Abstract
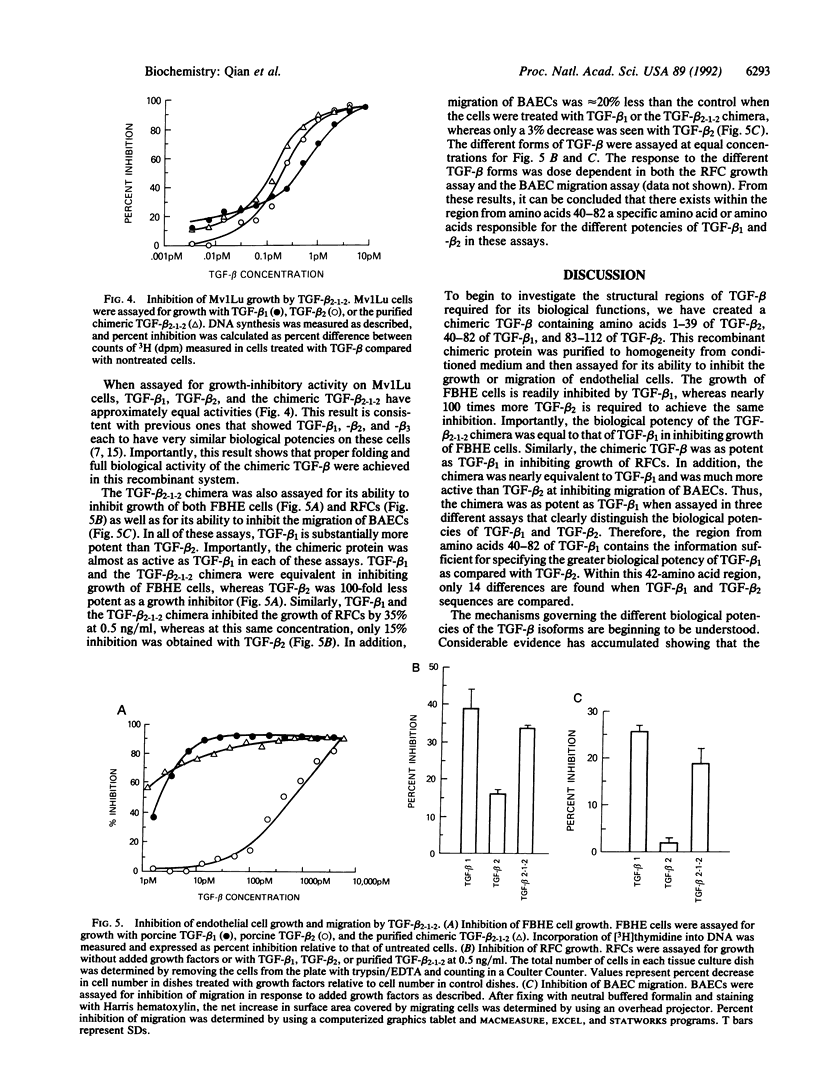
A chimeric transforming growth factor beta (TGF-beta) molecule has been synthesized to map the amino acids responsible for the substantially greater activity of TGF-beta 1 than TGF-beta 2 on growth and migration of endothelial cells. This chimera consists of a dimer of a monomeric unit composed of amino acids 1-39 of TGF-beta 2, 40-82 of TGF-beta 1, and 83-112 of TGF-beta 2. Structural identity of the purified recombinant protein has been confirmed by immunoblotting and NH2-terminal sequencing. The biological potency of the TGF-beta 2-1-2 chimera was equal to that of TGF-beta 1 in inhibition of growth of both fetal bovine heart endothelial cells and rat epididymal fat pad microvascular endothelial cells. Similarly, the TGF-beta 2-1-2 chimera was nearly equivalent to TGF-beta 1 and at least 10-fold more active than TGF-beta 2 in inhibiting migration of bovine aortic endothelial cells. These results identify the sequence between amino acids 40-82 as an important region within TGF-beta that functions to specify a TGF-beta 1- or TGF-beta 2-like activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunner A. M., Marquardt H., Malacko A. R., Lioubin M. N., Purchio A. F. Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor beta 1 precursor. Expression and characterization of mutant proteins. J Biol Chem. 1989 Aug 15;264(23):13660–13664. [PubMed] [Google Scholar]
- Cheifetz S., Bassols A., Stanley K., Ohta M., Greenberger J., Massagué J. Heterodimeric transforming growth factor beta. Biological properties and interaction with three types of cell surface receptors. J Biol Chem. 1988 Aug 5;263(22):10783–10789. [PubMed] [Google Scholar]
- Cheifetz S., Hernandez H., Laiho M., ten Dijke P., Iwata K. K., Massagué J. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J Biol Chem. 1990 Nov 25;265(33):20533–20538. [PubMed] [Google Scholar]
- Cheifetz S., Massagué J. Isoform-specific transforming growth factor-beta binding proteins with membrane attachments sensitive to phosphatidylinositol-specific phospholipase C. J Biol Chem. 1991 Nov 5;266(31):20767–20772. [PubMed] [Google Scholar]
- Cheifetz S., Weatherbee J. A., Tsang M. L., Anderson J. K., Mole J. E., Lucas R., Massagué J. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987 Feb 13;48(3):409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Sporn M. B. Differential inhibition of transforming growth factor beta 1 and beta 2 activity by alpha 2-macroglobulin. J Biol Chem. 1990 Apr 25;265(12):6973–6977. [PubMed] [Google Scholar]
- Flanders K. C., Roberts A. B., Ling N., Fleurdelys B. E., Sporn M. B. Antibodies to peptide determinants in transforming growth factor beta and their applications. Biochemistry. 1988 Jan 26;27(2):739–746. doi: 10.1021/bi00402a037. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Armour R., Baldwin J. H., Maldonado F., Spiess J., Holley R. W. Amino acid sequence of the BSC-1 cell growth inhibitor (polyergin) deduced from the nucleotide sequence of the cDNA. Proc Natl Acad Sci U S A. 1988 Jan;85(1):79–82. doi: 10.1073/pnas.85.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J. C., Mohan S., Linkhart T. A., Widstrom R., Baylink D. J. Comparison of the biological actions of TGF beta-1 and TGF beta-2: differential activity in endothelial cells. J Cell Physiol. 1988 Oct;137(1):167–172. doi: 10.1002/jcp.1041370120. [DOI] [PubMed] [Google Scholar]
- Jhappan C., Stahle C., Harkins R. N., Fausto N., Smith G. H., Merlino G. T. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990 Jun 15;61(6):1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- Kondaiah P., Van Obberghen-Schilling E., Ludwig R. L., Dhar R., Sporn M. B., Roberts A. B. cDNA cloning of porcine transforming growth factor-beta 1 mRNAs. Evidence for alternate splicing and polyadenylation. J Biol Chem. 1988 Dec 5;263(34):18313–18317. [PubMed] [Google Scholar]
- LaRochelle W. J., Giese N., May-Siroff M., Robbins K. C., Aaronson S. A. Molecular localization of the transforming and secretory properties of PDGF A and PDGF B. Science. 1990 Jun 22;248(4962):1541–1544. doi: 10.1126/science.2163109. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Dreyer B., Pitlick F. A., Furthmayr H. The collagenous components of the subendothelium. Correlation of structure and function. Lab Invest. 1980 Oct;43(4):303–315. [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Boyd F. T., Andres J. L. TGF-beta receptors and TGF-beta binding proteoglycans: recent progress in identifying their functional properties. Ann N Y Acad Sci. 1990;593:59–72. doi: 10.1111/j.1749-6632.1990.tb16100.x. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Merwin J. R., Newman W., Beall L. D., Tucker A., Madri J. Vascular cells respond differentially to transforming growth factors beta 1 and beta 2 in vitro. Am J Pathol. 1991 Jan;138(1):37–51. [PMC free article] [PubMed] [Google Scholar]
- Mitchell E. J., Fitz-Gibbon L., O'Connor-McCourt M. D. Subtypes of betaglycan and of type I and type II transforming growth factor-beta (TGF-beta) receptors with different affinities for TGF-beta 1 and TGF-beta 2 are exhibited by human placental trophoblast cells. J Cell Physiol. 1992 Feb;150(2):334–343. doi: 10.1002/jcp.1041500217. [DOI] [PubMed] [Google Scholar]
- Mitchell E. J., O'Connor-McCourt M. D. A transforming growth factor beta (TGF-beta) receptor from human placenta exhibits a greater affinity for TGF-beta 2 than for TGF-beta 1. Biochemistry. 1991 Apr 30;30(17):4350–4356. doi: 10.1021/bi00231a034. [DOI] [PubMed] [Google Scholar]
- O'Connor-McCourt M. D., Wakefield L. M. Latent transforming growth factor-beta in serum. A specific complex with alpha 2-macroglobulin. J Biol Chem. 1987 Oct 15;262(29):14090–14099. [PubMed] [Google Scholar]
- Ohta M., Greenberger J. S., Anklesaria P., Bassols A., Massagué J. Two forms of transforming growth factor-beta distinguished by multipotential haematopoietic progenitor cells. Nature. 1987 Oct 8;329(6139):539–541. doi: 10.1038/329539a0. [DOI] [PubMed] [Google Scholar]
- Ostman A., Andersson M., Hellman U., Heldin C. H. Identification of three amino acids in the platelet-derived growth factor (PDGF) B-chain that are important for binding to the PDGF beta-receptor. J Biol Chem. 1991 Jun 5;266(16):10073–10077. [PubMed] [Google Scholar]
- Ottmann O. G., Pelus L. M. Differential proliferative effects of transforming growth factor-beta on human hematopoietic progenitor cells. J Immunol. 1988 Apr 15;140(8):2661–2665. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Kondaiah P., Rosa F., Watanabe S., Good P., Danielpour D., Roche N. S., Rebbert M. L., Dawid I. B., Sporn M. B. Mesoderm induction in Xenopus laevis distinguishes between the various TGF-beta isoforms. Growth Factors. 1990;3(4):277–286. doi: 10.3109/08977199009003670. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Rosa F., Roche N. S., Coligan J. E., Garfield M., Rebbert M. L., Kondaiah P., Danielpour D., Kehrl J. H., Wahl S. M. Isolation and characterization of TGF-beta 2 and TGF-beta 5 from medium conditioned by Xenopus XTC cells. Growth Factors. 1990;2(2-3):135–147. doi: 10.3109/08977199009071500. [DOI] [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Segarini P. R., Roberts A. B., Rosen D. M., Seyedin S. M. Membrane binding characteristics of two forms of transforming growth factor-beta. J Biol Chem. 1987 Oct 25;262(30):14655–14662. [PubMed] [Google Scholar]
- Segarini P. R., Rosen D. M., Seyedin S. M. Binding of transforming growth factor-beta to cell surface proteins varies with cell type. Mol Endocrinol. 1989 Feb;3(2):261–272. doi: 10.1210/mend-3-2-261. [DOI] [PubMed] [Google Scholar]
- Sha X., Yang L., Gentry L. E. Identification and analysis of discrete functional domains in the pro region of pre-pro-transforming growth factor beta 1. J Cell Biol. 1991 Aug;114(4):827–839. doi: 10.1083/jcb.114.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. TGF-beta: problems and prospects. Cell Regul. 1990 Nov;1(12):875–882. doi: 10.1091/mbc.1.12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta. Multiple actions and potential clinical applications. JAMA. 1989 Aug 18;262(7):938–941. doi: 10.1001/jama.262.7.938. [DOI] [PubMed] [Google Scholar]