Abstract
Thymosins have been highly conserved during evolution. These hormones exist in many animal species and play an essential role in many biological events. However, little is known regarding the physiological function of silkworm Bombyx mori thymosin (BmTHY). In this study, we investigated the expression pattern of BmTHY in a Bombyx mori larval ovarian cell line (BmN) challenged with Bombyx mori nuclear polyhydrosis virus (BmNPV) and the antiviral effect of recombinant BmTHY (rBmTHY) for Bombyx mori against BmNPV. Western-blot assay and qRT-PCR analysis revealed that the level of BmTHY protein expression and transcription decreased over time when BmN cells were infected by BmNPV. Treatment with endotoxin-free rBmTHY led to a significant reduction in viral titer in the supernatant of BmN cells challenged with BmNPV. The results from antiviral tests performed in vitro and in vivo showed that endotoxin-free rBmTHY improved the survival rate of Bombyx mori infected with BmNPV. These findings suggest that BmTHY exerts immunomodulatory effects on Bombyx mori, rendering them resistant to viral infection.
Keywords: thymosin, Bombyx mori, BmNPV, antivirus
Thymosins (THY) are highly conserved polypeptides that were originally isolated and identified from calf thymus gland extract in 1966 (Goldstein et al. 1966) and have subsequently been demonstrated to exert a considerable function on the development and maintenance of a competent immune system (Law et al. 1968; Horowitz et al. 1977; Li et al. 2015). These hormones have been extensively described as the immunomodulatory activity on innate immune cells, such as polymorphonuclear leucocytes, dendritic cells, and macrophages (Knutsen et al. 1999). Thymosins are divided into three main groups, α-, β-, and γ-thymosin according to their isoelectric points (Low and Goldstein 1979; Low et al. 1979). They have been highly conserved during evolution and found in a broad phylogenetic distribution from invertebrates to vertebrates (Oates et al. 1988; Oates and Erdos 1989; Bodey et al. 2000; Manuel et al. 2000; Zhang et al. 2012). Emerging evidence reveals that vertebrates thymosins have been widely used as pharmacological agents in clinical practice to treat some diseases including immunodeficiency related diseases, chronic hepatitis and certain types of cancers (Rustgi 2004; Cheng et al. 2006; Kellici and Burazeri 2009; Maio et al. 2010; Kim et al. 2012).
However, studies examining insectile thymosins are very limited so far. Only 18 related proteins composed of thymosin conservative structural domains from lepidoptera can be found in NCBI website (Holt et al. 2002; Clark et al. 2007). These proteins are highly homologous to β-thymosin (Tβ) and are distributed in Drosophila melanogaster, Aedes aegypti, Bombyx mori and other species (Zhang et al. 2012). Ciboulot, a homologous thymosin protein characterized in Drosophila, has been implicated in axonal growth and brain metamorphosis (Boquet et al. 2000). Zhang et al. (2013) reported that up-regulation of thymosin reduced F-actin synthesis, but silencing thymosin by dsRNA led to increased replication of Autographa californica multiple nuclear polyhedrosis virus (AcMNPV) in Ha-shl-t (a cell line from the pupal testis of Helicoverpa armigera) cells.
In a previous study, the gene encoding a Bombyx mori thymosin (BmTHY) protein was identified from the cDNA library of silkworm pupae, which is highly homologous to Tβ (Zhang et al. 2012). However, the physiological function of BmTHY in Bombyx mori immunity remains unknown. Although the silkworm only has an innate immune system in defense against pathogens, the majority of the components or factors in the immune system are widely conserved among species (Ishii et al. 2015). Converging lines of evidence have shown that thymosins from other species played an important role in antiviral immune response (Jiang et al. 2010; Mosoian et al. 2010; Shi et al. 2015). Therefore, the present study aimed to investigate the relationship between BmTHY and BmNPV infection, as well as to identify antiviral activity of recombinant BmTHY (rBmTHY) against BmNPV.
Materials and Methods
Materials
We have constructed the recombinant plasmid pET-28a- BmTHY and preserved it in our lab (Zhang et al. 2012). Escherichia coli strains TG1 and BL21(DE3) were cultured in LB medium (5 g of yeast extract, 10 g of tryptone and 10 g of NaCl per liter, pH 7.5) at 37 °C. BmN cell, a Bombyx mori larval ovarian cell line, was preserved in our lab and cultured at 27 °C in Sf-900IImedium (Sigma) containing 10% (v/v) fetal bovine serum (FBS, Gibco BRL). Wild-type BmNPV (WT BmNPV, maintained in our lab) was propagated in BmN cells. Bombyx mori strains, Qingsong × Haoyue, were obtained from the laboratory animal center in Zhejiang Sci-Tech University, China. The fifth instar larvae were cultivated on mulberry leaves under standard conditions. We performed animal experiments according to the protocol authorized by the Institutional Animal Care and Use Committee (IACUC) of Institute of Laboratory Animal Sciences, Chinese Academy of Medical Science (No.: N-07-6001). The protocol meets with internationally recognized animal welfare guidelines.
The following antibodies were used in this study: anti-BmTHY IgG (prepared as previously described; Zhang et al. 2012); anti-β-Tubulin (Beyotime, China); HRP-labeled anti-rabbit IgG (DingGuo, China). ToxinEraser Endotoxin Removal Kit and ToxinSensor Gel Clot Endotoxin Assay Kit were purchased from GenScript Co., Ltd. The analytically pure chemical reagents used in this study were commercially available.
RNA Extraction and cDNA Synthesis From BmN Cells
BmN cells were cultured in Sf-900 medium with 10% (v/v) FBS in atmosphere of 5% carbon dioxide at 27 °C. The BmN cells infected with 1 × 108 pfu ml − 1 BmNPV served as the experimental group, whereas uninfected cells served as the control group. BmN cells were harvested for subsequent assay.
The total RNAs were extracted from BmN cells in each group using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol; the concentration and absorbance of which were determined by a Nanodrop ND-1000 spectrophotometer. Purified RNA samples dissolved in DEPC water were stored at −80 °C and prepared for cDNA synthesis. Total RNA was transcribed into cDNA using the SuperScript IIIFirst-Strand Synthesis System for qRT-PCR (Invitrogen) according to the manufacturer’s instructions.
Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
qRT-PCR primers for BmTHY were designed by the DNA STAR software. A pair of primers was designed to amplify β-actin used as an endogenous control for gene transcription analysis. The following primer pairs were used:
-(BmTHY) Forward primer, 5′-TTCTATCCCC TTCCTCA TCA A A AA-3′, Reverse primer, 5′-TCAGCA GACGGA AGCACAATC-3′; -(β-actin rRNA) Forward primer, 5′-CGATC CGCCGACG TTACTACA-3′, Reverse primer, 5′-GTCCGG GCCTGGT GAGATTT-3′.
We used qRT-PCR to detect BmTHY gene transcription levels in various groups with SuperScriptIII Platinum SYBR Green One-Step qRT-PCR Kit (Invitrogen) using ABI Prism 7300 Sequence Detection System (Applied Bio Systems) as previously described (Zhang et al. 2012). Data analysis was carried out by ABI Prism 7300 SDS Software V1.3.1 (Applied Biosystems). Transcription level of the target gene was normalized against that ofβ-actin rRNA gene. The relative gene transcription level was calculated using 2−ΔΔCT, where ΔCT = CT(BmTHY)−CT(β-actin rRNA), ΔΔCT = ΔCT(BmTHY) −ΔCT(maximum). Each assay was performed in triplicate.
Western-Blotting Analysis
To detect intracellular proteins, BmN cells were rinsed with PBS and then lysed immediately in a lysis buffer (50 mM tris (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate). The cell homogenates were centrifuged at 12,000×g for 15 min at 4 °C. The bicinchoninic acid (BCA) method (with reagents from Pierce) was performed to analyze protein concentrations. Subsequently, the total protein content between different samples was equalized for Western-blotting analysis. Protein samples were loaded onto 10% SDS–polyacrylamide gel, electrophoresed and electrotransfered to polyvinylidene difluoride (PVDF) membranes (Millipore) by a semi-dry method with steady current of 90 mA per SDS–polyacrylamide gel for 90 min. After blocking with 5% milk in TBST (pH 7.5) for 1 h at room temperature, PVDF membranes were probed with purified anti-BmTHY IgG at 4 °C overnight. Next, membranes were washed with TBST and incubated with HRP-labeled anti-rabbit IgG as secondary antibody according to a common protocol. Membranes were scanned using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE) at 700 nm. Proteins were finally visualized simultaneously, and the bands were measured using ImageJ ver. 1.46 software.
Preparation of rBmTHY for Antiviral Experimentation
The recombinant plasmid pET-28a- BmTHY was transformed into E. coli BL21 (DE3) competent cells using the heat-shock procedure. The cells were subsequently incubated at 37 °C in liquid LB culture media containing 50 mg/liter kanamycin. Expression of the His-tag fusion protein was induced at an A600 of 0.6 with 0.5 mM isopropyl thiogalactoside for 4 h incubation in large scale. Recombinant BmTHY was purified using Ni2+-SephadexTM G-25 Superfine column (Amersham) as instructed by the manufacturer. In addition, endotoxins were removed from the purified proteins using ToxinEraser endotoxin removal kit (GenScript). The endotoxin content in purified proteins was analyzed using the ToxinSensor Endotoxin Detection System (GenScript). Protein concentration was later determined using BCA protein assay (Pierce), and recombinant BmTHY samples were stored at −80 °C for antiviral experimentation.
Detection of Viral Titer in the Supernatant of BmN Cells Infected With BmNPV
To analyze the influence of rBmTHY on virus proliferation, 4μg·ml − 1 of endotoxin-free rBmTHY was added to BmN cells in the experimental group, and the same volume of PBS was added to BmN cells in the control group. After incubating 24 h at 27 °C, BmN cells were infected with BmNPV for 60 h. Then, the supernatant of BmN cells was harvested and diluted from 10 − 1 to 10 − 8 using TC-100 medium to investigate viral titer as follows: (1) Using flat-bottomed 96-well plates, 1× 103 BmN cells were seeded per well and incubated at 27 °C overnight. (2) BmN cells were inoculated with gradient dilutions (10 − 1∼10 − 8) of supernatant containing virus (100 μl per well), octuplicate in each dilution, and unvaccinated virus cells were served as negative control. (3) BmN cells were cultured at 27 °C in incubator for consecutive 6 d. The infection status of BmN cells were observed under microscope to obtain the accounts of well in which cells were damaged or dead for each dilution every day. (4) In the end, the viral titer was calculated according to the method of TCID50 (Martin and Croizier 1997).
Antiviral Test of Endotoxin-Free rBmTHY on BmN Cells
Using flat-bottomed 96-well test plates, 6 × 104 BmN cells were seeded per well in 200 μl of TC-100 medium containing 10% FBS and incubated at 27 °C. One column was used for negative control in which only medium was added. Another column was used for virus control in which cells were challenged with BmNPV at a multiplicity of infection (MOI) of 1 for 48 or 72 h. In the drug treatment group, BmN cells were exposed to different concentrations (0.04, 0.4, 4 μg ml − 1) of endotoxin-free recombinant BmTHY and infected with BmNPV at an MOI of 1 at select time intervals (48 and 72 h) in triplicate. After 48 or 72 h of incubation, the cell morphology in each group was examined under inverted electron microscope (NIKON, Japan). Cell viability was measured using the MTT assay described by Mosmann (1983). Optical Density was measured using an automatic microplate reader (BioTek EL 800) at 490 nm. Untreated cells were used as negative controls for further normalization and determination of cell viability for each stimulation. Cell viability = OD490 (experiment group)/OD490 (negative group).
Antiviral activities of different concentrations of rBmTHY were calculated according to the following formula (Pauwels et al. 1988): antiviral activity (%) = ((ODT490)v−(ODC490)v)/((ODC490) mock− (ODC490)v) × 100%. (ODT490)v and (ODC490)v represents the optical density of cells infected with BmNPV in presence of and in the absence of rBmTHY, respectively (index: T = treated, index: C = control). (ODC490)mock is the optical density of normal cell in negative group.
Antiviral Test of Endotoxin-Free rBmTHY on Larvae
To investigate antiviral activity of endotoxin-free rBmTHY on larvae, 250 newly exuviated fifth-instar larvae were randomly divided into five groups of 50 animals each. The five groups were as follows: (1) vehicle control: treatment with PBS; (2) virus control: subcutaneous inoculation with wild-type BmNPV (106 OB per larva); (3) treatment with high dose (20 μg ml − 1) of rBmTHY and infection with BmNPV (106 OB per larva); (4) treatment with an intermediate dose (2 μg ml − 1) of rBmTHY and infection with BmNPV (106 OB per larva); and (5) treatment with low dose (0.2 μg ml − 1) of rBmTHY and infection with BmNPV (106 OB per larva).
Fresh mulberry leaves were cut into round pieces 1 cm in diameter and fed to the larvae. For the drug treatment group, larvae were subcutaneously inoculated with OBs. Afterward, larvae were fed with mulberry leaves sprayed with different concentrations of rBmTHY for three consecutive days, transferred to fresh mulberry leaves and reared at 25 °C. For the virus control group, larvae were subcutaneously injected with OBs, whereas for vehicle control, larvae were inoculated with PBS instead of OBs. Four independent experiments were performed under the same conditions, and every repeat had 50 larvae for each group. Cumulative survival rates were calculated daily from infection time to moth stage.
Statistical Analysis
All data were presented as the mean ± SEM. Statistical analysis was performed with GraphPad Prism 5 software and significant differences were evaluated using specific statistical tests described in the figure legends. A probability (P) <0.05 was considered to be statistically significant.
Results
Transcription and Expression Level of BmTHY in BmN Cells Infected With BmNPV
We first detected expression changes of two housekeeping genes GAPDH and Tublin in BmN cells before and after BmNPV infection. The results of Western-blotting analysis in the Supp Data S1 (online only) showed the housekeeping gene Tublin used in this study was stable under infection, which is consistent with those of previous studies (Zhang et al. 2014a, Zhang et al. 2014b, Govindaraj et al. 2016). After confirming the internal control gene is indeed stable, we investigated transcription and expression level of BmTHY in BmN cells infected with BmNPV as follows. The experimental group of BmN cells was infected with identical quantities of wild-type BmNPV using 6 × 105 OB. Normal BmN cells served as control. Western blotting and qRT-PCR analysis detected target protein and mRNA expression in BmN cells.
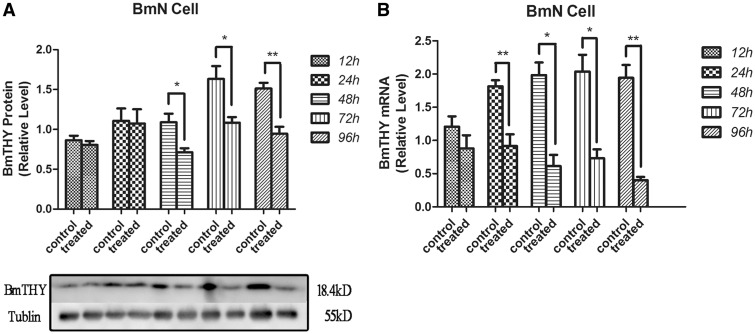
The protein expression level of BmTHY in the control group was set as 100%, and the values of the infected BmN cells were standardized against this value. Western blotting demonstrated that BmTHY protein was detected from 12 to 96 h after BmN cells were infected with BmNPV; however, there were no significant difference between the control group and experimental groups from 12 to 24 h. Significant changes were detected in experimental groups after 48 h. BmTHY expression level in experimental groups at 48, 72 and 96 h declined to 65.89, 67.00 and 69.98% of the corresponding control group values, respectively. The data (Fig. 1A) suggested that BmTHY expression was down-regulated during wild-type BmNPV infected BmN cells from 48 to 96 h, and the expression level of BmTHY at 96 h reached the lowest point detected by Western blotting.
Fig. 1.
Western blotting and qRT-PCR analysis of BmTHY expression and transcription in BmN cells challenged by wild-type BmNPV. (A) Western blotting was used to analyze the expression of BmTHY after being challenged with BmNPV in BmN cells. Ten micrograms of total protein extracts were loaded per lane. Tubulin was used as the control. (B) The mRNA expression of the target genes challenged with BmNPV in BmN cells. RNA were extracted from each group of BmN cells. Error bars represent the SEM in three replicates. Asterisk indicates significant difference between control group and experimental group (t-test, P<0.05).
The mRNA content of BmTHY in BmN cells prior to infection with BmNPV was set as 100%, and the values of the BmNPV-infected BmN cells were standardized against this value. qRT-PCR (Fig. 1B) demonstrated that the mRNA expression levels of BmTHY in BmN cells infected with BmNPV at 12 and 24 h were 72.98 and 50.46% relative to that of control group, respectively. While the mRNA expression levels of BmTHY in BmN cells infected with BmNPV at 48, 72 and 96 h were 30.89, 35.88 and 20.63% relative to that of control group, respectively, which were significant decreased (P < 0.05, Fig. 1B).
The results indicated that exposure to BmNPV results in down-regulation in the expression of BmTHY. This may be one of the mechanisms in which BmNPV results in BmN cell death.
Expression, Purification of Recombinant BmTHY and Endotoxin Detection
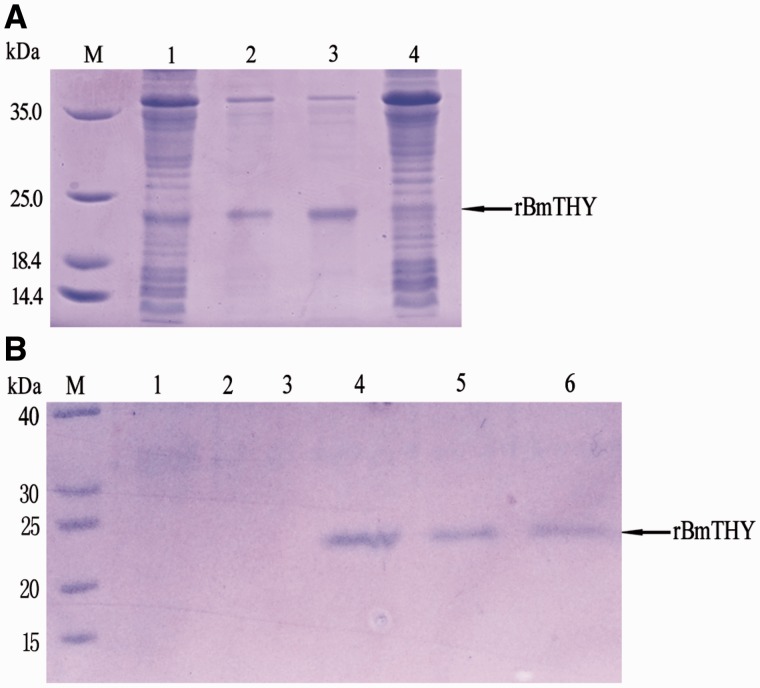
The recombinant E. coli BL21 (DE3) competent cells containing recombinant plasmid pET-28a- BmTHY were incubated at 37°C in liquid LB culture media. The His-tag fusion proteins were induced at an A600 of 0.6 followed by adding IPTG before another 5-h incubation. Expression of recombinant BmTHY in E. coli was investigated by SDS–PAGE shown in Fig. 2, which revealed a protein of expected size in the recombinant bacteria. Moreover, the rBmTHY were primarily observed in the supernatant of cell lysates by ultrasonication. His-tag rBmTHY proteins were purified by HiTrap Chelating HP and identified by mass spectrometry, as previously described (Zhang et al. 2012). Subsequently, the endotoxin content was analyzed using ToxinSensor Endotoxin Detection System (GenScript) after endotoxins were removed from the purified proteins. The endotoxin content in proteins was adjusted to <1.0 EU ml − 1. rBmTHY samples were stored at −80 °C for antiviral experimentation.
Fig. 2.
Expression and purification of rBmTHY protein. (A) M: Protein Marker: (1) BL21 (pET-28a (+)–BmTHY) induced by 0.5 mmol/liter IPTG at 37 °C for 5 h; (2) the deposition of supersonic fragmentation of BL21 (pET-28a (+)–BmTHY) induced by 0.5 mmol/liter IPTG at 37 °C for 5 h; (3) the supernate of supersonic fragmentation of BL21 (pET-28a (+)–BmTHY) induced by 0.5 mmol/liter IPTG at 37 °C for 5 h; (4) BL21 (pET-28a (+)–BmTHY) without IPTG at 37 °C for 5 h and (B) M: Protein Marker: 1,2,3: samples collected from Wash Buffer and 4,5,6: samples collected from Elution Buffer.
BmNPV Titer in the Supernatant of BmN Cells Treated With Endotoxin-Free rBmTHY
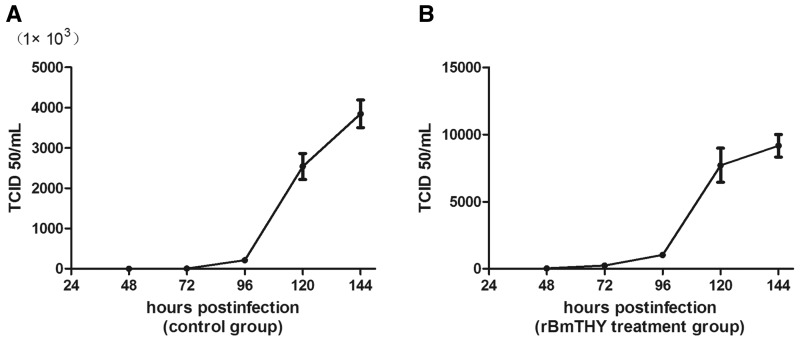
After treatment with endotoxin-free rBmTHY or PBS (control), BmN cells were infected with BmNPV for 60 h and the supernatant was harvested for viral titer detection. The result in Fig. 3 showed that viral titer increased significantly with lastingness of infection in both groups. However, the value of BmNPV titer in control group was much larger than that of rBmTHY treatment group. For instance, the mean value of TCID50 at 120-h post-infection in PBS control group and rBmTHY treatment group were 2.54 × 106 and 7.71 × 103, respectively. Therefore, treatment with rBmTHY led to a significant reduction in viral titer in the supernatant of BmN cells challenged with BmNPV. The results indicate that rBmTHY improved BmN cells the ability of resistance to BmNPV infection through antiviral activity (Raberg et al. 2009).
Fig. 3.
Titration curve of extracellular BmNPV virus present after BmN cells infected with BmNPV. The titer was determined on BmN cells by end-point dilution in a 96-well plate according to the method of TCID50.(A) BmN cells treated with PBS control and (B) BmN cells treated with endotoxin-free rBmTHY. Data represent mean±SEM of three independent experiments.
Antiviral Effects of Recombinant Protein BmTHY on BmN Cells Against BmNPV
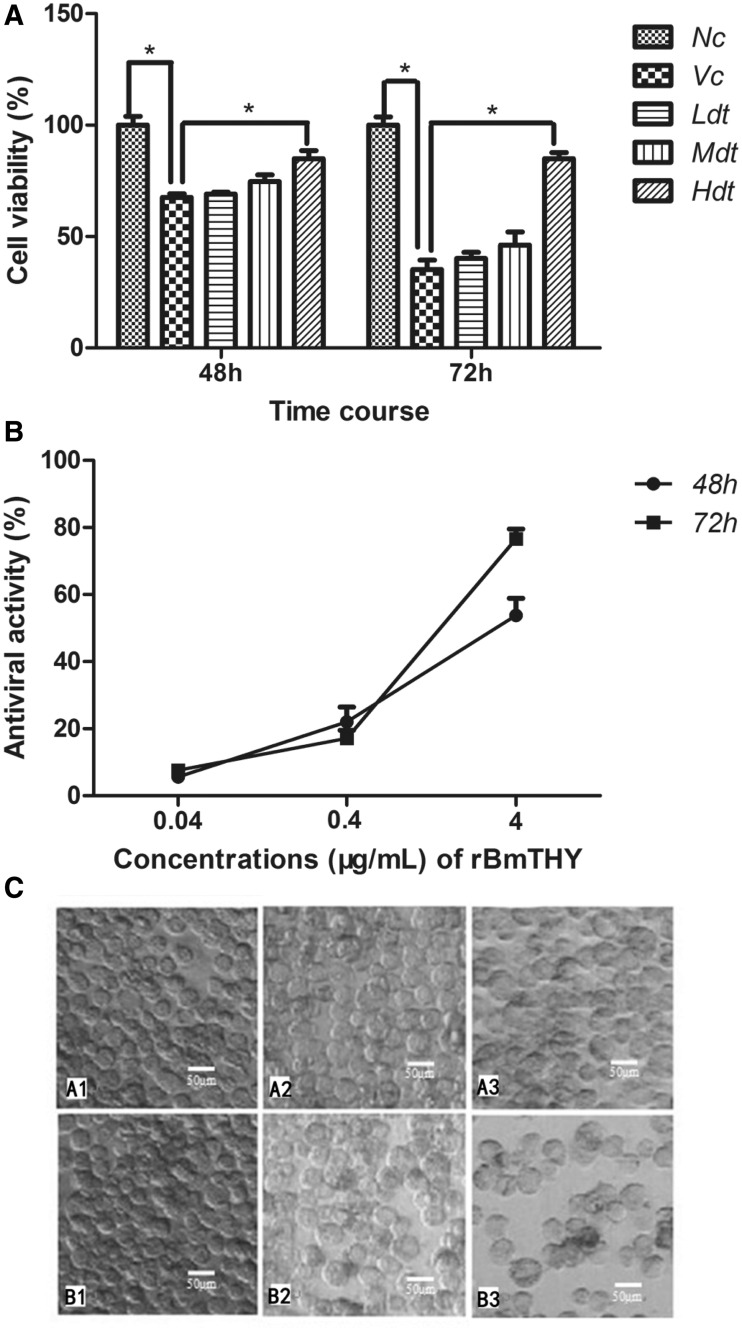
To determine the antiviral effects of rBmTHY against BmNPV infection, BmN cells were infected with BmNPV (MOI, 1) and subsequently treated with rBmTHY at different concentrations (0.04, 0.4, 4 μg ml − 1). The cell viability in each group and antiviral activities of rBmTHY were evaluated by MTT assays. After normalization using the absorbencies obtained with nonstimulated cells, the cell viability decreased to ∼67.5 ± 1.7%, 35.2 ± 4.2% after exposure to BmNPV for 48 and 72 h, respectively. However, the viability of BmNPV-infected cells treated with rBmTHY at different concentrations (0.04, 0.4, 4 μg ml − 1) were 69 ± 0.9%, 74.7 ± 3.1%, 84.9 ± 3.7% at 48 h of incubation, respectively, and 40.2 ± 2.8%, 46.2 ± 5.9%, 84.9 ± 2.7% at 72 h, respectively (Fig. 4A). The data noted a dose-dependent effect of an increase in BmNPV-infected cell survival associated with rBmTHY treatment. Furthermore, the results shown in Fig. 4B revealed that anti-BmNPV activity of rBmTHY increased as dosage increased from 0.04 to 4 μg ml − 1. Under an electron microscope, images of BmN cells untreated (A1, B1), treated with BmNPV (A3, B3) or treated with BmNPV followed by 4 μg ml − 1 of rBmTHY (A2, B2) revealed morphological changes (Fig. 4C) at 48 or 72 h of incubation. Untreated cells had well-defined, intact shapes with smooth surfaces. Cells infected with BmNPV showed considerable morphological alterations including deformation and shrinkage. However, cell morphologies, such as shape intactness, light transmission, and surface smoothness, in the drug treatment group were better than that of the viral control group.
Fig. 4.
Evaluation of antiviral effects of rBmTHY on BmN cells against BmNPV infection. (A) Effects of rBmTHY on BmNPV-infected cell viability. BmN cells were infected with BmNPV and then treated with rBmTHY. Cell viability in each group was evaluated by MTT assays. Cell viability, OD490 (experiment group)/OD490 (negative group); Nc, negative control group; Vc, viral control group; Ldt, low dose rBmTHY (0.04 μg ml−1) treatment group; Mdt, middle dose rBmTHY (0.4 μg ml−1); Hdt, high dose rBmTHY (4 μg ml−1) treatment group. Data represent mean±SEM of three independent experiments (two-way ANOVA * denotes P<0.05 vs. Vc). (B) Antiviral activity of rBmTHY on BmN cells against BmNPV infection. The antiviral activities of rBmTHY were calculated according to the formula: antiviral activity (%) = (ODT490)v − (ODC490)v/(ODC490)mock − (ODC490)v×100%. Data are presented as the mean ±SEM of three independent experiments. (C) Morphologies of BmN cells under electron microscope (scale bar=50 μm). (A1 and B1) Cells in negative control group at 48 or 72 h of incubation. (A2 and B2) Cells in drug treatment group (infected with BmNPV and then treated with 4 μg ml−1 rBmTHY) at 48 or 72 h of incubation. (A3 and B3) Cells in viral control group (infected with BmNPV) at 48 or 72 h of incubation. Untreated cells had well-defined, intact shapes with smooth surfaces. Cell morphologies, such as integrity, transmission of light, and surface smoothness, in the drug treatment group were better than that of the viral control group.
In conclusion, these results indicate that rBmTHY displayed an antiviral effect in a dose-dependent manner for BmN cells against BmNPV infection.
Antiviral Effects of rBmTHY on Fifth Instar Larvae
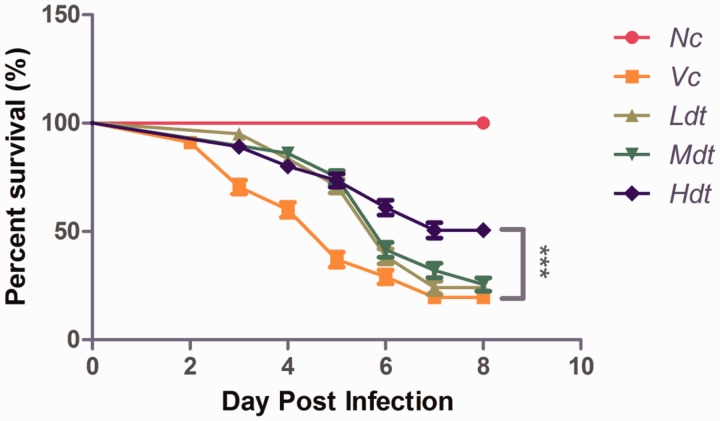
We evaluated safety margin of endotoxin-free rBmTHY and demonstrated that rBmTHY was safe and had no any phenotypic effect on uninfected larvae under dose of 50 μg ml − 1 (see the Supp Data S2 [online only]). As described under Materials and Methods, to investigate antiviral effect of endotoxin-free rBmTHY on silkworm against BmNPV infection, newly exuviated fifth-instar larvae were randomly divided into five groups. About 99% of untreated silkworms (vehicle control) survived and pupated normally. However, over 50% of BmNPV-infected silkworms (group B,C,D,E) died before the moth stage. For most of the dead larvae, death occurred primarily in the second and fourth day post-infection of BmNPV (106 OB per larva), and few deaths occurred in the subsequent stages. The survival rates of larvae in group A (vehicle control) was ∼100%, whereas that of group B (virus control) was only 19.50%. Interestingly, the survival rates of larvae in groups (C,D, E) treatment with different dose of rBmTHY (20, 2, 0.2 μg ml − 1) were 50.00, 25.00, and 24.00%, respectively, which are higher than that of group B. Thus, the mortality of larvae in drug treatment groups decreased dramatically compared with that in group B (virus control). The results shown in Fig. 5 indicate that endotoxin-free rBmTHY exerted antiviral effects on fifth-instar larvae against BmNPV infection in a dose-dependent manner. The survival statistics suggested that rBmTHY indeed has an increase in tolerance to BmNPV, which is attributed to the finding that infection of BmNPV led to decreased BmTHY expression.
Fig. 5.
Survival curve of BmNPV-infected fifth-instar larvae treatment with endotoxin-free rBmTHY. Nc, negative control: treatment with PBS; Vc, virus control: subcutaneous inoculation with wild-type BmNPV; Ldt, treatment with low dose (0.2 μg ml−1) of rBmTHY and infection with BmNPV; Mdt, treatment with middle dose (2 μg ml−1) of rBmTHY and infection with BmNPV; Hdt, treatment with high dose (20 μg ml−1) of rBmTHY and infection with BmNPV. The experiments were carried out in quadruplicate, and each repeat consisted of 50 larvae for each group. Data are presented as the mean±SEM. Asterisk indicates significant difference between control group and experimental group (Gehan–Breslow–Wilcoxon Test, ***P<0.001).
Discussion
Thymosins are small peptides that are highly conserved and exist in many different animal species (Zhang et al. 2012). Emerging evidence has revealed that thymosins participate in many important biological events, including immunoregulation, cell differentiation and migration, angiogenesis (Koutrafouri et al. 2001; Cha et al. 2003), wound healing (Malinda et al. 1998; Philp et al. 2004), and inflammation (Sosne et al. 2001, 2002, 2007). In a previous study, we characterized a protein homologous to thymosin betas (Tβ) from Bombyx mori, BmTHY (Zhang et al. 2012). However, it is still not known about its physiological function, especially immunoregulation for silkworm. Therefore, the present study investigated the role of BmTHY in innate immunity of Bombyx mori, specifically the relationship between BmTHY and BmNPV infection. In this study, the protein BmTHY expression profiles were analyzed by Western blotting in BmN cells prior to or after infection with BmNPV. Using qRT-PCR, the mRNA levels of BmTHY were compared between BmNPV-infected and no BmNPV-infected BmN cells. Recombinant BmTHY was expressed in Escherichia coli and purified. After removing endotoxins from the purified proteins, rBmTHY was prepared for antiviral experimentation. Antiviral activity and the effect of rBmTHY were evaluated both in BmN cells and the fifth instar larvae challenged with BmNPV.
Results from Western blotting and qRT-PCR showed that BmTHY protein expression and transcription were down-regulated when BmN cells were infected with BmNPV as time in culture increased, which is similar to the result of the recently reported study (Ma et al. 2015). However, these results are not consistent with those of previous studies on β-thymosin in Helicoverpa armigera (HaTHY), which demonstrated that HaTHY is up-regulated in response to H. armigera single nucleocapsid nucleopolyhedrovirus (HaSNPV) infection (Zhang et al. 2011). Thus, the expression pattern of thymosin in lepidopterous insects challenged with nucleopolyhedrovirus is likely to be controversial, requiring further investigation by biologists. Reduction in expression of BmTHY during BmNPV infection indicates that BmTHY may be degraded and may play a critical role in the virus infection of silkworms. We have reported that BmTHY is highly homologous to Tβ (Zhang et al. 2012). In human, Tβ can be degraded by angiotensin converting enzyme (ACE) (Cavasin et al. 2004),and the homologue of ACE was also found to be existed in silkworm (Quan et al. 2001). Consequently, BmTHY may also be degraded by ACE. In addition, a wealth of evidence has demonstrated that Tβs are critical immune-related factors and involve in progresses of anti-viral and anti-inflammatory (Wu and Wu 2009; Henkel et al. 2011; Zhang et al. 2011). BmTHY down-regulation may be a mechanism involved in the death of silkworm resulting from BmNPV infection. Therefore, we hypothesize that BmTHY gene is important for silkworm resistance to virus infection and addition of rBmTHY is conducive to preventation of virosis. Interestingly, the result of viral titer test demonstrated that rBmTHY could be reducing viral load through antiviral activity. Moreover, results from antiviral in vitro tests revealed that the viability of BmNPV-infected cells treated with rBmTHY was higher than that of BmNPV-infected cells. Similarly, antiviral test performed in vivo demonstrated that the mortality of fifth-instar larvae in drug treatment groups decreased dramatically compared with that in the virus control group. These results indicate that rBmTHY has an antiviral effect against BmNPV infection in silkworm. The results from these studies can be used to promote further investigations on the development of veterinary medicines for use in silkworm cultivation.
The present study data also prompted us to consider the following questions carefully. (1) What will happen in BmN cell or larvae if BmTHY expression is up-regulated or down-regulated by genetic and cell engineering? (2) Can we construct over-expression systems and inhibited-expressions systems of BmTHY? Also, what are the influences of BmTHY on BmNPV proliferation, replication, gene transcription and expression? (3) Can we breed a new antiviral variety of transgenic silkworm based on BmTHY? (4) Can the recombinant BmTHY be developed into a potential antiviral medicine to protect silkworm from BmNPV infection? These questions are the focus of our future experimental goals.
Taken together, these results indicate that the BmTHY gene is important for silkworm resistance to virus infection. The results provide a solid foundation on which to promote rBmTHY as a potential antiviral medicine for Bombyx mori against BmNPV.
Supplementary data
Supplementary data are available at Journal of Insect Science online.
Acknowledgments
This work was financially supported by the Public Technology Application Research Project from Science Technology Bureau of Zhejiang Province (2016C33080), the Zhejiang Provincial Top Key Discipline of Biology and the National High Technology Research and Development Program (2012ZX09102301-009). We are grateful to the editor and reviewers for their critical review, valuable suggestions, and favorable comments on our article. We also thank Elsevier Language Editing for revising the language of this article.
References Cited
- Bodey B., Bodey B., Jr., Siegel S. E., Kaiser H. E. 2000. Review of thymic hormones in cancer diagnosis and treatment. Int. J. Immunopharmacol. 22: 261–273. [DOI] [PubMed] [Google Scholar]
- Boquet I., Boujemaa R., Carlier M. F., Preat T. 2000. Ciboulot regulates actin assembly during Drosophila brain metamorphosis. Cell 102: 797–808. [DOI] [PubMed] [Google Scholar]
- Cavasin M. A., Rhaleb N. E., Yang X. P., Carretero O. A. 2004. Prolyl oligopeptidase is involved in release of the antifibrotic peptide Ac-SDKP. Hypertension 43: 1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha H. J., Jeong M. J., Kleinman H. K. 2003. Role of thymosin beta4 in tumor metastasis and angiogenesis. J. Natl. Cancer Inst. 95: 1674–1680. [DOI] [PubMed] [Google Scholar]
- Cheng S. Q., Wu M. C., Chen H., Shen F., Yang J. H., Cong W. M., Yin Z. F., Zhao Y. X., Wang P. J. 2006. Antiviral therapy using lamivudine and thymosin alpha(1) for hepatocellular carcinoma coexisting with chronic hepatitis B infection. Hepato-Gastroenterology 53: 249–252. [PubMed] [Google Scholar]
- Clark A. G., Eisen M. B., Smith D. R., Bergman C. M., Oliver B., Markow T. A., Kaufman T. C., Kellis M., Gelbart W., Iyer V. N., et al. 2007. Evolution of genes and genomes on the Drosophila phylogeny. Nature 450: 203–218. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., Slater F. D., White A. 1966. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc. Natl. Acad. Sci. USA. 56: 1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraj L., Gupta T., Esvaran V. G., Awasthi A. K., Ponnuvel K. M. 2016. Genome-wide identification, characterization of sugar transporter genes in the silkworm Bombyx mori and role in Bombyx mori nucleopolyhedrovirus (BmNPV) infection. Gene 579: 162–171. [DOI] [PubMed] [Google Scholar]
- Henkel C., Schwamborn K., Zimmermann H. W., Tacke F., Kuhnen E., Odenthal M., Groseclose M. R., Caprioli R. M., Weiskirchen R. 2011. From proteomic multimarker profiling to interesting proteins: thymosin-beta(4) and kininogen-1 as new potential biomarkers for inflammatory hepatic lesions. J. Cell. Mol. Med. 15: 2176–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. A., Subramanian, Halpern G. M. A., Sutton G. G., Charlab R., Nusskern D. R., Wincker P., Clark A. G., Ribeiro J. M., Wides R., et al. 2002. The genome sequence of the malaria mosquito Anopheles gambiae. Science 298: 129–149. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Borcherding W., Moorthy A. V., Chesney R., Schulte-Wisserman H., Hong R. 1977. Induction of suppressor T cells in systemic lupus erythematosus by thymosin and cultured thymic epithelium. Science 197: 999–1001. [DOI] [PubMed] [Google Scholar]
- Ishii K., Hamamoto H., Sekimizu K. 2015. Studies of host-pathogen interactions and immune-related drug development using the silkworm: interdisciplinary immunology, microbiology, and pharmacology studies. Drug Discov. Ther. 9: 238–246. [DOI] [PubMed] [Google Scholar]
- Jiang Y. F., Ma Z. H., Zhao P. W., Pan Y., Liu Y. Y., Feng J. Y., Niu J. Q. 2010. Effect of thymosin-alpha(1) on T-helper 1 cell and T-helper 2 cell cytokine synthesis in patients with hepatitis B virus e antigen-positive chronic hepatitis B. J. Int. Med. Res. 38: 2053–2062. [DOI] [PubMed] [Google Scholar]
- Kellici S., Burazeri G. 2009. Thymosin alpha1: a promising molecule for important clinical applications. Med. Arh. 63: 48–50. [PubMed] [Google Scholar]
- Kim B. H., Lee Y. J., Kim W., Yoon J. H., Jung E. U., Park S. J., Kim Y. J., Lee H. S. 2012. Efficacy of thymosin alpha-1 plus peginterferon alpha-2a combination therapy compared with peginterferon alpha-2a monotherapy in HBeAg-positive chronic hepatitis B: a prospective, multicenter, randomized, open-label study. Scand. J. Gastroenterol. 47: 1048–1055. [DOI] [PubMed] [Google Scholar]
- Knutsen A. P., Freeman J. J., Mueller K. R., Roodman S. T., Bouhasin J. D. 1999. Thymosin-alpha1 stimulates maturation of CD34+ stem cells into CD3 + 4+ cells in an in vitro thymic epithelia organ coculture model. Int. J. Immunopharmacol. 21: 15–26. [DOI] [PubMed] [Google Scholar]
- Koutrafouri V., Leondiadis L., Avgoustakis K., Livaniou E., Czarnecki J., Ithakissios D. S., Evangelatos G. P. 2001. Effect of thymosin peptides on the chick chorioallantoic membrane angiogenesis model. Biochim. Biophys. Acta 1568: 60–66. [DOI] [PubMed] [Google Scholar]
- Law L. W., Goldstein A. L., White A. 1968. Influence of thymosin on immunological competence of lymphoid cells from thymectomized mice. Nature 219: 1391–1392. [DOI] [PubMed] [Google Scholar]
- Li C., Bo L., Liu Q., Jin F. 2015. Thymosin alpha1 based immunomodulatory therapy for sepsis: a systematic review and meta-analysis. Int. J. Infect. Dis. 33: 90–96. [DOI] [PubMed] [Google Scholar]
- Low T. L., Goldstein A. L. 1979. The chemistry and biology of thymosin. II. Amino acid sequence analysis of thymosin alpha1 and polypeptide beta1. J. Biol. Chem. 254: 987–995. [PubMed] [Google Scholar]
- Low T. L., Thurman G. B., McAdoo M., McClure J., Rossio J. L., Naylor P. H., Goldstein A. L. 1979. The chemistry and biology of thymosin. I. Isolation, characterization, and biological activities of thymosin alpha1 and polypeptide beta1 from calf thymus. J. Biol. Chem. 254: 981–986. [PubMed] [Google Scholar]
- Ma S., Kang Z., Lu P., Yang Y., Yao Q., Xia H., Chen K. 2015. Molecular and physiological characterization of two novel multirepeat beta-thymosins from silkworm, Bombyx mori. PloS One 10: e0140182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio M., Mackiewicz A., Testori A., Trefzer U., Ferraresi V., Jassem J., Garbe C., Lesimple T., Guillot B., Gascon P., et al. 2010. Large randomized study of thymosin alpha 1, interferon alfa, or both in combination with dacarbazine in patients with metastatic melanoma. J. Clin. Oncol. 28: 1780–1787. [DOI] [PubMed] [Google Scholar]
- Malinda K. M., Sidhu G. S., Banaudha K. K., Gaddipati J. P., Maheshwari R. K., Goldstein A. L., Kleinman H. K. 1998. Thymosin alpha 1 stimulates endothelial cell migration, angiogenesis, and wound healing. J. Immunol. 160: 1001–1006. [PubMed] [Google Scholar]
- Manuel M., Kruse M., Muller W. E., Le Parco Y. 2000. The comparison of beta-thymosin homologues among metazoa supports an arthropod-nematode clade. J. Mol. Evol. 51: 378–381. [DOI] [PubMed] [Google Scholar]
- Martin O., Croizier G. 1997. Infection of a Spodoptera frugiperda cell line with Bombyx mori nucleopolyhedrovirus. Virus Res. 47: 179–185. [DOI] [PubMed] [Google Scholar]
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65: 55–63. [DOI] [PubMed] [Google Scholar]
- Mosoian A., Teixeira A., Burns C. S., Sander L. E., Gusella G. L., He C., Blander J. M., Klotman P., Klotman M. E. 2010. Prothymosin-alpha inhibits HIV-1 via Toll-like receptor 4-mediated type I interferon induction. Proc. Natl. Acad. Sci. USA. 107: 10178–10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates K. K., Erdos M. 1989. Biochemical identification of thymosin alpha-1: its phylogenetic distribution and evolutionary implications. Comp. Biochem. Physiol. B. 94: 759–763. [DOI] [PubMed] [Google Scholar]
- Oates K. K., Ginsburg G. T., Naylor P. H., Affronti L. F., Goldstein A. L. 1988. Identification and distribution of thymosin alpha 1-like immunoreactivity. Dev. Comp. Immunol. 12: 397–402. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20: 309–321. [DOI] [PubMed] [Google Scholar]
- Philp D., Goldstein A. L., Kleinman H. K. 2004. Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development. Mech. Ageing Dev. 125: 113–115. [DOI] [PubMed] [Google Scholar]
- Quan G. X., Mita K., Okano K., Shimada T., Ugajin N., Xia Z., Goto N., Kanke E., Kawasaki H. 2001. Isolation and expression of the ecdysteroid-inducible angiotensin-converting enzyme-related gene in wing discs of Bombyx mori. Insect Biochem. Mol. Biol. 31: 97–103. [DOI] [PubMed] [Google Scholar]
- Raberg L., Graham A. L., Read A. F. 2009. Decomposing health: tolerance and resistance to parasites in animals. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustgi V. 2004. Combination therapy of thymalfasin (thymosin-alpha 1) and peginterferon alfa-2a in patients with chronic hepatitis C virus infection who are non-responders to standard treatment. J. Gastroenterol. Hepatol. 19: S76–S78. [PubMed] [Google Scholar]
- Shi X. Z., Shi L. J., Zhao Y. R., Zhao X. F., Wang J. X. 2015. beta-Thymosins participate in antiviral immunity of red swamp crayfish (Procambarus clarkii). Dev. Comp. Immunol. 51: 213–225. [DOI] [PubMed] [Google Scholar]
- Sosne G., Chan C. C., Thai K., Kennedy M., Szliter E. A., Hazlett L. D., Kleinman H. K. 2001. Thymosin beta 4 promotes corneal wound healing and modulates inflammatory mediators in vivo. Exp. Eye Res. 72: 605–608. [DOI] [PubMed] [Google Scholar]
- Sosne G., Hafeez S., Greenberry A. L., 2nd, Kurpakus-Wheater M. 2002. Thymosin beta4 promotes human conjunctival epithelial cell migration. Curr. Eye Res. 24: 268–273. [DOI] [PubMed] [Google Scholar]
- Sosne G., Qiu P., Kurpakus-Wheater M. 2007. Thymosin beta 4: a novel corneal wound healing and anti-inflammatory agent. Clin. Ophthalmol. 1: 201–207. [PMC free article] [PubMed] [Google Scholar]
- Wu L., Wu X. 2009. Molecular cloning and expression analysis of a beta-thymosin homologue from a gastropod abalone, Haliotis diversicolor supertexta. Fish Shellfish Immunol. 27: 379–382. [DOI] [PubMed] [Google Scholar]
- Zhang F. X., Shao H. L., Wang J. X., Zhao X. F. 2011. beta-Thymosin is upregulated by the steroid hormone 20-hydroxyecdysone and microorganisms. Insect Mol. Biol. 20: 519–527. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen X. M., Zhang C. D., He Q., Dong Z. Q., Cao M. Y., Dong X. L., Pan C. X., Lu C., Pan M. H. 2014a. Differential susceptibilities to BmNPV infection of two cell lines derived from the same silkworm ovarian tissues. PloS One 9: e105986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., He Q., Zhang C. D., Chen X. Y., Chen X. M., Dong Z. Q., Li N., Kuang X. X., Cao M. Y., Lu C., et al. 2014b. Inhibition of BmNPV replication in silkworm cells using inducible and regulated artificial microRNA precursors targeting the essential viral gene lef-11. Antiviral Res. 104: 143–152. [DOI] [PubMed] [Google Scholar]
- Zhang M. J., Cheng R. L., Lou Y. H., Ye W. L., Zhang T., Fan X. Y., Fan H. W., Zhang C. X. 2012. Disruption of Bombyx mori nucleopolyhedrovirus ORF71 (Bm71) results in inefficient budded virus production and decreased virulence in host larvae. Virus Genes. 45: 161–168. [DOI] [PubMed] [Google Scholar]
- Zhang X., Chen M., Ma X., Zhao X., Wang J., Shao H., Song Q., Stanley D. 2013. Suppression of AcMNPV replication by adf and thymosin protein up-regulation in a new testis cell line, Ha-shl-t. Arch. Insect Biochem. Physiol. 82: 158–171. [DOI] [PubMed] [Google Scholar]