Abstract
Part of the exoskeleton of some wood-inhabiting insects is modified to form a mycangium, which is a specialized organ used to convey fungal spores or yeasts to their offspring. Although most stag beetles (Coleoptera: Lucanidae) are known to have female-specific mycangia and associated yeast symbionts, the evolutionary origin of the mycangium in this group remains unresolved. Here, we report the presence of a mycangium and associated yeast symbionts in the European horned stag beetle Sinodendron cylindricum (L.), which belongs to an ancestral clade of the Lucanidae. The mycangium of S. cylindricum is shown to be female-specific and have the same developmental origin as that of other stag beetles. A total of five yeast strains were isolated from adult mycangia and larval gut of S. cylindricum. Of these, we suggest that SICYAM1 is an undescribed yeast with taxonomic novelty, and have identified SICYLG3 as the xylose-fermenting yeast Scheffersomyces insectosa using nuclear ribosomal RNA and ITS sequences. The remaining three yeast strains, SICYAM2, SICYLG1, and SICYLG2, were assigned to the genus Sugiyamaella. Yeast density in the adult mycangium was lower than that of the more evolutionarily advanced stag beetles, the European Lucanus cervus (L.) and Dorcus parallelipipedus (L.), which were also examined in this study. No living yeasts were isolated from the adult guts. However, a third instar larva of S. cylindricum harbored 104–106 living yeasts in each gut region, which suggests that gut yeasts play an important role in these wood-feeding larvae.
Keywords: evolution, gut flora, insect–fungal associations, saproxylic insects, vertical transmission
The mycangium is an exoskeletal cavity or saccate structure that harbors symbiotic fungi or yeasts, and can be found in some wood-boring beetles and bark beetles, wood wasps, leaf-rolling weevils, and a bamboo-inhabiting lizard beetle (Batra 1963, Madden and Coutts 1979, Beaver 1989, Six 2003, Kobayashi et al. 2008, Toki et al. 2012, Davis 2015). Recently, a female-specific mycangium was independently discovered in the stag beetle Lucanus cervus (L.) (Coleoptera: Lucanidae) (Hawes 2009, 2010, 2013) and 12 genera of Lucanidae in Japan (Tanahashi et al. 2010). Yeast symbionts present in the mycangium of Lucanus and Dorcus species are closely related to the xylose-fermenting yeasts, Scheffersomyces stipitis (Pignal) Kurtzman et Suzuki (formerly, Pichia stipitis) (Tanahashi et al. 2010, Hawes 2013, Tanahashi and Fremlin 2013). Xylose-fermenting yeasts are commonly found in the digestive tracts and/or feeding tunnels of many xylophagous insects, suggesting an association with wood digestion (Suh et al. 2003, 2006). Stag beetle larvae of some genera feed on subterranean, decaying wood of trees and shrubs, or subterranean woody material of humus-rich soil, while others feed on moist, decaying wood above soil level (Holloway 2007), whereas adults utilize food material that is more nutritive such as fermented tree sap and over-ripe fruits, however a few species are known to exhibit carnivory (Mori and Chiba 2009), and some rarely feed after eclosion (Sadaki et al. 2014). Thus, the biochemical environment of the adult gut is likely to be very different from that of the wood-feeding larvae, where xylose-fermenting yeasts play an essential role. Completely separate from the digestive tract, the saccate mycangium provides a reservoir for yeast symbionts (Tanahashi et al. 2010, Fremlin and Tanahashi 2015). Mycangium evolution probably occurred only once in the ancestral stag beetle clade and might have been the starting point for the evolution of the larval and adult dimorphic food habits.
The presence of a mycangium has been reported in nine genera of the subfamily Lucaninae (Dorcus, Prosopocoilus, Aegus, Lucanus, Neolucanus, Prismognathus, Figulus, Nigidius, and Platycerus), two genera of Aesalinae (Aesalus and Nicagus), and one genus of Sindesinae (Ceruchus). Of the Scarabaeoidea, mycangia have only been found in the family Lucanidae. Consequently, the distribution of mycangia on the phylogenetic tree is markedly similar to the phylogenetic distribution of the family Lucanidae (Krajcik 2001, Hosoya and Araya 2005, Holloway 2007, Fujita 2010). However, because some supposedly ancestral genera have not been investigated for the presence of a mycangium, together with the phylogenetic ambiguity in the ancestral clades of Lucanidae, the evolutionary origin of the mycangium remains unclear.
The genus Sinodendron is currently placed in the subfamily Syndesinae, which is described as one of the ancestral clades of Lucanidae (Kim and Farrell 2015). Sinodendron species are found in the United Kingdom, Europe, and North America, where they are known as ‘rhinoceros beetles’ or ‘horned stag beetles’ due to the characteristic, prominent horn on the head of the males. Of these, only the species Sinodendron cylindricum (Linnaeus, 1758) is present in Europe and the United Kingdom, where its distribution is widespread (Zahradnik and Chvala 1989, Alexander 2002, Sutton 2003, Klausnitzer and Sprecher-Uebersax 2008, GBIF 2016, NBN 2016). Glossy-black, 10–18-mm long and coarsely punctate, the adult beetles are active nocturnally and often found gathering around fermented sap of hardwood trees. Both sexes collaborate to excavate a burrow in decaying wood. Perhaps uniquely, the male guards the burrow and removes the debris while the female is excavating. Some 20 eggs are laid in tunnel branches, each of which is then packed with wood particles. A male will often continue guarding the burrow entrance after the female has completed oviposition (Chapman 1868, Arrow 2005), possibly to deter predators, parasitoids, and/or conspecific competitors. Larvae feed on the decaying wood and pupate within it.
As adult and larval feeding behavior of S. cylindricum is similar to that of other stag beetle species, we hypothesized that (1) S. cylindricum females have a specialized mycangium, similar to that of other stag beetle species, or (2) females and/or males store symbiotic microorganisms in their digestive tracts. In this study, we examined S. cylindricum for (1) presence or absence of a mycangium in females and males, (2) presence or absence of yeast symbionts in the mycangium, (3) presence or absence of yeast symbionts in the adult digestive system, and (4) presence or absence of yeast symbionts in the larval digestive system. In addition, (5) we analyzed the yeasts from the European stag beetles, Dorcus parallelipipedus and L. cervus, which are known to possess a female-specific mycangium and associated xylose-fermenting Scheffersomyces yeasts (Hawes 2013, Tanahashi and Fremlin 2013).
Materials and Methods
Isolation of Yeasts From Adult Insects
Two adult males and two adult females of S. cylindricum (Fig. 1a and b) were collected from decaying logs of Fagus sylvatica L. or Carpinus betulus L. at Grandfontaine, Bas-Rhin, France (48.49°N, 7.16°E) on 4 September 2014. Specimens were anesthetized using gaseous carbon dioxide, then dissected in sterile phosphate-buffered saline (PBS) and examined for the presence or absence of a mycangium, as described in Tanahashi et al. (2010). The mycangium found in both females was removed from its underlying tissue and weighed using an electronic microbalance (MT5, Mettler Toledo). The midgut and hindgut of both sexes were also removed and weighed. These organs were placed separately in 1.5 ml tubes containing 160 μl PBS and then homogenized using pellet pestles (Kimble Kontes, Fischer Science). The total volume was adjusted to 200 μl by adding PBS, and a fivefold dilution series was made by transferring 40 μl of the suspension to a new 1.5 ml tube containing 160 μl of PBS. For quantitative isolation of yeasts, 40 μl of each dilution (i.e., 1/5, 1/25,…, 1/55 equivalent of the original homogenate) was loaded separately onto five potato dextrose agar (PDA) (BD) plates (9-cm diameter), which contained 50 μg/ml rifampicin, and spread uniformly using a glass spreader. The plates were incubated at 20°C for 4 d.
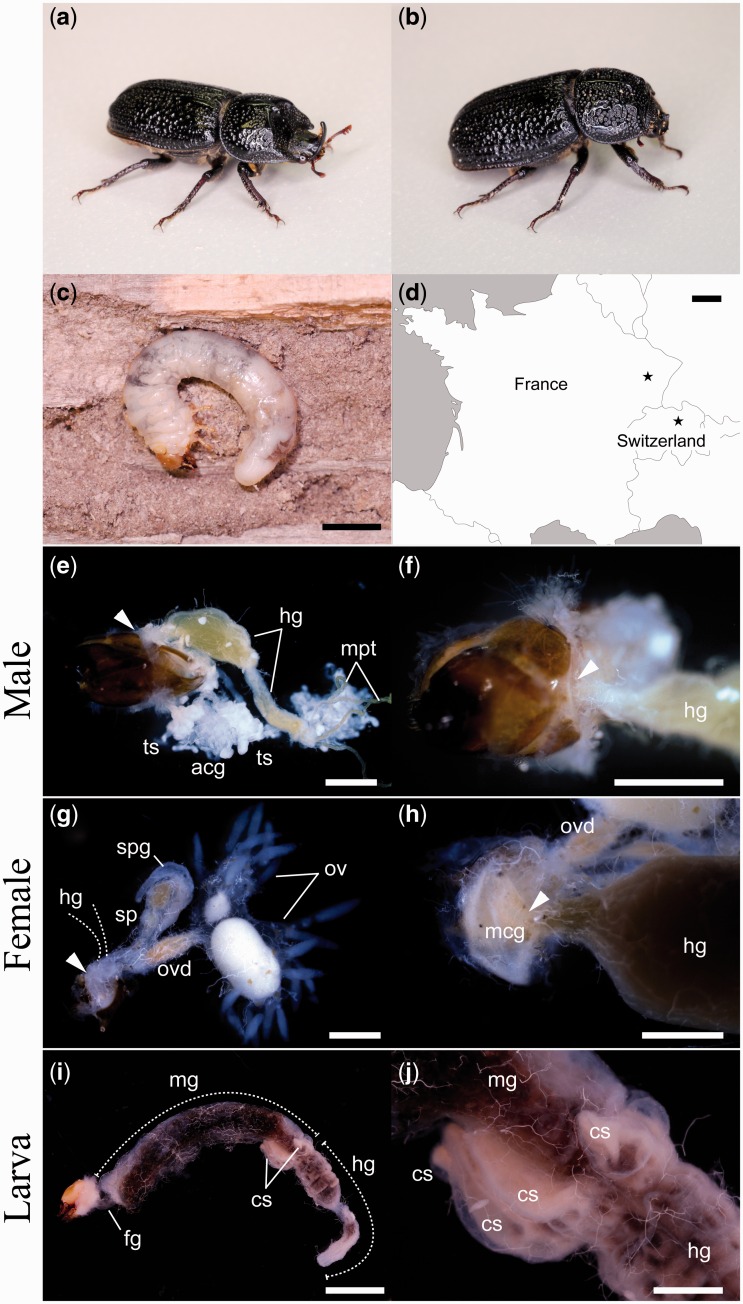
Fig. 1.
Adults and a larva of S. cylindricum. (a) Male, (b) female, (c) third instar larva, (d) sampling locations in France and Switzerland. (e, f) Male reproductive organs and the absence of mycangium. Arrow indicates the location of a mycangium in a female. (g, h) Female reproductive organs and the presence of mycangium (arrows). Removed hindgut is indicated by dotted lines. (I, j) Larval gut and cecum-like sacs. ag, accessory gland; cs, cecum-like sac; fg, foregut; hg, hindgut; mcg, mycangium; mg, midgut; mpt, Malpighian tubule, ov, ovary; ovd, (main) oviduct; sp, spermatheca; spg, spermathecal gland; ts, testis. Scale bars: (c, i) 5 mm; (d) 100 km; (e–h, j) 1 mm.
To compare the density and diversity of yeast symbionts in the mycangium between different insect species, we analyzed three adult female Dorcus parallelipipedus (L.), one of which was collected from sycamore (Acer pseudoplatanus L.) tree stump in Bentley, Ipswich, Suffolk, United Kingdom (51.988°N, 1.076°E, 40 m alt.) on 12 March 2014 and others which were collected from a decaying log in Basel, Switzerland (47.553N, 7.604E, 317 m alt.) on 16 June 2014. A newly emerged female of L. cervus collected in Stutton, Ipswich, Suffolk, United Kingdom (51.970N, 1.134E, 9.5 m alt.) on 10 June 2011 was also added to the analysis.
Isolation of Yeasts From Larvae
Four lucanid-like larvae (Fig. 1c) were collected from a decaying beech log at the northern slope of Lägern, Zurich, Switzerland (47.48°N, 8.39°E, 850 m alt.) on 15 June 2014. As Lägern is a protected area, insect collection was permitted as part of the field survey program in the 8th Symposium on the Conservation of Saproxylic Beetles (Basel, Switzerland, 13–15 June 2014). The larvae were maintained in the same decaying wood at 10–15°C until they were required for examination. For isolation of yeasts, a healthy larva was washed with distilled water and dissected in sterile PBS. The whole gut was removed and washed twice with sterile PBS. The midgut, hindgut and cecum-like sacs were separated from the whole gut, weighed and then homogenized in 1.5-ml tubes. The total volume in each tube was adjusted to 200 μl, and a tenfold dilution series made by transferring 20 μl of the suspension to a new 1.5 ml tube containing 180 μl PBS. Finally, 20 μl of each dilution was spread on a PDA plate, as described above. The plates were incubated at 20°C for 4 d. The remaining three larvae were reared to adults for future analysis.
Quantification of Yeasts
The yeast colonies from adults and a larva were roughly classified according to their morphological traits (morphotype), and each morphotype colony was counted on every plate. Sixteen colonies were randomly selected for each morphotype from each isolation source and these were transferred to new PDA plates for later DNA analysis. Although one morphotype is likely to contain multiple species or strains, such pre-classification is helpful in elucidating species diversity, especially when there are minor but morphologically different colonies. Colony forming unit (CFU) was calculated for each morphotype by using the plate where an adequate number of colonies (usually, 30–300 colonies per plate) appeared. When the estimated CFU per organ was <10, it was determined as ‘no colonies’.
DNA Sequencing and Haplotyping of Yeasts
For DNA extraction, small pellets of the isolated yeast colonies were suspended in 50 μl lyticase solution (0.4 U/μl lyticase and 50 mM EDTA) and incubated at 37°C for 2 h. The spheroplast yeast cells were collected by centrifugation and then processed using Wizard (R) Genomic DNA Purification Kit (Promega). For the first DNA screening analysis, internal transcribed spacer (ITS) regions of the nucleus ribosomal RNA (rRNA) gene were amplified by PCR using the primer pair NS7-NL4 (White et al. 1990), which covers the full length of ITS1, 5.8S, and ITS2. The PCR fragments were sequenced with an internal primer ITS5 to obtain complete nucleotide sequences of those regions. Both ends of the sequences were trimmed to contain only those three ITS regions. The total length of the trimmed sequence varied from 463 to 547 bases. ITS haplotype was determined for each isolate according to the trimmed sequence. For the second intensive DNA analysis, a representative isolate was randomly chosen for each ITS haplotype for each insect specimen (i.e., isolates that had the same ITS haplotype from the same specimen were assumed to be the same strain, whereas those from a different specimen were treated as different strains). Approximately 2,900 bp of the nucleus ribosomal RNA genes, including 18S rRNA, ITS1, 5.8S rRNA, ITS2, and 26S rRNA, were amplified by PCR with NS1-FS2 and NS7-NL4 primer sets (White et al. 1990), which were designed to have an overlapping region of 170 bases. The PCR fragments were sequenced with multiple sequencing primers; NS1, NS2, NS3, FS2, NS7, ITS5, and NL1 (White et al. 1990). These short nucleotide sequences (∼700 bases) were combined on ContigExpress (Invitrogen, USA) software.
DNA Sequencing of Reference Yeasts
Type strains of five yeast species, S. stipitis JCM 10742, Scheffersomyces segobiensis (Santa Marïa et Garcïa) Kurtzman et Suzuki JCM10740, Scheffersomyces shehatae (Buckley et van Uden) Urbina et Blackwell JCM9840, Scheffersomyces insectosa (Kurtzman) Urbina et Blackwell JCM9842, and Sugiyamaella novakii (Péter et al.) Urbina et Blackwell ATCC 201508, and an identified yeast strain Wickerhamomyces anomalus (Hansen) Kurtzman et al. DBL01s1Shirosato (Toki et al. 2012) were subjected to the sequencing analysis as described above, since there were no continuous, long sequences from 18S to 26S in the GenBank database at that time. Yeasts that had been isolated from the adult mycangium of two Japanese lucanid species D. rectus (Motschulsky) and L. maculifemoratus Motschulsky (Tanahashi et al. 2010) were sequenced in the same way.
Identification of Yeast Species by BLAST
Even though we obtained relatively long, continuous sequences of the rRNA genes of the yeasts, most of the yeast sequences in the GenBank database are short (∼600 bases). In this situation, BLAST search usually scoops up longer but weaker-matching sequences (typically, genome sequences of several well-known yeasts), when using a long query sequence with default search parameters. Therefore, we divided our sequence data into three regions, namely, 18S, ITS, and D1/D2, which have been traditionally used for identification and phylogenetic analysis of yeasts. The three regions were then separately subjected to BLAST search (Altschul et al. 1997) using the procedure obtained from the NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Carbon Assimilation Test
The representative isolates were cultured aerobically in 4 ml of yeast nitrogen base (YNB) (BD) containing 0.5% glucose at 25°C for 24 h. The culture media were centrifuged and the cell pellets were suspended in sterile water, in which the OD600 was adjusted to 0.5. 1 μl of the cell suspension was spread onto each of ten nutrient medium plates containing YNB, 1.5% agar and one of the following carbon sources: glucose, galactose, mannose, xylose, arabinose, glucuronic acid, cellobiose, xylan (from oat spelt), glucomannan (from Konjac), and carboxymethyl cellulose. The concentration of each carbon source was 0.5 g/liter, except for insoluble xylan at 1.5 g/liter. The plates were incubated at 25°C for 5 d to determine the growth of each strain.
DNA Sequencing of Host Insects
To identify the species of the lucanid-like larvae from Switzerland, DNA sequencing analysis was conducted on the adult females from France and one of the larvae. Insect DNA was extracted from muscles of the adult prothorax and larval head using a QIAamp (R) DNA Mini Kit (Qiagen) following manufacturer’s instructions. PCR was performed to amplify mitochondrial 16S ribosomal RNA gene with the primers, mt16SB and mt16SC (Hosoya and Araya 2005), and ITS2 region of the nucleus ribosomal RNA gene with the primers, 5.8S38F (5′-CGATGAAGAACGCAGCTAATTG-3′) and ITS4col (5′-TCCTCCGCTTAGTAATATGC-3′) (developed in this study). These PCR products were sequenced with mt16SA, mt16SB and mt16SC primers for mt16S, and 5.8S38F and ITS4col for ITS2 region.
Phylogenetic Analysis of Yeasts and Host Insects
The sequence data were subjected to multiple DNA sequence alignment program ClustalW (Larkin et al. 2007). The alignments were then inspected and corrected manually, from which ambiguously aligned sites were removed. Phylogenetic analyses were conducted using the maximum likelihood (ML) method, program Mega 6 (Tamura et al. 2013). We selected the Tamura-Nei + G + I model for the yeast phylogeny and GTR + I + G model for the insect phylogeny, on the basis of the Akaike criterion estimated by the program Modeltest 3.06. Bootstrap tests were performed with 1,000 replications. Since the yeasts from the three D. parallelipipedus females had an identical sequence, we used that from the United Kingdom.
Results
Species Identification of Insects Using DNA Analysis
There was no difference in the nucleotide sequence of mt16S (977 bases) and ITS2 (999 bases) (accession numbers: LC119078–LC119081) between the adult female of S. cylindricum from France and the unidentified larva from Switzerland. Since other Sinodendron species are not found in Europe, we conclude that the larva has been correctly identified as S. cylindricum.
Morphological Observation of Symbiotic Organs
In adult females of S. cylindricum, a mycangium was found under the eighth tergite (Fig. 1h), at the same location reported for other lucanid species (Tanahashi et al. 2010, Hawes 2013, Tanahashi and Fremlin 2013). The two lateral oviducts of S. cylindricum were expanded, as in other lucanid species, and function as storage organs for mature eggs. Twelve ovarioles were observed at the anterior end of each oviduct and yellow bodies were observed at the posterior end of each oviduct that had produced at least one egg. Female #1 had eggs in the lateral oviducts and yellow bodies were present in all ovarioles (Fig. 1g), which suggests that it had been sexually mature and active the previous summer. Female #2 contained no eggs, or yellow bodies. In contrast, no mycangium-like structure was located in the males (Fig. 1f).
The intestine of the S. cylindricum larva consisted of a short, thin foregut, a long, cylindrical midgut, and an enlarged, segmented hindgut (Fig. 1i). Cecum-like sacs were found at the posterior end of the midgut (Fig. 1j). Although such cecum-like structures are known in most Scarabaeoidea larvae, their function has yet to be fully investigated.
Yeasts From Adult Insects
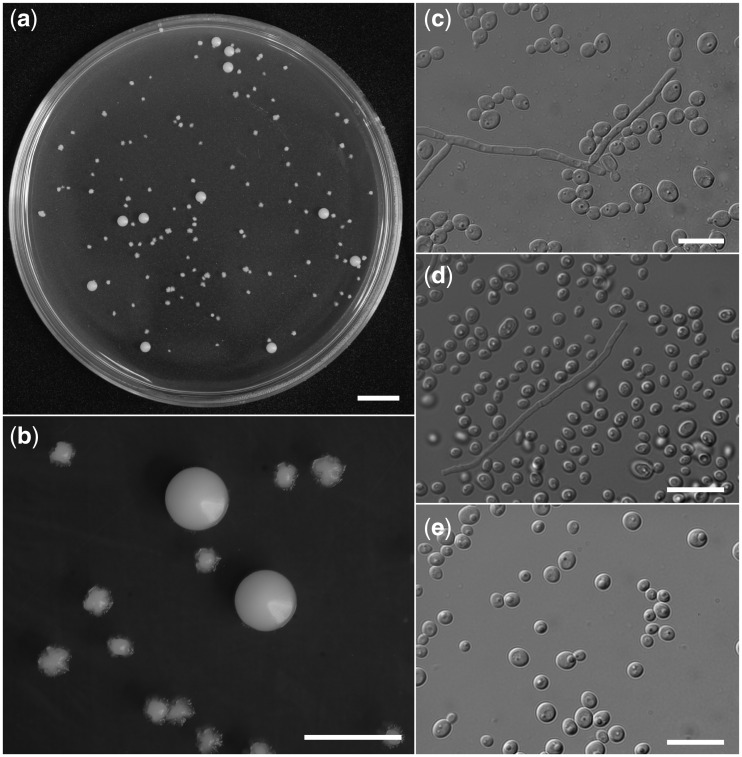
Two types of yeast colony (M1 and M2) grew on the PDA plates where the mycangium homogenate of female #1 was spread (Fig. 2a), while no yeasts appeared from the mycangium homogenate of female #2 (Table 1). No living yeasts were found from any of the gut samples. M1 was the dominant colony type, with an estimated CFU of 9.8 × 102 per mycangium. The growth at 20°C was slow and the surface of the colonies was wrinkled (Fig. 2b). All eight M1 isolates shared the same ITS haplotype (h1) and one of these was chosen for the standard strain, namely, SICYAM1 (yeast from S. cylindricum adult mycangium, isolate 1). SICYAM1 formed hyphae on the yeast morphology agar after a 5-d incubation period at 25°C (Fig. 2c). The maximum sequence identity values for 18S rRNA, ITS, and 26S rRNA were 96, 91, and 89%, respectively (Table 2). Since values of conspecific strains are usually no less than 99% (i.e., 1% nucleotide difference between two strains) in yeasts (Kurtzman and Suzuki 2010), SICYAM1 is thought to be a novel yeast species. M2 appears to be a minor morphotype, with an estimated CFU of 9.1 × 101 per mycangium. It showed moderate growth and a smooth colony edge and surface on PDA medium (Fig. 2b). All M2 isolates shared the same ITS haplotype (h2) and a second strain SICYAM2 was established. SICYAM2 rarely formed hyphae on the yeast morphology agar (Fig. 2c), but gradually extended pseudohyphae around the colonies after long-term incubation. DNA analysis showed 99 and 98% sequence identity with Sugiyamaella lignohabitans (Kurtzman) Urbina et Blackwell ATCC MYA-4663 by 18S and 26S rRNA, whereas the ITS region showed only 89% identity with ATCC MYA-4663 or any known strains of S. lignohabitans (Table 2). Therefore, we conclude that SICYAM2 is an undescribed species of Sugiyamaella.
Fig. 2.
Morphological traits of the yeasts isolated from S. cylindricum. (a, b) Colonies on a PDA plate medium where the homogenate of female mycangium was spread. Two types of colonies are found on the plate. (c) SICYAM1 (unclassified yeast from adult mycangium), (d) SICYAM2 (Sugiyamaella sp. from adult mycangium), (e) SICYLG3 (Scheffersomyces sp. from larval gut). Scale bars: (a) 10 mm; (b) 5 mm; (c–e) 50 μm.
Table 1.
Yeasts isolated from S. cylindricum, D. parallelipipedus, and L. cervus
| Species | Stage | Sex/specimen | Body weight (mg) | Organ | Weight (mg) |
CFU |
||
|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | ||||||
| S. cylindricum | Adult | Female #1 | 131.9 | Mycangium | 0.60 | 9.8 × 102 | 9.1 × 101 | – |
| Gut | 4.89 | – | – | – | ||||
| female #2 | 156.6 | Mycangium | 0.87 | – | – | – | ||
| Gut | 1.64 | – | – | – | ||||
| male #1 | 115.2 | Gut | 0.96 | – | – | – | ||
| male #2 | 160.1 | Gut | 0.80 | – | – | – | ||
| larva | unknown | 371.7 | Midgut | 96.3 | – | 2.5 × 104 | 1.0 × 104 | |
| Hindgut | 31.8 | – | 1.3 × 106 | 2.3 × 105 | ||||
| Cecum | 24.7 | – | 2.7 × 105 | 2.3 × 104 | ||||
| D. parallelipipedus | Adult | Females a (n = 3) | 646.0 (± 151.0) | Mycangium | 1.11 (± 0.16) | – | – | 1.1 × 105 (±0.2 × 105) |
| Gut | 19.6 (± 5.6) | – | – | – | ||||
| L. cervus | Adult | Female | 2647.0 | Mycangium | 5.20 | – | – | 7.6 × 105 |
| Gut | n/a | – | – | – | ||||
M1, M2, and M3 indicate different colony types on PDA plate media.
–, not detected (<10 CFU); n/a, data not available.
aData shown as mean ± SD.
Table 2.
Species or taxon of the yeasts isolated from S. cylindricum inferred from NCBI BLASTn search
| Host species | Morphotype | ITS haplotype | Yeast straina | Accession no. |
Maximum partial similarity (%)b |
Estimated species/taxon | ||
|---|---|---|---|---|---|---|---|---|
| 18S | ITS | 26S | ||||||
| S. cylindricum | M1 | h1 | Y1028-SICYAM1 | LC119082 | 96 | 91 | 89 | (Novel clade) |
| M2 | h2 | Y1028-SICYAM2 | LC119083 | 99 | 89 | 98 | Sugiyamaella sp. | |
| h2 | Y1053-SICYLG1 | LC119084 | 99 | 89 | 98 | Sugiyamaella sp. | ||
| h2v | Y1053-SICYLG2 | LC119085 | 99 | 89 | 98 | Sugiyamaella sp. | ||
| M3 | h3 | Y1053-SICYLG3 | LC119086 | 100 | 100 | 100 | S. insectosa | |
| D. parallelipipedus | M3 | h4 | Y719-DOPAAM | LC120356 | 100 | 100 | 100 | S. stipitis |
| L. cervus | M3 | h5 | Y718-LUCEAM | LC120355 | 99 | 99c | 100c | Scheffersomyces sp.c |
aUnder ‘Yeast strain’, the leading ‘Y’ and the following group of three or four numbers represent the host specimen (abbreviated in the main text and Table 3). The four letters following the hyphen represent the host species, the last two letters indicate the source organ (AM, adult mycangium; LG, larval guts).
bPercentages show the maximum similarity values to the nucleotide collection nr/nt database.
cITS and 26S rRNA sequences gave maximum similarity to the yeast symbiont isolated from mycangium of L. maculifemoratus in Japan (Tanahashi et al. 2010).
Yeasts From the Larval Gut
Two types of yeast colony (M2 and M3) appeared on the PDA plates where the separate homogenates of larval midgut, hindgut and cecum-like sacs were spread (Table 1). M2 colonies from the larva had a similar appearance to those from the adult mycangium. The maximum CFU was obtained from the hindgut (1.3 × 106), followed by the cecum-like sacs (2.7 × 105) and then the midgut (2.5 × 104). Most of the M2 isolates shared the ITS haplotype (h2) however, a minor genetic variant (one nucleotide replacement and one insertion), namely h2v, was determined from the midgut (two of eight colonies) and the cecum (one of eight colonies). Therefore, the representative isolates SICYLG1 and SICYLG2 were established from h2 and h2v colonies, respectively. There was no sequence difference in 18S or 26S rRNAs between the two isolates. M3 colonies exhibited the fastest growth and smoothness on the PDA plate, and did not produce hyphae on the yeast morphology agar (Fig. 2e). The CFU of M3 was approximately one order of magnitude smaller than M2 in every organ (Table 1). All eight isolates shared the same ITS haplotype (h3), thus the representative isolate SICYLG3 was established as a third yeast strain. BLAST search gave 100% identity to the xylose-fermenting yeast, S. insectosa (Table 2).
Yeasts From Other Lucanid Species
Uniform colonies that were similar to M3 from the Sinodendron larva were isolated from the mycangia of D. parallelipipedus and L. cervus (Table 1), and the strains DOPAAM and LUCEAM with different ITS haplotypes h4 and h5 were established, respectively (Table 2). The rRNA sequences (2,932 bases) of the yeast from D. parallelipipedus were identical to those of S. stipitis CBS 6054, the complete genome of which has been sequenced (Jeffries et al. 2007) (Table 2). The yeast from L. cervus was also assigned to the genus Scheffersomyces; however, ITS and 26S rRNA sequences indicated that this strain is close to the yeast symbiont of L. maculifemoratus in Japan, which has been reported in an earlier study (Tanahashi et al. 2010).
Phylogenetic Analysis of Yeasts
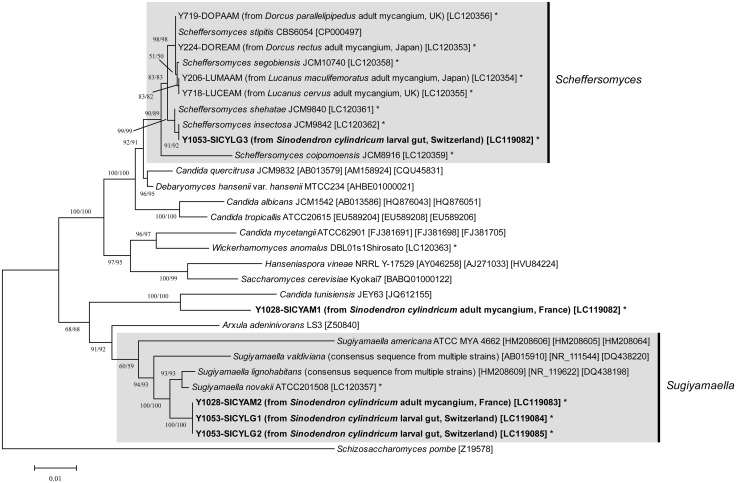
SICYAM1, which was dominant in adult mycangia, was not compatible with any clades of previously known Saccharomycotina yeasts (Fig. 3). It was also far removed from Scheffersomyces yeasts that are known symbionts in lucanids. SICYAM2, SICYLG1, and SICYLG2 were placed in the Sugiyamaella clade, although the ITS sequences indicate that they are different from any known species of Sugiyamaella yeasts.
Fig. 3.
Phylogenetic placement of the yeasts isolated from S. cylindricum and other lucanid species. The yeast strains isolated in this study are shown in bold. Asterisks after the accession numbers indicate the DNA sequences determined in this study. The two numbers on each branch represent bootstrap values (1,000 replications) by using neighbor-joining (NJ) and ML methods. See also the footnotes in Table 2 for the definition of strain names of yeast symbionts.
Carbon Assimilation Test
All five yeast isolates from S. cylindricum were able to utilize glucose, mannose, xylose, and cellobiose (Table 3). SICYAM1 did not assimilate galactose or glucuronic acid. Three Sugiyamaella isolates (SICYAM2, SICYLG1, and SICYLG2) assimilated glucuronic acid (Table 3).
Table 3.
Growth of the five yeast strains isolated from S. cylindricum on different carbon sources
| Carbon source | SICYAM1 | SICYAM2 | SICYLG1 | SICYLG2 | SICYLG3 |
|---|---|---|---|---|---|
| Glucose | + | + | + | + | + |
| Galactose | – | w | w | w | + |
| Mannose | + | + | + | + | + |
| Xylose | + | + | + | + | + |
| Arabinose | w | w | w | w | w |
| Galacturonic acid | – | + | + | + | – |
| Cellobiose | + | + | + | + | + |
| Xylan | w | w | w | w | w |
| Mannan | – | – | – | – | – |
| Carboxymethyl cellulose | – | – | – | – | – |
+, moderate growth; w, weak growth; –, no growth.
Discussion
In this study, we confirmed the presence of a female-specific mycangium in the genus Sinodendron, which is described as an ancestral clade of the Lucanidae. The mycangium of S. cylindricum originates from the intersegmental membrane that connects the eighth and ninth tergites, as it does in more evolutionarily advanced lucanids (Fremlin and Tanahashi 2015). As far as we know, this homologous structure has never been reported in other Scarabaeoidea families (Tanahashi et al. 2010). Our results therefore strengthen the view that the mycangium is a common and ancient characteristic of the Lucanidae. We obtained a total of five yeast strains from the adult mycangia and larval gut: a supposedly novel yeast from the mycangium, SICYAM1, three conspecific yeast strains that belong to Sugiyamaella clade, SICYAM2, SICYLG1 and SICYLG2, and the yeast identified as S. insectosa, SICYLG3. All of these yeast strains could utilize xylose, and therefore are considered to play a part in the digestion of wood components, especially hardwood hemicelluloses, which consist mainly of xylose and glucose (Sjostrom 1993). Sugiyamaella yeasts are common in the gut of wood-feeding passalids (Coleoptera: Passalidae) (Urbina and Blackwell 2012) and they were present in both adult mycangium and larval guts of S. cylindricum. However, the novel yeast SICYAM1 was found only in the adult mycangium. Since SICYAM1 grew slowly, it might have been overlooked on the larval plate where the fast-growing Sugiyamaella and Scheffersomyces formed their colonies, this being one of the major problems in such a culture-dependent analysis. Males do not possess a mycangium, nor are there any living yeasts in their digestive tract, which suggests that they are unlikely to be involved in the vertical transmission of the yeast symbionts. Moreover, the absence of living yeasts in the female digestive tract indicates that the mycangium alone acts as a storage organ for the yeast symbionts.
Microbial Environment of Adult Mycangium
Although the mycangium of S. cylindricum harbored yeasts, the density of these was low (no more than 103 CFU), compared with those found in the mycangium of L. cervus and D. parallelipipedus (more than 105 CFU), which often share the same ecological niche as S. cylindricum in Europe and the UK. The mycangium of S. cylindricum was almost transparent and difficult to distinguish from the surrounding intersegmental membrane, perhaps because of the small amount of mycangial secretion present. The low density of yeasts in the mycangium of S. cylindricum is unlikely to be due to the insect’s immaturity as the dissected beetle showed it to be sexually mature. Co-existence of multiple yeast species in the mycangium, contrasts with the single-yeast monoculture in the mycangium of higher lucanids (Tanahashi et al. 2010, Hawes 2013, Fremlin and Tanahashi 2013), and probably reflects a less specialized insect–yeast association in this species. This suggests that the mycangium of S. cylindricum represents an early stage in the evolution of insect-yeast symbiosis in the Lucanidae. Bacterial symbionts were not examined in this study; however, bacteria also play important roles in some higher lucanids, which at the same time harbour monoculture xylose-fermenting Scheffersomyces yeasts in their mycangia. Kuranouchi et al. (2006), for example, demonstrated nitrogen-fixing chemical activity in the gut of Dorcus rectus larvae, suggesting the presence of nitrogen-fixing bacteria, while both Tanahashi et al. (2009) and Tanahashi and Kubota (2013) suggest the probable symbiotic function of D. rectus gut bacteria is to assist digestion of fungal mycelia in decaying wood. More recently, Miyashita et al. (2015) discovered antibiotic-producing bacteria from mycangia of some Dorcus species. We suggest that the presence and roles of bacterial symbionts in S. cylindricum need further investigation.
Microbial Environment of the Larval Gut
In lucanid larvae, the midgut forms a straight, cylindrical tube, which contains dark-brown homogenates of ingested wood and digestive fluid. This rather liquid midgut fluid is highly alkaline, ranging from pH 10.2–10.4 in third instar larvae of D. rectus (M. Tanahashi, unpublished data). Such high alkalinity is also common in soil- or humus-feeding Scarabaeoidea (Lemke et al. 2003). In contrast, the hindgut of lucanid larvae is expanded when filled with digested food or feces, and usually constricted into two or more sections that will finally form each fecal pellet. The hindgut content has an almost neutral pH (6.7–7.8) in D. rectus larvae, and is rather solid and light-colored compared with that of the midgut. In this study, the midgut of the S. cylindricum larva contained considerably fewer living yeasts (3.6 × 102 CFU/mg) than the hindgut (1.1 × 105 CFU/mg), which suggests that the yeasts proliferate in the hindgut.
Similar to other lucanids, cecum-like sacs are located at the junction between midgut and hindgut of S. cylindricum larva. Although these seem to be a prominent internal structure, the function of the cecum-like sacs has not been fully investigated. The sacs, which are somewhat harder than other intestinal organs, were seen to contain fine wood particles and many yeast cells, when observed under the microscope. The inner surface of these sacs seems to be lined with a thick cuticle, suggesting that it originated from ectodermal hindgut. Their yeast content is similar to that of the midgut and hindgut, even though the net capacity of the sacs is small. It is suggested that these sacs act as a refuge for the hindgut symbionts, where they can be retained safely against the gut flow until released into the hindgut. However, gut yeasts in passalid larvae are sometimes found adhering to the gut wall by means of branched filaments (holdfasts), which helps prevent them washing out (Nardi et al. 2006).
Difference in Microbial Environment Between Adult and Larva
In an earlier study, Tanahashi et al. (2010) put forward the hypothesis that the evolution of mycangia in Lucanidae is related to the biological difference in food eaten by larvae compared with that eaten by adults. Adult females of most lucanid species deposit eggs on decaying wood, although oviposition preference for decay type varies among species (Araya 1993, 2002). Wood is one of the most indigestible organic materials; it contains high levels of wood polymers such as cellulose, hemicelluloses and lignin, and a very low level of nitrogen (Haack and Slansky 1987). Degradation by wood-decaying fungi may enhance the nutrient value of wood to some extent, but nitrogen level remains low (usually, no more than 0.3%) (Tanahashi and Togashi 2009). Today, microbial symbionts are widely known from many wood-feeding or wood-inhabiting insect groups, and are thought to help digestion of wood polymers such as cellulose and lignin (Breznak and Brune 1994, Geib et al. 2009), detoxify the woody chemicals (Doud 1992), have an ability to fix nitrogen (Benemann 1973) or serve as food for insects (Beaver 1989). Lucanid larvae, including those of S. cylindricum referred to in this study, may also benefit from the xylose-utilizing ability of yeast symbionts.
In contrast, adult lucanids feed mainly on fermented tree sap (slime flux). Tree sap is rich in free sugars, amino acids, and vitamins (Kallio et al. 1989, Jiang et al. 2001). Free-living yeasts that are also commonly found in slime flux (Lachance et al. 2001) may improve its nutrient value. This suggests that adult stag beetles do not require specialist yeast symbionts in their food or digestive system, a suggestion supported by S. cylindricum adult beetles harboring no living yeasts or fungi, even though some food materials were still present in their digestive system. The same data were obtained from adults of D. parallelipipedus and L. cervus in this study, and also from Japanese stag beetles D. rectus and L. maculifemoratus when they were examined at least 1 d after feeding (M. Tanahashi, unpublished data). The absence of living yeasts in sap-feeding adults is somewhat puzzling, because their food is likely to contain a large number of living yeasts and moulds. A possible answer to this question is that these yeasts have been completely digested to provide additional nutrient in their passage through the gut. Thus the gut of S. cylindricum adults, as well as adults of more evolutionarily advanced lucanids, seem never to act as storage organs for yeast symbionts, which we suggest has resulted in the evolution of a mycangium, an organ that is used for temporary storage of specialist yeast symbionts during the adult phase. In contrast to lucanids, the majority of wood-feeding insects rely on wood or woody tissues of trees throughout their lives, and some develop subsociality, where parents prepare food for their larvae. The Passalidae (Coleoptera) the major wood-feeding family in the Scarabaeoidea exhibits subsociality. Adults and larvae of passalids live in the same piece of decaying wood, which is also used as food by the larvae (Schuster and Schuster 1997, Ento et al. 2008); xylose-fermenting Scheffersomyces yeasts are commonly found in the gut of adult beetles (Suh et al. 2003) as well as larvae of this group (M. Tanahashi unpublished). We therefore hypothesize that evolution of the mycangium in the ancestral Lucanidae might have led to the development of adults and larvae using different food sources, sap-feeding in the former and wood-feeding in the latter.
Limitations and Future Issues
We are aware that our sample size is small and that these results need to be confirmed. Although S. cylindricum is widespread in Europe and the United Kingdom, it is very local and difficult to find, especially as this species spends its life almost entirely within decaying wood.
The evolution of a mycangium is possibly highly associated with the evolution of stag beetles. However, the phylogenetic placement of Sinodendron seems to be unresolved. For example, the recent phylogenetic study (Kim and Farrell 2015) showed only weak branch support values for the ancestral clades of Lucanidae, including Sinodendron. Further studies are required to resolve these issues, and to elucidate the evolutionary origin of the insect-yeast symbiosis in Lucanidae.
Geolocation Information, Bioethics, and Data Availability
Samples of wild organisms used in this study were collected in the United Kingdom, France, Switzerland, and Japan. The biological and statistical analyses were conducted in the United Kingdom and Japan. International agreements were followed, protected areas were respected and bioethical standards met. All nucleotide sequences are available in GenBank (accession nos. LC119078–LC119086 and LC120353–LC120363). Yeast strains are being preserved as frozen stocks or plate cultures by the authors.
Acknowledgments
We greatly thank Eva Sprecher-Uebersax and the staff of Naturhistorisches Museum Basel for making the arrangements for insect sampling in Switzerland, and Ludovic Fuchs (French Forestry National Office) for sampling in France. We also thank Wataru Toki for providing Wickerhamomyces yeast strains and technical advice. Special thanks are due to Professor Kunio Araya for detailed comments and suggestions. We are indebted to Martin Sanford (Suffolk Biological Records Centre, United Kingdom) for suggesting local areas in Suffolk (United Kingdom) to search for S. cylindricum.
Funding
This study was supported by the Japan Society for the Promotion of Science Fellowship for Young Scientists.
References Cited
- Alexander K. N. A. 2002. The invertebrates of living and decaying timber in Britain and Ireland. Engl. Nat. Res. Rep. 467: 9–142. [Google Scholar]
- Altschul S. F., Madden T. L., Schaffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya K. 1993. Relationship between the decay types of dead wood and occurrence of lucanid beetles (Coleoptera: Lucanidae). Appl. Entomol. Zool. 28: 27–33. [Google Scholar]
- Araya K. 2002. Effects of variation in dead wood on resource utilization patterns and fitness of lucanid beetles (Insecta, Coleoptera, Lucanidae). Jpn. J. Ecol. 52: 89–98. [Google Scholar]
- Arrow G. J. 2005. Horned beetles: a study of the fantastic in nature. Natural History Publications (Borneo) Sdn. Bhd, Kota Kinabalu. [Google Scholar]
- Batra L. R. 1963. Ecology of ambrosia fungi and their dissemination by beetles. Trans. Kans. Acad. Sci. 66: 213–236. [Google Scholar]
- Beaver R. A. 1989. Insect-fungus relationship in the bark and ambrosia beetles pp. 121–143. In Wilding N., Collins N.M., Hammond P.M., Webber J.F. (eds), Insect–fungus interactions. Academic Press, London. [Google Scholar]
- Benemann J. R. 1973. Nitrogen fixation in termites. Science 181: 164–165. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Brune A. 1994. Role of microorganisms in the digestion of lignocellulose by termites. Annu. Rev. Entomol. 39: 453–487. [Google Scholar]
- Chapman T. A. 1868. Note on the habits of Sinodendron cylindricum during oviposition. Entomol. Month. Mag. 5: 139–141. [Google Scholar]
- Davis T. S. 2015. The ecology of yeasts in the bark beetle holobiont: a century of research revisited. Microb. Ecol. 69: 723–732. [DOI] [PubMed] [Google Scholar]
- Doud P. F. 1992. Insect fungal symbionts: a promising source of detoxifying enzymes. J. Ind. Microbiol. 9: 149–161. [Google Scholar]
- Ento K., Araya K., Kudo S. I. 2008. Trophic egg provisioning in a passalid beetle (Coleoptera). Eur. J. Entomol. 105: 99–104. [Google Scholar]
- Fremlin M., Tanahashi M. 2015. Sexually-dimorphic post-eclosion behaviour in the European stag beetle Lucanus cervus (L.) (Coleoptera: Lucanidae). Bull. Soc. Entomol. Suisse. 8: 29–38. [Google Scholar]
- Fujita H. 2010. The Lucanid Beetles of the World. Mushi-Sha, Tokyo [Google Scholar]
- GBIF 2016. Global biodiversity information facility. www.gbif.org.
- Geib S. M., Filley T. R., Hatcher P. G., Hoover K., Carlson J. E., Jimenez-Gasco M. M., Nakagawa-Izumi A., Sleighter R. L., Tien M. 2009. Lignin degradation in wood-feeding insects. Proc. Natl Acad. Sci. U S A. 105: 12932–12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack R. A., Slansky F. 1987. Nutritional ecology of wood-feeding Coleoptera, Lepidoptera, and Hymenoptera pp. 449–486. In Slansky F., Rodriguez J.G. (eds), Nutritional ecology of insects, mites, and spiders. John Wiley, New York. [Google Scholar]
- Hawes C. J. 2009. Discovery of a mycangium in Lucanus cervus. Report 1: people's trust for endangered species. PTES, London. [Google Scholar]
- Hawes C. J. 2010. Discovery of a mycangium in Lucanus cervus. Report 2: people's trust for endangered species PTES, London. [Google Scholar]
- Hawes C. J. 2013. Discovery of a mycangium and associated yeasts in the stag beetle Lucanus cervus (Coleoptera: Lucanidae). White Admiral. 85: 22–3. Erratum in: White Admiral 2013; 86: 12. [Google Scholar]
- Holloway B. A. 2007. Lucanidae (Insecta: Coleoptera). Fauna of New Zealand 61. Manaaki Whenua Press, Lincoln. [Google Scholar]
- Hosoya T., Araya K. 2005. Phylogeny of Japanese stag beetles (Coleoptera: Lucanidae) inferred from 16S mtrRNA gene sequences, with reference to the evolution of sexual dimorphism of mandibles. Zool. Sci. 22: 1305–1318. [DOI] [PubMed] [Google Scholar]
- Jeffries T. W., Grigoriev I. V., Grimwood J., Laplaza J. M., Aerts A., Salamov A., Schmutz J., Lindquist E., Dehal P., Shapiro H.,et al. 2007. Genome sequence of the lignocellulose-bioconverting and xylose-fermenting yeast Pichia stipitis. Nat. Biotechnol. 25: 319–326. [DOI] [PubMed] [Google Scholar]
- Jiang H., Sakamoto Y., Tamai Y., Terazawa M. 2001. Proteins in the exudation sap from birch trees, Betula platyphylla Sukatchev var. japonica Hara and Betula verrucosa Her. Eur. J. For. Res. 2: 59–64. [Google Scholar]
- Kallio H., Teerinen T., Ahtonen S., Suihko M., Linko R. R. 1989. Composition and properties of birch syrup (Betula pubescens). J. Agric. Food Chem. 37: 51–54. [Google Scholar]
- Kim S. I., Farrell B. D. 2015. Phylogeny of world stag beetles (Coleoptera: Lucanidae) reveals a Gondwanan origin of Darwin’s stag beetle. Mol. Phylogenet. E. 86: 35–48. [DOI] [PubMed] [Google Scholar]
- Klausnitzer B., Sprecher-Uebersax E. 2008. Die Hirschkafer oder Schroter (Lucanidae), Die Neue Brehm-Bucherei 551 Westarp Wissenschaften, Hohenwarsleben. [Google Scholar]
- Kobayashi C., Fukasawa Y., Hirose D., Kato M. 2008. Contribution of symbiotic mycangial fungi to larval nutrition of a leaf-rolling weevil. Evol. Ecol. 22: 711–722. [Google Scholar]
- Krajcik M. 2001. Lucanidae of the world, Catalogue. Part 1. Checklist of the stag beetles of the world (Coleoptera: Lucanidae). Milan Krajcik, Czech. [Google Scholar]
- Kuranouchi T., Nakamura T., Shimamura S., Kojima H., Goka K., Okabe K., Mochizuki A. 2006. Nitrogen fixation in the stag beetle, Dorcus (Macrodorcus) rectus (Motschulsky) (Col., Lucanidae). J. Appl. Entomol. 130: 471–472. [Google Scholar]
- Kurtzman C. P., Suzuki M. 2010. Phylogenetic analysis of ascomycete yeasts that form coenzyme Q-9 and the proposal of the new genera Babjeviella, Meyerozyma, Millerozyma, Priceomyces and Scheffersomyces. Mycoscience. 51: 2–14. [Google Scholar]
- Lachance M. A., Klemens J. A., Bowles J. M., Janzen D. H. 2001. The yeast community of sap fluxes of Costa Rican Maclura (Chlorophora) tinctoria and description of two new yeast species, Candida galis and Candida ortonii. FEMS Yeast Res. 1: 87–92. [DOI] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R. 2007. ClustalW and ClustalX version 2. Bioinformatics. 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- Lemke T., Stingl U., Egert M., Friedrich M. W., Brune A. 2003. Physicochemical conditions and microbial activities in the highly alkaline gut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 69: 6650–6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden J. L., Coutts M. 1979. The role of fungi in the biology and ecology of woodwasps (Hymenoptera: Siricidae) pp. 165–174. In Batra L. R. (ed.), Insect–fungus symbiosis: nutrition, mutualism, and commensalism. John-Willy and Sons, New York. [Google Scholar]
- Miyashita A., Hirai Y., Sekimizu K., Kaito C. 2015. Antibiotic-producing bacteria from stag beetle mycangia. Drug. Discov. Ther. 9: 33–37. [DOI] [PubMed] [Google Scholar]
- Mori H., Chiba S. 2009. Sociality improves larval growth in the stag beetle Figulus binodulus (Coleoptera: Lucanidae). Eur. J. Entomol. 106: 379–383. [Google Scholar]
- Nardi J. B., Bee C. M., Miller L. A., Nguyen N. H., Suh S. O., Blackwell M. 2006. Communities of microbes that inhabit the changing hindgut landscape of a subsocial beetle. Arthropod. Struct. Dev. 35: 57–68. [DOI] [PubMed] [Google Scholar]
- NBN 2016, National Biodiversity Network. http://www.nbn.org.uk/.
- Sadaki R., Hayashi T., Tsuchiya T. 2014. Neolucanus in Japan. Mushi-sha, Tokyo. [Google Scholar]
- Schuster J. C., Schuster L. B. 1997. The evolution of social behavior in Passalidae (Coleoptera) pp. 260–269. In Choe J.C., Crespi B.J. (eds.), The evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge. [Google Scholar]
- Six D. L. 2003. A comparison of mycangial and phoretic fungi of individual mountain pine beetles. Can. J. For. Res. 33: 1331–1334. [Google Scholar]
- Sjostrom E. 1993. Wood chemistry. Fundamentals and applications, 2nd ed. Academic Press, San Diego. [Google Scholar]
- Suh S. O., Marshall C. J., McHugh J. V., Blackwell M. 2003. Wood ingestion by passalid beetles in the presence of xylose-fermenting gut yeasts. Mol. Ecol. 12: 3137–3145. [DOI] [PubMed] [Google Scholar]
- Suh S. O., McHugh J. V., Pollock D. D., Blackwell M. 2006. The beetle gut: a hyperdiverse source of novel yeasts. Mycol. Res. 109: 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton P. G. 2003. The British stag beetle family (Coleoptera: Lucanidae). Bull. Amateur Entomol. Soc.. 62: 248–261. [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. E. 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi M., Fremlin M. 2013. The mystery of the lesser stag beetle Dorcus parallelipipedus (L.) (Coleoptera: Lucanidae) mycangium yeasts. Bull. Amateur Entomol. Soc. 72: 146–152. [Google Scholar]
- Tanahashi M., Kubota K., Matsushita N., Togashi K. 2010. Discovery of mycangia and associated xylose-fermenting yeasts in stag beetles (Coleoptera: Lucanidae). Naturwissenschaften 97: 311–317. [DOI] [PubMed] [Google Scholar]
- Tanahashi M., Kubota K. 2013. Utilization of the nutrients in the soluble and insoluble fractions of fungal mycelium by larvae of the stag beetle, Dorcus rectus (Coleoptera: Lucanidae). Eur. J. Entomol. 110: 611–615. [Google Scholar]
- Tanahashi M., Matsushita N., Togashi T. 2009. Are stag beetles fungivorous? J. Insect Physiol. 55: 983–988. [DOI] [PubMed] [Google Scholar]
- Tanahashi M., Togashi K. 2009. Interference competition and cannibalism by Dorcus rectus (Motschulsky) (Coleoptera: Lucanidae) larvae in the laboratory and field. Coleopt. Bull. 63: 301–310. [Google Scholar]
- Toki W., Tanahashi M., Togashi K., Fukatsu T. 2012. Fungal farming in a non-social beetle. PLoS One 7: e41893.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina H., Blackwell M. 2012. Multilocus phylogenetic study of the Scheffersomyces yeast clade and characterization of the N-terminal region of xylose reductase gene. PLoS One 7: e39128.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. J., Bruns T., Lee S., Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, pp. 315–322. In Innis M.A., Gelfand D.H., Sninsky J.J., White T.J. (eds.), PCR protocols—a guide to methods and applications. Academic Press, San Diego. [Google Scholar]
- Zahradnik J., Chvala M. 1989. Insects: a comprehensive guide to the insects of Britain and Europe. Hamlyn, London. [Google Scholar]