Abstract
Sound plays an important role in the mating behavior of mosquitoes, including Aedes aegypti (L). Males orient to the fundamental wing beat frequency of females, and both sexes actively modulate their flight tone before mating to converge at harmonic frequencies. The majority of studies on mosquito mating acoustics have been conducted in the laboratory using tethered individuals. In this study, we present the first free-flight recording of naturally forming Ae. aegypti swarms in Thailand. We describe mating behaviors and present results on the flight tone frequency and dynamics of wild pairs in free flight. To assess the importance of these behaviors in vector control programs, especially those using genetically modified mosquitoes, it will be critical to use methods, such as those described in this work, to measure mosquito mating behaviors in the field.
Keywords: Aedes aegypti, mating, bioacoustics
Although mosquitoes are widely studied in their capacity as disease vectors, there are surprisingly little data on their basic life history and behavior (Ferguson et al. 2010). Mating behavior is one of the most neglected areas of mosquito biology (Ferguson et al. 2010, Takken et al. 2006). Control strategies based on disruption of mosquito reproduction, especially those relying on the mating success of genetically modified males, require a better understanding of the mating systems of these insects (Scott et al. 2002).
Aedes aegypti (L.) form swarms in response to host cues (Hartberg 1971). These swarms are composed predominately of males, with females entering singly to be mated (Hartberg 1971, Yuval 2006). Mating occurs on the wing, with males approaching the female from behind and then rotating 180 degrees to position themselves venter-to-venter (Roth 1948, Hartberg 1971).
Sound has long been known to be important for male localization and orientation to females in Ae. aegypti (Roth 1948). The male Johnston’s organ actively amplifies the fundamental flight tone frequency of females (Göpfert and Robert 2001). Recently, new precopulatory acoustic interactions have been described. In Ae. aegypti, tethered males and females alter their flight tone frequency to converge at harmonic components of their flight tone signals (Cator et al. 2009). This behavior, which we call “harmonic convergence,” now has been described in several mosquito species and has been implicated as a potential mate choice mechanism (Cator et al. 2009, 2010; Warren et al. 2009; Pennetier et al. 2010).
To date, the majority of studies investigating mosquito bioacoustics have been conducted in the laboratory using males and females tethered into position. Cator et al. (2009) conducted recordings of field-collected Ae. aegypti and found that they also displayed convergence behavior, and Warren et al. (2009) described the neurophysiology of field-collected Culex pipiens. Despite laboratory investigations of field-collected insects, there are very few data on free-flying mosquitoes. Duhrkopf and Hartberg (1992) collected recordings of a free-flying laboratory population of Ae. aegypti and Aedes albopictus. Wekesa et al. (1998) used short clips of free-flying mosquitoes held in small Plexiglas tubes to determine average flight tone frequencies of Anopheles gambiae and Anopheles arabiensis. Some studies have measured free-flight frequency using light deflection (Moore et al. 1986, Tripet et al. 2004). Again, these methods required that the test mosquitoes be held individually in small plastic cages. There is no record of the acoustic behavior of swarming mosquitoes in nature.
To clarify the role of bioacoustics in mosquito mating, it is important to observe swarming dynamics in the field. In this study, we describe an approach for recording the natural bioacoustics of mosquito swarms. We also present data collected using this method from naturally forming swarms of Ae. aegypti in Thailand.
Materials and Methods
Recording Apparatus
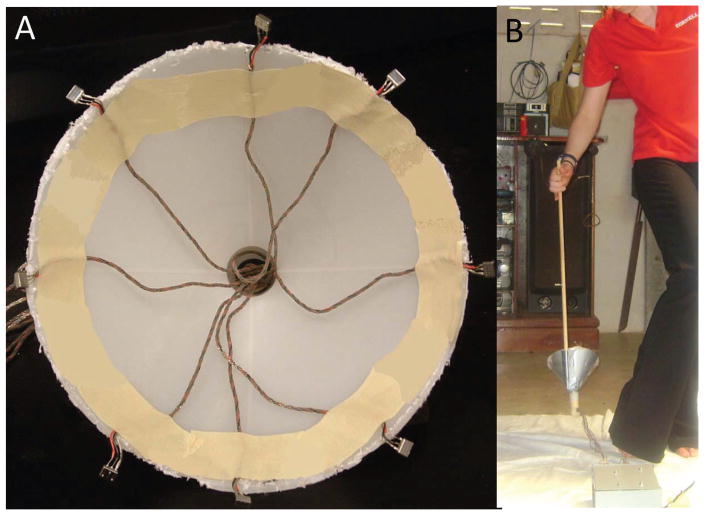
Six particle velocity (Knowles NR-23158, Itasca, IL) and two pressure-sensitive microphones (Knowles EK-23132, Itasca, IL) were positioned around the perimeter of a funnel (15 cm diameter) to create a spatially separated microphone array (Fig. 1A). A custom electronic circuit was designed to power the microphones as well as amplify and filter their output signal (B.J.A., unpublished data). An analog-to-digital converter (779676-01 NI USB-6211 Bus-Powered M Series, National Instruments, Austin, TX) was used to transfer data to be stored on a laptop computer.
Fig. 1.
Recording procedure. (A) The observer acted as the swarm marker. The microphone array positioned along the funnel was moved in and out of swarming Ae. aegypti. (B) Overview of microphone array. Six particle velocity and two pressure-sensitive microphones were positioned equidistantly around a 15-cm-diameter funnel. Microphone positioning alternated between being pointed into the center of the funnel and upward (parallel with the ground). (Online figure in color.)
Recording Procedure
Recordings were taken from residential homes in Nai Muang subdistrict, Muang district, Kamphaeng Phet Province, Thailand (16°27′ 48”N, 99°31′ 47”E). Recordings were taken between 1300 and 1530 hours Indochina time. A total of 3.5 h of observation was conducted over 2 d. Temperature and humidity were recorded using a data logger (Hobo Pro Series, Onset, Pocasset, MA). The observer stood on a 0.6 m × 0.6-m white cloth to facilitate visual observations. The microphone array was attached to a 1-m-long wooden pole. Swarms of Ae. aegypti formed naturally within the house around the observer. The observer moved the microphone array in and out of the swarming area. The array funnel was positioned so that the funnel was under the flying individual (Fig. 1B).
Recording Analysis
Each of the eight simultaneously recorded channels from the array was analyzed individually in Raven (version 1.0, Cornell University Laboratory of Ornithology, Ithaca, NY). Recordings initially were viewed in 17.2 Hz resolution to distinguish the very short fly-by clips. After clip identification, we configured the analysis software to enhance resolution of frequency information to 4.95 Hz. At the time of the original recording session, the observer dictated behavioral observations in real time. Females and males recorded while flying alone were classified as female or male solo fly-bys. We identified paired flights visually. Paired flight was classified as either male with male paired flight, when two males were in the acoustic scope of the array simultaneously, or as male-female paired flight. Male-female paired flight was reported any time males and females were in contact, including precopulatory, copulatory, and postcopulatory flight. We did not observe female-female pairs in flight.
StatisticalAnalysis
The average flight tone frequencies of individuals engaged in different types of flight (male-female paired, male-male paired, or solo) were compared using a Student’s t test when appropriate, and nonparametric tests when data were not normally distributed.
Results
Behavioral Observations
Males appeared around the observer within 5 min. Males began flying in a figure 8 pattern, as reported previously for this species (Hartberg 1971). Swarms formed within 1 m of the experimenter’s legs (in the horizontal plane) and ≈30 cm from the ground. Swarms consisted of 12–30 individuals, the majority of which were male. The average temperature during the recording period was 32.6 ± 0.5 (SE)° C with a R.H. of 49.9 ± 1.9 (SE)%.
We made behavioral observations of 23 copulating pairs using the methods described above. Once a pair was positioned in copula, both males and females continued beating their wings to stay aloft. Interestingly, once copulating pairs had assumed the venter-to-venter position, they moved rapidly away from the host. It was not clear whether this movement was a function of male behavior, female behavior, or both. We described pairs that are in the venter-to-venter orientation in flight as being in copula.
Frequency of Flight
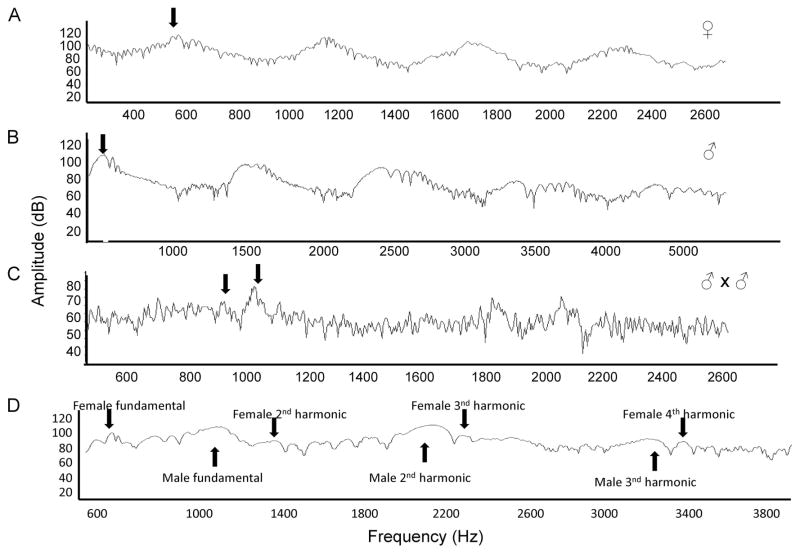
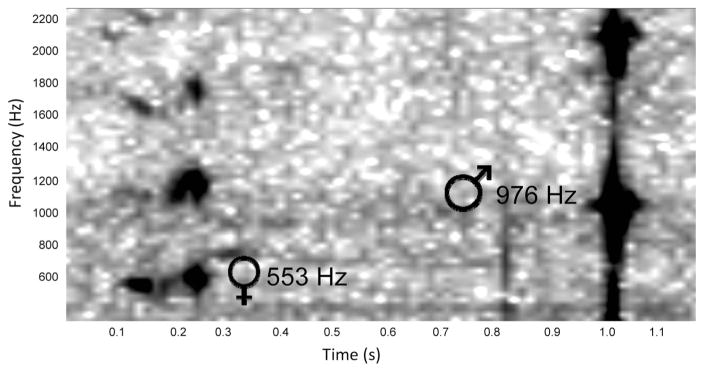
We captured recordings of 114 separate fly-by events (Fig. 2). These included 23 instances of solo female (Fig. 2A), 82 instances of male solo flight (Fig. 2B), and 13 instances of paired flight (Fig. 2C). Three of the paired flights were between males and females, whereas the remainder were between males (Fig. 2D). The average duration of sound clips was 365 ± 6 ms. Average fundamental components of the flight tone frequency of these groups are presented in Table 1. There was no significant difference between the flight tone frequencies of paired and unpaired males (Mann-Whitney U test, P = 0.102). Males in paired flight with other males had slightly lower flight tone frequencies (975.08 ± 8.09 Hz) than males recorded in paired flight with females (1010.9 ± 45.51 Hz). This difference was not statistically significant (Student’s t test, df = 11, t = −0.582, P = 0.572). In one recording clip, we were able to observe a male in pursuit of a female (Fig. 3). The male crossed the array ≈0.8 s after the female. In another clip (Fig. 2D), we observed a paired flight in which a male and female appeared to be in the process of convergence. The clip only lasted 0.04 s, but even in this short interval, we saw the female’s third harmonic and male’s second harmonic were at frequencies near to convergence.
Fig. 2.
Power spectra of natural flight behavior within a swarm of Ae. aegypti. (A) Female solo flight. (B) Male solo flight. (C) Two males in paired flight. Fundamental frequencies are indicated by black arrows. (D) Paired flight between male and female. Male second and female third harmonic appear to be approaching convergence.
Table 1.
Average flight tone frequency measured in Hz ± SE of free-flying Ae. aegypti in swarms in Kamphaeng Phet, Thailand
| Recording | N | Flight tone |
|---|---|---|
| Male solo | 12 | 982.0 ± 1.0 |
| Female solo | 27 | 664.3 ± 4.6 |
| Male paired | 78 | 989.3 ± 7.4 |
| Female paired | 3 | 609.1 ± 48.5 |
Fig. 3.
Spectrogram of male engaged in an in-flight pursuit of a female. Female flies past the microphone first, and male follows 0.8 s later.
Discussion
We were able to observe mating swarms of Ae. aegypti and recorded flight tones from swarming individuals in their natural habitat in Thailand. The average flight tone frequencies from males and females were higher than those typically described in laboratory experiments (Brogdon 1994, Duhrkopf and Hartberg 1992, Wishart and Riordan 1959). This is likely because of the temperatures experienced in the field. The wing beat frequency of Diptera has been found to increase with temperature (for review, see Belton 1986). Some data also suggest that tethering may decrease the flight tone (B.J.A., unpublished data). Others have found that tethering increases the load on the flight mechanism and actually increases wing beat frequency (Belton and Costello 1979, Chadwick 1953). Further studies to confirm that laboratory and field populations produce similar flight tone frequencies under identical conditions would clarify whether there are any differences between these populations. Differences would be important because future studies will undoubtedly use laboratory data to evaluate the behavior of males used in transgenic or sterile male release programs.
Our behavioral observations largely support those of Hartberg (1971). Male Ae. aegypti were attracted to human host stimuli and flew in a characteristic figure 8 pattern. Females paired with males as they came to the host to feed. It has been suggested that swarming is a vestigial behavior and not associated with mating (Nielsen et al. 1960). However, we were able to readily observe mating in swarms.
In one instance, Hartberg (1971) described a male-female pair moving away from human hosts in flight. We observed this behavior in all pairs in the venter-to-venter position in the field (n = 23). Similar behaviors have been reported in field observations of Culex (Reisen et al. 1985) and Anopheles (Reisen and Aslamkhan 1976). Among these, Ae. aegypti is unique in that its swarms occur in close proximity to a defensive human host. Rapid movement away from the host and swarm may serve as an avoidance response evolved in Ae. aegypti to limit exposure to host-defensive behaviors while in copula. Pairs flying in copula appear to be less agile than individuals. This type of response has been reported in the water strider, Gerris remigis, threatened by sun fish predation. Mating durations were shortened to increase the speed of escape (Sih et al. 1990). Alternatively, movement away from aggregations may be a male-driven action. Males may be able to decrease interference from other males and increase the likelihood of successful insemination. This type of interference has been described in other swarming mosquito species (Reisen et al. 1985), and similar behavior has been reported in several swarming Dipteran species (for review, see Thornhill and Alcock 1983). We have observed male interference in the laboratory (L.C.H., unpublished data). Although we did observe males flying in proximity to one another (male-male paired flights), we did not observe male-male interference during copula formations in the field.
As a result of the rapid retreat of pairs, it was difficult to capture the entire acoustic sequence of paired flight between males and females. Our low sample size of male-female pair auditory data (n = 3) did not allow us to conclude with confidence that harmonic convergence was occurring in these pairs. We did, however, observe at least one intriguing instance in which convergence may have been occurring (Fig. 2D). Future field studies with greater sample sizes may lead to recordings of convergence between mating pairs.
We observed 23 pairs in copula, but were only able to acoustically record a small proportion of these because of the speed of the mosquitoes in free flight. Ae. aegypti flight movement is temperature dependent and can be very fast during hot periods (Belton and Costello 1979, Sotavalta 1947, Tamarina et al. 1980). To compensate for the increased speed of flight, the size and scope of the recording array should be increased in future studies. This could be accomplished by increasing the number of microphones and decreasing the spatial separation of microphones.
Recent laboratory studies have revealed a greater level of complexity of mosquito mating behavior (Cator et al. 2009, 2010; Pennetier et al. 2010; Warren et al. 2009) than was previously known (for review, see Yuval 2006). In this work, we have described an innovative new technique for recording acoustic data in field observations of mosquito swarms. This methodology can be used to more accurately assess the role of acoustics in laboratory, as well as be used in further applications in the field.
Currently, most of the work on mosquito acoustics has involved measurements of behavior for two tethered individuals. Working with free-flying laboratory and field populations will enable better comparisons between acoustic behaviors of these two groups. Cator et al. (2009) reported lower harmonic convergence response to playbacks in mated compared with virgin female. Measuring such differences in free flight, in which not only acoustic, but mating attempt outcomes can be measured would be particularly interesting. Finally, coupling this type of recording array with a video data would allow us, for the first time, to dissect the timing of convergence in relation to male pursuit and copula formation. Innovation of this kind will enhance our ability to study field behavior and will ultimately lead to a better understanding of mosquito mating behavior.
Acknowledgments
We thank the staff of the United States Army Medical Component of the Armed Forces Research Institute of the Medical Sciences (Bangkok, Thailand) and the field crew in Kamphaeng Phet, Thailand. We also thank the residents of Kamphaeng Phet for allowing us to conduct recordings in their homes. This work was supported by Centers for Disease Control and Prevention Dissertations in Public Health Grant 1R36CK000130-01 (to L.J.C.) and National Institutes of Health, Health and Human Services, Grant 2R01 DC000103 (to B.J.A.).
Footnotes
The presence of the experimenter (L.J.C.) in Ae. aegypti swarms was approved by the Institutional Review Board of Cornell University (Protocol 06-03-043).
References Cited
- Belton P. Sounds of insects in flight. In: Danthanarayana W, editor. Insect Flight: Dispersal and Migration. Springer; Berlin, Germany: 1986. pp. 60–70. [Google Scholar]
- Belton P, Costello RA. Flight sounds of the females of some mosquitoes of Western Canada. Entomol Exp Appl. 1979;26:105–114. [Google Scholar]
- Brogdon WG. Measurement of flight tone differences between female Aedes aegypti and A. albopictus (Diptera: Culicidae) J Med Entomol. 1994;31:700–703. doi: 10.1093/jmedent/31.5.700. [DOI] [PubMed] [Google Scholar]
- Cator LJ, Arthur BJ, Harrington LC, Hoy RR. Harmonic convergence in the love songs of the dengue vector mosquito. Science. 2009;323:1077–1079. doi: 10.1126/science.1166541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator LJ, Ng’Habi KR, Hoy RR, Harrington LC. Sizing up a mate: variation in production and response to acoustic signals in Anopheles gambiae. Behav Ecol. 2010;21:1033–1039. [Google Scholar]
- Chadwick LE. The flight muscles and their control. In: Roeder KD, editor. Insect Physiology. Wiley; New York: 1953. [Google Scholar]
- Duhrkopf RE, Hartberg WK. Differences in male mating response and female flight sounds in Aedes aegypti and Ae. albopictus (Diptera: Culicidae) J Med Entomol. 1992;29:796– 801. doi: 10.1093/jmedent/29.5.796. [DOI] [PubMed] [Google Scholar]
- Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, Gimnig JE, Fish D, Killeen GF. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, Robert D. Active auditory mechanics in mosquitoes. Proc R Soc B. 2001;268:333–339. doi: 10.1098/rspb.2000.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartberg WK. Observations on the mating behaviour of Aedes aegypti in nature. Bull WHO. 1971;45:847– 850. [PMC free article] [PubMed] [Google Scholar]
- Moore A, Miller JR, Tabashnik BE, Gage SH. Automated identification of flying insects by analysis of wingbeat frequencies. J Econ Entomol. 1986;79:1703–1706. [Google Scholar]
- Nielsen ET, Bell RT, Haeger JS. Swarming and mating in mosquitoes. Ann Entomol Soc Am. 1960;1:71–95. [Google Scholar]
- Pennetier C, Warren B, Dabiré KR, Russell IJ, Gibson G. “Singing on the wing“ as a mechanism for species recognition in the malarial mosquito Anopheles gambiae. Curr Biol. 2010;20:131–136. doi: 10.1016/j.cub.2009.11.040. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Aslamkhan M. Observation on the swarming and mating behavior of Anopheles culcifacies Giles in nature. Bull WHO. 1976;54:155–158. [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Knop NF, Peloguin JJ. Swarming and mating behavior of laboratory and field strains of Culex tarsalis (Diptera: Culicidae) Ann Entomol Soc Am. 1985;78:667– 673. [Google Scholar]
- Roth LM. A study of mosquito behavior: an experimental laboratory study of the sexual behavior of Aedes aegypti (Linnaeus) Am Midl Nat. 1948;40:265–352. [Google Scholar]
- Scott TW, Takken W, Knols BG, Boëte C. The ecology of genetically modified mosquitoes. Science. 2002;298:117–119. doi: 10.1126/science.298.5591.117. [DOI] [PubMed] [Google Scholar]
- Sih A, Krupa J, Travers S. An experimental study on the effects of predation risk and feeding regime on the mating behavior of the water strider. Am Nat. 1990;135:284–290. [Google Scholar]
- Sotavalta O. The flight-tone (wing-stroke frequency) of insects. Acta Entomol Fenn. 1947;4:111–117. [Google Scholar]
- Takken W, Costantini C, Dolo G, Hassanali A, Sagnon N, Osir E. Mosquito mating behaviour. In: Knols BGJ, Louis C, editors. Bridging Laboratory and Field Research for Genetic Control of Disease Vectors. Springer; Dordrecht, the Netherlands: 2006. pp. 183–188. [Google Scholar]
- Tamarina NA, Zhantiev RD, Fedorova MV. Frequency characteristics of flight sounds and Johnston’s organs of sympatric mosquitoes of the genus Aedes (Culicidae) Parazitologiya. 1980;14:398– 402. [PubMed] [Google Scholar]
- Thornhill R, Alcock J. The Evolution of Insect Mating Systems. Harvard University Press; New York, NY: 1983. [Google Scholar]
- Tripet F, Dolo G, Traoré S, Lanzaro GC. The “wingbeat hypothesis” of reproductive isolation between members of the Anopheles gambiae complex (Diptera: Culicidae) does not fly. J Med Entomol. 2004;41:375–384. doi: 10.1603/0022-2585-41.3.375. [DOI] [PubMed] [Google Scholar]
- Warren B, Gibson G, Russell IJ. Sex recognition through midflight mating duets in Culex mosquitoes is mediated by acoustic distortion. Curr Biol. 2009;19:485– 491. doi: 10.1016/j.cub.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Wekesa JW, Brogdon WG, Hawley WA, Besansky NJ. Flight tone of field-collected populations of Anopheles gambiae and An. arabiensis (Diptera: Culicidae) Phys Entomol. 1998;23:289–294. [Google Scholar]
- Wishart G, Riordan DF. Flight responses to various sounds by adult males of Aedes aegypti (L.) (Diptera: Culicidae) Can Entomol. 1959;91:181–191. [Google Scholar]
- Yuval B. Mating systems of blood-feeding flies. Annu Rev Entomol. 2006;51:413– 440. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]