Abstract
It is well established that cancer development ensues based on reciprocal interactions between genomically altered neoplastic cells and diverse populations of recruited “host” cells co-opted to support malignant progression. Among the host cells recruited into tumor microenvironments, several subtypes of myeloid cells, including macrophages, monocytes, dendritic cells, and granulocytes contribute to tumor development by providing tumor-promoting factors as well as a spectrum of molecules that suppress cytotoxic activities of T lymphocytes. Based on compelling preclinical data revealing that inhibition of critical myeloid-based programs leads to tumor suppression, novel immune-based therapies and approaches are now entering the clinic for evaluation. This review discusses mechanisms underlying protumorigenic programming of myeloid cells and discusses how targeting of these has potential to attenuate solid tumor progression via the induction and of mobilization CD8+ cytotoxic T cell immunity.
Keywords: B cell, CD8+ T cell, chemotherapy, dendritic cell, eosinophil, immunotherapy, lymphocyte, macrophage, myeloid, neutrophil, tumor microenvironment
The tumor microenvironment (TME) regulates all aspects of tumorigenesis via complex paracrine signaling programs involving initiated and/or frankly neoplastic cells, soluble and insoluble components of extracellular matrix, and resident and recruited “host” cells, where the contributions of immune cells to TMEs are now well appreciated.1 Utilizing a variety of methodologies to define immune cell complexity and functionality in combination with immune-competent mouse models of cancer development, we now understand that cancer-associated inflammation is sculpted by tissue and TMEs. This process, while representing a fundamental hallmark of cancer,2 does not represent a generic process. Instead, both the complexity and functional bioactivities of immune cell types differ within a tumor (with advancing progression) and between different tumor types.3 While myeloid cells are generally the most abundant immune cells in murine solid tumors,4 human tumors differ considerably in that lymphocytes are often more prevalent. 3,5 However, most tumors are endowed with cellular and molecular mechanisms to functionally repress productive antitumor T cell responses. Thus, identifying functionally significant targets to ameliorate these repressive mechanisms may translate into effective therapeutic strategies for treatment.
The TME: Role of Myeloid Cells
Diverse subsets of immune cells populate solid tumor TMEs. Myeloid cells, including macrophages, dendritic cells (DCs), neutrophils, monocytes, and granulocytes, dynamically regulate tumor growth and progression.3,6,7 Macrophages and/or monocytes are generally the most populous of myeloid lineage cells in developing solid tumors and play important roles in regulating both protumor and antitumor immune responses.8–10 Simply contextualized, macrophages found within TMEs represent a spectrum of variably polarized phenotypes existing within the M1/M2 paradigm.11 Although it is important to recognize that macrophage polarization is a dynamic process continually shaped by local signals, in general, immune-stimulatory macrophages variably express TH1-type mediators, including nitric oxide, interleukin 12 (IL-12) and interferon γ (IFN-γ), whereas immunesuppressive and protumorigenic macrophages tend to reflect a more TH2-skewed phenotype expressing IL-10, IL-13, IL-4, proangiogenic growth factors, and transforming growth factor β.8,12,13 Similar to tumor-promoting macrophages, tumor-associated monocytes, neutrophils, and DCs also exist within a spectrum of phenotypes encompassing both tumor-promoting and tumor-suppressive functionality.14–17 Further stratifying these subsets, the presence of mature DCs in a number of solid tumors correlates with favorable clinical outcomes, likely owing to cross-presentation capabilities and increased immunogenicity.18,19 Targeted therapies aimed at repolarizing/programming TMEs to favor TH1 effector pathways have now entered the clinic and are at the forefront of modern clinical cancer research. Because myeloid cells orchestrate much of their protumorigenic biology in concert with select lymphocyte populations,20 this review explores aspects of myeloid-lymphocyte interaction to better understand how myeloid-based targeted therapy may be beneficial in mitigating immune-suppressive TMEs to instead foster cytotoxic T cell activities.
Macrophages, Malignancy, and Response to Therapy
Macrophages populate TMEs, and although not absolute, poor patient prognosis has been correlated with increased macrophage presence in breast, uterine, liver, and bladder carcinoma.4,21 Conversely, favorable prognosis has been associated with increased macrophage infiltration in non–small cell lung cancer, prostate, colorectal, and gastric cancers.21,22 Whether these distinctions reflect true differences in macrophage biology or conversely arise because of discordant detection techniques is unclear. In breast cancers (BCs), multiple studies have reported that macrophage presence in stroma correlates with aggressive disease23 and outcome.24,25 Macrophages are recruited into tumors following activation of colony-stimulating factor-1 receptor (CSF-1R) by either CSF-1 or IL-34, two high-affinity ligands for CSF-1R.26 In addition, there is evidence indicating that the chemokine CCL2 also plays a role in macrophage recruitment.27,28 Notably, a CSF-1-response gene expression signature has been identified in 17% to 25% of BCs associated with decreased expression of estrogen receptor and progesterone receptor.29 In addition, in two independent BC cohorts, a correlation between intratumoral macrophage presence and specific tumor features (high grade, hormone receptor negativity, basal-like subtype, and increased risk of death) has been observed.25 Perhaps unsurprisingly, serum concentrations of CSF-1 correlate positively with tumor size and predict poor survival in women with BC.30
Activation of CSF-1R modulates a number of biological programs regulated by macrophages including angiogenesis, lymphogenesis, matrix remodeling, and fibrosis.8,9 Importantly, early studies from the Pollard laboratory revealed a critical role for macrophages in also regulating mammary cancer metastasis,31 thus providing biological rationale for targeting macrophages to minimize late-stage disease progression. To test this hypothesis, we evaluated the use of CSF-1 neutralizing monoclonal antibodies (αCSF-1) and small molecule CSF-1R inhibitors to suppress macrophage survival and/or presence in mammary tumors in combination with chemotherapy or radiation therapy. Results from these studies revealed reduced primary tumor growth, 85% reduction in pulmonary metastases, and increased overall survival in mice receiving combination therapy.24,32–34 Furthermore, increased chemosensitivity and radiation sensitivity using combined therapy were associated with induction of antitumor immune responses and CD8+ T cell infiltration of tumors.24,32,33 A role for macrophage signaling protein acting through its transmembrane receptor kinase, RON, in BC has also been elucidated wherein activation of RONin macrophages regulates tumor growth, angiogenesis, and metastasis by favoring conversion of micrometastatic lesions to overt metastases by suppressing antitumor immune responses. Blockade of RON potentiates tumor-specific CD8+ T cell responses indicating that RON inhibitors may improve outcomes for BC patients.35–37 Collectively, these findings highlight an exciting opportunity: Therapies that reduce presence or immunosuppressive status of macrophages by blocking CSF-1/CSF-1R or macrophage signaling protein/RON signaling may trigger antitumor immunity to suppress tumor growth when administered in combination with cytotoxic therapies. Additional support for this approach comes from preclinical studies where macrophage targeting improved outcomes as monotherapy or in combination with cytotoxic therapy in glioma,38 prostate,39 and pancreas cancer.40–42 Colony-stimulating factor-1 receptor blockade also improved antitumor efficacy of immune checkpoint blockade therapies in pancreatic cancer43 as well as adoptive T cell therapy in melanoma.44 Importantly, administration of RG7155, a monoclonal antibody inhibiting CSF-1R activation, to patients with diffuse-type giant cell tumors reduced CSF-1R+CD163+ macrophages and translated into clinical objective responses.45 Because acquired resistance to cytotoxic therapies remains a significant and common clinical issue in oncology, the possibility of reversing this process is extremely attractive, and in contrast to reports of increased metastasis following CCL2-blockade,46 targeted inhibition of CSF-1/CSF-1R signaling does not appear to drive adverse rebound tumor growth.
While targeting macrophages with CSF-1R or RON antagonists represents a significant therapeutic opportunity, they are unlikely to be effective for all macrophage-rich tumors. As we have reported,5 individual tumors vary with respect to their balance of immune-suppressive versus immunestimulatory cells. Thus, understanding immune contexture represents a major goal for these approaches going forward. We reported that human BCs vary with respect to proportions of CD68+ macrophages, CD4+ and CD8+ T cells wherein ratios correlate with overall survival, progression-free survival, and pathologic complete response to therapy with the most significant stratification in HER2+ and basal/triple-negative disease.24,32 Thus, patients with high proportions of CD8+ T cells exhibit improved overall and progression-free survival over tumors with high CD68+ macrophages and CD4+ T cells.24 Similar results have been reported for esophageal cancers where high densities of macrophages correlate with poor response to chemotherapy.47
Immunohistochemical analyses evaluating immune contexture have emerged as a significant integrating concept to understand tumors.48 However, complete characterization of immune complexity requires single cell-level quantitation of multiple epitopes.5 As such, quantitative Immunoscore identification has emerged as a powerful prognostic tool to evaluate tumors and predict response to therapy.49 Indeed, a recent meta-analysis revealed that increased CD8+ T cell tumor infiltration is a strong prognostic factor predicting disease-free and overall survival for various solid tumors including melanoma, breast, bladder, pancreatic, lung, head and neck, and ovarian carcinoma.50 These data, in combination with the fact that macrophage infiltration of solid tumors is often associated with poor prognosis19 and that macrophages also play a role in inhibiting cytotoxic T cell functions,24,51 emphasize the importance of myeloid-lymphocyte interactions in cancer.
Functional Roles for DCs
Tumor-associated DCs can be directly impacted by factors secreted by neoplastic cells that render these professional antigen-presenting cells immature and refractory to stimulation via mechanisms linked to IL-10.52,53 Similar to macrophages, DCs represent a target for developing immunotherapeutic strategies because of their ability to elicit potent T cell responses directed at tumor antigens.52,54 For example, exogenous stimulation of dectin 1, an innate immune receptor on DCs associated with TH1 polarization, successfully reprogrammed tumor-infiltrating DCs and inhibited tumor progression in a humanized mouse model of BC.14 In this model, DC-derived transforming growth factor β led to homing and expansion of mucosal CD103+ T cells resulting in stimulation of T cell–mediated antitumor immunity.14 Indeed, engagement of CD103+ cytotoxic T lymphocytes with tumor cell expression of E-cadherin is linked to antitumor lytic function of T cells.55 Generation of tumor-specific T cells relies on the ability of mature DCs to cross-present tumor antigens.52 Recent studies revealed that a sparse population of CD103+ tumor-associated DCs were significant cytotoxic T lymphocyte stimulators in a murine model of mammary carcinoma and were essential for controlling T cell– mediated tumor regression in a murine EG7.1 thymoma model.19 Perhaps most importantly, FLT3 ligand–expressing B16 melanoma drove expansion of rare CD103+ cells, revealing potential for targeted therapy to enhance therapeutic immunity against cancer.19 Supporting the significance of CD103+ DCs in tumors, we reported that IL-4–activated macrophages express high levels of IL-10, which directly suppresses IL-12 production by intratumoral DCs, DC maturation, antigen cross presentation, and CD8+ T cell functionality.32 Therapeutic blockade of CSF-1/CSF-1R24,32,34 improved response to chemotherapy and radiation therapy and was associated with expansion of mature CD103+ DCs in tumor stroma by IL-12 and CD8+ T cell–dependent mechanisms.32 The importance of this population is further reinforced by the fact that an increased mRNA ratio of CD103+ to CD103− is associated with increased overall survival across all human cancers.19 However, contrary to the antitumor role of this rare population of mature DCs, a protumorigenic role for mature DCs in promoting cancer progression has also been reported. Studies from Aspord and colleagues revealed a novel mechanism whereby BC directs DC function to promote protumor polarization of IL-13–secreting CD4+ T cells.56
Neutrophils and T Cells
Neutrophil abundance in solid tumors predicts poor survival in patients with various solid tumor types, including BC57,58 and head and neck squamous cell carcinoma (SCC).59 Although typically not as abundant as macrophages, neutrophils are present in TMEs and are important in orchestrating T cell responses.60 For example, although neutrophils can function as antigen-presenting cells to stimulate T cell immunity,61 degranulation results in the release of factors that suppress ex vivo T cell function.62 Using a de novo murine model of spontaneous mammary cancer metastasis, Coffelt and colleagues recently revealed that IL-1β elicits IL-17 expression from γδ T cells, resulting in systemic, granulocyte CSF (G-CSF)–dependent expansion and polarization of neutrophils in mice bearing mammary tumors63. These tumor-associated neutrophils acquired the ability to suppress cytotoxic T lymphocytes and therefore facilitated establishment of metastases. Although neutralization of IL-17 and granulocyte CSF, G-CSF or depletion of γδ T cells did not influence primary tumor progression, these therapeutic strategies prevented neutrophil accumulation, minimized T cell suppression, and were associated with reduced metastatic burden.63 These data indicate that biological targeting of this axis may represent a novel strategy to limit latestage disease. Similarly, protumorigenic and immunosuppressive properties have been attributed to various populations of immature myeloid-derived cells (reviewed elsewhere).64 Taken together, these data collectively reveal that strategies to bolster T cell–mediated antitumor immunity can be achieved by targeting protumorigenic myeloid-based pathways acting to functionally suppress immunogenic T cell responses. Importantly, a number of the molecular mediators described above are targetable and provide an opportunity for therapeutic intervention.
A Role for Eosinophils Emerges
Conventionally, eosinophils are mediators of the innate immune response with important roles in wound repair and resolution of parasitic infection.65 Eosinophils, like monocytes and macrophages, are cytotoxic immune effector cells present within TMEs and play divergent roles in cancer progression. There is emerging evidence that tumor-associated tissue eosinophilia is associated with improved prognosis for a number of malignancies including gastrointestinal cancers66, bladder67 and prostate carcinomas.68 Conversely, the presence of tumor-associated tissue eosinophilia in Hodgkin lymphoma,69 cervical carcinoma,70 and oral SCC71 is associated with poor outcome. The root of this discordance may be the fact that, like macrophages, eosinophils exist in variably polarized states and produce TH1- and TH2-type factors,72 and thus, evaluating their presence without also knowing the complexity of the TME diminishes predictive value.73
The mechanisms attributed to the tumoricidal properties of eosinophils have not been extensively investigated. Traditionally, degranulation and release of cytotoxic proteins have been proposed as a method by which eosinophils mediate tumor rejection.74,75 However, the importance of eosinophils in regulating both the innate and adaptive immune response,75 coupled with the importance of myeloid-lymphocyte crosstalk in mediating cancer progression, provides support for studying the role of eosinophils in regulating cancer progression.65 Indeed, it was recently reported that tumor-associated eosinophils mediate cancer rejection via orchestrating tumor-specific CD8+ T cell infiltration. In addition to recruiting lymphocytes, eosinophils contribute to tumor stasis by normalizing tumor vasculature and repolarizing macrophages toward an M1 phenotype.73 Therapeutic targets promoting generation of TH1-like tumor-homing eosinophils may therefore be a novel immunotherapeutic strategy to bolster CD8+ T cell–mediated tumor cytotoxicity.
Lymphocyte-Directed Myeloid Programming CD4+ T Cells and Myeloid Cells
The association between CD4+ T cell populations and cancer progression is less definitive as there are reports of both beneficial and poor clinical outcomes associated with CD4+ T cell infiltration, even within the same solid tumor type.50 These divergent phenotypes are likely a result of the unique lineage heterogeneity of CD4+ T cells imparting different effects on tumor growth in context- and tissue-dependent manners.76 Whereas TH1-polarized CD4+ cells aid in orchestration of antitumor responses by enhancing cytotoxicity of CD8+ T cells and macrophages,77 TH2-polarized CD4+ T cells function to suppress cell-mediated antitumor immunity, thereby promoting cancer development.33 CD4+ T cells heavily infiltrate BCs5 and express IFN-γ and type 2 cytokines, IL-4 and IL-13, in response to DC-derived factors.56,78 In this context, IL-4/IL-13–expressing CD4+ T cells promote metastasis of mammary carcinomas by activating protumor and TH2 properties of macrophages and monocytes79 by cathepsin-dependent mechanisms.80,81 Therapeutic blockade of either IL-4 or IL-13 significantly improved response to chemotherapy and radiation therapy by minimizing macrophage TH2-effector functions, resulting in CD8+ T cell–dependent antitumor immunity.33 On the other hand, therapeutic efficacy of extracorporeal photochemotherapy involves CD4+ T cell–induced secretion of monocyte-derived IL-8.82 In addition to fostering T cell chemotaxis, IL-8 suppresses CD4+ T cell–derived IL-4,83 thus indicating that cross-talk between CD4+ T cells and myeloid cells can modulate humoral versus cell-mediated responses. Moreover, cell-to-cell contact between activated CD4+ T cells and blood-derived monocytes inhibits monocyte-derived IL-12 release, thereby leading to impaired TH1 responses.84 Together, these data reveal that CD4+ lymphocytes are capable of influencing myeloid cells and that this cross-talk can either promote or quell programs contributing to malignancy.
B Cell and Humoral Regulation of Myeloid-Associated Protumorigenic Programs
As a component of the adaptive immune system, B cells have traditionally been recognized for their role in immunoglobulin (Ig) production, cytokine release, and antigen presentation. Indeed, contributions of B cells to solid tumor progression have only recently begun to be investigated.85,86 Interestingly, a 1993 study, which sought to investigate the role of B cells in suppression of transplantation immunity, revealed that tumor growth in B cell– deficient mice was slowed.87 Later, murine studies using anti-IgM–mediated partial B-cell depletion revealed a role for B cells in colon carcinoma and metastasis, although the mechanism by which B cells contributed to malignancy remained obscure.88 A more complete understanding of the mechanisms involved in B cell–mediated carcinogenesis was revealed using mouse models of SCC development wherein genetic deficiency of B lymphocytes slowed malignant progression and limited recruitment of myeloid cells.89,90 Importantly, adoptive transfer of educated B cells or serum from tumor-prone mice into B and T cell–deficient mice restored characteristics of myeloid cell inflammation and reestablished malignancy, thereby revealing an important role for B cells and their soluble mediators in SCC development.89,90 Indeed, B cell–derived circulating IgGs activate Ig receptors (FcγR) on resident and recruited myeloid cells and promote TH2-like properties.91 B cells promoting tumor development have similarly been identified in melanoma92 as well as prostate cancer.93,94
The notion that an effective immunotherapy would act to repolarize immune cell contributions of TMEs toward TH1-like phenotypes is enticing and presents an exciting opportunity to investigate targeted, combinatorial therapies. Importantly, therapies impacting B cells may also have a significant role in solid tumors. Indeed, recent studies in preclinical mouse cancer models have revealed efficacy of B cell depletion as either monotherapy93,95 or in combination with cytotoxic therapy96 by mechanisms involving reprogramming myeloid cells toward TH1-effector phenotypes and bolstering CD8+ T cell–dependent responses.
The concept that T cells become TH1 polarized in the absence of B cells is well documented,97–99 although a mechanism through which this occurs in the context of tumor immunity requires further elucidation. In vitro tumor cell challenge via coculture with splenic cells from B cell–deficient versus B cell–proficient mice revealed that IFN-γ release from CD8+ and natural killer cells was increased when B cells were absent, whereas presence of B cells or B cell–derived IL-10 was associated with reduced IFN-γ.97 Although these in vitro studies indicate a direct effect for B cells in directing T cell responses, the role of myeloid cells as mediators of these responses is now clear91,96 and indicates that therapies targeting common pathways in B cells and/or myeloid cells, such as spleen tyrosine kinase, Bruton tyrosine kinase (Btk), or phosphatidylinositol 3-kinase (PI3K) may be efficacious in solid tumors.85 Indeed, targeted inhibition of Btk, using a small molecule inhibitor,100 although an effective therapy for several B cell malignancies,101–104 has also been reported to provide a survival advantage in mouse models of de novo pancreatic adenocarcinoma105 and neuroendocrine cancer,106 as well as in several subcutaneous tumor models107 where a common feature is reduced inflammation and inflammatory desmoplasia, and evidence of macrophage repolarization.108
The δ isoform of PI3K (PI3Kδ) is an attractive target for hematologic malignancies with the specific inhibitor idelalisib showing clinical benefit for patients with relapsed/refractory chronic lymphocytic leukemia109 and lymphoma.110 The restricted expression of PI3Kδ to immune cells111 renders this enzyme an appealing target for immunotherapy as compared with use of pan-PI3K inhibitors, which may elicit nonspecific and undesirable effects. Whether PI3Kδ plays a role in solid tumor malignancy remains to be definitively established, but syngeneic models of mammary carcinoma, Lewis lung carcinoma, melanoma, thymoma, and pancreatic ductal adenocarcinoma revealed that genetic inactivation of PI3Kδ yielded reduction of tumor burden and metastases associated with suppression of T-regulatory function and increased CD8+ T cell–mediated immunity.112 Given that the PI3Kγ isoform is trophic for myeloid cells and regulates their effector functions in tumors,113 it will be interesting to reveal how targeted blockade of individual or multiple PI3K enzymes translates to solid tumor therapy.
Looking Forward to Myeloid-Targeted Therapies
There is now overwhelming evidence to support the notion that therapeutic targeting of critical myeloid-based molecules impacts solid tumor progression and may provide survival benefits in the clinic (Fig. 1). Whether these approaches will be efficacious as monotherapies or instead will require combinatorial approaches remains unclear (Table 1). Based on the experimental data at hand, it seems reasonable to anticipate that combinatorial approaches will be more efficacious, especially considering the fact that antigens are released by dying tumor cells following cytotoxic therapy114,115 and could be critical for intratumoral antigen presentation to CD8+ T cells for durable tumor repression. Given that some myeloid-targeted approaches (e.g., CSF-1 blockade) elicit distinct effects dependent on tumor type, an important aspect to be considered as these approaches enter the clinic is to not only identify tumor types likely to respond, but also identify stratification biomarkers for patient selection so as to enroll patients most likely to benefit. In so far as targeting microenvironmental pathways is concerned, much can be learned from historic approaches targeting TME components; protease inhibitors entered the clinic with compelling preclinical data but failed to demonstrate efficacy, largely owing to lack of tissue/tumor-type selectivity or validated biomarker assessments to guide their use.116 And while angiogenesis inhibitors have seen some positive responses leading to limited Food and Drug Administration approval, these are limited to select tumor types and not broadly efficacious.117–119
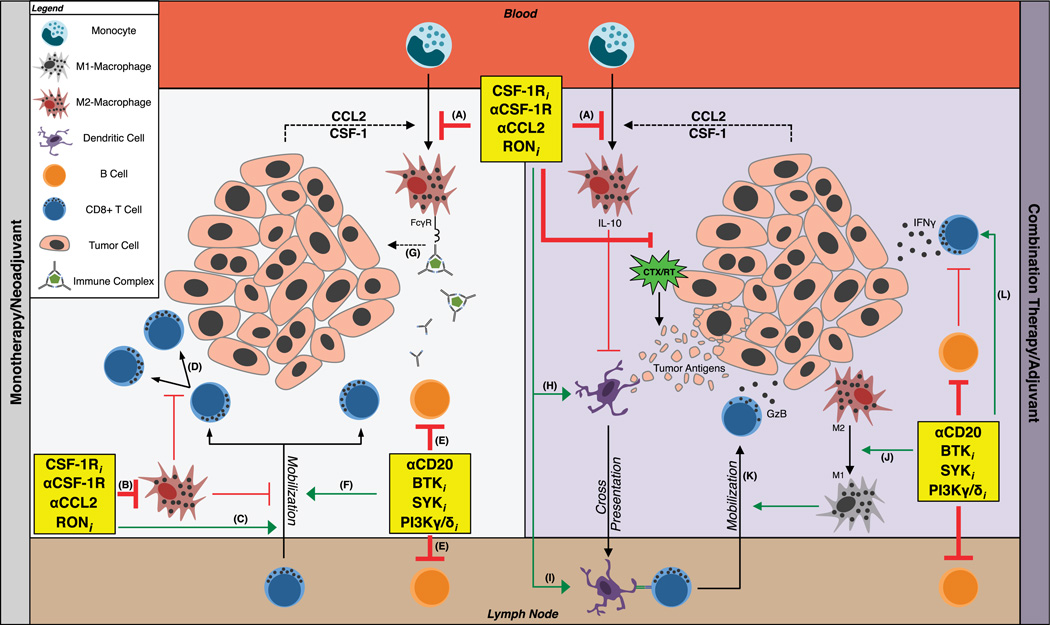
FIGURE 1.
Strategies targeting myeloid cell–lymphocyte interactions to promote antitumor immunity. A protumorigenic TME is fostered by signals (dashed arrows) originating from tumor cells and myeloid-lymphocyte interactions. Therapies designed to prevent myeloid cell recruitment from blood (red box; A) to TMEs or inhibit macrophage survival (B) lead to mobilization (C) and proliferation (D) of CD8+ T cells, which function to inhibit tumor growth. Inhibition of B cell signaling (E) slows malignant progression via mobilization of antitumor CD8+ T cells (F) and through inhibition of myeloid-associated tumor-promoting pathways (G). The antitumor efficacy of these therapies may be amplified when used in combination with chemotherapy/radiation (CTX/RT) therapy (purple box) as compared with monotherapy (gray box). In the absence of tumor-associated macrophages (A, B), CTX/RT-induced release of tumor antigens stimulates DC maturation (H) and trafficking to regional lymph nodes (brown box) where cross-presentation (I) to CD8+ T cells occurs. In this context, macrophage depletion (A, B) and/or therapies associated with M2-to-M1 repolarization (J) mobilize granzyme B (K) and interferon-secreting (L) CD8+ T cells to TMEs whereupon these cells mount cytolytic attacks on tumor cells. Green arrows indicate propagation of therapy-induced antitumor programs via mechanisms denoted by thick red lines. Thin red lines represent pre-established inhibitory pathways. FcγR indicates Fc γ receptor; GzB, granzyme B; CTX, chemotherapy; RT, radiation therapy; IL-10, interleukin-10; IFN-γ, interferon γ; RONi, receptor tyrosine kinase RON inhibitor; SYKi, spleen tyrosine kinase inhibitor; Btki, Btk inhibitor; CSF-1Ri, CSF-1R inhibitor; PI3Kγ/δi, PI3Kγ/δ inhibitor.
TABLE 1.
Preclinical Data Supporting Myeloid Cell–Targeted Therapy as Therapeutic Strategies in Solid Tumors
| Name | Target | Mechanism | Effective as Monotherapy? | Tumor Type | Antitumor Effects | Ref. |
|---|---|---|---|---|---|---|
| BLZ945 | CSF-1R inhibition | Repolarized MΦ to M1-like | Y | GBM | Blocked glioma progression and improved survival |
(Pyonteck, 38) |
| GW2580 | CSF-1R inhibition | TIM depletion | N: combined with radiotherapy |
Prostate | Enhanced radiation-induced suppression of tumor growth |
(Xu, 39) |
| PLX3397 | CSF-1R inhibition | Macrophage depletion enhanced antitumor CD8+ T cell–dependent chemotherapy cytotoxicity |
N: combined with paclitaxel |
PyMT mammary | Enhanced chemotherapy-induced slowing of tumor growth, reduced lung metastasis, and increased survival |
(DeNardo, 24) |
| BLZ945 | CSF-1R inhibition | Slowed TAM turnover, reduced macrophage recruitment, and increased CD8+ T cell tumor infiltration |
Y | PyMT mammary, HPV16 cervical |
Inhibited tumor growth | (Strachan, 34) |
| PLX3397 | CSF-1R inhibition | Reduced TIMs and skewed population of MΦ to MHC1Ihi |
N: combined with ACT |
Melanoma | Improved ACT and reduced tumor growth due to improved T cell effector function |
(Mok, 44) |
| GW2580 PLX3397 αCSF1 |
CSF-1/CSF-1R blockade |
Depleted TAMs, enhanced antigen presentation and induced T cell responses |
N: combined with PD-1 and CTLA4 antagonists |
PDAC | Enhanced checkpoint-based immunotherapies to limit PDAC progression |
(Zhu, 43) |
| GW2580 PLX3397 PF-04136309 |
CSF-1R inhibition + CCR2 inhibition |
Depleted TAMs and inflammatory monocytes |
N: combined with gemcitabine |
PDAC | TAM depletion enhanced response to chemotherapy and reduced metastasis via increased antitumor T cell responses |
(Mitchem, 41) |
| αCCL2 | CCL2/CCR2 signaling blockade |
Reduced PSA levels and slowed tumor growth in bone |
Y/N: antitumor effect greater when combined with docetaxel |
Prostate cancer growth in bone |
Enhanced docetaxel-mediated inhibition of prostate cancer cell growth in bone |
(Kirk, 126) |
| αCCL2 | CCL2/CCR2 signaling blockade |
Blocked inflammatory monocyte recruitment to metastatic sites |
Y | PyMT mammary | Reduced metastatic burden and increased survival |
(Qian, 27) |
| Ibrutinib | Btk | Enhanced antitumor CD8+ T cell-mediated immunity induced by checkpoint blockade |
N: combined with anti–PD-L1 therapy |
4T1 mammary, CT26 colon |
Enhanced anti–PD-L1–mediated reduction in tumor growth and metastasis; combination therapy induced long-term memory and blunted tumor regrowth in rechallenge studies |
(Sagiv-Barfi, 107) |
| PI-3065 | PI3Kδ | Reduced Treg population and increased tumor CD8+ T cell infiltration |
Y | 4T1 mammary, KPC pancreatic |
Suppressed tumor growth and metastasis | (Ali, 112) |
| TG100-115 AS605240 |
PI3Kγ | Inhibited integrin α4β1 activation; impaired myeloid/macrophage trafficking; reduced inflammation and angiogenesis |
Y | Lewis lung carcinoma, PyMT mammary |
Suppressed inflammation, angiogenesis, and tumor growth |
(Schmid, 127) |
ACT indicates adoptive cell therapy; Btk, Bruton tyrosine kinase; CCL2, chemokine (C-C motif) ligand 2; CCR2, C-C chemokine receptor type 2; CTLA4, cytotoxic T-lymphocyte—associated protein 4; GBM, glioblastoma multiforme; HPV, human papillomavirus; MΦ, macrophage; MHC II, major histocompatibility complex class II; PD-1, programmed death-1; PDAC, pancreatic ductal adenocarcinoma; PD-L1, programmed death ligand-1; PI3K, phosphatidylinositide 3-kinase; PSA, prostate-specific antigen; PyMT, polyoma middle T; TAM, tumor associated macrophage; Treg, regulatory T cell; TIM, tumor-infiltrating myeloid cell; Y, yes; N, no.
Other significant issues that must be addressed with myeloid-based approaches involve timing and duration of therapy. Neoadjuvant versus adjuvant responses may be quite distinct because primary tumor responses are different when compared with responses of metastatic tumors. Given that immune responses vary based on organ-specific rules hardwired into tissues to regulate acute versus chronic inflammation and control autoimmune pathology,3 it is imperative to determine the optimal approach for administration of these myeloid-based therapies to elicit maximum clinical benefit. Because several of these agents are now undergoing clinical evaluation,120 these are significant issues to be addressed if successful deployment of these approaches is to be achieved going forward.
The recent clinical successes of immune checkpoint inhibitors, including ipilimumab (targeting cytotoxic T-lymphocyte– associated protein 4), nivolumab, and pembrolizumab (targeting the programmed death- 1 pathways), for treatment of melanoma and non–small cell lung cancer establish immunotherapy as tangible therapeutic approaches.121–125 A major issue to be resolved is to determine if myeloid-based therapeutics will be efficacious as monotherapies or will instead require combination with standard-of-care cytotoxics or checkpoint inhibitors. This endeavor will reveal which scenario results in durable long-term responses that will significantly improve the lives of patients with cancer over the available therapeutic options.
Acknowledgments
The authors thank members of the Coussens laboratory for critical insight and discussions and all authors contributing to studies discussed herein but not mentioned because of space consideration.
T.R.M. was supported by the American Cancer Society-Friends of Rob Kinas, the Medical Research Foundation, and the Cathy and Jim Rudd Career Development Award for Cancer Research. L.M.C. acknowledges support from the NIH/NCI, DOD BCRP Era of Hope Scholar Expansion Award, Susan B. Komen Foundation, Brenden-Colson Center for Pancreatic Health, and Stand Up To Cancer - Lustgarten Foundation Pancreatic Cancer Convergence Dream Team Translational Research Grant (Grant Number: SU2C–AACR-DT14-14). The authors also acknowledge support from the OHSU Knight Cancer Institute.
Footnotes
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 5.Ruffell B, Au A, Rugo HS, et al. Leukocyte composition of human breast cancer. Proc Natl Acad Sci USA. 2012;109:2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer developement. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 7.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills CD, Kincaid K, Alt JM, et al. M-1/M-2 macrophages and the TH1/ TH2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 12.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu TC, Xu K, Banchereau R, et al. Reprogramming tumor-infiltrating dendritic cells for CD103+CD8+ mucosal T-cell differentiation and breast cancer rejection. Cancer Immunol Res. 2014;2:487–500. doi: 10.1158/2326-6066.CIR-13-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilliams M, Ginhoux F, Jakubzick C, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palucka K, Ueno H, Fay J, et al. Dendritic cells and immunity against cancer. J Intern Med. 2011;269:64–73. doi: 10.1111/j.1365-2796.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Shurin GV, Peiyuan Z, et al. Dendritic cells in the cancer microenvironment. J Cancer. 2013;4:36–44. doi: 10.7150/jca.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014;105:1–8. doi: 10.1111/cas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DW, Min HS, Lee KH, et al. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br J Cancer. 2008;98:1118–1124. doi: 10.1038/sj.bjc.6604256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin DN, Boersma BJ, Yi M, et al. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One. 2009;4:e4531. doi: 10.1371/journal.pone.0004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell MJ, Tonlaar NY, Garwood ER, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011;128:703–711. doi: 10.1007/s10549-010-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chihara T, Suzu S, Hassan R, et al. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ. 2010;17:1917–1927. doi: 10.1038/cdd.2010.60. [DOI] [PubMed] [Google Scholar]
- 27.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Beck AH, Espinosa I, Edris B, et al. The macrophage colony-stimulating factor 1 response signature in breast carcinoma. Clin Cancer Res. 2009;15:778–787. doi: 10.1158/1078-0432.CCR-08-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aharinejad S, Salama M, Paulus P, et al. Elevated CSF1 serum concentration predicts poor overall survival in women with early breast cancer. Endocr Relat Cancer. 2013;20:777–783. doi: 10.1530/ERC-13-0198. [DOI] [PubMed] [Google Scholar]
- 31.Lin EY, Nguyen AV, Russell RG, et al. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruffell B, Chang-Strachan D, Chan V, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiao SL, Ruffell B, DeNardo DG, et al. TH2-polarized CD4+ T cells and macrophages limit efficacy of radiotherapy. Cancer Immunol Res. 2015;3:518–525. doi: 10.1158/2326-6066.CIR-14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strachan DC, Ruffell B, Oei Y, et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology. 2013;2:e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kretschmann KL, Eyob H, Buys SS, et al. The macrophage stimulating protein/RON pathway as a potential therapeutic target to impede multiple mechanisms involved in breast cancer progression. Curr Drug Targets. 2010;11:1157–1168. doi: 10.2174/138945010792006825. [DOI] [PubMed] [Google Scholar]
- 36.Eyob H, Ekiz HA, Welm AL. RON promotes the metastatic spread of breast carcinomas by subverting antitumor immune responses. Oncoimmunology. 2013;2:e25670. doi: 10.4161/onci.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eyob H, Ekiz HA, Derose YS, et al. Inhibition of RON kinase blocks conversion of micrometastases to overt metastases by boosting antitumor immunity. Cancer Discov. 2013;3:751–760. doi: 10.1158/2159-8290.CD-12-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Escamilla J, Mok S, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amit M, Gil Z. Macrophages increase the resistance of pancreatic adenocarcinoma cells to gemcitabine by upregulating cytidine deaminase. Oncoimmunology. 2013;2:e27231. doi: 10.4161/onci.27231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pyonteck SM, Gadea BB, Wang HW, et al. Deficiency of the macrophage growth factor CSF-1 disrupts pancreatic neuroendocrine tumor development. Oncogene. 2012;31:1459–1467. doi: 10.1038/onc.2011.337. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mok S, Koya RC, Tsui C, et al. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 2014;74:153–161. doi: 10.1158/0008-5472.CAN-13-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Bonapace L, Coissieux MM, Wyckoff J, et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 47.Sugimura K, Miyata H, Tanaka K, et al. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111:752–759. doi: 10.1002/jso.23881. [DOI] [PubMed] [Google Scholar]
- 48.Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Galon J, Pages F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 51.Lepique AP, Daghastanli KR, Cuccovia IM, et al. HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin Cancer Res. 2009;15:4391–4400. doi: 10.1158/1078-0432.CCR-09-0489. [DOI] [PubMed] [Google Scholar]
- 52.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vicari AP, Chiodoni C, Vaure C, et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196:541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palucka K, Coussens LM, O’Shaughnessy J. Dendritic cells, inflammation, and breast cancer. Cancer J. 2013;19:511–516. doi: 10.1097/PPO.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Floc’h A, Jalil A, Vergnon I, et al. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aspord C, Pedroza-Gonzalez A, Gallegos M, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 58.Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013;16:55–59. doi: 10.4048/jbc.2013.16.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trellakis S, Bruderek K, Dumitru CA, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer. 2011;129:2183–2193. doi: 10.1002/ijc.25892. [DOI] [PubMed] [Google Scholar]
- 60.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 61.Abi Abdallah DS, Egan CE, Butcher BA, et al. Mouse neutrophils are professional antigen-presenting cells programmed to instruct TH1 and TH17 T-cell differentiation. Int Immunol. 2011;23:317–326. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sippel TR, White J, Nag K, et al. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin Cancer Res. 2011;17:6992–7002. doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- 63.Coffelt SB, Kersten K, Doornebal CW, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Condamine T, Ramachandran I, Youn JI, et al. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110. doi: 10.1146/annurev-med-051013-052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2:1–8. doi: 10.1158/2326-6066.CIR-13-0196. [DOI] [PubMed] [Google Scholar]
- 66.Pretlow TP, Keith EF, Cryar AK, et al. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res. 1983;43:2997–3000. [PubMed] [Google Scholar]
- 67.Flamm J. Tumor-associated tissue inflammatory reaction and eosinophilia in primary superficial bladder cancer. Urology. 1992;40:180–185. doi: 10.1016/0090-4295(92)90524-z. [DOI] [PubMed] [Google Scholar]
- 68.Luna-More S, Florez P, Ayala A, et al. Neutral and acid mucins and eosinophil and argyrophil crystalloids in carcinoma and atypical adenomatous hyperplasia of the prostate. Pathol Res Pract. 1997;193:291–298. doi: 10.1016/S0344-0338(97)80006-4. [DOI] [PubMed] [Google Scholar]
- 69.von Wasielewski R, Seth S, Franklin J, et al. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin’s disease, allowing for known prognostic factors. Blood. 2000;95:1207–1213. [PubMed] [Google Scholar]
- 70.van Driel WJ, Hogendoorn PC, Jansen FW, et al. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum Pathol. 1996;27:904–911. doi: 10.1016/s0046-8177(96)90216-6. [DOI] [PubMed] [Google Scholar]
- 71.Horiuchi K, Mishima K, Ohsawa M, et al. Prognostic factors for well-differentiated squamous cell carcinoma in the oral cavity with emphasis on immunohistochemical evaluation. J Surg Oncol. 1993;53:92–96. doi: 10.1002/jso.2930530209. [DOI] [PubMed] [Google Scholar]
- 72.Liu LY, Bates ME, Jarjour NN, et al. Generation of TH1 and TH2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol. 2007;179:4840–4848. doi: 10.4049/jimmunol.179.7.4840. [DOI] [PubMed] [Google Scholar]
- 73.Carretero R, Sektioglu IM, Garbi N, et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 74.Huland E, Huland H. Tumor-associated eosinophilia in interleukin-2-treated patients: evidence of toxic eosinophil degranulation on bladder cancer cells. J Cancer Res Clin Oncol. 1992;118:463–467. doi: 10.1007/BF01629431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gatault S, Legrand F, Delbeke M, et al. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. 2012;61:1527–1534. doi: 10.1007/s00262-012-1288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Nishimura T, Iwakabe K, Sekimoto M, et al. Distinct role of antigen-specific T helper type 1 (TH1) and TH2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–627. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bell D, Chomarat P, Broyles D, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gocheva V, Wang HW, Gadea BB, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shree T, Olson OC, Elie BT, et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25:2465–2479. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tokura Y, Seo N, Tomida M, et al. Augmentation of monocyte interleukin-8 production by psoralen/UVA-treated CD4+ T cells. Exp Dermatol. 2002;11:564–572. doi: 10.1034/j.1600-0625.2002.110609.x. [DOI] [PubMed] [Google Scholar]
- 83.Gesser B, Lund M, Lohse N, et al. IL-8 induces T cell chemotaxis, suppresses IL-4, and up-regulates IL-8 production by CD4+ T cells. J Leukoc Biol. 1996;59:407–411. doi: 10.1002/jlb.59.3.407. [DOI] [PubMed] [Google Scholar]
- 84.Wittmann M, Alter M, Stunkel T, et al. Cell-to-cell contact between activated CD4+ T lymphocytes and unprimed monocytes interferes with a TH1 response. J Allergy Clin Immunol. 2004;114:965–973. doi: 10.1016/j.jaci.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 85.Gunderson AJ, Coussens LM. B cells and their mediators as targets for therapy in solid tumors. Exp Cell Res. 2013;319:1644–1649. doi: 10.1016/j.yexcr.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 87.Monach PA, Schreiber H, Rowley DA. CD4+ and B lymphocytes in transplantation immunity. II. Augmented rejection of tumor allografts by mice lacking B cells. Transplantation. 1993;55:1356–1361. [PubMed] [Google Scholar]
- 88.Barbera-Guillem E, Nelson MB, Barr B, et al. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother. 2000;48:541–549. doi: 10.1007/PL00006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 90.Schioppa T, Moore R, Thompson RG, et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andreu P, Johansson M, Affara NI, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong SC, Puaux AL, Chittezhath M, et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–2307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 93.Ammirante M, Luo JL, Grivennikov S, et al. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woo JR, Liss MA, Muldong MT, et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med. 2014;12:30. doi: 10.1186/1479-5876-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shalapour S, Font-Burgada J, Di Caro G, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Affara NI, Ruffell B, Medler TR, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Inoue S, Leitner WW, Golding B, et al. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 98.Bradley LM, Harbertson J, Biederman E, et al. Availability of antigen-presenting cells can determine the extent of CD4 effector expansion and priming for secretion of TH2 cytokines in vivo. Eur J Immunol. 2002;32:2338–2346. doi: 10.1002/1521-4141(200208)32:8<2338::AID-IMMU2338>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 99.Shah S, Divekar AA, Hilchey SP, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117:574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 100.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/ refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Byrd JC, Furman RR, Coutre SE, et al. Targeting Btk with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Byrd JC, O’Brien S, James DF. Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:1278–1279. doi: 10.1056/NEJMc1309710. [DOI] [PubMed] [Google Scholar]
- 104.Wang ML, Rule S, Martin P, et al. Targeting Btk with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Masso-Valles D, Jauset T, Serrano E, et al. Ibrutinib exerts potent antifibrotic and antitumor activities in mouse models of pancreatic adenocarcinoma. Cancer Res. 2015;75:1675–1681. doi: 10.1158/0008-5472.CAN-14-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soucek L, Buggy JJ, Kortlever R, et al. Modeling pharmacological inhibition of mast cell degranulation as a therapy for insulinoma. Neoplasia. 2011;13:1093–1100. doi: 10.1593/neo.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, et al. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both Btk and ITK. Proc Natl Acad Sci U S A. 2015;112:E966–E972. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ni Gabhann J, Hams E, Smith S, et al. Btk regulates macrophage polarization in response to lipopolysaccharide. PLoS One. 2014;9:e85834. doi: 10.1371/journal.pone.0085834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gopal AK, Kahl BS, deVos S, et al. PI3KS inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chantry D, Vojtek A, Kashishian A, et al. P110delta, a novel phos-phatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem. 1997;272:19236–19241. doi: 10.1074/jbc.272.31.19236. [DOI] [PubMed] [Google Scholar]
- 112.Ali K, Soond DR, Piñeiro R, et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–411. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmid MC, Franco I, Kang SW, et al. PI3-kinase 7 promotes Rap1a-mediated activation of myeloid cell integrin α4β1, leading to tumor inflammation and growth. PLoS One. 2013;8:e60226. doi: 10.1371/journal.pone.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 115.Ma Y, Conforti R, Aymeric L, et al. How to improve the immunogenicity of chemotherapy and radiotherapy. Cancer Metastasis Rev. 2011;30:71–82. doi: 10.1007/s10555-011-9283-2. [DOI] [PubMed] [Google Scholar]
- 116.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 117.Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60:222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kerbel R. Anti-angiogenesis in cancer; met and unmet goals—an interview with Robert Kerbel by Francesco Bertolini. Int J Dev Biol. 2011;55:395–398. doi: 10.1387/ijdb.103217fb. [DOI] [PubMed] [Google Scholar]
- 119.Cao Y, Arbiser J, D’Amato RJ, et al. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3:114rv3. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wolchok JD, Hodi FS, Weber JS, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann NY Acad Sci. 2013;1291:1–13. doi: 10.1111/nyas.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Robert C, Ribas A, Wolchok JD, et al. Anti–programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 124.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kirk PS, Koreckij T, Nguyen HM, et al. Inhibition of CCL2 signaling in combination with docetaxel treatment has profound inhibitory effects on prostate cancer growth in bone. Int J Mol Sci. 2013;14(5):10483–10496. doi: 10.3390/ijms140510483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmid MC, Avraamides CJ, Dippold HC, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19(6):715–727. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]