Abstract
Malignant peripheral nerve sheath tumors (MPNSTs) are aggressive sarcomas that can show overlapping features with benign neurofibromas as well as high-grade sarcomas. Additional diagnostic markers are needed to aid in this often challenging differential diagnosis.
Recently mutations in two critical components of the polycomb repressor 2 (PRC2) complex, SUZ12 and EED, were reported to occur specifically in MPNSTs while such mutations are absent in neurofibromas, both in the setting of neurofibromatosis (NF) and sporadic cases. Furthermore, both SUZ12 and EED mutations in MPNSTs were associated with loss of H3K27 tri-methylation, a downstream target of PRC2. Therefore we tested whether H3K27me3 immunohistochemistry is useful as a diagnostic and prognostic marker for MPNSTs.
We performed H3K27me3 immunohistochemistry in 162 primary MPNSTs, 97 neurofibromas and 341 other tumors using tissue microarray.
We observed loss of H3K27me3 in 34% (55/162) of all MPNSTs while expression was retained in all neurofibromas including atypical (n=8) and plexiform subtypes (n=24). Within other tumors we detected loss of H3K27me3 in only 7% (24/341). Surprisingly, 60% (9/15) of synovial sarcomas and 38% (3/8) of fibrosarcomatous dermatofibrosarcoma protuberans (DFSP) showed loss of H3K27 trimethylation. Only 1 out of 44 schwannomas showed loss of H3K27me3 and all 4 perineuriomas showed intact H3K27me3. Furthermore, MPNSTs with loss of H3K27 tri-methylation showed inferior survival compared to MPNSTs with intact H3K27 tri-methylation, which was validated in two independent cohorts.
Our results indicate that H3K27me3 immunohistochemistry is useful as a diagnostic marker in which loss of H3K27me3 favours MPNST above neurofibroma. However H3K27me3 immunohistochemistry is not suitable to distinguish MPNST from its morphological mimicker synovial sarcoma or fibrosarcomatous DFSP. Since loss of H3K27 tri-methylation was related to poorer survival in MPNST, chromatin modification mediated by this specific histone seems to orchestrate more aggressive tumour biology.
Introduction
Malignant peripheral nerve sheath tumors most commonly arise in the extremities and account for approximately 5-10% of soft tissue sarcomas. (1) Most malignant peripheral nerve sheath tumors are high grade sarcomas, with a high probability of producing local recurrence and distant metastasis. The prognosis is therefore still very poor, despite extensive oncologic surgery, often with adjuvant radiation or chemotherapy. (2;3)
Establishing the diagnosis of malignant peripheral nerve sheath tumor can be difficult, due to significant morphological overlap with other tumors such as cellular or atypical neurofibroma or high-grade sarcomas like synovial sarcoma, fibrosarcomatous dermatofibrosarcoma protuberans, myxofibrosarcoma, or undifferentiated pleomorphic or spindle cell sarcoma. In addition to S100 protein, SOX10 and p16 as immunohistochemical tools, no other easily applicable markers are available that are specific for malignant peripheral nerve sheath tumors. (4-7) Therefore, additional diagnostic markers are urgently needed to aid in this often difficult differential diagnosis.
About half of all malignant peripheral nerve sheath tumors arise in patients with the hereditary syndrome neurofibromatosis. Moreover, approximately 10% are radiation induced and the remainder affect individuals without a known genetic predisposition. (1) Neurofibromas in individuals with neurofibromatosis develop through germline mutations (first hit) and somatic inactivating mutations (second hit) in the NF1 gene. Also within sporadic and radiotherapy-associated malignant peripheral nerve sheath tumors, 87.5 % showed a nonsense mutation or homozygous deletion in NF1. (8) An additional third hit was proposed that could explain progression of neurofibromas towards malignant peripheral nerve sheath tumor, namely loss-of-function mutations in EED and SUZ12, both components of Polycomb Repressive Complex 2 (PRC2). (8;9) Mutations in EED or SUZ12 were mutually exclusive and detected in 36-92% of sporadic, 23-70% of NF1-associated and 90% of radiotherapy-associated malignant peripheral nerve sheath tumors. (8;9) Importantly, none of these mutations were observed in neurofibromas (n=17). (8;9)
SUZ12 is a gene encoding zeste homolog 12 protein and EED is a gene encoding embryonic ectoderm development protein. Both are chromatin-modifying proteins, which together with EZH1 and EZH2, establish and maintain the di- and trimethylation of lysine 27 of histone H3 (H3K27me2 and H3K27me3). (8) Using immunohistochemistry, it was shown in a series of 62 malignant peripheral nerve sheath tumors, that malignant peripheral nerve sheath tumors with PRC2 loss, caused by mutations in SUZ12 or EED, showed complete loss of H3K27me3. More specifically, 56% (19/34) of NF1-associated malignant peripheral nerve sheath tumors and >90% of sporadic (15/16) and radiotherapy-associated (11/12) malignant peripheral nerve sheath tumors show loss of H3K27 staining, compared to retained H3K27me3 immunostaining in 7 tested neurofibromas. (8)
The aim of our study was to investigate whether H3K27 tri-methylation is useful as a diagnostic and /or prognostic marker for malignant peripheral nerve sheath tumors. H3K27 immunohistochemistry was performed in a large series of 162 primary malignant peripheral nerve sheath tumors, 97 neurofibromas and 290 other tumors including undifferentiated pleomorphic (or spindle) sarcoma / sarcoma not otherwise specified, synovial sarcoma, fibrosarcomatous dermatofibrosarcoma protuberans and schwannoma.
Materials and methods
Tumor samples
Our 600 study cases were originally diagnosed and collected from three different Pathology departments, including Leiden University Medical Center (Leiden, the Netherlands), The University of Texas MD Anderson Cancer Center (Houston, USA) and Stanford University Medical Center, USA. In total, 162 malignant peripheral nerve sheath tumors were included, for which full clinicopathological data were available (Table 1). The samples from malignant peripheral nerve sheath tumors Cohort 1 MDACC (n=80) were originally diagnosed in a multidisciplinary setting between 1986 and 2006. The malignant peripheral nerve sheath tumors from Cohort 2 (n=66) were originally diagnosed in a multidisciplinary setting between 1979 and 2006 and the malignant peripheral nerve sheath tumors from Cohort 3 LUMC (n=16) were originally diagnosed in a multidisciplinary setting between 2001 and 2007.
Table 1. Clinical parameters of MPNST cohorts.
| MPNST Cohort 1 MDACC (n=80) |
MPNST Cohort 2 Stanford (n=66) |
MPNST Cohort 3 LUMC (n=16) |
|
|---|---|---|---|
| Age at diagnosis | |||
| -median (range) | 40 (3-73) | n.a. | 28 (15-68) |
| Gender | |||
| -Female | 27 (34%) | n.a. | 9 (56%) |
| -Male | 37 (46%) | n.a. | 7 (44%) |
| -Not available | 16 (20%) | ||
| MPNSTs | |||
| -NF1 associated | 34 (43%) | 43 (65%) | 4 (25%) |
| -Sporadic | 22 (27%) | 21 (32%) | 12 (75%) |
| -Radiation associated | 5 (6%) | 0 (0%) | 0 (0%) |
| -Data n.a. | 19 (24%) | 2 (3%) | 0 (0%) |
| Treatment | |||
| -Resection | 75 (93%) | n.a. | 16 (100%) |
| -Radiotherapy | 6 (8%) | n.a. | 10 (63%) |
| -Chemotherapy | 16 (20%) | n.a. | 4 (25%) |
| -Not available | 48 (60%) | 1 (6%) | |
| Mean survival Time (years) | 4,9 | n.a. | 5,8 |
| Events | 35 (56%) | n.a. | 9 (56%) |
MPNST=malignant peripheral nerve sheath tumor; MDACC= The University of Texas MD Anderson Cancer Center; SUMC= Stanford University Medical Center; LUMC= Leiden University Medical Center. Events = death due to disease. n.a.=not available.
In addition, 97 neurofibromas and 341 other tumors were used, including undifferentiated pleomorphic (or spindle) sarcoma / sarcoma not otherwise specified (n=203), angiosarcoma (n=21), myxofibrosarcoma (n=17), synovial sarcoma (n=15), melanoma (histologically spindle type) (n=9), fibrosarcomatous dermatofibrosarcoma protuberans (n=8), clear cell sarcoma (n=5), leiomyosarcoma (n=5), pleomorphic liposarcoma (n=5), rhabdomyosarcoma (n=2), dedifferentiated liposarcoma (n=1), osteosarcoma (n=2), schwannoma (n=44) and perineurioma (n=4) (no clinicopathological data available).
Tissue micro array construction
The LUMC tissue microarrays included tissue cores taken from tumor areas selected by a pathologist on the basis of a haematoxylin and eosin (H&E)-stained slide (Beecher Instruments, Silver Springs, MD, USA). Tissue microarrays were constructed with tissue cores of a 1.5 mm diameter from each paraffin-embedded tumor tissue sample using a TMA Master (3DHISTECH, Budapest, Hungary). At least three cores from each tumor were sampled in order to outweigh intra-tumoral heterogeneity. Each tissue array contained additional cores from other tissue types, both as internal control for immunohistochemistry and for orientation purposes. Details for the MDACC (10) and the Stanford TMAs have been published previously. (11-13)
Immunohistochemistry
All tissue microarrays were stained at the LUMC pathology laboratory using the H3K27 antibody, H3K27-trimethyl (Millipore, Billerica, USA) with tonsil as positive control, using standard laboratory procedures as described previously. (14) After antigen retrieval by exposure to microwave heating in Tris–EDTA buffer pH 9.0 at 100°C for 10 min, the sections were incubated overnight at 4°C with 1:8000 diluted H3K27me3 antibody.
Evaluation of staining
Immunohistochemistry was evaluated by two observers independently (AHGC and JVMGB), blinded towards clinicopathological data, and discrepancies were discussed. Nuclear staining for H3K27me3 was evaluated for staining intensity (0 = negative, 1 = weak, 2 = moderate, 3 = strong). In addition, the percentage of positive tumor cell nuclei was scored (0 = 0%, 1 = 1-24%, 2 = 25-49%, 3 = 50-74% and 4 = 75-100%). In cases where the tumor cells were positive, the percentage of positive cells was always greater than 50%.Therefore, for statistical analysis we classified the staining patterns as ‘loss of’ H3K27me3 if negative or very weak intensity (with positive internal control (lymphocytes, endothelial cells) was observed, or ‘intact’ H3K27me3 if moderate or strong intensity of staining was observed.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics 20. All correlations were identified using the Pearson Chi-Square test. Disease-specific survival (DSS) was defined as time from diagnosis to death from Malignant Peripheral Nerve Sheath Tumor, with patients censored if death was due to other causes. The method of Kaplan-Meier and the log-rank test were used to compare DSS between groups. Independent variables predicting survival were evaluated in a multivariable model using Cox Regression analyses. The Cox-regression model included H3K27me3, age, sex, sporadic/NF associated disease and metastasis at diagnosis. All 2-sided p values of <0.05 were considered statistically significant.
Results
Loss of H3K27me3 staining in all malignant peripheral nerve sheath tumors subtypes
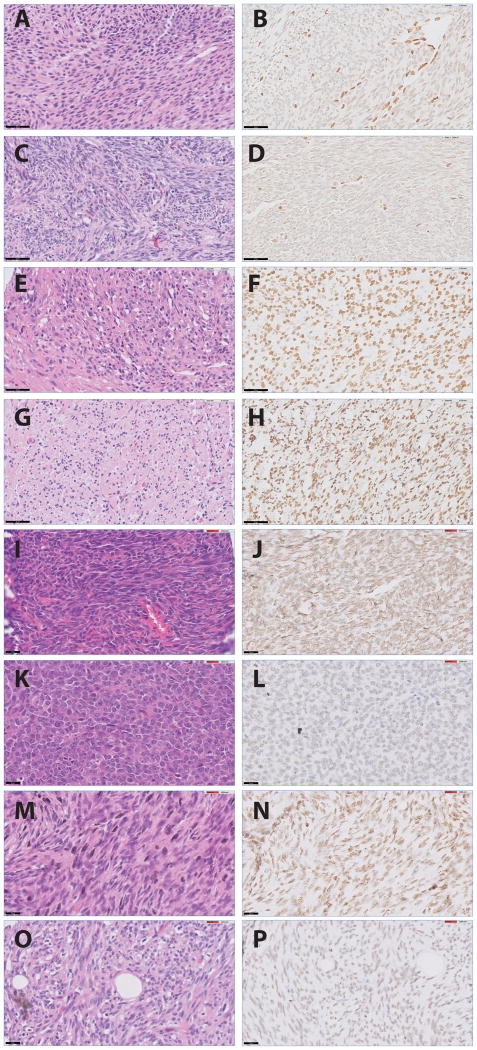
Of all analysed malignant peripheral nerve sheath tumors, 34% (55 out of 162) showed complete absence or weak H3K27me3 nuclear staining (7 out of 55 and 48 out of 55, respectively) in more than 75% of the tumor cells, with a positive internal control (Fig. 1 A,B). These cases were considered as malignant peripheral nerve sheath tumors with loss of H3K27 tri-methylation. Sixty-six percent (107 out of 162) of malignant peripheral nerve sheath tumors showed retention of H3K27 tri-methylation, as they demonstrated moderate or strong nuclear staining in 75-100% of the tumor cells (Fig. 1C). We did not see any significant difference in frequency of loss of H3K27 tri-methylation between sporadic and neurofibromatosis-associated malignant peripheral nerve sheath tumors (Table 2). We included two epithelioid malignant peripheral nerve sheath tumors of which both showed intact H3K27me3. For the 22 metastatic and 18 recurrent malignant peripheral nerve sheath tumors that were included, there was a 100% concordance in H3K27me3 status between primary tumors, recurrent tumors and / or metastases from the same patient (data not shown).
Figure 1.
Staining patterns of H3K27 tri-methylation: A, malignant peripheral nerve sheath tumor NF1-associated (H&E) with loss of H3K27me3 (B) as well as sporadic malignant peripheral nerve sheath tumor case (C (H&E) +D)) with lymphocytes and vessel walls as positive internal controls; E, sporadic malignant peripheral nerve sheath tumor case (H&E) with Intact H3K27me3 (F); G, plexiform type neurofibroma (H&E) with retention of H3K27me3 (H); I, synovial sarcoma (H&E) with retention of H3K27me3 (J); K, synovial sacoma (H&E) with loss of H3K27me3 (L); M, fibrosarcomatous dermatofibrosarcoma protuberans (H&E) with retention of H3K27me3 (N); O, fibrosarcomatous dermatofibrosarcoma protuberans (H&E) with loss of H3K27me3 (P). (A-H black bar = 50 μm; I-P black bar = 20 μm)
Table 2. Distribution of loss or intact H3K27me3 according to tumor subtype.
| Loss of H3K27me3 | Intact H3K27me3 | |
|---|---|---|
| MPNSTs | 55 (34%) | 107 (66%) |
| -Triton | 0 (0%) | 5 (100%) |
| -NF1 associated | 33(41%) | 47(59%) |
| -Sporadic | 17(32%) | 37(68%) |
| Neurofibroma | 0 (0%) | 97 (100%) |
| -Atypical | 0 (0%) | 8 (100%) |
| -Plexiform | 0 (0%) | 24 (100%) |
| Schwannoma | 1 (2%) | 43 (98%) |
| Perineurioma | 0 (0%) | 4 (100%) |
| Sarcoma NOS | 0 (0%) | 26 (100%) |
| Undifferentiated pleomorphic or spindle cell sarcoma | 5 (3%) | 172 (97%) |
| Angiosarcoma | 2 (10%) | 19 (90%) |
| Myxofibrosarcoma | 0 (0%) | 17 (100%) |
| Synovial sarcoma | 9 (60%) | 6 (40%) |
| Melanoma | 1 (11%) | 8 (89%) |
| DFSP | 3 (38%) | 5 (62%) |
| Clear cell sarcoma | 2 (40%) | 3 (60%) |
| Leiomyosarcoma | 0 (0%) | 5 (100%) |
| Dedif. Liposarcoma | 0 (0%) | 1 (100%) |
| Pleomorphic liposarcoma | 0 (0%) | 5 (100%) |
| Rhabdomyosarcoma | 0 (0%) | 2 (100%) |
| Osteosarcoma | 1 (50%) | 1 (50%) |
MPNST=malignant peripheral nerve sheath tumor; NOS= not otherwise specified; DFSP=dermatofibrosarcoma protuberans.
Loss of H3K27me3 is not observed in neurofibromas
Loss of H3K27me3 immunoreactivity was not observed in all 97 neurofibromas (Table 2), since 38% (37/97) showed moderate and 62% (60/97) showed strong H3K27 tri-methylation staining (Fig. 1D). This group of neurofibromas included sporadic cases as well as patients with hereditary neurofibromatosis. Moreover, different morphological subtypes of neurofibroma such as atypical (n=8) and plexiform neurofibroma (n=24) were included (Table 2). Of 44 patients for which both a neurofibroma and a malignant peripheral nerve sheath tumor were available, retention of H3K27me3 immunoreactivity was seen in all forty-four neurofibromas. In patients with more than one neurofibroma, all tumors demonstrated retention of H3K27 tri-methylation (n=42).
Loss of H3K27me3 is not specific for malignant peripheral nerve sheath tumors
Seven percent (24 out of 341) of other mesenchymal tumors demonstrated loss of H3K27 tri-methylation. Loss was frequently seen in synovial sarcomas (60% (9 out of 15) and fibrosarcomatous dermatofibrosarcoma protuberans (38% (3 out of 8)) (Figure 1E-H), which show significant morphological overlap with malignant peripheral nerve sheath tumors (Table 2).
Other high grade sarcomas that are difficult to distinguish from malignant peripheral nerve sheath tumor solely based on morphology, such as sarcoma not otherwise specified or undifferentiated pleomorphic (or spindle cell) sarcoma, demonstrated loss of H3K27 tri-methylation in a very low frequency (0% (0/26), and 2,8 % (5/177), respectively (Table 2)). Furthermore, loss of H3K27 tri-methylation was observed in small subsets of angiosarcomas (10% (2/21)), clear cell sarcomas (40% (2/5)), melanoma (11% (1/9)), osteosarcoma (50% (1/2)) and schwannoma (2% (1/44)). Intact H3K27 tri-methylation was detected in myxofibrosarcoma (n=17), pleomorphic liposarcoma (n=5), rhabdomyosarcoma (n=2), dedifferentiated liposarcoma (n=1), leiomyosarcoma (n=5) and perineurioma (n=4) (Table 2).
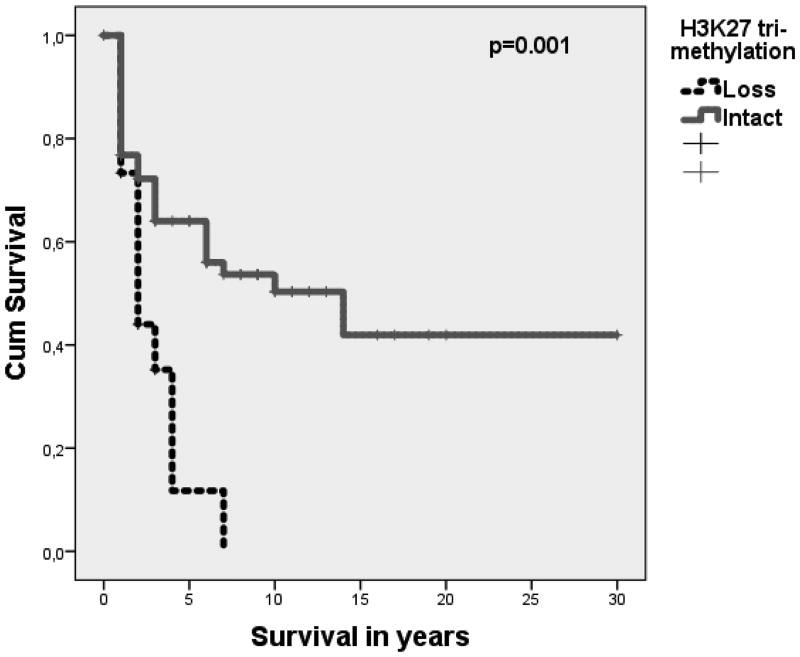
Loss of H3K27me3 predicts poor survival in malignant peripheral nerve sheath tumors
Loss of H3K27 tri-methylation was an independent prognostic factor for a poor survival compared to intact H3K27me3 among malignant peripheral nerve sheath tumor patients of Cohort 1 MDACC (n=62) (Fig. 2, Kaplan Meier, p=001), with a HR of 2.6 (Cox-regression, CI 1.2-5.7, p=0.017). In a second cohort of malignant peripheral nerve sheath tumors of Cohort 3 LUMC (n=16) with available follow-up data, cases with loss of H3K27 tri-methylation showed a higher frequency of disease specific mortality compared to cases with intact H3K27, respectively 75% (3/4) and 50% (6/12). Due to the low number of cases with available follow-up data in Cohort 3, additional survival analyses were not performed.
Figure 2.
Loss of H3K27me3 is associated with poor outcome: Poorer disease specific survival (in years) of MPNSTs with loss of H3K27 tri-methylation (n=14) compared to MPNSTs with intact H3K27 tri-methylation (n=48), p=0.001.
Discussion
The distinction between neurofibroma and malignant peripheral nerve sheath tumor can be extremely difficult based on histology alone, and so far no good immunohistochemical or molecular markers are available to assist in the differential diagnosis. We show here, in a multicentre study, that loss of H3K27 tri-methylation can be used as a diagnostic and prognostic marker for malignant peripheral nerve sheath tumors. Loss of H3K27 tri-methylation was found in 34% of malignant peripheral nerve sheath tumors compared to intact H3K27 tri-methylation in all neurofibromas, including morphological variants like atypical and plexiform neurofibromas, indicating that loss of H3K27 tri-methylation is highly specific for malignant peripheral nerve sheath tumors in the differential diagnosis with neurofibroma.
Our results regarding the specificity of H3K27 tri-methylation are in concordance with previous studies. In a study by Schaefer et al loss of H3K27 tri-methylation was shown in 51% (51/100) of malignant peripheral nerve sheath tumors and in another study by Prieto-Granada et al, loss of H3K27 tri-methylation was found in 69% (47/68) of malignant peripheral nerve sheath tumors. (15;16) In a study by Zhang et al, 29% (6/21) of malignant peripheral nerve sheath tumors showed loss of H3K27 tri-methylation, more specifically 8% (1/12) of neurofibromatosis-associated malignant peripheral nerve sheath tumors and 55% (5/9) of sporadic malignant peripheral nerve sheath tumors. In the second study by Lee et al, loss of H3K27 tri-methylation was observed with a higher frequency, respectively 56% (19/34) of NF1-associated malignant peripheral nerve sheath tumors and 93% (15/16) of sporadic malignant peripheral nerve sheath tumors. Although the specificity of loss of H3K27 tri-methylation for malignant peripheral nerve sheath tumors is high, the prevalence H3K27 tri-methylation loss ranged from 20% up to as high as 93%. A possible explanation might be that the frequency of NF1 associated versus sporadic/radiotherapy-associated malignant peripheral nerve sheath tumors are heterogeneous between these cohorts. Progression of disease and sequence of genetic inactivation of PRC2 complex and thus H3K27 tri-methylation status is thought to be different in malignant peripheral nerve sheath tumors that are NF1 associated compared to sporadic and radiotherapy-associated malignant peripheral nerve sheath tumors. However there were no statistical significant differences in distribution of NF1-associated versus sporadic malignant peripheral nerve sheath tumors between our study cohorts and those published by others. Furthermore it is not likely that differences in antibodies between the studies explain the range in sensitivity. We used the same antibody as Schaefer et al and Lee et al, and although we used a higher dilution (1:8.000 versus 1:500 and 1:250), this did not result in a higher frequency of loss of H3K27 tri-methylation in our current study.
Based on morphological criteria including high cellularity, nuclear atypia and mitotic activity, pathologists try to differentiate malignant peripheral nerve sheath tumors from plexiform neurofibromas and atypical neurofibromas. Logically a greater degree of cellularity, pleomorphism and higher mitotic activity are criteria that are used to classify the lesion as malignant. The problem with these histological features is that they are prone to interobserver variability. Additionally, the use of immunohistochemistry in the differential diagnosis has been of limited value, due to lack of sensitivity and specificity to establish the diagnosis of malignant peripheral nerve sheath tumor with certainty. S100 protein has been widely used to prove nerve sheath differentiation in a monomorphic appearing spindle cell sarcoma, but it is expressed on occasion in a variety of other lesions. Therefore, in the past, many efforts have been undertaken to identify additional molecular markers that could differentiate between these tumors. (17;18) For example, expression profiling in matched malignant peripheral nerve sheath tumors and neurofibroma samples did identify more than 500 kinase genes that are differentially expressed between both entities, with mitotic regulators BUB1B, PBK and NEK2 being overexpressed in malignant peripheral nerve sheath tumors. (19) However, the patterns of these changes turned out to be complex and heterogeneous, without a single unifying alteration that could be used as a diagnostic marker. In contrast, staining for H3K27me3 seems to be a more promising and easy to use diagnostic tool, in which loss of H3K27me3 favours progression to malignant peripheral nerve sheath tumors above plexiform or atypical neurofibroma.
Besides neurofibromas, cellular schwannomas and high-grade undifferentiated sarcomas, synovial sarcomas and fibrosarcomatous dermatofibrosarcoma protuberans belong to the differential diagnosis at the opposite end of the spectrum of malignant peripheral nerve sheath tumors which may be difficult to distinguish from each other solely based on morphological criteria. Malignant peripheral nerve sheath tumors has a broad morphologic range (including in the well defined set of those present in the setting of neurofibromatosis), composed of a relatively monomorphic spindle cell proliferation, to pleomorphic and even rarely to epithelioid cytomorphology. Mitoses are usually readily identified and necrosis is often present, but these features vary and are not distinct. Diffuse S100 positive staining favours a diagnosis of schwannoma, and since 98% of the schwannomas in our study showed intact H3K27me3, loss of this staining almost excludes the diagnosis of a cellular schwannoma. In line with this, according to our study, loss of H3K27 tri-methylation is a negligible finding in undifferentiated sarcomas that could mimic malignant peripheral nerve sheath tumors. Therefore, in the difficult differential diagnosis of high grade spindle cell lesions, loss of H3K27me3 is strongly indicative of malignant peripheral nerve sheath tumor and does not favor a cellular schwannoma or, undifferentiated sarcoma, or melanoma.
On the other hand, loss of H3K27 tri-methylation was frequently observed within synovial sarcomas and fibrosarcomatous dermatofibrosarcoma protuberans. These lesions have overlapping histological and immunohistochemical features. Our results show that H3K27me3 immunohistochemistry is not useful to differentiate between these two entities and demonstration of the t(X;18) translocation remains the cornerstone for the diagnosis of synovial sarcoma while dermatofibrosarcoma protuberans is characterized by COL1A1-PDGFB gene fusions.
SS18 and SSX are transcriptional cofactors involved in activation (SS18) and repression (SSX) of gene transcription. SS18 interacts with SWI/SNF, whereas SSX is associated with the polycomb chromatin remodelling complex. Indeed, Garcia et al have shown H3K27me3 to be the dominant epigenetic marker associated with SYT-SSX2 binding and gene expression (20), which may explain the loss of H3K27 tri-methylation which we found in 60% of synovial sarcomas. In contrast to malignant peripheral nerve sheath tumors, mutations in SUZ12, EED, EZH1 and EZH2 are not described in synovial sarcomas and therefore could not explain the observed loss of H3K27 tri-methylation. Likewise, our finding of loss of H3K27 tri-methylation in 38% of dermatofibrosarcoma protuberans also needs to be further explored in a larger group, since no data have been published about a possible mechanistic link between H3K27 tri-methylation and the specific fusion protein, COL1A1-PDGFB, which is characteristic of dermatofibrosarcoma protuberans. (21)
Our finding that malignant peripheral nerve sheath tumors with loss of H3K27 tri-methylation showed inferior survival compared to malignant peripheral nerve sheath tumors with intact H3K27 tri-methylation are in line with the results of Schaefer et al, who demonstrated increased loss of H3K27 with increasing histological grade. (15) We do not have sufficient data regarding histological grading available for the three cohorts we studied, which is a limitation in our study. The value of grading malignant peripheral nerve sheath tumors is unclear and its relationship with clinical outcome has been long debated in the literature.
In the study by Lee et al and Zhang et al, it was shown that loss of H3K27 tri-methylation was caused by mutations in SUZ12 or EED. These two genes establish and maintain the di- and trimethylation of lysine 27 of histone H3 (H3K27me2 and H3K27me3) and methylation of this position results in transcriptional repression of target genes. (8) It was shown that the vast majority (95%) of differentially expressed genes (FOXN4, IGF2, PAX2, TLX1) were upregulated in malignant peripheral nerve sheath tumors with PRC2 loss, which is consistent with the role of PRC2 in transcriptional repression via histone H3. (8) Since genes such as IGF2 play an important role in the growth and development of cells and have been implicated in oncogenesis and tumor progression, differential upregulation of these genes in malignant peripheral nerve sheath tumors with loss of H3K27 tri-methylation, could explain the observed effect on survival in our study.
Besides using H3K27me3 staining as a diagnostic and prognostic marker, loss of H3K27me3 may predict response to histone deacetylase (HDAC) inhibitors. In a study performed within malignant peripheral nerve sheath tumor cell lines, histone deacetylase 8 specific inhibitor induced cell growth inhibition and marked S-phase cell cycle arrest. (22) However, resistance to histone deacetylase inhibitors has also been shown in malignant peripheral nerve sheath tumors (23) and likely mutations in chromatin remodelling genes like SUZ12 or EED and thus H3K27 tri-methylation, play a mechanistic role in level of response to histone deacetylase inhibitors. This is an important area for more preclinical modeling.
In conclusion, our results show that loss of H3K27 favours the diagnosis of malignant peripheral nerve sheath tumor over neurofibroma, undifferentiated pleomorphic (or spindle) sarcoma / sarcoma not otherwise specified and melanoma but is not suitable to distinguish malignant peripheral nerve sheath tumors from its morphological mimickers, namely synovial sarcoma and fibrosarcomatous dermatofibrosarcoma protuberans. Since loss of H3K27 tri-methylation was related to poorer survival in malignant peripheral nerve sheath tumor, chromatin modification mediated by this specific histone might explain the more aggressive tumour biology.
Acknowledgments
The authors acknowledge Torsten Nielsen (Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada) and Marieke de Graaff (Department of Pathology, LUMC) for their contribution to the TMAs.
Footnotes
Disclosure/conflict of interest: All authors have no disclosure or conflict of interest.
Reference List
- 1.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. Malignant peripheral nerve sheath tumour. WHO Classification of Tumours of Soft Tissue and Bone. 2013:187. [Google Scholar]
- 2.Goldblum JR, Folpe AL, Weiss SW. Malignant Peripheral Nerve Sheath Tumors. Soft Tissue Tumors. (sixth) 2014:867. [Google Scholar]
- 3.Kroep JR, Ouali M, Gelderblom H, Le CA, Dekker TJ, Van GM, Hogendoorn PC, Hohenberger P. First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: an EORTC soft tissue and bone sarcoma group study. Ann Oncol. 2011;22:207–14. doi: 10.1093/annonc/mdq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nonaka D, Chiriboga L, Rubin BP. Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol. 2008;35:1014–9. doi: 10.1111/j.1600-0560.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 5.Hirose T, Hasegawa T, Kudo E, Seki K, Sano T, Hizawa K. Malignant peripheral nerve sheath tumors: an immunohistochemical study in relation to ultrastructural features. Hum Pathol. 1992;23:865–70. doi: 10.1016/0046-8177(92)90396-k. [DOI] [PubMed] [Google Scholar]
- 6.Sabah M, Cummins R, Leader M, Kay E. Loss of p16 (INK4A) expression is associated with allelic imbalance/loss of heterozygosity of chromosome 9p21 in microdissected malignant peripheral nerve sheath tumors. Appl Immunohistochem Mol Morphol. 2006;14:97–102. doi: 10.1097/01.pai.0000143787.80564.f5. [DOI] [PubMed] [Google Scholar]
- 7.Pekmezci M, Reuss DE, Hirbe AC, Dahiya S, Gutmann DH, von DA, Horvai AE, Perry A. Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod Pathol. 2015;28:187–200. doi: 10.1038/modpathol.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee W, Teckie S, Wiesner T, Ran L, Prieto Granada CN, Lin M, Zhu S, Cao Z, Liang Y, Sboner A, Tap WD, Fletcher JA, Huberman KH, Qin LX, Viale A, Singer S, Zheng D, Berger MF, Chen Y, Antonescu CR, Chi P. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46:1227–32. doi: 10.1038/ng.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M, Wang Y, Jones S, Sausen M, McMahon K, Sharma R, Wang Q, Belzberg AJ, Chaichana K, Gallia GL, Gokaslan ZL, Riggins GJ, Wolinksy JP, Wood LD, Montgomery EA, Hruban RH, Kinzler KW, Papadopoulos N, Vogelstein B, Bettegowda C. Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46:1170–2. doi: 10.1038/ng.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou C, Smith KD, Liu J, Lahat G, Myers S, Wang WL, Zhang W, McCutcheon IE, Slopis JM, Lazar AJ, Pollock RE, Lev D. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–22. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen TO, Hsu FD, O'Connell JX, Gilks CB, Sorensen PH, Linn S, West RB, Liu CL, Botstein D, Brown PO, van de RM. Tissue microarray validation of epidermal growth factor receptor and SALL2 in synovial sarcoma with comparison to tumors of similar histology. Am J Pathol. 2003;163:1449–56. doi: 10.1016/S0002-9440(10)63502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terry J, Saito T, Subramanian S, Ruttan C, Antonescu CR, Goldblum JR, Downs-Kelly E, Corless CL, Rubin BP, Van de Rijn M, Ladanyi M, Nielsen TO. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol. 2007;31:240–6. doi: 10.1097/01.pas.0000213330.71745.39. [DOI] [PubMed] [Google Scholar]
- 13.Karamchandani JR, Nielsen TO, Van de Rijn M, West RB. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol. 2012;20:445–50. doi: 10.1097/PAI.0b013e318244ff4b. [DOI] [PubMed] [Google Scholar]
- 14.Baranski Z, Booij TH, Cleton-Jansen AM, Price LS, van de Water B, Bovee JV, Hogendoorn PC, Danen EH. Aven-mediated checkpoint kinase control regulates proliferation and resistance to chemotherapy in conventional osteosarcoma. J Pathol. 2015;236:348–59. doi: 10.1002/path.4528. [DOI] [PubMed] [Google Scholar]
- 15.Schaefer IM, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4–13. doi: 10.1038/modpathol.2015.134. [DOI] [PubMed] [Google Scholar]
- 16.Prieto-Granada CN, Wiesner T, Messina JL, Jungbluth AA, Chi P, Antonescu CR. Loss of H3K27me3 Expression Is a Highly Sensitive Marker for Sporadic and Radiation-induced MPNST. Am J Surg Pathol. 2015 doi: 10.1097/PAS.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian S, Thayanithy V, West RB, Lee CH, Beck AH, Zhu S, Downs-Kelly E, Montgomery K, Goldblum JR, Hogendoorn PCW, Corless CL, Oliveira AM, Dry SM, Nielsen TO, Rubin BP, Fletcher JA, Fletcher CD, Van de Rijn M. Genome-wide transcriptome analyses reveal p53 inactivation mediated loss of miR-34a expression in malignant peripheral nerve sheath tumours. J Pathol. 2010;220:58–70. doi: 10.1002/path.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirbe AC, Dahiya S, Miller CA, Li T, Fulton RS, Zhang X, McDonald S, DeSchryver K, Duncavage EJ, Walrath J, Reilly KM, Abel HJ, Pekmezci M, Perry A, Ley TJ, Gutmann DH. Whole Exome Sequencing Reveals the Order of Genetic Changes during Malignant Transformation and Metastasis in a Single Patient with NF1-plexiform Neurofibroma. Clin Cancer Res. 2015;21:4201–11. doi: 10.1158/1078-0432.CCR-14-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stricker TP, Henriksen KJ, Tonsgard JH, Montag AG, Krausz TN, Pytel P. Expression profiling of 519 kinase genes in matched malignant peripheral nerve sheath tumor/plexiform neurofibroma samples is discriminatory and identifies mitotic regulators BUB1B, PBK and NEK2 as overexpressed with transformation. Mod Pathol. 2013;26:930–43. doi: 10.1038/modpathol.2012.242. [DOI] [PubMed] [Google Scholar]
- 20.Garcia CB, Shaffer CM, Eid JE. Genome-wide recruitment to Polycomb-modified chromatin and activity regulation of the synovial sarcoma oncogene SYT-SSX2. BMC Genomics. 2012;13:189. doi: 10.1186/1471-2164-13-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llombart B, Monteagudo C, Sanmartin O, Lopez-Guerrero JA, Serra-Guillen C, Poveda A, Jorda E, Fernandez-Serra A, Pellin A, Guillen C, Llombart-Bosch A. Dermatofibrosarcoma protuberans: a clinicopathological, immunohistochemical, genetic (COL1A1-PDGFB), and therapeutic study of low-grade versus high-grade (fibrosarcomatous) tumors. J Am Acad Dermatol. 2011;65:564–75. doi: 10.1016/j.jaad.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Lopez G, Bill KL, Bid HK, Braggio D, Constantino D, Prudner B, Zewdu A, Batte K, Lev D, Pollock RE. HDAC8, A Potential Therapeutic Target for the Treatment of Malignant Peripheral Nerve Sheath Tumors (MPNST) PLoS ONE. 2015;10:e0133302. doi: 10.1371/journal.pone.0133302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez G, Torres K, Liu J, Hernandez B, Young E, Belousov R, Bolshakov S, Lazar AJ, Slopis JM, McCutcheon IE, McConkey D, Lev D. Autophagic survival in resistance to histone deacetylase inhibitors: novel strategies to treat malignant peripheral nerve sheath tumors. Cancer Res. 2011;71:185–96. doi: 10.1158/0008-5472.CAN-10-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]