Abstract
Background
Cerebral blood flow (CBF) regulation is a critical element in cerebrovascular pathophysiology, particularly in large vessel disease, but the best method to use for hemodynamic assessment is not clear. We examined 4 different blood-flow related measures in patients with unilateral high-grade carotid artery disease, assessing asymmetry between the occluded vs non-occluded side, and the correlations among the measures.
Methods
Thirty-three patients (age 50-93, 19M) with unilateral 80-100% ICA occlusion but no stroke underwent: 1) mean flow velocity (MFV) in both middle cerebral arteries by transcranial Doppler (TCD), 2) quantitative resting CBF using pseudo-continuous arterial spin labeling (pCASL) MRI, 3) Vasomotor reactivity (VMR) in response to 5% CO2 inhalation, and 4) Dynamic cerebral autoregulation (DCA) assessing the counter-regulation of blood flow to spontaneous changes in blood pressure using TCD monitoring and finger photoplethysmography. Paired T-tests and Pearson correlations assessed side-to-side differences within each measure, and correlations between measures.
Results
CBF (p=.001), MFV (p<.001), VMR (p=.008), and DCA (p=.047) all showed significantly lower values on the occluded side. The 4 measures were independent of each other on correlation analysis, even when controlling for age and anterior circle of Willis collateral (all partial correlations <.233 and p-values >.468).
Conclusions
These 4 measures showed high sensitivity to the occluded carotid artery, but their dissociation suggests that any given measure only partially characterizes the hemodynamic state. Additional research is needed to explore the multifaceted biology of cerebral blood flow regulation.
Keywords: carotid artery stenosis, cerebral blood flow, cerebral hemodynamics, autoregulation, transcranial Doppler, arterial spin labeling
1. Introduction
Adequate blood flow regulation is required for normal brain function and to prevent cerebral ischemia. Blood flow and its correlates can be measured using a variety of techniques, and there are a number of conditions, such as high-grade carotid occlusive disease, in which several hemodynamic measures have been promoted for diagnostic and prognostic use. There has been no consensus as to the best method for hemodynamic assessment, however, and there is continued debate about whether abnormalities on different measures may in fact represent different aspects of hemodynamic dysfunction(1). In order to address the relationship between measures, we assessed cerebral hemodynamics in a group of patients with asymptomatic high grade carotid occlusive disease using four different measures of hemodynamics, each of which has been used to characterize carotid artery disease. Our first measure -- resting mean flow velocity (MFV) by transcranial Doppler (TCD) -- has been shown to be reduced in severe carotid stenosis(2), and to predict stroke risk(3). Our second -- resting cortical cerebral blood flow (CBF) using arterial spin labeling (ASL) MRI -- has been shown to be altered ipsilaterally in patients with unilateral carotid artery stenosis, and to correlate with PET CBF, long considered the gold standard for cerebral hemodynamics.(4) Our third measure -- vasomotor reactivity (VMR) using TCD with hypercapnia -- has been used to predict risk of subsequent ischemia in high grade carotid artery disease(5-7). And our fourth measure -- dynamic cerebral autoregulation (DCA) -- has been offered as an alternative to VMR, measuring the counter-regulation of blood flow to induced or spontaneous changes in blood pressure with TCD(8-11). Whereas each of these measures has been used to assess cerebral hemodynamics in carotid disease, it is unlikely that they are all measuring the same hemodynamic property. We hypothesized that each measure would show hemispheral asymmetry because of the presence of the unilateral severe carotid disease, but the measures would show low correlation with each other to indicate that the measures represent independent aspects of cerebral hemodynamics. Such a result would suggest that the measures are not interchangeable, but may provide unique information about hemodynamic function, either for prediction of stroke risk or for characterizing the functional consequences of hemodynamic failure.(5, 12-15).
2. Materials and Methods
2.1 Subjects
Thirty-three patients, age 50-93, 19 male, with unilateral 80-100% ICA occlusion but no stroke were enrolled in the study. Inclusion criteria were: ≥80% carotid stenosis or complete occlusion by carotid Doppler, MRA, or CTA, with <40% stenosis in the contralateral ICA. Other inclusion criteria were: asymptomatic status or TIA-only referable to the ICA stenosis, fluent in English, and able to give informed consent. Exclusion criteria were prior clinical stroke (defined as a clinical event with MRI correlate), diagnosis of dementia, history of head trauma, current substance abuse, major psychiatric disease, NYHA Stage 3/4 congestive heart disease, or contraindication to MRI. Clinically silent small subcortical infarcts ≤3mm in diameter were allowed, as well as leukoariosis, defined as confluent white matter hyperintensity abutting any portion of the lateral ventricles. The study was approved by the Institutional Review Board of Columbia University and all patients signed informed consent.
2.2. Measures of blood flow
2.2.1 MCA mean flow velocity
Hemispheral resting flow was estimated with transcranial Doppler (DWL-Multidop-X, Sipplingen, Germany). MFV in each MCA was measured with a 2 MHz probe at a depth of 50 to 56mm on the occluded and unoccluded sides using the temporal window.
2.2.2 Resting Cortical Blood Flow (CBF)
Tissue-specific pseudo-Continuous Arterial Spin Labeling (ts-pCASL) fMRI(16, 17) was used to acquire CBF images with the following parameters: labeling duration = 2s; post-labeling delay (PLD) adjusted to account for the inter-slice acquisition time as PLD = (acquisition slice −1) × (60ms) + 1900ms; number of slices = 12, slice thickness = 8mm/1mm gap. ASL images were processed using a partial volume correction method that yields tissue-specific flow density values in gray and white matter tissues independently. We used gray matter only in our analysis. This method has been shown to have lower intersubject variability, thus higher (~ 2.9 times) SNR at the group level analysis(18). CBF images were computed using a two-compartment formula(16).
2.2.3. Vasomotor reactivity
VMR was determined using TCD monitoring with the standard head frame and 2 MHz probes on each temporal window. We measured MFV at baseline and in response to 2 minutes of 5% CO2 inhalation delivered by non-rebreathing face mask, using a Doppler-integrated inline capnometer (Spencer Technologies). Magnitude of response was calculated as the percent rise in MCA MFV per mmHg increase in PCO2. A total PCO2 rise of at least 10 mmHg was required for a valid measurement. The VMR CO2 inhalation challenge was performed in each patient after the 10-minute monitoring period for the DCA.
2.2.4. Dynamic Cerebral Autoregulation
DCA measurements were performed in supine position with slight elevation of the upper body while the subjects were breathing spontaneously. The proximal MCA was insonated through the temporal window with a 2 MHz probe attached to a standard head frame at an insonation depth of 50-56mm. Blood pressure was recorded simultaneously using servo-controlled finger plethysmography (Finometer Pro, Amsterdam, Netherlands). The appropriate finger cuff (Size: small, medium or large) was placed on the middle phalanx of the left or right middle finger. After establishing a stable recording, the intermittently occurring calibration procedure was turned off. Measurements were recorded for 10 minutes. All analog signals were digitized and stored for editing and offline analysis. Data sampling frequency was 100 Hz.
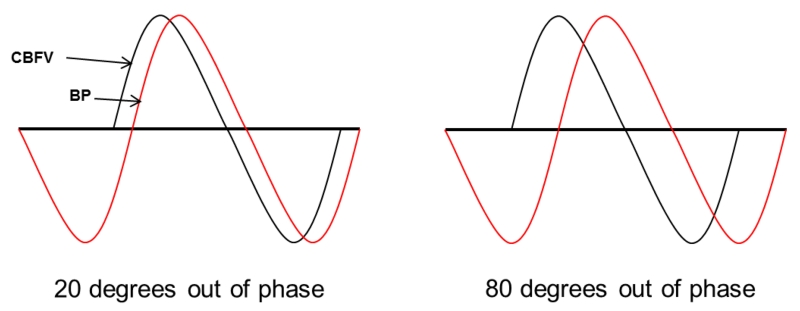
DCA Data analysis: The editing process included temporal synchronization of the blood pressure and blood flow velocity wave forms using ICUpilot software (Dipylon Medical, Solna, Sweden),followed by visual inspection and removal of all major artifacts. The data were then analyzed using Matlab (MathWorks, Natick, USA) with an in-house written program. The relationship between changes in oscillatory arterial pressure and CBFV was assessed with transfer function analysis, which quantifies the extent to which the input signal (ABP) is reflected in the output signal (CBFV) in a given oscillatory frequency range.(19) The result was a characterization of the efficacy of the autoregulatory system. The primary outcome measure was phase shift (PS), the relative separation of signals in a frequency range in which auto regulation is known to occur. The more efficient the counter-regulation of flow, the more CBFV “leads” the fluctuating pressure wave (Figure 1). Lower degree of PS indicated worse autoregulation.
Figure 1.
Dynamic Cerebral Autoregulation (DCA) curves showing counterregulatory cerebral blood flow velocity (CBFV) curve “leading” the blood pressure (BP) curve with low phase shift (left) indicating abnormal autoregulation, and normal phase shift (right), indicating normal autoregulation.
2.3. Statistical analysis
A two-tailed paired t-test was used to compare mean values in the occluded versus unoccluded hemisphere for each of the 4 blood flow measures. The Pearson correlation test was used to look for correlations among the 4 measures, controlling for age and circle of Willis collateral cross-filling (a dichotomous variable determined by reversed flow direction in the anterior circle of Willis on the side of the occluded carotid). All calculations were done using SPSS v.22.
3. Results
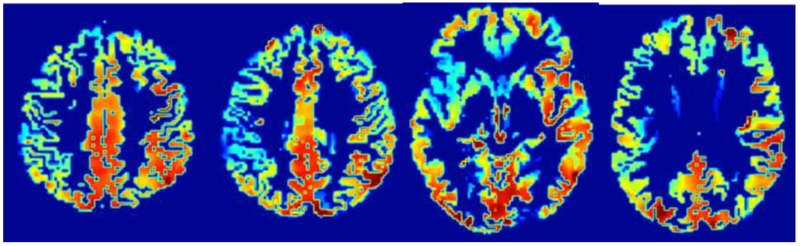
Among the 33 patients in this cohort, 4 had insufficient temporal windows to obtain mean flow velocities on one or both sides and 5 were unable to complete the CO2 inhalation testing. An additional 4 had poor Finapres signal and so could not provide DCA data. Ten either could not complete the MRI, had a contraindication for MRI, or the ASL signal was not adequate for analysis. For the 23 with adequate MRI data 11 had leukoariosis and 16 had small silent subcortical infarcts. Taking all patient data for those who completed each test, all 4 tests showed a statistically significant asymmetry by hemisphere, with the lower value on the occluded side (see Table 1). An illustration of the pCASL asymmetry is illustrated in Figure 2. None of the measures showed a statistical difference in side-to-side asymmetry between those with high-grade stenosis versus those with complete occlusion, nor between those with left versus right-sided occlusion.
Table 1.
Hemispheral asymmetry by blood flow test.
| Mean (±SD) | ||||
|---|---|---|---|---|
|
| ||||
| Test (units) | N | UNOCCL | OCCL | P |
| MFV (cm/sec) | 29 | 55.0 (13.7) | 39.1 (11.1) | <.001 |
| CBF (mL/100g*min) | 23 | 97.6 (24.8) | 87.8 (25.6) | 0.001 |
| VMR (% increase) | 24 | 2.5 (0.7) | 1.9(1.2) | 0.008 |
| DCA (deg of PS) | 19 | 45.7(21.5) | 32.0(25.9) | 0.047 |
MFV=mean flow velocity, CBF= Gray Matter Cerebral Blood Flow extracted from PVEc ASL, VMR=vasomotor reactivity, DCA=dynamic cerebral autoregulation, OCCL=measurement on the side of the occluded carotid artery, UNOCCL= measurement on the side of the unoccluded carotid artery, PS=phase shift
Figure 2.
pCASL output showing cortical resting cerebral blood flow asymmetry due to carotid occlusion. Red color indicates higher CBF.
On correlation analysis, there was no evidence that the four blood flow differences were correlated, controlling for age and anterior circle of Willis collateral (see Table 2).
Table 2.
Correlation among blood flow measures.
| VMR_DIFF | DCA_DIFF | CBF_DIFF | ||
|---|---|---|---|---|
| MFV_DIFF | Corr | 0.120 | 0.000 | 0.112 |
| P | 0.610 | 0.999 | 0.669 | |
| VMR_DIFF | Corr | 0.085 | 0.149 | |
| P | 0.764 | 0.596 | ||
| DCA_DIFF | Corr | 0.233 | ||
| P | 0.468 | |||
|
|
||||
MFV=mean flow velocity, VMR=vasomotor reactivity, DCA=dynamic cerebral autoregulation, CBF= gray matter cerebral blood flow, DIFF=difference between measurement on hemisphere of the unoccluded –occluded carotid artery, Corr=correlation coefficient. P-values in boldface.
4. Discussion
Among a cohort of patients with asymptomatic high grade carotid occlusive disease we showed that four commonly used measures of cerebral hemodynamics -- resting mean flow velocity, cortical CBF, vasomotor reactivity, and dynamic cerebral autoregulation -- all showed significantly lower values in the hemisphere supplied by the affected carotid artery. The asymmetry was not unexpected. Many studies of carotid disease have used the same or similar tests and have reported asymmetries related to the stenosis.(2, 5-8, 20-23) Yet when we assessed the four tests for concordance with each other, there was a very low correlation. Previous studies have addressed the multifaceted biology of cerebral blood flow regulation(1, 11) and differences have been shown to exist even within specific aspects of autroregulation(24). What makes our study unique is the concurrent examination of four commonly used measures of cerebral hemodynamics in a homogeneous population of patients who were likely to have hemodynamic abnormalities, Assuming that the low correlation represents true biological divergence rather than measurement or statistical variability, this finding has important implications for our understanding of cerebral hemodynamics. Our findings suggest that these tests are not interchangeable, but rather, provide unique information about different mechanisms controlling blood flow in the brain.
The brain is one of the only organs in the body to regulate its own blood flow, and the only organ whose function is critically dependent on this capacity. Loss of blood flow regulation occurs in a variety of cerebrovascular conditions, both in acute stroke(25-27), and in chronic cerebrovascular conditions such as small vessel disease(28, 29), metabolic syndrome(30, 31), and high grade carotid artery stenosis(32-34). Poor blood flow is an important predictor of stroke risk. The mechanisms, time course and additional variables that create abnormal blood flow remain crucial questions, both for an understanding of cerebrovascular physiology, and for the clinical management of cerebrovascular patients.
Each of the hemodynamic measurements examined in this study has a known association with carotid artery stenosis. Of the two measures of resting flow, MCA mean flow velocity by TCD may be considered the most direct measure. A blunted waveform in the MCA is common in high grade carotid stenosis(2), and the reduced flow velocity occurs as a direct result of the proximal ICA constriction. MFV may therefore be a sensitive but non-specific indicator of hemodynamic impairment. Our pCASL measurement specifically assesses cortical blood flow(35). Although CASL depends for its calculation on “arterial transit time”(36, 37) -- a variable related mathematically to MFV -- its final output of cortical blood flow is influenced by distinct physiological variables such as cortical blood volume,(16) collateral patterns,(38) cortical network connectivity,(39) and local neurotransmitter effects(40, 41). Impaired cortical flow may thus have functional implications for the cortex that are distinct from the non-specific reduced flow velocity identified by TCD. Cognitive function is dependent on cortical function, and can be influenced by alterations of blood flow, either directly(42) or as a marker of underlying metabolic dysfunction.(43)
The two measures of cerebral autoregulation – VMR and DCA – can also be considered distinct from one another.(44) Vasomotor reactivity is commonly tested in carotid disease with a vasodilatory challenge using CO2 inhalation, acetazolamide, or breath holding. Vasoconstriction can also be assessed with hyperventilation to lower pCO2. The vasoconstrictive or vasodilatory response is thought to be due to decrease or increase in pH, respectively, and is also modulated by adrenergic factors.(45) VMR assesses how much vasodilatory and vasoconstrictive reserve is available to protect the brain from extremes of hypo- and hypertension. In a state of chronic low perfusion pressure such as high grade carotid stenosis, the VMR with CO2 inhalation demonstrates whether the system is close to maximum vasodilation and therefore to stroke risk. A recent data pooling analysis included 754 carotid stenosis patients from 9 studies who underwent CO2 vasoreactivity as a predictor of stroke risk.(7) This study showed a hazard ratio = 3.69 for stroke for both symptomatic and asymptomatic patients for those with low VMR. VMR may thus be considered a measure of “static” autoregulation. DCA on the other hand, although influenced by pCO2(46, 47), assesses the counterregulation of blood flow to spontaneous or induced changes of intracranial perfusion pressure. Although a variety of procedures and analytical approaches have been proposed for DCA,(24) we chose the transfer function analysis phase shift output because it has emerged recently as a metric to assess carotid disease.(10, 32) DCA is appropriately named “dynamic” cerebral autoregulation because it reflects real-time changes in flow. As measured in this study, it may be considered a reflection of homeostasis, as flow changes are assessed in an ongoing, short time frame, with low amplitude counter-regulatory responses. DCA has been shown to be abnormal for several days following acute embolic stroke.(25) It may be more useful in predicting stroke outcomes rather than stroke risk.
Limitations of this study include the number of hemodynamic techniques we compared. Many others are available, but were beyond the scope of this study. We also had a relatively small numbers of subjects, which could have contributed to the low inter-test correlation. This outcome seems unlikely, however, since each of the measures had sufficient sensitivity to show a statistically significant difference by hemisphere. Another possible reason for the low correlation is that the four measures have different sensitivities to a common cerebral hemodynamic factor.. Another limitation is that not all subjects were able to complete all tests. Poor TCD bone windows are present in about 10-15% of older subjects in general, which was found in our cohort. The additional complexity of using the CO2 inhalation and finger plethysmography equipment reduced the numbers who were able to complete the VMR and DCA tests, respectively, and there were several individuals who were unable to complete the MRIs.
5. Conclusions. Toward a multidimensional model of cerebral blood flow regulation.
The consistent hemispheral asymmetry across our four measures of cerebral blood flow supports their sensitivity to unilateral carotid occlusive disease, yet the dissociation among them suggests that each needs to be considered for its unique contribution to the patient’s hemodynamic state. Reduced MFV is rarely seen in carotid stenosis less than 80%, and therefore may be used as a screening tool for consideration of more specific hemodynamic testing. VMR appears to be a good test for assessing stroke risk among those who have some hemodynamic compromise as might be demonstrated by low MFV. Whereas MFV and VMR may both be relevant to stroke risk stratification, they may not be sufficiently sensitive or specific as biomarkers for brain function such as cognition. Although VMR has been shown by some investigators to correlate with cognitive impairment in carotid stenosis, other methods such as pCASL can have specificity for cortical blood flow and therefore may be more useful for assessing hemodynamic impact on cortical brain function. Finally, DCA addresses integrity of the real-time autoregulatory system in the normal perfusion range, and may be most useful as marker of autoregulation in a pathologically changing setting such as acute stroke. Our findings suggest that additional research is needed to explore cerebral blood flow measurements as distinct entities, with unique information to elucidate cerebrovascular pathophysiology and guide clinical management.
Highlights.
Cerebral hemodynamics can be measured in several ways in unilateral high-grade carotid stenosis
Each measure showed significant asymmetry, but there was low correlation among them
Low correlation among hemodynamic measures suggests unique physiological contributions
Choice of hemodynamic measurement techniques depends on the outcome of interest
Acknowledgments
Funding sources: This work was supported by the National Institutes of Health: NINDS R01NS076277 (RSM, RML); and the Richard and Jenny Levine endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Derdeyn CP, Grubb RL, Jr., Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology. 1999 Jul 22;53(2):251–9. doi: 10.1212/wnl.53.2.251. PubMed PMID: 10430410. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann A, Mast H, Thompson JL, Sia RM, Mohr JP. Transcranial Doppler waveform blunting in severe extracranial carotid artery stenosis. Cerebrovasc Dis. 2000 Jan-Feb;10(1):33–8. doi: 10.1159/000016022. PubMed PMID: 10629344. [DOI] [PubMed] [Google Scholar]
- 3.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Transcranial Doppler hemodynamic parameters and risk of stroke: the Rotterdam study. Stroke. 2007 Sep;38(9):2453–8. doi: 10.1161/STROKEAHA.107.483073. PubMed PMID: 17673712. [DOI] [PubMed] [Google Scholar]
- 4.Bokkers RP, Bremmer JP, van Berckel BN, Lammertsma AA, Hendrikse J, Pluim JP, et al. Arterial spin labeling perfusion MRI at multiple delay times: a correlative study with H(2)(15)O positron emission tomography in patients with symptomatic carotid artery occlusion. J Cereb Blood Flow Metab. 2010 Jan;30(1):222–9. doi: 10.1038/jcbfm.2009.204. PubMed PMID: 19809464. Pubmed Central PMCID: 2949108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke. 1999 Mar;30(3):593–8. doi: 10.1161/01.str.30.3.593. PubMed PMID: 10066857. [DOI] [PubMed] [Google Scholar]
- 6.Marshall RS, Lazar RM, Young WL, Solomon RA, Joshi S, Duong DH, et al. Clinical utility of quantitative cerebral blood flow measurements during internal carotid artery test occlusions. Neurosurgery. 2002 May;50(5):996–1004. doi: 10.1097/00006123-200205000-00012. discussion -5. PubMed PMID: 11950402. [DOI] [PubMed] [Google Scholar]
- 7.Reinhard M, Schwarzer G, Briel M, Altamura C, Palazzo P, King A, et al. Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology. 2014 Oct 14;83(16):1424–31. doi: 10.1212/WNL.0000000000000888. PubMed PMID: 25217057. Pubmed Central PMCID: 4206163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diehl RR, Linden D, Lucke D, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. Stroke. 1995 Oct;26(10):1801–4. doi: 10.1161/01.str.26.10.1801. PubMed PMID: 7570728. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998 Jan;274(1 Pt 2):H233–41. doi: 10.1152/ajpheart.1998.274.1.h233. PubMed PMID: 9458872. [DOI] [PubMed] [Google Scholar]
- 10.Reinhard M, Hetzel A, Lauk M, Lucking CH. Dynamic cerebral autoregulation testing as a diagnostic tool in patients with carotid artery stenosis. Neurol Res. 2001 Jan;23(1):55–63. doi: 10.1179/016164101101198299. PubMed PMID: 11210431. Epub 2001/02/24. eng. [DOI] [PubMed] [Google Scholar]
- 11.Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovascular engineering. 2008 Mar;8(1):42–59. doi: 10.1007/s10558-007-9044-6. PubMed PMID: 18041584. [DOI] [PubMed] [Google Scholar]
- 12.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003 Dec 23;61(12):1667–72. doi: 10.1212/01.wnl.0000098934.18300.be. PubMed PMID: 14694027. [DOI] [PubMed] [Google Scholar]
- 13.Birns J, Markus H, Kalra L. Blood pressure reduction for vascular risk: is there a price to be paid? Stroke. 2005 Jun;36(6):1308–13. doi: 10.1161/01.STR.0000165901.38039.5f. PubMed PMID: 15860749. [DOI] [PubMed] [Google Scholar]
- 14.Hadjiev DI, Mineva PP. Antihypertensive treatment with cerebral hemodynamics monitoring by ultrasonography in elderly hypertensives without a history of stroke may prevent or slow down cognitive decline. A pending issue. Med Hypotheses. 2011 Mar;76(3):434–7. doi: 10.1016/j.mehy.2010.11.014. PubMed PMID: 21134723. [DOI] [PubMed] [Google Scholar]
- 15.Balestrini S, Perozzi C, Altamura C, Vernieri F, Luzzi S, Bartolini M, et al. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology. 2013 Jun 4;80(23):2145–50. doi: 10.1212/WNL.0b013e318295d71a. PubMed PMID: 23624562. [DOI] [PubMed] [Google Scholar]
- 16.Asllani I, Borogovac A, Brown TR. Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn Reson Med. 2008 Dec;60(6):1362–71. doi: 10.1002/mrm.21670. PubMed PMID: 18828149. [DOI] [PubMed] [Google Scholar]
- 17.Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008 Dec;60(6):1488–97. doi: 10.1002/mrm.21790. PubMed PMID: 19025913. Pubmed Central PMCID: 2750002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borogovac A, Habeck C, Small SA, Asllani I. Mapping brain function using a 30-day interval between baseline and activation: a novel arterial spin labeling fMRI approach. J Cereb Blood Flow Metab. 2010 Oct;30(10):1721–33. doi: 10.1038/jcbfm.2010.89. PubMed PMID: 20648039. Pubmed Central PMCID: 3023398. Epub 2010/07/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen NH, Ortega-Gutierrez S, Reccius A, Masurkar A, Huang A, Marshall RS. Comparison of Non-invasive and Invasive Arterial Blood Pressure Measurement for Assessment of Dynamic Cerebral Autoregulation. Neurocrit Care. 2013 Nov 14; doi: 10.1007/s12028-013-9898-y. PubMed PMID: 24233812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bokkers RP, van Osch MJ, van der Worp HB, de Borst GJ, Mali WP, Hendrikse J. Symptomatic carotid artery stenosis: impairment of cerebral autoregulation measured at the brain tissue level with arterial spin-labeling MR imaging. Radiology. 2010 Jul;256(1):201–8. doi: 10.1148/radiol.10091262. PubMed PMID: 20574097. [DOI] [PubMed] [Google Scholar]
- 21.Powers WJ, Zazulia AR. The use of positron emission tomography in cerebrovascular disease. Neuroimaging Clin N Am. 2003 Nov;13(4):741–58. doi: 10.1016/s1052-5149(03)00091-1. PubMed PMID: 15024958. [DOI] [PubMed] [Google Scholar]
- 22.Teng MM, Cheng HC, Kao YH, Hsu LC, Yeh TC, Hung CS, et al. MR perfusion studies of brain for patients with unilateral carotid stenosis or occlusion: evaluation of maps of “time to peak” and “percentage of baseline at peak”. J Comput Assist Tomogr. 2001 Jan-Feb;25(1):121–5. doi: 10.1097/00004728-200101000-00022. PubMed PMID: 11176306. [DOI] [PubMed] [Google Scholar]
- 23.Kluytmans M, van der Grond J, van Everdingen KJ, Klijn CJ, Kappelle LJ, Viergever MA. Cerebral hemodynamics in relation to patterns of collateral flow. Stroke. 1999 Jul;30(7):1432–9. doi: 10.1161/01.str.30.7.1432. PubMed PMID: 10390319. [DOI] [PubMed] [Google Scholar]
- 24.Tzeng YC, Ainslie PN, Cooke WH, Peebles KC, Willie CK, MacRae BA, et al. Assessment of cerebral autoregulation: the quandary of quantification. Am J Physiol Heart Circ Physiol. 2012 Sep 15;303(6):H658–71. doi: 10.1152/ajpheart.00328.2012. PubMed PMID: 22821992. [DOI] [PubMed] [Google Scholar]
- 25.Petersen NH, Ortega-Gutierrez S, Reccius A, Masurkar A, Huang A, Marshall RS. Dynamic cerebral autoregulation is transiently impaired for one week after large-vessel acute ischemic stroke. Cerebrovasc Dis. 2015;39(2):144–50. doi: 10.1159/000368595. PubMed PMID: 25661277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson SL, Blake MJ, Panerai RB, Potter JF. Dynamic but not static cerebral autoregulation is impaired in acute ischaemic stroke. Cerebrovasc Dis. 2000 Mar-Apr;10(2):126–32. doi: 10.1159/000016041. PubMed PMID: 10686451. [DOI] [PubMed] [Google Scholar]
- 27.Eames PJ, Blake MJ, Dawson SL, Panerai RB, Potter JF. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2002 Apr;72(4):467–72. doi: 10.1136/jnnp.72.4.467. PubMed PMID: 11909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo ZN, Xing Y, Wang S, Ma H, Liu J, Yang Y. Characteristics of dynamic cerebral autoregulation in cerebral small vessel disease: Diffuse and sustained. Scientific reports. 2015;5:15269. doi: 10.1038/srep15269. PubMed PMID: 26469343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gommer ED, Staals J, van Oostenbrugge RJ, Lodder J, Mess WH, Reulen JP. Dynamic cerebral autoregulation and cerebrovascular reactivity: a comparative study in lacunar infarct patients. Physiol Meas. 2008 Nov;29(11):1293–303. doi: 10.1088/0967-3334/29/11/005. PubMed PMID: 18843165. [DOI] [PubMed] [Google Scholar]
- 30.Giannopoulos S, Boden-Albala B, Choi JH, Carrera E, Doyle M, Perez T, et al. Metabolic syndrome and cerebral vasomotor reactivity. Eur J Neurol. 2010 Dec;17(12):1457–62. doi: 10.1111/j.1468-1331.2010.03087.x. PubMed PMID: 20500212. Epub 2010/05/27. eng. [DOI] [PubMed] [Google Scholar]
- 31.Huq R, Philbey CE, Mistri AK, Panerai RB, Robinson TG. Dynamic cerebral autoregulation assessed by respiratory manoeuvres in non-insulin-treated Type 2 diabetes mellitus. Diabetic medicine: a journal of the British Diabetic Association. 2012 May;29(5):609–13. doi: 10.1111/j.1464-5491.2011.03497.x. PubMed PMID: 22004530. [DOI] [PubMed] [Google Scholar]
- 32.Reinhard M, Roth M, Muller T, Czosnyka M, Timmer J, Hetzel A. Cerebral autoregulation in carotid artery occlusive disease assessed from spontaneous blood pressure fluctuations by the correlation coefficient index. Stroke. 2003 Sep;34(9):2138–44. doi: 10.1161/01.STR.0000087788.65566.AC. PubMed PMID: 12920261. Epub 2003/08/16. eng. [DOI] [PubMed] [Google Scholar]
- 33.Roc AC, Wang J, Ances BM, Liebeskind DS, Kasner SE, Detre JA. Altered hemodynamics and regional cerebral blood flow in patients with hemodynamically significant stenoses. Stroke. 2006 Feb;37(2):382–7. doi: 10.1161/01.STR.0000198807.31299.43. PubMed PMID: 16373653. [DOI] [PubMed] [Google Scholar]
- 34.Klijn CJ, Kappelle LJ, van Huffelen AC, Visser GH, Algra A, Tulleken CA, et al. Recurrent ischemia in symptomatic carotid occlusion: prognostic value of hemodynamic factors. Neurology. 2000 Dec 26;55(12):1806–12. doi: 10.1212/wnl.55.12.1806. PubMed PMID: 11134377. [DOI] [PubMed] [Google Scholar]
- 35.Yujie Q, Borogovac A, Laine A, Hirsch J, Asllani I. Tissue specific arterial spin labeling fMRI: a superior method for imaging cerebral blood flow in aging and disease. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6687–90. doi: 10.1109/EMBC.2014.6945162. PubMed PMID: 25571530. [DOI] [PubMed] [Google Scholar]
- 36.Yoshiura T, Hiwatashi A, Yamashita K, Ohyagi Y, Monji A, Takayama Y, et al. Simultaneous measurement of arterial transit time, arterial blood volume, and cerebral blood flow using arterial spin-labeling in patients with Alzheimer disease. AJNR Am J Neuroradiol. 2009 Aug;30(7):1388–93. doi: 10.3174/ajnr.A1562. PubMed PMID: 19342545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bokkers RP, van der Worp HB, Mali WP, Hendrikse J. Noninvasive MR imaging of cerebral perfusion in patients with a carotid artery stenosis. Neurology. 2009 Sep 15;73(11):869–75. doi: 10.1212/WNL.0b013e3181b7840c. PubMed PMID: 19752454. [DOI] [PubMed] [Google Scholar]
- 38.Leoni RF, Paiva FF, Kang BT, Henning EC, Nascimento GC, Tannus A, et al. Arterial spin labeling measurements of cerebral perfusion territories in experimental ischemic stroke. Translational stroke research. 2012 Mar;3(1):44–55. doi: 10.1007/s12975-011-0115-z. PubMed PMID: 24323754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avirame K, Lesemann A, List J, Witte AV, Schreiber SJ, Floel A. Cerebral autoregulation and brain networks in occlusive processes of the internal carotid artery. J Cereb Blood Flow Metab. 2015 Feb;35(2):240–7. doi: 10.1038/jcbfm.2014.190. PubMed PMID: 25388676. Pubmed Central PMCID: 4426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hess DC, Hoda MN, Khan MB. Humoral Mediators of Remote Ischemic Conditioning: Important Role of eNOS/NO/Nitrite. Acta Neurochir Suppl. 2016;121:45–8. doi: 10.1007/978-3-319-18497-5_8. PubMed PMID: 26463921. [DOI] [PubMed] [Google Scholar]
- 41.Franklin TR, Wang Z, Sciortino N, Harper D, Li Y, Hakun J, et al. Modulation of resting brain cerebral blood flow by the GABA B agonist, baclofen: a longitudinal perfusion fMRI study. Drug and alcohol dependence. 2011 Sep 1;117(2-3):176–83. doi: 10.1016/j.drugalcdep.2011.01.015. PubMed PMID: 21333466. Pubmed Central PMCID: 3348615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jing Z, Shi C, Zhu L, Xiang Y, Chen P, Xiong Z, et al. Chronic cerebral hypoperfusion induces vascular plasticity and hemodynamics but also neuronal degeneration and cognitive impairment. J Cereb Blood Flow Metab. 2015 Aug;35(8):1249–59. doi: 10.1038/jcbfm.2015.55. PubMed PMID: 25853908. Pubmed Central PMCID: 4528009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wirth M, Pichet Binette A, Brunecker P, Kobe T, Witte AV, Floel A. Divergent regional patterns of cerebral hypoperfusion and gray matter atrophy in mild cognitive impairment patients. J Cereb Blood Flow Metab. 2016 Apr 1; doi: 10.1177/0271678X16641128. PubMed PMID: 27037094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen TS, Larsen B, Herning M, Skriver EB, Lassen NA. Blood flow and vascular reactivity in collaterally perfused brain tissue. Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983 May-Jun;14(3):332–41. doi: 10.1161/01.str.14.3.332. PubMed PMID: 6658900. [DOI] [PubMed] [Google Scholar]
- 45.Kawamura Y, Meyer JS, Hiromoto H, Aoyagi M, Hashi K. Neurogenic control of cerebral blood flow in the baboon. Effects of alpha adrenergic blockade with phenoxybenzamine on cerebral autoregulation and vasomotor reactivity to changes in PaCO2. Stroke. 1974 Nov-Dec;5(6):747–58. doi: 10.1161/01.str.5.6.747. PubMed PMID: 4432254. [DOI] [PubMed] [Google Scholar]
- 46.Panerai RB, Deverson ST, Mahony P, Hayes P, Evans DH. Effects of CO2 on dynamic cerebral autoregulation measurement. Physiol Meas. 1999 Aug;20(3):265–75. doi: 10.1088/0967-3334/20/3/304. PubMed PMID: 10475580. [DOI] [PubMed] [Google Scholar]
- 47.Carrera E, Lee LK, Giannopoulos S, Marshall RS. Cerebrovascular reactivity and cerebral autoregulation in normal subjects. J Neurol Sci. 2009 Oct 15;285(1-2):191–4. doi: 10.1016/j.jns.2009.06.041. PubMed PMID: 19608202. Epub 2009/07/18. eng. [DOI] [PubMed] [Google Scholar]