Abstract
Imaging photoplethysmography (IPPG) is a recently developed technique for noncontact assessment of cardiovascular function. However, its wide use is limited by low signal-to-noise ratio due to motion artifacts. The aim of this work is to estimate the polarization-filtration impact on discriminating artifacts in IPPG measurements. Experiments were carried out in-vivo by almost simultaneous illumination of subject’s palm with polarized and non-polarized light during video recording of 41 subjects. It was found that the light-polarization filtration efficiently reduces motion artifacts compared to the non-polarized illumination while the pulsation amplitude measured at the heartbeat frequency remains unaffected. The polarization filtration improves reliability of IPPG system in non-contact monitoring of subject’s heart rate and its variability.
OCIS codes: (170.0170) Medical optics and biotechnology, (170.3880) Medical and biological imaging, (280.0280) Remote sensing and sensors, (170.3660) Light propagation in tissues
1. Introduction
Advances in the areas of microelectronics and image processing techniques resulted recently in fast growing interest in development of optical imaging technologies for vital sign monitoring. These technologies include laser Doppler imaging (LDI) [1], laser speckle imaging (LSI) [2], tissue viability imaging (TiVi) [3], and imaging photoplethysmography (IPPG) [4]. Although physical principles of the techniques differ, there are two primary components in common: a light source and digital camera. Whereas LDI, LSI, and TiVi techniques require subject’s illumination by the polarized light, the most of IPPG systems operate under non-polarized illumination (see, for example [5–7]) with only few exceptions [8,9] in which polarization filtration was applied. It is suggested that filtering of orthogonal polarization suppresses the diffusely backscattered light and improves imaging of sub-epidermal tissues [10,11] including imaging blood vessels [3,12–14]. However, no direct comparison of the polarization filtration impact on imaging photoplethysmography has been carried out.
In this paper, we compare performance of an IPPG system with and without polarization filtration. Such a comparison was carried out in-vivo by illuminating subject’s palm with linearly polarized light during each even camera frame and with non-polarized light during each odd frame. The time shift between different types of illumination was 23 ms. Since typical period of cardiac activity is much slower (about 1 s), we assume that both types of illumination read out information from almost the same state of blood vessels.
2. Subjects and methods
2.1 Experimental setup
Experiments were carried out in a typical configuration of IPPG system in reflectance geometry as shown in Fig. 1. A digital black-and-white CMOS camera (8-bit model GigE uEye UI-5220SE of Imaging Development Systems GmbH) was used to collect images of a subject’s palm. The palm was illuminated alternately by light-emitting diodes (LED) operating at center wavelength of 525 nm with bandwidth of 60 nm and output power of 10 W (model BL-HPEPGC-10W of Betlux Electronics Co, Ltd.). This wavelength was used because IPPG at green light is characterized with better signal-to-noise ratio (SNR) [6,15–17]. Polarization filtering was implemented only for one of the LEDs by placing a thin-film polarizer in front of it. Another polarizer was attached to the camera lens. Its transmission axis was adjusted to be orthogonal with that of the first polarizer. Extinction ratio of the polarizers was 90:1 (State Optical Institute, USSR). While the first LED illuminated a subject’s hand with linearly polarized light, the second one provided non-polarized illumination.
Fig. 1.
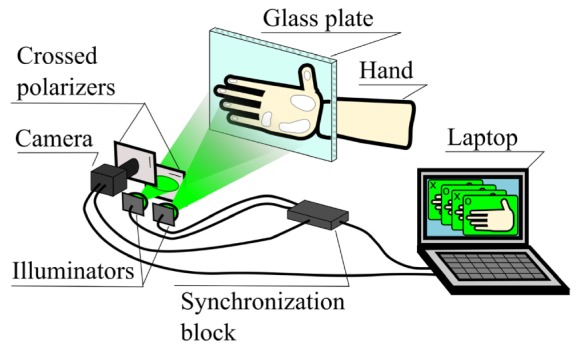
Sketch of the experimental setup for estimation of influence of the polarization filtration on blood pulsations imaging.
Alternating illumination provided by the LEDs was synchronized with the frame grabber of the camera. Each sync pulse at the beginning of every frame switched the power from one LED to another by means of a synchronizer. This way, each even frame was recorded with polarization filtration, and each odd frame – without. The frames were recorded with the spatial resolution of 752 × 480 pixels and frame rate of 44 frames per second (fps). Therefore, after single measurements we obtained two video sequences (with and without polarization filtration) with effective frame rate of 22 fps. Since the time shift between these sequences is shorter than typical vascular processes, we may consider that both videos contain information about the same biological processes.
Each LED provided almost uniform illumination of the palm area so that the difference of the light intensity between two arbitrary chosen subareas does not exceed 30%. Uniformity of illumination was controlled before the experiment by measuring spatial distribution of pixel values within a frame recorded with white paper instead of a palm. The distance between each LED and the palm was about 60 cm whereas the camera was installed at the distance of 1 m from the hand. Before each experiment we measured a mean pixel value over the palm of each subject and adjusted the electric current of one of the LEDs to achieve approximate equality of the camera responses (within 15%) for polarized and non-polarized illumination.
2.2 Experimental procedure
We have studied 41 healthy subjects (19 – 72 years old, 24 men and 17 women) without any cardiovascular, neurologic, or skin diseases. All subjects gave their written informed consent of participation in the experiment. Each subject was asked to relax in a quiet laboratory room for 15 minutes prior to measurements. During video recordings, the subject’s palm was touching the vertically fixed, 4-mm thick glass plate (see Fig. 1). The palm was adhered to the glass by an adhesive tape from the palm’s backside. Contact of the palm with the glass plate had double goal. First, it stabilized the image by diminishing movement artefacts. Second, it significantly increased the amplitude of PPG waveforms due to more efficient modulation of the capillary density by pulsating arteries [18,19]. Video of a palm under alternating polarized / non-polarized illumination was recorded during 30 s. Thereafter, the same recording was executed for another subject's palm. Recorded frames were stored in the portable network graphics format on the hard drive of a computer. Raw images of a palm from two adjacent frames taken under polarized and non-polarized illumination are shown in Fig. 2.
Fig. 2.
An example of raw images of the same palm recorded a) with polarization filtration and b) without.
2.3 Algorithm of data processing
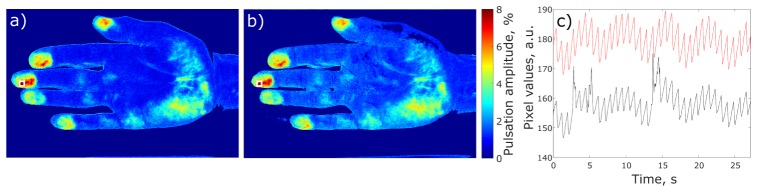
Recorded data were processed offline by using custom software implemented in the MATLAB platform. First, images recorded under different type of illumination (odd and even frames) were separated and stored into different folders. Second, we calculated spatial distribution of the PPG amplitude by using synchronous (lock-in) amplification of each set of the recorded frames with a heartbeat frequency, which was described in details in our previous papers [16,20]. Briefly, at the first stage we defined the beginning and duration of each cardiac cycle from the PPG waveform which is frame-to-frame evolution of the mean pixel value calculated in an area of 9 × 9 pixels. In current experiments this area was chosen in the distal phalanx of the index finger where the elevated amplitude of blood pulsations is typically observed. Duration of the cycle is enough to define the complex reference function of the sinusoidal type for every cardiac cycle, which is required for lock-in amplification [20]. At the second stage we calculated the correlation matrix between each pixel value in the frame and the reference function for arbitrary chosen four sequential cardiac cycles. The absolute value of the correlation matrixes averaged over four cycles represents the spatial distribution of blood pulsations amplitude (BPA) [16,20]. Typical examples of BPA mapping with and without polarization filtration for one of the subjects are shown in Figs. 3(a) and 3(b), respectively. It is worth noting similarity of spatial distribution of blood pulsations amplitude obtained under polarized and non-polarized illumination (Fig. 3(a) vs Fig. 3(b)).
Fig. 3.
Distribution of blood pulsation amplitude calculated from the set of frames with (a) and without (b) polarization filtration. The hot spot with the maximal BPA was found in the middle finger and marked by white squares. The plot (c) shows unprocessed, raw PPG waveforms calculated from the hot spot in the case with (red curve) and without (black curve) polarization filtration.
BPA maps are needed to find regions with the highest blood pulsation amplitude referred to as hot spots. In both sets, related to polarized and non-polarized light, the frames were recorded with the same lens and photosensitive matrix. Therefore, the spatial coordinates of the palm’s topology coincide for both types of illumination. For the particular subject shown in Fig. 3, the position of the hot spot was found to be in the middle finger and it is marked by the white square in the BPA maps. Red and black curves in Fig. 3(c) show the PPG waveforms obtained in the region of interest (ROI) placed in the selected hot spot for the case with and without polarization filtration, respectively. Plots in Fig. 3(c) are the raw PPG waveforms without any filtration either in time or frequency domain.
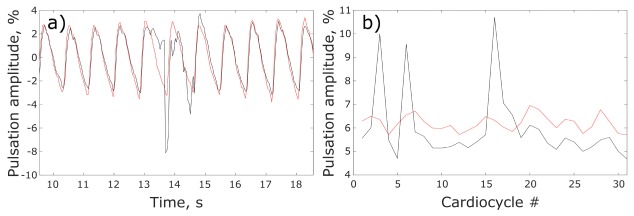
To compare quantitatively the effect of the polarization filtration on the measured blood pulsations amplitude, we estimated the relative difference in BPA (in percent) in the hot spot for signals from the polarized and non-polarized sets of recorded frames. First, BPA in each set was calculated as an AC/DC ratio where AC and DC refer to the peak-to-peak value and the mean value of the PPG waveform in every cardiac cycle, respectively. Second, BPA difference was calculated for each cycle and then averaged over all cardiac cycles. Figure 4(a) shows fragments of both PPG waveforms between 10th to 18th s for clarity of presentation. Variability of BPA calculated with and without polarization filtration is shown in Fig. 4(b) for the complete number of recorded cycles.
Fig. 4.
Comparison of PPG waveforms in the hot spot measured with and without polarization filtration. (a) Normalized (AC / DC ratio) PPG waveforms obtained under polarized (red curve) and non-polarized (black curve) illumination. (b) Pulsations amplitude as a function of the cardiac cycle for polarized (red curve) and non-polarized (black curve) illumination.
Note several spikes which are clearly seen in the PPG waveform (black curve in Fig. 3(c)) obtained from the set of frames without polarization filtration. These spikes are absent in the red curve measured with polarization filtration. They contain frequency components which are higher than the heartbeat frequency. Most probably they originate from accidental motion of palm’s parts. To estimate quantitatively the effect of polarization filtration on the high-frequency components, we applied a high-pass filter with the cut-off frequency of 5 Hz to the PPG waveform. By this way, the heartbeat frequency and its higher (up to 4th) harmonics were filtered out. To compensate non-uniformity of the palm illumination, the signals were normalized with respect to their mean value. Since the component at the heartbeat frequency is the main source of information about cardiovascular activity, the higher-frequency components are considered as the noise. Ratio of the root-mean-square deviation of the high-frequency noise measured under non-polarized illumination to that under polarized illumination was chosen as a parameter for comparative estimation of the polarization filtration impact.
3. Experimental results
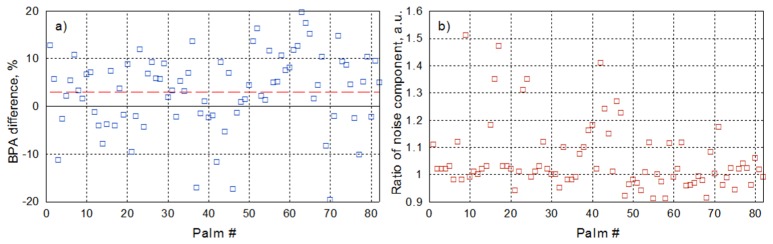
As one can see in Figs. 3 and 4, blood pulsation amplitude measured in the hot spot for a particular subject is almost the same for the sets with and without polarization filtration. The average BPA difference calculated over 32 cardiac cycles is 2.6% in this particular case. Here the positive sign of the difference means that the BPA obtained with polarization filtration is larger. We have applied the same algorithm of BPA difference estimation for both palms of each subject. Considering recordings of left and right hands as independent experiments, the total number of statistically independent measurements was twice the number of the subjects. The results of measurements are shown in Fig. 5(a). It is clearly seen that the relative BPA difference can be either positive or negative, and it does not exceed 20%. The mean difference between the pulsation amplitude measured under polarized and non-polarized illumination is 3.1 ± 1.7% (calculated with the confidence level of 95% for the whole data set). Therefore, influence of the polarization filtration on the readout of information from cutaneous vessels pulsating at the heartbeat frequency is insignificant.
Fig. 5.
Comparison of the parameters of PPG waveforms measured in the hot spots found in a palm of different subjects with and without polarization filtration. a) Relative difference in the amplitude of blood pulsations at the heartbeat frequency. b) Ratio of the root-mean-square of the high-frequency noise measured without polarization filtration to that with polarization filtration. The red dashed line in the graph (a) indicates the mean BPA difference.
The ratio of the standard deviation of the high-frequency noise component of the PPG waveform measured under non-polarized illumination to that measured under polarized light is shown in Fig. 5(b). For the majority of measurements, deviation of this ratio from the unity is less than 10%. However, in 22 palms, which is approximately 27% of measurements, the ratio of high-frequency noises exceeds the level of 1.1. In all these palms, we observed the accidental signal irregularities similar to those shown by black curve in Fig. 3(c). The irregularities were observed only in raw PPG signals obtained from the set of frames without polarization filtration. It is worth noting that the respective PPG signals with polarization filtration were free of the spikes.
4. Discussion
The initial polarization state of the polarized light is changing after multiple scattering in biological tissues [12]. However, the polarization state of specularly reflected or weakly scattered light returned from the tissue surface retains its original polarization state [21]. This difference creates the basis for information extraction from deeper layers of tissue, which contain the pulsatile blood vessels, by means of the polarization filtration. Application of polarization filtration to the technique of Laser Doppler Perfusion measurements revealed that it reduces the perfusion signal significantly [13]. It was found that the perfusion signal is strongly influenced by motion not related to blood flow, which is most probably attributed with movement artifacts [13]. In our experiments, the movement artifacts were minimized by adhering a subject’s palm to the fixed glass plate. However, complete exclusion of accidental displacement of superficial skin layer of living hand was impossible. In 27% of cases (22 of 82 measured palms), we clearly observed high-frequency irregularities of the raw PPG signal obtained under non-polarized illumination while these irregularities were significantly diminished in simultaneously recorded signal under polarized illumination (compare black and red curves in Fig. 3(c)). Nevertheless, it was found that for the duration of single measurement (30 s), the average difference between the amplitude of the PPG-signal modulation at the heartbeat frequency measured with and without polarization filtration is insignificant (see Fig. 5(a)).
Red curve in Fig. 4(a) shows the PPG waveform calculated as AC/DC ratio from the set of frames recorded under polarized illumination. Its shape resembles modulation of the arterial blood pressure. While waveforms obtained from the sets of frames recorded under polarized illumination maintain well-defined structure, simultaneously recorded waveforms from non-polarized sets were often distorted (22 out of 82 palms) by spikes originated from the irregularities in the raw PPG signal (e.g., black curve in Fig. 4(a)). These spikes may lead to incorrect estimation of the beginning and duration of cardiac cycles. Since spikes and irregularities occur in much shorter time scale than the processes of cardiac activity, we assume their origin from accidental skin-surface displacement. As one can see from Fig. 5(b), polarization filtration diminishes this kind of noise.
5. Conclusion
In this study, we have compared PPG waveforms simultaneously recorded from subject’s palm under polarized and non-polarized illumination. On the one hand, experimental results clearly demonstrate that the polarization filtration insignificantly affects the amplitude of the PPG waveform originated from the pulsatile cutaneous blood vessels. On the other hand, polarization filtration efficiently washes out the high-frequency noise related to movement artifacts and improves the shape of the PPG waveform allowing us to identify the parameters of cardiac cycles with higher reliability.
Acknowledgments
Financial support by the Russian Science Foundation (grant 15-15-20012) is acknowledged. The authors are grateful to Alexander Lvov for his help in carrying out the experiments.
References and links
- 1.Serov A., Steinacher B., Lasser T., “Full-field laser Doppler perfusion imaging and monitoring with an intelligent CMOS camera,” Opt. Express 13(10), 3681–3689 (2005). 10.1364/OPEX.13.003681 [DOI] [PubMed] [Google Scholar]
- 2.Fujii H., Asakura T., Nohira K., Shintomi Y., Ohura T., “Blood flow observed by time-varying laser speckle,” Opt. Lett. 10(3), 104–106 (1985). 10.1364/OL.10.000104 [DOI] [PubMed] [Google Scholar]
- 3.O’Doherty J., Henricson J., Anderson C., Leahy M. J., Nilsson G. E., Sjöberg F., “Sub-epidermal imaging using polarized light spectroscopy for assessment of skin microcirculation,” Skin Res. Technol. 13(4), 472–484 (2007). 10.1111/j.1600-0846.2007.00253.x [DOI] [PubMed] [Google Scholar]
- 4.Wu T., Blazek V., Schmitt H. J., “Photoplethysmography imaging: a new noninvasive and noncontact method for mapping of the dermal perfusion changes,” Proc. SPIE 4163, 62–70 (2000). 10.1117/12.407646 [DOI] [Google Scholar]
- 5.Wieringa F. P., Mastik F., van der Steen A. F. W., “Contactless multiple wavelength photoplethysmographic imaging: a first step toward “SpO2 camera” technology,” Ann. Biomed. Eng. 33(8), 1034–1041 (2005). 10.1007/s10439-005-5763-2 [DOI] [PubMed] [Google Scholar]
- 6.Sun Y., Papin C., Azorin-Peris V., Kalawsky R., Greenwald S., Hu S., “Use of ambient light in remote photoplethysmographic systems: comparison between a high-performance camera and a low-cost webcam,” J. Biomed. Opt. 17(3), 037005 (2012). 10.1117/1.JBO.17.3.037005 [DOI] [PubMed] [Google Scholar]
- 7.Allen J., Howell K., “Microvascular imaging: Techniques and opportunities for clinical physiological measurements,” Physiol. Meas. 35(7), R91–R141 (2014). 10.1088/0967-3334/35/7/R91 [DOI] [PubMed] [Google Scholar]
- 8.Kamshilin A. A., Teplov V., Nippolainen E., Miridonov S., Giniatullin R., “Variability of microcirculation detected by blood pulsation imaging,” PLoS One 8(2), e57117 (2013). 10.1371/journal.pone.0057117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu S., Sun L., Rohde G. K., “Robust efficient estimation of heart rate pulse from video,” Biomed. Opt. Express 5(4), 1124–1135 (2014). 10.1364/BOE.5.001124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demos S. G., Papadopoulos A. J., Savage H., Heerdt A. S., Schantz S., Alfano R. R., “Polarization filter for biomedical tissue optical imaging,” Photochem. Photobiol. 66(6), 821–825 (1997). 10.1111/j.1751-1097.1997.tb03231.x [DOI] [PubMed] [Google Scholar]
- 11.Nothdurft R., Yao G., “Expression of target optical properties in subsurface polarization-gated imaging,” Opt. Express 13(11), 4185–4195 (2005). 10.1364/OPEX.13.004185 [DOI] [PubMed] [Google Scholar]
- 12.Sankaran V., Walsh J. T., Jr, Maitland D. J., “Comparative study of polarized light propagation in biologic tissues,” J. Biomed. Opt. 7(3), 300–306 (2002). 10.1117/1.1483318 [DOI] [PubMed] [Google Scholar]
- 13.Karlsson M. G. D., Wårdell K., “Polarized laser Doppler perfusion imaging--reduction of movement-induced artifacts,” J. Biomed. Opt. 10(6), 064002 (2005). 10.1117/1.2120467 [DOI] [PubMed] [Google Scholar]
- 14.O’Doherty J., McNamara P., Clancy N. T., Enfield J. G., Leahy M. J., “Comparison of instruments for investigation of microcirculatory blood flow and red blood cell concentration,” J. Biomed. Opt. 14(3), 034025 (2009). 10.1117/1.3149863 [DOI] [PubMed] [Google Scholar]
- 15.Verkruysse W., Svaasand L. O., Nelson J. S., “Remote plethysmographic imaging using ambient light,” Opt. Express 16(26), 21434–21445 (2008). 10.1364/OE.16.021434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamshilin A. A., Miridonov S., Teplov V., Saarenheimo R., Nippolainen E., “Photoplethysmographic imaging of high spatial resolution,” Biomed. Opt. Express 2(4), 996–1006 (2011). 10.1364/BOE.2.000996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bal U., “Non-contact estimation of heart rate and oxygen saturation using ambient light,” Biomed. Opt. Express 6(1), 86–97 (2015). 10.1364/BOE.6.000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamshilin A. A., Mamontov O. V., Koval V. T., Zayats G. A., Romashko R. V., “Influence of a skin status on the light interaction with dermis,” Biomed. Opt. Express 6(11), 4326–4334 (2015). 10.1364/BOE.6.004326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamshilin A. A., Nippolainen E., Sidorov I. S., Vasilev P. V., Erofeev N. P., Podolian N. P., Romashko R. V., “A new look at the essence of the imaging photoplethysmography,” Sci. Rep. 5(5), 10494 (2015). 10.1038/srep10494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teplov V., Nippolainen E., Makarenko A. A., Giniatullin R., Kamshilin A. A., “Ambiguity of mapping the relative phase of blood pulsations,” Biomed. Opt. Express 5(9), 3123–3139 (2014). 10.1364/BOE.5.003123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan S. P., Stockford I. M., “Surface-reflection elimination in polarization imaging of superficial tissue,” Opt. Lett. 28(2), 114–116 (2003). 10.1364/OL.28.000114 [DOI] [PubMed] [Google Scholar]