Abstract
The bone marrow is the site of hematopoesis and contains mixed population of blood cells including erythrocytes, granulocytes, monocytes, dendritic cells, lymphocytes and hematopoietic stem cells. The following protocol provides a simple and fast method for isolation of bone marrow immune cells (no erythrocytes) from mouse femurs with a yield of approximate 8 × 107 cells in 5 ml culture media (1.6 × 104 cells/μl). Further isolation or flow cytometric analysis might be required for study of specific immune cell types.
Materials and Reagents
Sterile paper towel
Sterile surgical pad (Direct Resource, catalog number: 19015742)
23-gauge (or 25-/26-gauge) needle (BD Biosciences, catalog number: 305145)
10 ml syringe (BD Biosciences, catalog number: 309604)
-
70 μm nylon cell strainer (Falcon, catalog number: 352350)
Note: Currently, it is “Corning, Falcon®, catalog number: 11995-065”.
-
50 ml conical tube (Falcon, catalog number: 21008-940)
Note: Currently, it is “Corning, Falcon®, catalog number: 21008-940”.
5 ml syringe plunger (BD Biosciences, catalog number: 309646)
Adult mice (> 6 weeks, any strain) (e.g. C57BL/6)
-
Hank’s balanced salt solution (HBSS), no Calcium, no Magnesium, no phenol red (Life Technologies, catalog number: 14175095)
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 14175095”.
-
DMEM medium, high glucose, pyruvate, L-glutamine (Life Technologies, catalog number: 11995-065)
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 11995-065”.
70% ethanol
Fetal bovine serum heat inactivated (FBS) (Sigma-Aldrich, catalog number: F9665)
Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: 213330)
Potassium bicarbonate (KHCO3) (Sigma-Aldrich, catalog number: 237205)
Disodium edetate (Sigma-Aldrich, catalog number: D2900000)
RBC lysis buffer (see Recipes)
DMEM medium (see Recipes)
Equipment
Blunt-end sterile scissors (Thermo Fisher Scientific, Fisher Scientific, catalog number: 08-950)
Sharp sterile scissors (Thermo Fisher Scientific, Fisher Scientific, catalog number: 08-940)
Sterile forceps (Thermo Fisher Scientific, Fisher Scientific, catalog number: 08-890)
Hausser™ Levy™ Hemacytometer Chamber Set (Thermo Fisher Scientific, Fisher Scientific, catalog number: 02-671-55A) or coulter Z2 cell and particle counter (Beckman Coulter, catalog number: 383550)
Refrigerated centrifuge
Sterile culture hood
CO2 rodent euthanasia chamber
Procedure
Euthanize the mouse with CO2 and place mouse onto a sterile surgical pad in a sterile hood. Sterilize the mouse abdomen area and skin of hindlimbs with 70% ethanol (Figure 1).
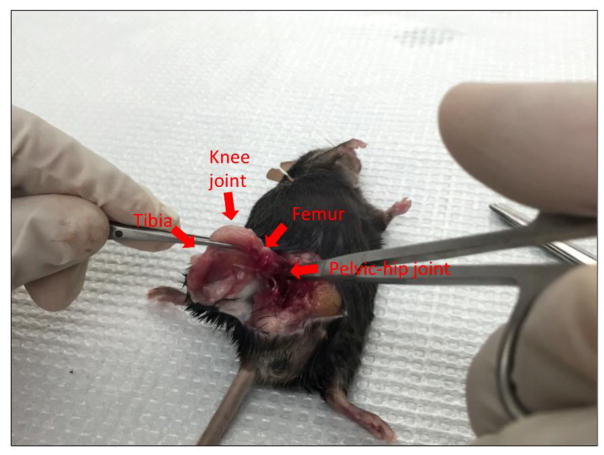
Open the abdominal cavity with blunt-end sterile scissors and remove the surface muscles and find the pelvic-hip joint (Figure 2).
Cut off the hind leg above the pelvic-hip joint with sharp sterile scissors (Figure 3). Cut off the tibia from the hind leg below the knee joint with sharp sterile scissors (Figure 4).
(Optional) If higher yield of bone marrow cells is needed, tibia can also be used for bone marrow cell isolation. Cut at the tibia ankle joint to dissect the tibia. The following procedures can be applied to both femur and tibia.
Remove the muscles and residue tissues surrounding the femur with sterile forceps and scissors (Figure 5).
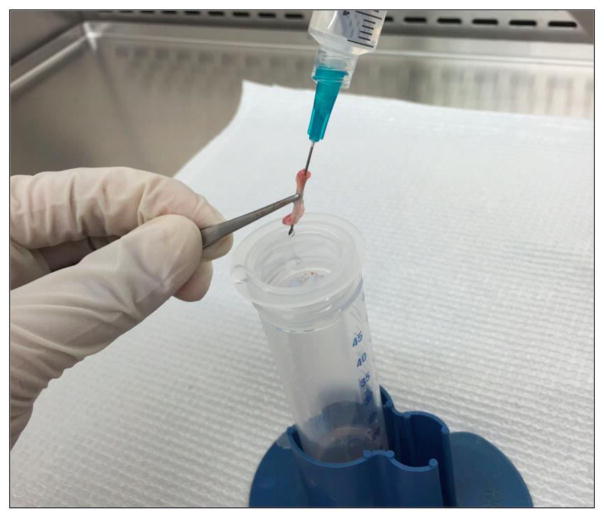
Cut the femurs at both ends with sharp sterile scissors (Figure 6). Use a 23-gauge (some literature suggests 25-or 26-gauge) needle and a 10 cc syringe filled with ice-cold HBSS to flush the bone marrow out onto a 70 μm nylon cell strainer placed in a 50 ml Falcon conical tube (Figure 7). Use all the 10 ml HBSS or until the flow through turns white.
(Optional) In case some residue bone marrow cells could not be flushed off (very few bone marrow visible in the flow through or the yield is significantly less, e.g. < 1 × 107 cells), scrape the inner surface of the femur with the needle and flush with extra ~5 ml HBSS (Figure 8).
Smash the bone marrow through the cell strainer with a 5 ml plunger (Figure 9). Wash the strainer with another ~5 ml HBSS.
Centrifuge cells at 1,500 rpm for 7 min at 4 °C. Discard the supernatant and blot on paper towel (Figure 10).
Resuspend the cell pellet with 1 ml RBC lysis buffer (for each mouse). Incubate for 5 min at room temperature or 2 min at 37 °C, and neutralize the lysis buffer by adding 5 ml FBS.
Centrifuge cells at 1,500 rpm for 7 min at 4 °C. Discard the supernatant and blot on paper towel. Resuspend the cell pellet with appropriate media for the next step of assay such as 5 ml DMEM medium containing 10% FBS. Cells are then placed on ice.
Count the bone marrow cells with a hemocytometer or a Beckman Z2 coulter counter. Cells are ready for assays or culture. Cells can stay viable on ice for at least 5 h. It is recommended to perform the experiment (culture or assays) right after isolation for best results.
Figure 1.
Sterilization of mouse abdomen area and skin of hindlimbs
Figure 2. Find the pelvic-hip joint.

Bone anatomy reference: http://www.informatics.jax.org/cookbook/figures/figure41.shtml
Figure 3.
Cut off the hind leg above the pelvic-hip joint
Figure 4.
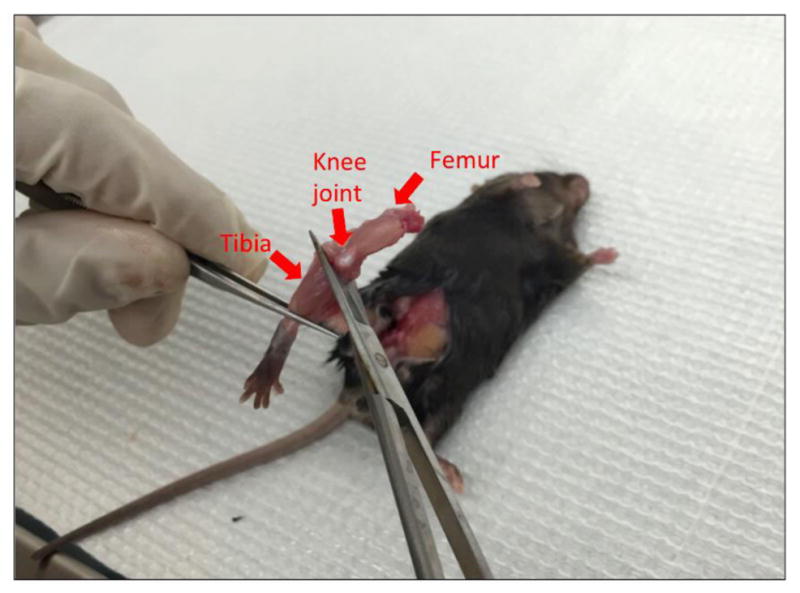
Cut off the tibia at knee joint
Figure 5.

Remove the muscles and residue tissues surrounding the femur
Figure 6.
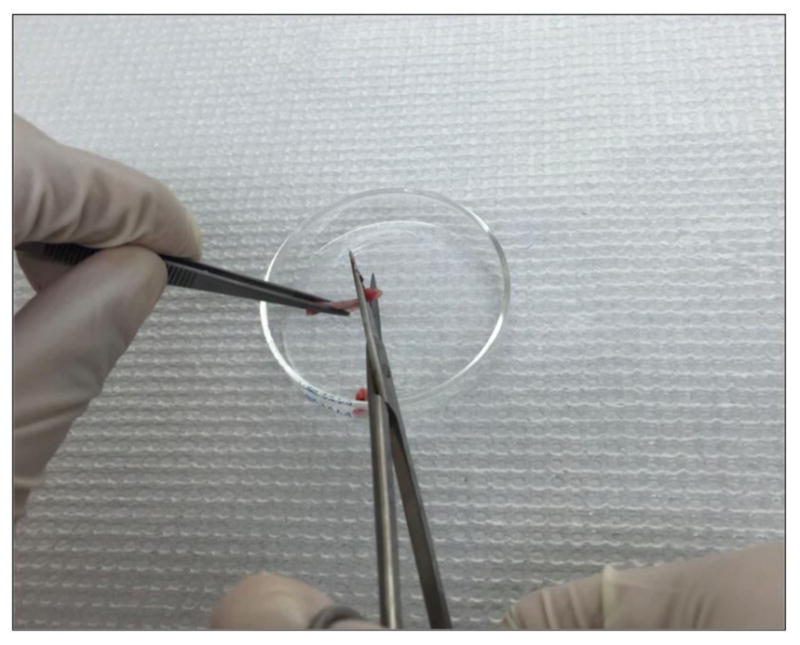
Cut femurs at both ends
Figure 7.
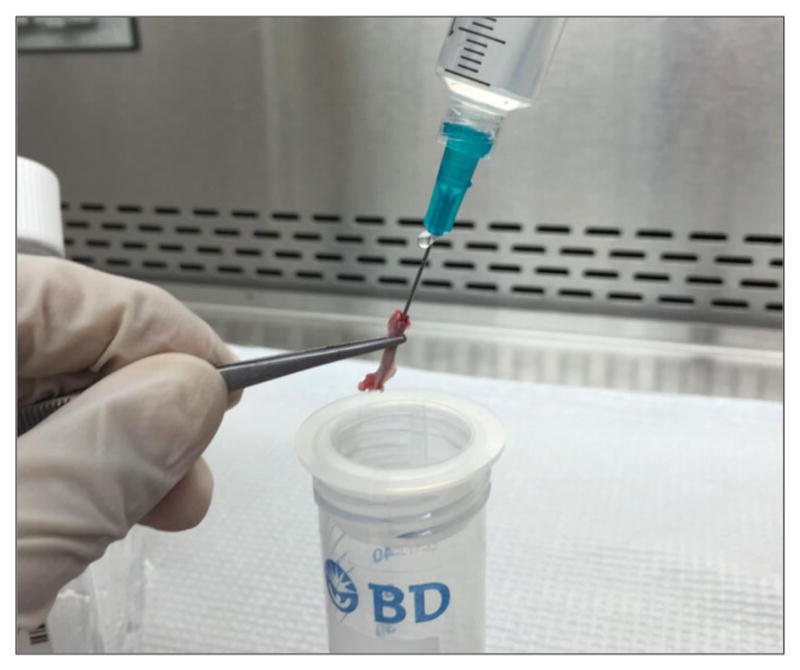
Flush the bone marrow onto the cell strainer with HBSS
Figure 8.
(Optional) Scrape the inner surface of femur with needle
Figure 9.
Smash the bone marrow cells through the cell strainer with a 5 ml plunger
Figure 10.
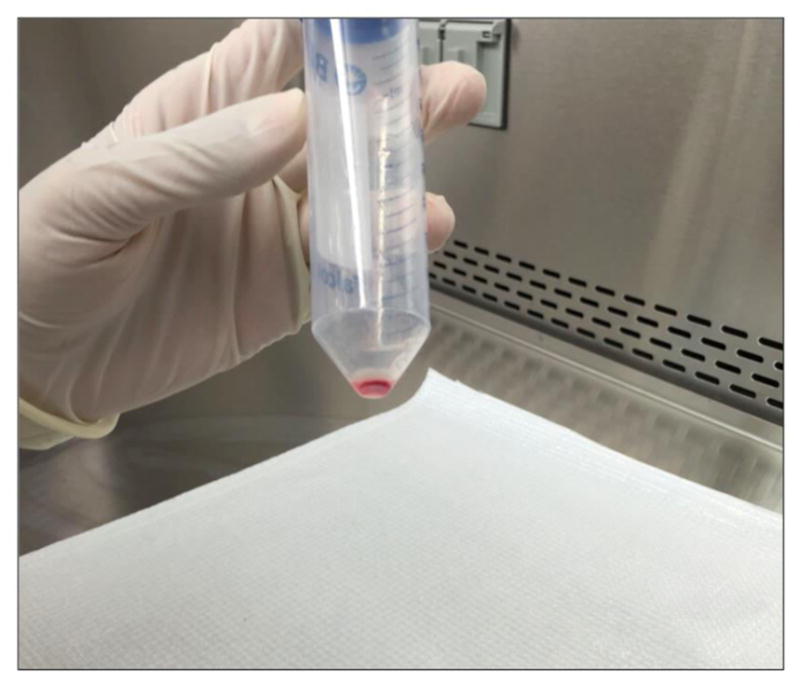
Cell pellet before RBC buffer resuspension
Recipes
-
RBC lysis buffer
0.16 M NH4Cl, 10 mM KHCO3, and 0.13 mM EDTA, dissolved in sterile H2O and stored at 4 °C.
For 500 ml, 4.28 g NH4Cl, 0.5 g KHCO3, 0.024 g Disodium EDTA
It is recommended to prepare fresh RBC lysis buffer for the experiment. RBC lysis buffer will be stable at 4 °C for at least 1 month.
-
DMEM medium
DMEM medium, high glucose, pyruvate, L-glutamine supplemented with 10% FBS
Stored at 4 °C
Acknowledgments
This protocol was revised based on previous studies including the referenced articles below and was supported by an NIH grant (R21 MH099482) to Ning Quan.
References
- 1.Madaan A, Verma R, Singh AT, Jain SK, Jaggi M. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. J Biol Methods. 2014;1(1):e1. [Google Scholar]
- 2.Weischenfeldt J, Porse B. Bone marrow-derived macrophages (BMM): Isolation and applications. CSH Protoc. 2008:2008. doi: 10.1101/pdb.prot5080. pdb prot5080. [DOI] [PubMed] [Google Scholar]