun PE, Vannan MA, Narula J, Macgregor GR, Wallace DC. A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science. 2008 Feb 15;319(5865):958–62. doi: 10.1126/science.1147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huttemann M, Lee I, Gao X, Pecina P, Pecinova A, Liu J, Aras S, Sommer N, Sanderson TH, Tost M, et al. Cytochrome c oxidase subunit 4 isoform 2-knockout mice show reduced enzyme activity, airway hyporeactivity, and lung pathology. FASEB J. 2012 Sep;26(9):3916–30. doi: 10.1096/fj.11-203273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shteyer E, Saada A, Shaag A, Al-Hijawi FA, Kidess R, Revel-Vilk S, Elpeleg O. Exocrine pancreatic insufficiency, dyserythropoeitic anemia, and calvarial hyperostosis are caused by a mutation in the COX4I2 gene. Am J Hum Genet. 2009 Mar;84(3):412–7. doi: 10.1016/j.ajhg.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radford NB, Wan B, Richman A, Szczepaniak LS, Li JL, Li K, Pfeiffer K, Schagger H, Garry DJ, Moreadith RW. Cardiac dysfunction in mice lacking cytochrome-c oxidase subunit VIaH. Am J Physiol Heart Circ Physiol. 2002 Feb;282(2):H726–33. doi: 10.1152/ajpheart.00308.2001. [DOI] [PubMed] [Google Scholar]
- 66.Lee I, Huttemann M, Liu J, Grossman LI, Malek MH. Deletion of heart-type cytochrome c oxidase subunit 7a1 impairs skeletal muscle angiogenesis and oxidative phosphorylation. J Physiol. 2012 Oct 15;590(Pt 20):5231–43. doi: 10.1113/jphysiol.2012.239707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huttemann M, Klewer S, Lee I, Pecinova A, Pecina P, Liu J, Lee M, Doan JW, Larson D, Slack E, et al. Mice deleted for heart-type cytochrome c oxidase subunit 7a1 develop dilated cardiomyopathy. Mitochondrion. 2012 Mar;12(2):294–304. doi: 10.1016/j.mito.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diaz F, Garcia S, Hernandez D, Regev A, Rebelo A, Oca-Cossio J, Moraes CT. Pathophysiology and fate of hepatocytes in a mouse model of mitochondrial hepatopathies. Gut. 2008 Feb;57(2):232–42. doi: 10.1136/gut.2006.119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diaz F, Thomas CK, Garcia S, Hernandez D, Moraes CT. Mice lacking COX10 in skeletal muscle recapitulate the phenotype of progressive mitochondrial myopathies associated with cytochrome c oxidase deficiency. Hum Mol Genet. 2005 Sep 15;14(18):2737–48. doi: 10.1093/hmg/ddi307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noe N, Dillon L, Lellek V, Diaz F, Hida A, Moraes CT, Wenz T. Bezafibrate improves mitochondrial function in the CNS of a mouse model of mitochondrial encephalopathy. Mitochondrion. 2013 Sep;13(5):417–26. doi: 10.1016/j.mito.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang H, Brosel S, Acin-Perez R, Slavkovich V, Nishino I, Khan R, Goldberg IJ, Graziano J, Manfredi G, Schon EA. Analysis of mouse models of cytochrome c oxidase deficiency owing to mutations in Sco2. Hum Mol Genet. 2010 Jan 1;19(1):170–80. doi: 10.1093/hmg/ddp477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Acin-Perez R, Salazar E, Brosel S, Yang H, Schon EA, Manfredi G. Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol Med. 2009 Nov;1(8–9):392–406. doi: 10.1002/emmm.200900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mallipattu SK, Horne SJ, D’Agati V, Narla G, Liu R, Frohman MA, Dickman K, Chen EY, Ma’ayan A, Bialkowska AB, et al. Kruppel-like factor 6 regulates mitochondrial function in the kidney. J Clin Invest. 2015 Mar 2;125(3):1347–61. doi: 10.1172/JCI77084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bénit P, Goncalves S, Dassa EP, Brière JJ, Rustin P. The variability of the Harlequin mouse phenotype resembles that of human mitochondrial-complex I-deficiency syndromes. Plos One. 2008;3:e3208. doi: 10.1371/journal.pone.0003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature. 2002 Sep 26;419(6905):367–74. doi: 10.1038/nature01034. [DOI] [PubMed] [Google Scholar]
- 76.Barrientos A, Barros MH, Valnot I, Rotig A, Rustin P, Tzagoloff A. Cytochrome oxidase in health and disease. Gene. 2002 Mar 6;286(1):53–63. doi: 10.1016/s0378-1119(01)00803-4. [DOI] [PubMed] [Google Scholar]
- 77.Goldenberg PC, Steiner RD, Merkens LS, Dunaway T, Egan RA, Zimmerman EA, Nesbit G, Robinson B, Kennaway NG. Remarkable improvement in adult Leigh syndrome with partial cytochrome c oxidase deficiency. Neurology. 2003 Mar 11;60(5):865–8. doi: 10.1212/01.wnl.0000049460.72439.7f. [DOI] [PubMed] [Google Scholar]
- 78.Uusimaa J, Jungbluth H, Fratter C, Crisponi G, Feng L, Zeviani M, Hughes I, Treacy EP, Birks J, Brown GK, et al. Reversible infantile respiratory chain deficiency is a unique, genetically heterogenous mitochondrial disease. J Med Genet. 2011 Oct;48(10):660–8. doi: 10.1136/jmg.2011.089995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bacman SR, Williams SL, Pinto M, Peralta S, Moraes CT. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med. 2013 Sep;19(9):1111–3. doi: 10.1038/nm.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meyerson C, Van Stavern G, McClelland C. Leber hereditary optic neuropathy: current perspectives. Clin Ophthalmol. 2015;9:1165–76. doi: 10.2147/OPTH.S62021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ellouze S, Augustin S, Bouaita A, Bonnet C, Simonutti M, Forster V, Picaud S, Sahel JA, Corral-Debrinski M. Optimized Allotopic Expression of the Human Mitochondrial ND4 Prevents Blindness in a Rat Model of Mitochondrial Dysfunction. Am J Hum Genet. 2008 Sep 3; doi: 10.1016/j.ajhg.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cwerman-Thibault H, Augustin S, Lechauve C, Ayache J, Ellouze S, Sahel JA, Corral-Debrinski M. Nuclear expression of mitochondrial ND4 leads to the protein assembling in complex I and prevents optic atrophy and visual loss. Mol Ther Methods Clin Dev. 2015;2:15003. doi: 10.1038/mtm.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koilkonda RD, Yu H, Chou TH, Feuer WJ, Ruggeri M, Porciatti V, Tse D, Hauswirth WW, Chiodo V, Boye SL, et al. Safety and effects of the vector for the Leber hereditary optic neuropathy gene therapy clinical trial. JAMA Ophthalmol. 2014 Apr 1;132(4):409–20. doi: 10.1001/jamaophthalmol.2013.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dimauro S, Rustin P. A critical approach to the therapy of mitochondrial respiratory chain and oxidative phosphorylation diseases. Biochim Biophys Acta. 2008 Nov 5;1792:1159–67. doi: 10.1016/j.bbadis.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 85.El-Hattab AW, Adesina AM, Jones J, Scaglia F. MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab. 2015 Jun 15; doi: 10.1016/j.ymgme.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 86.Chabrol B, Mancini J, Chretien D, Rustin P, Munnich A, Pinsard N. Valproate-induced hepatic failure in a case of cytochrome c oxidase deficiency. Eur J Pediatr. 1994 Feb;153(2):133–5. doi: 10.1007/BF01959226. [DOI] [PubMed] [Google Scholar]
- 87.Berger I, Segal I, Shmueli D, Saada A. The effect of antiepileptic drugs on mitochondrial activity: a pilot study. J Child Neurol. 2010 May;25(5):541–5. doi: 10.1177/0883073809352888. [DOI] [PubMed] [Google Scholar]
- 88.Geromel V, Kadhom N, Cebalos-Picot I, Ouari O, Polidori A, Munnich A, Rotig A, Rustin P. Superoxide-induced massive apoptosis in cultured skin fibroblasts harboring the neurogenic ataxia retinitis pigmentosa (NARP) mutation in the ATPase-6 gene of the mitochondrial DNA. Hum Mol Genet. 2001 May 15;10(11):1221–8. doi: 10.1093/hmg/10.11.1221. [DOI] [PubMed] [Google Scholar]
- 89.Diaz F, Garcia S, Padgett KR, Moraes CT. A defect in the mitochondrial complex III, but not complex IV, triggers early ROS-dependent damage in defined brain regions. Hum Mol Genet. 2012 Dec 1;21(23):5066–77. doi: 10.1093/hmg/dds350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Casarin A, Giorgi G, Pertegato V, Siviero R, Cerqua C, Doimo M, Basso G, Sacconi S, Cassina M, Rizzuto R, et al. Copper and bezafibrate cooperate to rescue cytochrome c oxidase deficiency in cells of patients with SCO2 mutations. Orphanet J Rare Dis. 2012;7:21. doi: 10.1186/1750-1172-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viscomi C, Bottani E, Civiletto G, Cerutti R, Moggio M, Fagiolari G, Schon EA, Lamperti C, Zeviani M. In Vivo Correction of COX Deficiency by Activation of the AMPK/PGC-1alpha Axis. Cell Metab. 2011 Jul 6;14(1):80–90. doi: 10.1016/j.cmet.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gray KA, Daugherty LC, Gordon SM, Seal RL, Wright MW, Bruford EA. Genenames.org: the HGNC resources in 2013. Nucleic Acids Res. 2013 Jan;41(Database issue):D545–52. doi: 10.1093/nar/gks1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lamperti C, Diodato D, Lamantea E, Carrara F, Ghezzi D, Mereghetti P, Rizzi R, Zeviani M. MELAS-like encephalomyopathy caused by a new pathogenic mutation in the mitochondrial DNA encoded cytochrome c oxidase subunit I. Neuromuscul Disord. 2012 Nov;22(11):990–4. doi: 10.1016/j.nmd.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 94.Valente L, Piga D, Lamantea E, Carrara F, Uziel G, Cudia P, Zani A, Farina L, Morandi L, Mora M, et al. Identification of novel mutations in five patients with mitochondrial encephalomyopathy. Biochim Biophys Acta. 2009 May;1787(5):491–501. doi: 10.1016/j.bbabio.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 95.Kollberg G, Moslemi AR, Lindberg C, Holme E, Oldfors A. Mitochondrial myopathy and rhabdomyolysis associated with a novel nonsense mutation in the gene encoding cytochrome c oxidase subunit I. J Neuropathol Exp Neurol. 2005 Feb;64(2):123–8. doi: 10.1093/jnen/64.2.123. [DOI] [PubMed] [Google Scholar]
- 96.Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci U S A. 2005 Jan 18;102(3):719–24. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karadimas CL, Greenstein P, Sue CM, Joseph JT, Tanji K, Haller RG, Taivassalo T, Davidson MM, Shanske S, Bonilla E, et al. Recurrent myoglobinuria due to a nonsense mutation in the COX I gene of mitochondrial DNA. Neurology. 2000 Sep 12;55(5):644–9. doi: 10.1212/wnl.55.5.644. [DOI] [PubMed] [Google Scholar]
- 98.Comi GP, Bordoni A, Salani S, Franceschina L, Sciacco M, Prelle A, Fortunato F, Zeviani M, Napoli L, Bresolin N, et al. Cytochrome c oxidase subunit I microdeletion in a patient with motor neuron disease. Ann Neurol. 1998 Jan;43(1):110–6. doi: 10.1002/ana.410430119. [DOI] [PubMed] [Google Scholar]
- 99.Pereira L, Soares P, Radivojac P, Li B, Samuels DC. Comparing phylogeny and the predicted pathogenicity of protein variations reveals equal purifying selection across the global human mtDNA diversity. Am J Hum Genet. 2011 Apr 8;88(4):433–9. doi: 10.1016/j.ajhg.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Varlamov DA, Kudin AP, Vielhaber S, Schroder R, Sassen R, Becker A, Kunz D, Haug K, Rebstock J, Heils A, et al. Metabolic consequences of a novel missense mutation of the mtDNA CO I gene. Hum Mol Genet. 2002 Aug 1;11(16):1797–805. doi: 10.1093/hmg/11.16.1797. [DOI] [PubMed] [Google Scholar]
- 101.Gattermann N, Retzlaff S, Wang YL, Hofhaus G, Heinisch J, Aul C, Schneider W. Heteroplasmic point mutations of mitochondrial DNA affecting subunit I of cytochrome c oxidase in two patients with acquired idiopathic sideroblastic anemia. Blood. 1997 Dec 15;90(12):4961–72. [PubMed] [Google Scholar]
- 102.D’Aurelio M, Pallotti F, Barrientos A, Gajewski CD, Kwong JQ, Bruno C, Beal MF, Manfredi G. In vivo regulation of oxidative phosphorylation in cells harboring a stop-codon mutation in mitochondrial DNA-encoded cytochrome c oxidase subunit I. J Biol Chem. 2001 Dec 14;276(50):46925–32. doi: 10.1074/jbc.M106429200. [DOI] [PubMed] [Google Scholar]
- 103.Nishigaki Y, Ueno H, Coku J, Koga Y, Fujii T, Sahashi K, Nakano K, Yoneda M, Nonaka M, Tang L, et al. Extensive screening system using suspension array technology to detect mitochondrial DNA point mutations. Mitochondrion. 2010 Apr;10(3):300–8. doi: 10.1016/j.mito.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clark KM, Taylor RW, Johnson MA, Chinnery PF, Chrzanowska-Lightowlers ZM, Andrews RM, Nelson IP, Wood NW, Lamont PJ, Hanna MG, et al. An mtDNA mutation in the initiation codon of the cytochrome C oxidase subunit II gene results in lower levels of the protein and a mitochondrial encephalomyopathy. Am J Hum Genet. 1999 May;64(5):1330–9. doi: 10.1086/302361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abu-Amero KK, Bosley TM. Mitochondrial abnormalities in patients with LHON-like optic neuropathies. Invest Ophthalmol Vis Sci. 2006 Oct;47(10):4211–20. doi: 10.1167/iovs.06-0295. [DOI] [PubMed] [Google Scholar]
- 106.Rahman S, Taanman JW, Cooper JM, Nelson I, Hargreaves I, Meunier B, Hanna MG, Garcia JJ, Capaldi RA, Lake BD, et al. A missense mutation of cytochrome oxidase subunit II causes defective assembly and myopathy. Am J Hum Genet. 1999 Oct;65(4):1030–9. doi: 10.1086/302590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei YL, Yu CA, Yang P, Li AL, Wen JY, Zhao SM, Liu HX, Ke YN, Campbell W, Zhang YG, et al. Novel mitochondrial DNA mutations associated with Chinese familial hypertrophic cardiomyopathy. Clin Exp Pharmacol Physiol. 2009 Sep;36(9):933–9. doi: 10.1111/j.1440-1681.2009.05183.x. [DOI] [PubMed] [Google Scholar]
- 108.Uusimaa J, Finnila S, Vainionpaa L, Karppa M, Herva R, Rantala H, Hassinen IE, Majamaa K. A mutation in mitochondrial DNA-encoded cytochrome c oxidase II gene in a child with Alpers-Huttenlocher-like disease. Pediatrics. 2003 Mar;111(3):e262–8. doi: 10.1542/peds.111.3.e262. [DOI] [PubMed] [Google Scholar]
- 109.Horvath R, Schoser BG, Muller-Hocker J, Volpel M, Jaksch M, Lochmuller H. Mutations in mtDNA-encoded cytochrome c oxidase subunit genes causing isolated myopathy or severe encephalomyopathy. Neuromuscul Disord. 2005 Dec;15(12):851–7. doi: 10.1016/j.nmd.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 110.Abu-Amero KK, Bosley TM, Morales J. Analysis of nuclear and mitochondrial genes in patients with pseudoexfoliation glaucoma. Mol Vis. 2008;14:29–36. [PMC free article] [PubMed] [Google Scholar]
- 111.Siwar BG, Myriam G, Afif BM, Emna MR, Nozha C, Afifa S, Faiza F, Leila AK. Two novel mutations in COII and tRNA(His) mitochondrial genes in asthenozoospermic infertiles men. Biochem Biophys Res Commun. 2014 Jul 18;450(1):610–5. doi: 10.1016/j.bbrc.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 112.McFarland R, Taylor RW, Chinnery PF, Howell N, Turnbull DM. A novel sporadic mutation in cytochrome c oxidase subunit II as a cause of rhabdomyolysis. Neuromuscul Disord. 2004 Feb;14(2):162–6. doi: 10.1016/j.nmd.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 113.Tabebi M, Mkaouar-Rebai E, Mnif M, Kallabi F, Ben Mahmoud A, Ben Saad W, Charfi N, Keskes-Ammar L, Kamoun H, Abid M, et al. A novel mutation MT-COIII m.9267G>C and MT-COI m.5913G>A mutation in mitochondrial genes in a Tunisian family with maternally inherited diabetes and deafness (MIDD) associated with severe nephropathy. Biochem Biophys Res Commun. 2015 Apr 10;459(3):353–60. doi: 10.1016/j.bbrc.2015.01.151. [DOI] [PubMed] [Google Scholar]
- 114.Horvath R, Scharfe C, Hoeltzenbein M, Do BH, Schroder C, Warzok R, Vogelgesang S, Lochmuller H, Muller-Hocker J, Gerbitz KD, et al. Childhood onset mitochondrial myopathy and lactic acidosis caused by a stop mutation in the mitochondrial cytochrome c oxidase III gene. J Med Genet. 2002 Nov;39(11):812–6. doi: 10.1136/jmg.39.11.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baklouti-Gargouri S, Ghorbel M, Ben Mahmoud A, Mkaouar-Rebai E, Cherif M, Chakroun N, Sellami A, Fakhfakh F, Ammar-Keskes L. Mitochondrial DNA mutations and polymorphisms in asthenospermic infertile men. Mol Biol Rep. 2013 Aug;40(8):4705–12. doi: 10.1007/s11033-013-2566-7. [DOI] [PubMed] [Google Scholar]
- 116.Mkaouar-Rebai E, Ellouze E, Chamkha I, Kammoun F, Triki C, Fakhfakh F. Molecular-clinical correlation in a family with a novel heteroplasmic Leigh syndrome missense mutation in the mitochondrial cytochrome c oxidase III gene. J Child Neurol. 2011 Jan;26(1):12–20. doi: 10.1177/0883073810371227. [DOI] [PubMed] [Google Scholar]
- 117.Figueroa-Martinez F, Vazquez-Acevedo M, Cortes-Hernandez P, Garcia-Trejo JJ, Davidson E, King MP, Gonzalez-Halphen D. What limits the allotopic expression of nucleus-encoded mitochondrial genes? The case of the chimeric Cox3 and Atp6 genes. Mitochondrion. 2011 Jan;11(1):147–54. doi: 10.1016/j.mito.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 118.Bosley TM, Brodsky MC, Glasier CM, Abu-Amero KK. Sporadic bilateral optic neuropathy in children: the role of mitochondrial abnormalities. Invest Ophthalmol Vis Sci. 2008 Dec;49(12):5250–6. doi: 10.1167/iovs.08-2193. [DOI] [PubMed] [Google Scholar]
- 119.Marotta R, Chin J, Kirby DM, Chiotis M, Cook M, Collins SJ. Novel single base pair COX III subunit deletion of mitochondrial DNA associated with rhabdomyolysis. J Clin Neurosci. 2011 Feb;18(2):290–2. doi: 10.1016/j.jocn.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 120.Hanna MG, Nelson IP, Rahman S, Lane RJ, Land J, Heales S, Cooper MJ, Schapira AH, Morgan-Hughes JA, Wood NW. Cytochrome c oxidase deficiency associated with the first stop-codon point mutation in human mtDNA. Am J Hum Genet. 1998 Jul;63(1):29–36. doi: 10.1086/301910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lenaz G, Baracca A, Carelli V, D’Aurelio M, Sgarbi G, Solaini G. Bioenergetics of mitochondrial diseases associated with mtDNA mutations. Biochim Biophys Acta. 2004 Jul 23;1658(1–2):89–94. doi: 10.1016/j.bbabio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 122.Levin L, Zhidkov I, Gurman Y, Hawlena H, Mishmar D. Functional recurrent mutations in the human mitochondrial phylogeny: dual roles in evolution and disease. Genome Biol Evol. 2013;5(5):876–90. doi: 10.1093/gbe/evt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vondrackova A, Vesela K, Hansikova H, Docekalova DZ, Rozsypalova E, Zeman J, Tesarova M. High-resolution melting analysis of 15 genes in 60 patients with cytochrome-c oxidase deficiency. J Hum Genet. 2012 Jul;57(7):442–8. doi: 10.1038/jhg.2012.49. [DOI] [PubMed] [Google Scholar]
- 124.Ghezzi D, Sevrioukova I, Invernizzi F, Lamperti C, Mora M, D’Adamo P, Novara F, Zuffardi O, Uziel G, Zeviani M. Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor. Am J Hum Genet. 2010 Apr 9;86(4):639–49. doi: 10.1016/j.ajhg.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berger I, Ben-Neriah Z, Dor-Wolman T, Shaag A, Saada A, Zenvirt S, Raas-Rothschild A, Nadjari M, Kaestner KH, Elpeleg O. Early prenatal ventriculomegaly due to an AIFM1 mutation identified by linkage analysis and whole exome sequencing. Mol Genet Metab. 2011 Dec;104(4):517–20. doi: 10.1016/j.ymgme.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 126.Kettwig M, Schubach M, Zimmermann FA, Klinge L, Mayr JA, Biskup S, Sperl W, Gartner J, Huppke P. From ventriculomegaly to severe muscular atrophy: expansion of the clinical spectrum related to mutations in AIFM1. Mitochondrion. 2015 Mar;21:12–8. doi: 10.1016/j.mito.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 127.Diodato D, Tasca G, Verrigni D, D’Amico A, Rizza T, Tozzi G, Martinelli D, Verardo M, Invernizzi F, Nasca A, et al. A novel AIFM1 mutation expands the phenotype to an infantile motor neuron disease. Eur J Hum Genet. 2015 Jul 15; doi: 10.1038/ejhg.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ostergaard E, Weraarpachai W, Ravn K, Born AP, Jonson L, Duno M, Wibrand F, Shoubridge EA, Vissing J. Mutations in COA3 cause isolated complex IV deficiency associated with neuropathy, exercise intolerance, obesity, and short stature. J Med Genet. 2015 Mar;52(3):203–7. doi: 10.1136/jmedgenet-2014-102914. [DOI] [PubMed] [Google Scholar]
- 129.Huigsloot M, Nijtmans LG, Szklarczyk R, Baars MJ, van den Brand MA, Hendriksfranssen MG, van den Heuvel LP, Smeitink JA, Huynen MA, Rodenburg RJ. A mutation in C2orf64 causes impaired cytochrome c oxidase assembly and mitochondrial cardiomyopathy. Am J Hum Genet. 2011 Apr 8;88(4):488–93. doi: 10.1016/j.ajhg.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Valnot I, von Kleist-Retzow JC, Barrientos A, Gorbatyuk M, Taanman JW, Mehaye B, Rustin P, Tzagoloff A, Munnich A, Rotig A. A mutation in the human heme A:farnesyltransferase gene (COX10) causes cytochrome c oxidase deficiency. Hum Mol Genet. 2000 May 1;9(8):1245–9. doi: 10.1093/hmg/9.8.1245. [DOI] [PubMed] [Google Scholar]
- 131.Antonicka H, Leary SC, Guercin GH, Agar JN, Horvath R, Kennaway NG, Harding CO, Jaksch M, Shoubridge EA. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum Mol Genet. 2003 Oct 15;12(20):2693–702. doi: 10.1093/hmg/ddg284. [DOI] [PubMed] [Google Scholar]
- 132.Antonicka H, Mattman A, Carlson CG, Glerum DM, Hoffbuhr KC, Leary SC, Kennaway NG, Shoubridge EA. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am J Hum Genet. 2003 Jan;72(1):101–14. doi: 10.1086/345489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oquendo CE, Antonicka H, Shoubridge EA, Reardon W, Brown GK. Functional and genetic studies demonstrate that mutation in the COX15 gene can cause Leigh syndrome. J Med Genet. 2004 Jul;41(7):540–4. doi: 10.1136/jmg.2003.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Szklarczyk R, Wanschers BF, Nijtmans LG, Rodenburg RJ, Zschocke J, Dikow N, van den Brand MA, Hendriks-Franssen MG, Gilissen C, Veltman JA, et al. A mutation in the FAM36A gene, the human ortholog of COX20, impairs cytochrome c oxidase assembly and is associated with ataxia and muscle hypotonia. Hum Mol Genet. 2013 Feb 15;22(4):656–67. doi: 10.1093/hmg/dds473. [DOI] [PubMed] [Google Scholar]
- 135.Ghezzi D, Saada A, D’Adamo P, Fernandez-Vizarra E, Gasparini P, Tiranti V, Elpeleg O, Zeviani M. FASTKD2 nonsense mutation in an infantile mitochondrial encephalomyopathy associated with cytochrome c oxidase deficiency. Am J Hum Genet. 2008 Sep;83(3):415–23. doi: 10.1016/j.ajhg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Olahova M, Haack TB, Alston CL, Houghton JA, He L, Morris AA, Brown GK, McFarland R, Chrzanowska-Lightowlers ZM, Lightowlers RN, et al. A truncating PET100 variant causing fatal infantile lactic acidosis and isolated cytochrome c oxidase deficiency. Eur J Hum Genet. 2015 Jul;23(7):935–9. doi: 10.1038/ejhg.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lim SC, Smith KR, Stroud DA, Compton AG, Tucker EJ, Dasvarma A, Gandolfo LC, Marum JE, McKenzie M, Peters HL, et al. A founder mutation in PET100 causes isolated complex IV deficiency in Lebanese individuals with Leigh syndrome. Am J Hum Genet. 2014 Feb 6;94(2):209–22. doi: 10.1016/j.ajhg.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Valnot I, Osmond S, Gigarel N, Mehaye B, Amiel J, Cormier-Daire V, Munnich A, Bonnefont JP, Rustin P, Rotig A. Mutations of the SCO1 gene in mitochondrial cytochrome c oxidase deficiency with neonatal-onset hepatic failure and encephalopathy. Am J Hum Genet. 2000 Nov;67(5):1104–9. doi: 10.1016/s0002-9297(07)62940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Papadopoulou LC, Sue CM, Davidson MM, Tanji K, Nishino I, Sadlock JE, Krishna S, Walker W, Selby J, Glerum DM, et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet. 1999 Nov;23(3):333–7. doi: 10.1038/15513. [DOI] [PubMed] [Google Scholar]
- 140.Tiranti V, Hoertnagel K, Carrozzo R, Galimberti C, Munaro M, Granatiero M, Zelante L, Gasparini P, Marzella R, Rocchi M, et al. Mutations of SURF-1 in Leigh disease associated with cytochrome c oxidase deficiency. Am J Hum Genet. 1998 Dec;63(6):1609–21. doi: 10.1086/302150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Von Kleist-Retzow JC, Yao J, Taanman JW, Chantrel K, Chretien D, Cormier-Daire V, Rotig A, Munnich A, Rustin P, Shoubridge EA. Mutations in SURF1 are not specifically associated with Leigh syndrome. J Med Genet. 2001 Feb;38(2):109–13. doi: 10.1136/jmg.38.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weraarpachai W, Antonicka H, Sasarman F, Seeger J, Schrank B, Kolesar JE, Lochmuller H, Chevrette M, Kaufman BA, Horvath R, et al. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat Genet. 2009 Jul;41(7):833–7. doi: 10.1038/ng.390. [DOI] [PubMed] [Google Scholar]
- 143.Orr AL, Ashok D, Sarantos MR, Ng R, Shi T, Gerencser AA, Hughes RE, Brand MD. Novel inhibitors of mitochondrial sn-glycerol 3-phosphate dehydrogenase. PLoS One. 2014;9(2):e89938. doi: 10.1371/journal.pone.0089938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rehling P, Brandner K, Pfanner N. Mitochondrial import and the twin-pore translocase. Nat Rev Mol Cell Biol. 2004 Jul;5(7):519–30. doi: 10.1038/nrm1426. [DOI] [PubMed] [Google Scholar]
- 145.Mick DU, Dennerlein S, Wiese H, Reinhold R, Pacheu-Grau D, Lorenzi I, Sasarman F, Weraarpachai W, Shoubridge EA, Warscheid B, et al. MITRAC links mitochondrial protein translocation to respiratory-chain assembly and translational regulation. Cell. 2012 Dec 21;151(7):1528–41. doi: 10.1016/j.cell.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 146.Dennerlein S, Oeljeklaus S, Jans D, Hellwig C, Bareth B, Jakobs S, Deckers M, Warscheid B, Rehling P. MITRAC7 Acts as a COX1-Specific Chaperone and Reveals a Checkpoint during Cytochrome c Oxidase Assembly. Cell Rep. 2015 Sep 8;12(10):1644–55. doi: 10.1016/j.celrep.2015.08.009. [DOI] [PubMed] [Google Scholar]