Abstract
Omega-3 polyunsaturated fatty acids (PUFAs) exert an anticancer effect by affecting multiple cellular mechanisms leading to inhibition of proliferation and induction of apoptosis. It is well known that breast cancer comprises distinct molecular subtypes which differ in their responsiveness to therapeutic and preventive agents. We tested the hypothesis that n-3FA may preferentially affect triple-negative breast cancer cells for which no targeted intervention is presently available. The in vitro antiproliferative effects of n-3 PUFA docosahexaenoic acid (DHA) and its metabolite, 4-OH-DHA as well as its putative metabolite 4-OXO-DHA, were tested in five triple-negative human basal breast cell lines at different stages of transformation (MCF-10F, trMCF, bsMCF, MDA-MB-231, and BT-549) and three luminal breast cancer cell lines (MCF-7, T-47D, and SK-BR-3). Cell proliferation was measured with the tetrazolium MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay. DHA and its oxidized derivatives significantly inhibited cell proliferation (20–90% reduction) of both basal and luminal breast cancer cell lines. The inhibitory effect was more pronounced on triple-negative basal breast cancer cell lines as compared to luminal breast cancer cell lines after 4-OXO-DHA treatment. Our data provide novel information regarding the preferential antitumor effect of oxidized derivatives of DHA on basal type breast cancer.
Keywords: Triple-negative breast cancer, Fish oil, DHA
Introduction
The role of diet as a preventative measure for breast cancer remains controversial (Signori et al. 2011). Current literature suggests that the risk of developing breast cancer may decrease or increase with the intake of omega-3 (n-3) and omega-6 (n-6) fatty acids, respectively (Carroll and Braden 1984). n-6 polyunsaturated fatty acids (PUFAs) have been suggested to promote cancer while n-3 PUFAs have been implicated in cancer suppression (Chapkin et al. 2007; Larsson et al. 2004). It has been reported that n-3 PUFAs alter the cell membrane phospholipid composition and as a result, n-3 PUFAs may exert an anticancer effect by affecting multiple cellular mechanisms (Signori et al. 2011). Among these, the expression and function of multiple receptors, proteins, and lipid-derived signaling molecules may be affected. n-3 PUFAs may mediate mammary cancer prevention by affecting eicosanoid metabolism, oxidative stress, cell membrane structure, cell proliferation, and apoptosis (Signori et al. 2011). Altering these processes will eventually lead to the inhibition of cell proliferation and increased cell death (Signori et al. 2011). Currently, the molecular mechanisms of these alterations are not well understood (Berquin et al. 2008).
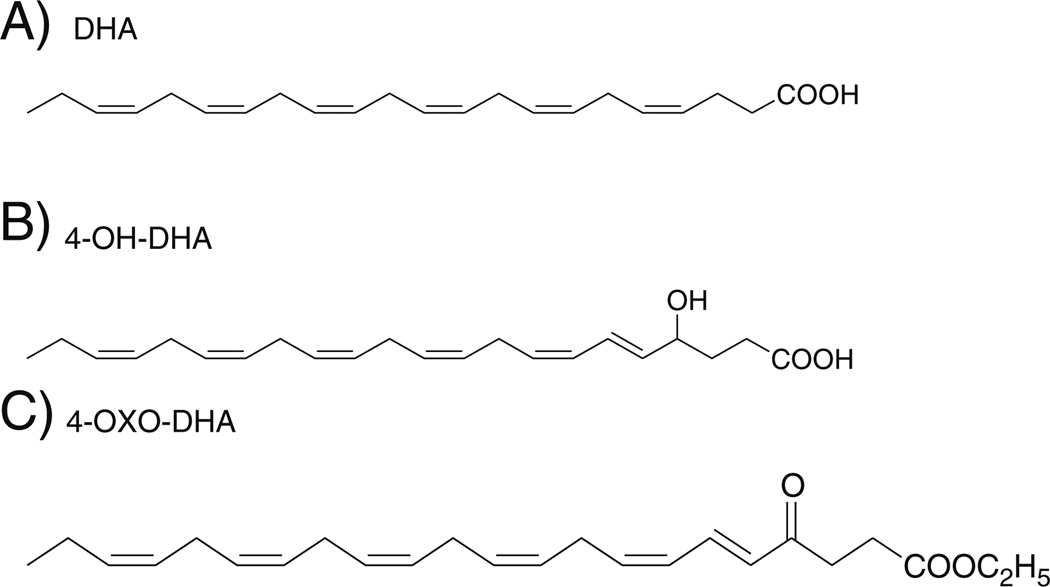
Dietary long-chain n-3 PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are found primarily in cold-water fish (Berquin et al. 2008). The amount of EPA and DHA varies from species of fish and geographical location. Fish such as mackerel, tuna, and salmon are from deep, cold water and tend to have the highest concentration of EPA and DHA (Larsson et al. 2004). DHA has been shown to promote an anticancer effect on multiple breast cancer cell lines in vitro (Liu et al. 2007; Schley et al. 2007; Sun et al. 2008). DHA has also been reported to induce apoptosis via multiple pathways (Berquin et al. 2008; Sun et al. 2008; Blanckaert et al. 2010; Kang et al. 2010; Ravacci et al. 2013). In vivo studies using animal models also found that n-3 PUFAs reduced tumor growth, slowed histopathological progression, and increased survival (Berquin et al. 2007). Indeed, epidemiologic studies show an inverse association between percent calories from fish and incidence of breast cancer, suggesting a protecting role (Kaizer et al. 1989). The protective effects of fish oil have been shown also in other cancer types, such as colorectal cancer (Anti et al. 1992). Nevertheless, analytic epidemiologic studies having a case-control or cohort design have not yielded clear conclusions concerning the protective effect of fish consumption or n-3 PUFAs intake against cancer. Some studies published in the literature have failed to show an inverse association between the intake of n-3 PUFAs or fish and cancer risk (Vatten et al. 1990; Chajes et al. 1999; Holmes et al. 1999). Previous studies conducted in vitro and in vivo models of mammary carcinogenesis clearly demonstrated that DHA (Fig. 1a) is a superior chemopreventive agent to EPA (Noguchi et al. 1997; Yuri et al. 2003; Kang et al. 2010; Rahman et al. 2013). Promising literature indicated that DHA and its metabolite 4-OH-DHA (Fig. 1b) may play a major role in the antitumor properties of omega-3 (Itoh et al. 2006; Sapieha et al. 2011).
Figure 1.
Chemical structures of a DHA, b 4-OH-DHA, and c 4-OXO-DHA.
The focus of the present work was to determine whether DHA and 4-OH-DHA exert a differential effect in at least two subtypes of breast cancer, the luminal and the basal type. Furthermore, because of its superior PPARγ agonistic activity to that of 4-OH-DHA, we included the putative metabolite 4-OXO-DHA (Fig. 1c) for comparison (Itoh et al. 2008). Our laboratory has developed a model of human breast epithelial cell transformation using the normal-like basal cell-type MCF-10F and 17-β-estradiol as the carcinogen (Russo et al. 2003, 2006a, b). This model represents the progression of basal breast cancer from normal cell (MCF-10F), transformed cell (trMCF), and invasive-metastatic (bsMCF) (Soule et al. 1990; Russo et al. 2006a, b; Huang et al. 2007). The uniqueness of this model is that all cells have the same genetic lineage and stable phenotypes. In addition to these cell lines, we included two basal (MDA-MB-231 and BT-549) and three luminal cell lines (MCF-7, T-47D, and SK-BR-3) well characterized in literature. Our results show that oxidized derivative of DHA, 4-OXO-DHA, preferentially inhibited the growth of basal-like breast cancer for which currently there is no targeted therapy available.
Materials and Methods
Cell cultures
The antiproliferative effect of DHA and its oxidized derivatives were tested on eight human breast-derived cell lines. Among the eight cell lines, one normal-like breast epithelial cell line (MCF-10F), one transformed breast epithelial cell line (trMCF), three basal breast cancer cell lines bsMCF, MDA-MD-231, and BT-549), and three luminal breast cancer cell lines (MCF7, T-47D, and SK-BR- 3) were chosen. Basal cell lines were classified as ER−, PR−, and HER2−. MCF7 and T-47D were classified as luminal A (ER+, PR+, and HER2−) while SK-BR-3 is classified as luminal B (ER+, PR+, HER2+). MCF10F, trMCF, and bsMCF cells were maintained in DMEM/F12 media (1:1 Gibco/BRL, Gaithersburg, MD) supplemented with 5% horse serum (Gibco), 100 ng/ml cholera toxin (ICN Biomedicals, Cleveland, OH), 10 µg/ml insulin (Sigma, St. Louis, MO), 0.5 µg/ml hydrocortisone (Sigma), 20 ng/ml epidermal growth factor (Gibco), 1.05 mM CaCl2, and antibiotics (penicillin, 100 U/ml; streptomycin, 100 µg/ml; amphotericin, 0.25 µg/ml; Sigma). MCF7 cells were maintained in DMEM media supplemented with 10% fetal bovine serum (FBS). MDA-MB-231, BT-549, and T-47D breast cancer cells were grown in RPMI-1640 media supplemented with 10% FBS. SK-BR-3 was grown in McCoy’s 5a media supplemented with 10% FBS. All cell lines were maintained in a 37°C, 5% CO2 incubator. All cell lines and media were obtained from the Tissue Culture Facility at Fox Chase Cancer Center.
Preparation of DHA and oxidized metabolites
Stock concentrations of 2.5 mM of DHA and oxidized derivatives were bovine serum albumin (BSA)-conjugated. A solution of 5 mg DHA/0.05 M Na2CO3 was prepared and flushed with nitrogen gas. This solution was then vortexed extensively and left at room temperature for 1 h. The vial was vortexed intermittently to insure that all DHA was dissolved completely. A 2.5-mM fatty acid (FA) BSA complex was prepared at FA/BSA 3:1 mole ratio using 15% BSA dissolved in normal cell culture medium. Solutions were prepared in a conical tube and were flushed with nitrogen gas before being placed on a shaker for 30 min. The FA-BSA stock solution was filter-sterilized under a sterile cell culture hood, aliquoted, and stored at −20°C.
Treatment
DHA was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). DHA derivatives, 4-OH-DHA and 4-OXO-DHA, were synthesized and provided by Dr. Karam El-Bayoumy laboratory using a previously established synthetic approach (Itoh et al. 2006). DHA and its metabolites were BSA-conjugated in culture medium as previously described. Stock solutions (2.5mM) were prepared and stored at −20°C. Based on published literature, five concentrations, 0, 10, 25, 50, and 100 µM, of DHA and its derivatives were tested for a total of 4 days. Medium was changed daily over the course of treatment time.
MTT assay
Cells were seeded in 100 µL culture medium into costar 96-well flat bottom tissue culture plates at an optimal density per cell line (1000–4000 cells/well) to have a 70–80% confluent culture by 5 days. Cells were treated in adherent conditions on the following day. Medium containing the tested treatment doses was changed daily.
Cell proliferation was measured with the tetrazolium MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay (Mosmann 1983). Cell proliferation was assessed every 24 h of treatment for a total of 96 h using Vybrant MTT Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR). Media was removed and replaced with 100 µL phenol-red-free media containing 12 mM MTT. The plate was incubated in a 37°C, 5% CO2 incubator for 4 h, followed by 4 h of incubation after the addition of HCl-SDS to solubilize the formazan product. Optical density was read at 570 nm using Epoch Microplate Spectrophotometer (Biotek, Winnoski, VT). Data was analyzed on Sigma Plot v12. A one-way analysis of variance (ANOVA) was performed using the Holm-Sidak method to calculate a relevant p value where p values less than 0.05 were considered to be statistically significant.
Statistics
For each of the treatments under consideration, 100 µM DHA, 4-OH-DHA, and 4-OXO-DHA, Kruskal-Wallis tests indicated a significant effect of cell line on growth relative to untreated control after treatment. Tukey honestly significant difference (HSD) tests with α=0.05 on the ranks of cell growth relative to untreated control after treatment was used to identify cell lines responsible for the significance of Kruskal-Wallis tests. Results of this post hoc analysis are shown in Tables 1 and 2; cell lines labeled with the same letter are not significantly different. For each treatment, the Tukey HSD test controls the overall type I error rate at 0.05; no adjustment for multiple comparisons was made between treatments for means of comparing individual treatment effect on cell lines.
Table 1.
Results of the post hoc analysis from MTT results of 96 h 100 µM DHA, 100 µM 4-OH-DHA, and 100 µM 4-OXO-DHA treatment
| DHA | 4-OH-DHA | 4-OXO-DHA | ||||||
|---|---|---|---|---|---|---|---|---|
| Labels | Cell line | Rank means | Labels | Cell line | Rank means | Labels | Cell line | Rank means |
| a | MCF-7 | 30.5 | a | MCF-7 | 30 | a | MCF-7 | 30.50 |
| ab | bsMCF | 21 | ab | SK-BR-3 | 23.25 | ab | T-47D | 25.50 |
| ab | T-47D | 20 | abc | T-47D | 20.5 | bc | SK-BR-3 | 23.50 |
| ab | BT-549 | 19.5 | abc | BT-549 | 17.25 | cd | MCF-10 F | 18.50 |
| bc | MCF-10 F | 16.75 | bcd | MDA-MB-231 | 15.25 | de | bsMCF | 13.25 |
| bc | SK-BR-3 | 15.25 | bcd | MCF-10 F | 13.5 | e | BT-549 | 9.50 |
| cd | trMCF | 6.5 | cd | bsMCF | 8.75 | e | trMCF | 8.75 |
| d | MDA-MB-231 | 2.5 | d | trMCF | 3.5 | f | MDA-MB-231 | 2.50 |
| (χ2(7)=24.32; p=0.0010) | (χ2(7)=22.00; p=0.0025) | (χ2(7)=29.36; p=0.0001) | ||||||
Kruskal-Wallis test indicated a significant effect of cell line on growth relative to untreated control after treatment. Tukey HSD tests with α=0.05 on the ranks of cell growth relative to untreated control after treatment was used to identify cell lines responsible for the significance of Kruskal-Wallis tests. This post hoc analysis yielded the following, where cell lines labeled with the same letter are not significantly different
Table 2.
Results of post hoc analysis from MTT results of 96 h DHA, 4-OH-DHA, and 4-OXO-DHA treatment
| Kruskal-Wallis test | ||||||||
|---|---|---|---|---|---|---|---|---|
| MCF-10F | trMCF | bsMCF | MDA-MB-231 | BT-549 | MCF-7 | T-47D | SK-BR-3 | |
| 0 µM control | a | a | a | a | a | b | a | a |
| 100 µM DHA | ab | b | b | c | a | a | b | b |
| 100 µM 4-OH-DHA | b | c | c | b | a | ab | b | b |
| 100 µM 4-OXO-DHA | b | c | c | d | b | ab | b | c |
Kruskal-Wallis test indicated a significant effect of cell line on growth relative to untreated control after treatment. Tukey HSD tests with α=0.05 on the ranks of cell growth relative to untreated control after treatment was used to identify cell lines responsible for the significance of Kruskal-Wallis tests. This post hoc analysis yielded the following, where cell lines labeled with the same letter are not significantly different. Labels in each column are determined independently, and in each case a>b>c>d, showing the directionality of significant differences in each case
Results
DHA, 4-OH-DHA, and 4-OXO-DHA inhibit in vitro cell proliferation of human breast cancer cells
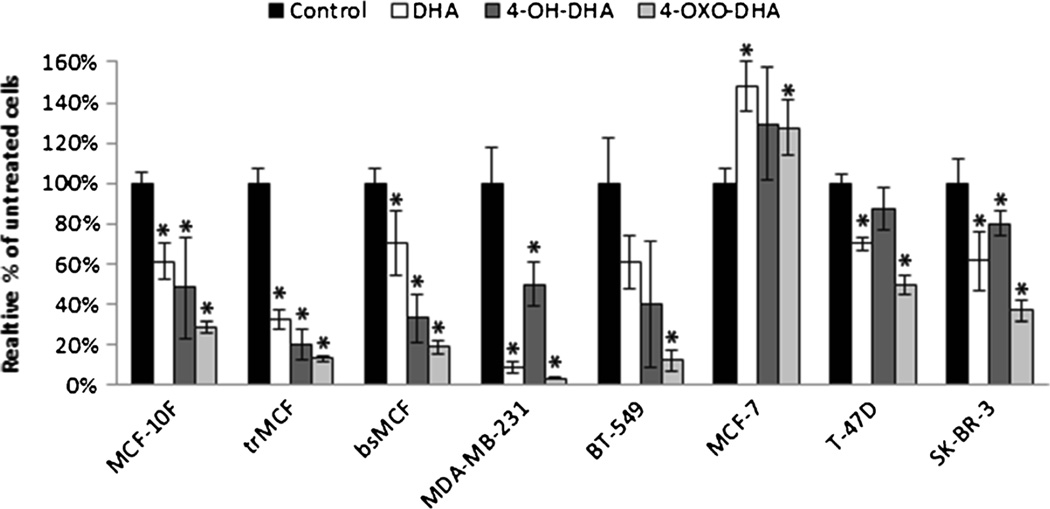
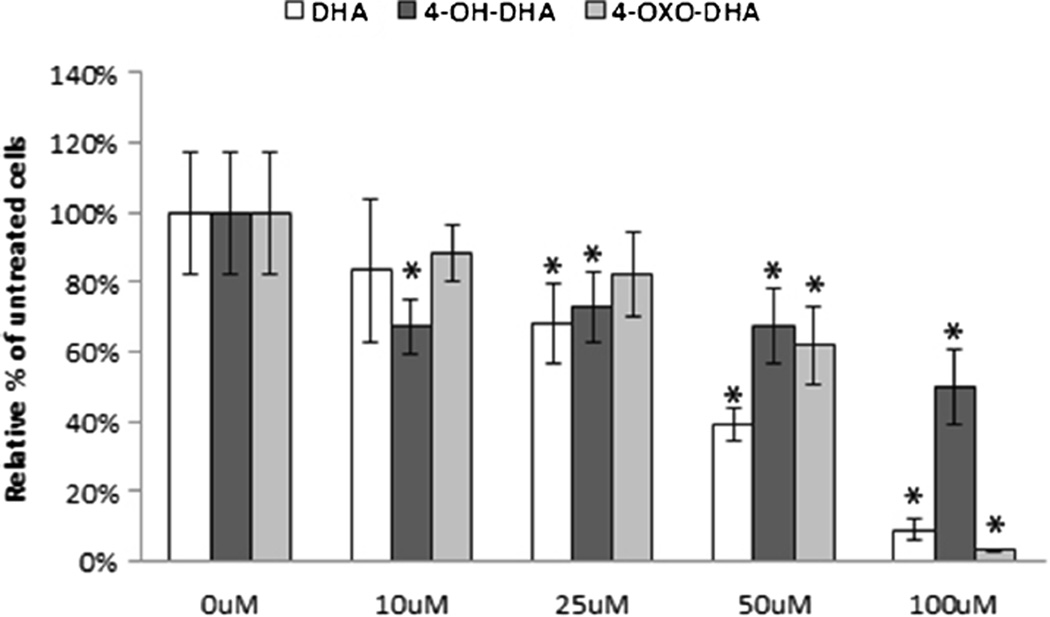
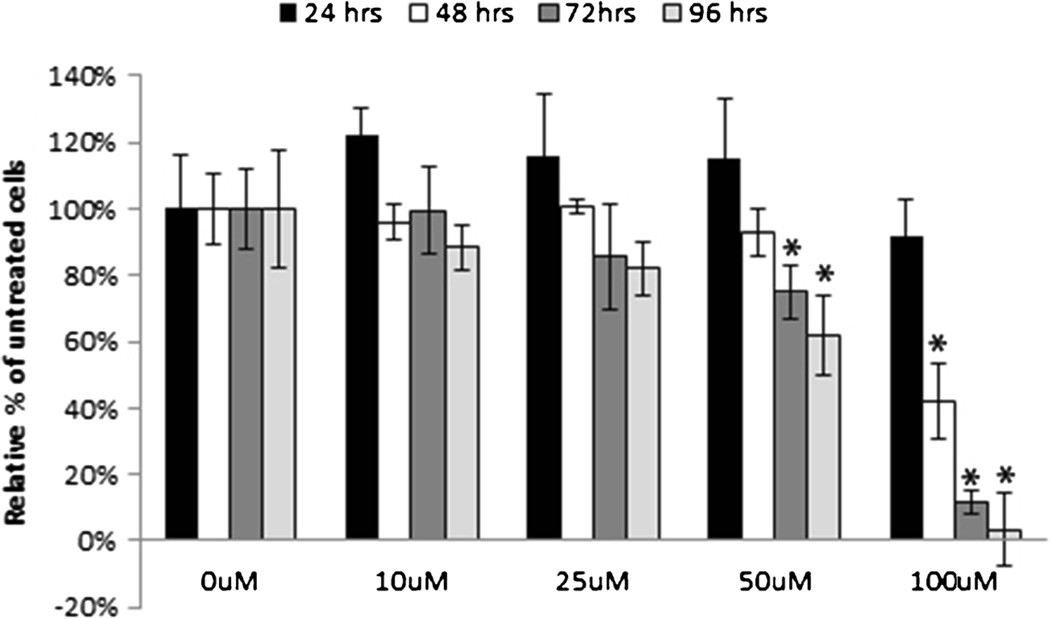
Treatment of DHA, 4-OH-DHA, and 4-OXO-DHA resulted in an inhibitory effect on the proliferation of MCF-10F, trMCF, bsMCF, MDA-MB-231, BT-549, T-47D, and SK-BR-3 (Fig. 2). Proliferation was significantly increased after DHA and 4-OXO-DHA treatment on MCF-7. DHA and its oxidized metabolites produced an inhibitory effect that was dose dependent after 96 h of treatment (Fig. 3). Cell proliferation was significantly inhibited within 48 h of 100 µM treatment, but the most prominent effect was observed after 96 h of 100 µM treatment (Fig. 4).
Figure 2.
The antiproliferative effect of DHA, 4-OH-DHA, and 4-OXO-DHA (100 µM) on a normal breast epithelial and breast cancer cell lines. Cell proliferation was measured by MTT assay 96 h after treatment as described in “Materials and Methods.” All values were compared to a relative percentage of the untreated control. Bars represent the mean± standard deviation of triplicate experiments with experimental quadruplicate replicates. *p≥0.05 indicates a significant difference compared to 0 µM control.
Figure 3.
The dose-dependent antiproliferative effect of DHA, 4-OH-DHA, and 4-OXO-DHA (100 µM) on MDA-MB-231 basal breast cancer cell line. Cell proliferation was measured by MTT assay 96 h after treatment as described in “Materials and Methods.” All values were compared to a relative percentage of the untreated control. Bars represent the mean±standard deviation of triplicate experiments with experimental quadruplicate replicates. *p≥0.05 indicates a significant difference compared to 0 µM control.
Figure 4.
The antiproliferative effect of 4-OXO-DHA on MDA-MB-231 basal breast cancer cell line. Cell proliferation was measured by MTT assay every 24 h after treatment for a total of 96 h as described in “Materials and Methods.” All values were compared to a relative percentage of the untreated control. Bars represent the mean±standard deviation of triplicate experiments with experimental quadruplicate replicates. *p≥0.05 indicates a significant difference compared to 0 µM control.
The effect of 100 µM 4-OXO-DHA on the proliferation of all cultured cell lines differed according to the cell type. The three luminal cell lines, MCF-7, T-47D, and SK-BR-3, were significantly different from the four basal cell lines tested, trMCF, bsMCF, BT-549, and MDA-MB-231 as described in Table 1. DHA and 4-OH-DHA treatment did not show a clear preferential effect. Treatment of 4-OXO-DHA was more effective than DHA and 4-OH-DHA in MDA-MB-231, BT-549, and SK-BR-3 cell lines (Table 2). 4-OXO-DHA was more potent than DHA in trMCF and bsMCF. 4-OH-DHA treatment was more potent than DHA in trMCF and bsMCF.
Discussion
Triple-negative breast cancer remains a difficult and aggressive cancer to treat. The possibility of preventing triple-negative breast cancer by dietary n-3 PUFAs supplements such as DHA or its metabolites may be a potential approach in cancer prevention or treatment. A new concept is emerging which is based on the combination of chemotherapy and nutrition intervention. Preclinical evidence of the preventive benefits of n-3 PUFAs in breast cancer continues to fuel interest in the potential role of dietary fat content in reducing breast cancer risk. There are several clinical trials that seek to gain knowledge of the role of dietary fatty acids in the prevention of breast cancer. Yee et al. tested different concentrations of n-3 PUFAs in a 6-month randomized open-label study with 48 women with increased breast cancer risk (Yee et al. 2010). The authors concluded that daily doses up to 7.56 g DHA+EPA were well tolerated with excellent compliance. Body mass index and baseline fatty acid concentrations modulated the dose-response effects of n-3 PUFAs supplements on serum EPA and DHA and breast adipose tissue DHA. In a recent report by our team, we demonstrated the expected rise in serum n-3 PUFAs in women supplemented with Lovaza (4 g daily) for 12 mo (Signori et al. 2012); these results further support the excellent compliance and the oral supplementation of n-3 PUFAs as a feasible strategy in future breast cancer chemoprevention trials. n-3 PUFA consumption has also been studied in colorectal cancer (CRC). Two large epidemiological observational studies have demonstrated significant inverse relationships between n-3 PUFA intake/levels and risk of colorectal neoplasia. The studies showed a significant dose-dependent reduction in CRC risk for total n-3 PUFA intake (Kim et al. 2010). Recent evidence supports a broader role of n-3 fatty acid supplements in cancer patients. Early administration of this nutritional supplement helps to preserve muscle tissue and prevent cachexia as well as tolerate better the chemotherapy treatment (Murphy et al. 2012). The dose of fish-oil/omega-3 PUFAs needed to achieve maximal target tissue effects for breast cancer prevention remains undefined. Our data show that DHA and its metabolites have a preferential antiproliferative effect on basal breast cancer compared to luminal breast cancer.
Our data show that DHA-oxidized derivative, 4-OXO-DHA, had a differential effect on the two main subtypes of breast cancer cell lines tested. Our results indicated that not only 4-OXO-DHA had an inhibitory effect on breast cancer, but also it produced a preferential inhibitory effect on basal cell lines over luminal cell lines. Under the experimental conditions used in this study, there was a stimulatory effect of DHA and metabolites on MCF7 cell line. However, the other luminal A cell line, T-47D, showed an inhibitory effect; thus, we cannot assume that DHA may enhance the growth of luminal A breast cancer. Recent studies demonstrated that DHA intake has been shown to promote an anticancer effect on multiple breast cancer cell lines in vitro (Liu et al. 2007; Schley et al. 2007; Sun et al. 2008). Specifically, Blankaert et al. reported that intake of DHA decreased cell proliferation, increased apoptosis, and decreased the invasive potential of the MDA-MB-231 cell line, a highly malignant triple-negative breast cancer cell line (Blanckaert et al. 2010). Multiple pathways have been proposed to explain how DHA has also been reported to induce apoptosis via multiple pathways (Berquin et al. 2008; Sun et al. 2008; Blanckaert et al. 2010; Kang et al. 2010; Ravacci et al. 2013). The mechanism of DHA remains unclear and controversial (Gago-Dominguez et al. 2003; Stripp et al. 2003; Schley et al. 2007; Zhang et al. 2012). Furthermore, no mechanism has been identified to explain why triple-negative breast cancer cells are more sensitive to DHA, 4-OH-DHA, and 4-OXO-DHA treatment. This finding warrants further investigation.
Using the MCF-10F model, we indicated that more transformed cells were more sensitive to DHA and metabolite treatment. For example, both trMCF and bsMCF, which represent a transformed and metastatic cell line, were more sensitive to DHA, 4-OH-DHA, and 4-OXO-DHA treatment compared to their parent normal-like MCF-10F cell line. This observation is consistent with a previous report where they showed that a transformed cell, MCF-7, responded better to DHA ethanolamine derivatives than the normal-like breast epithelial cell line MCF-10A (Rovito et al. 2013). MCF- 10A and MCF-10F were both derived from the same patient, and both express phenotypes of a normal-like breast epithelial cell line (Soule et al. 1990). The pronounced inhibitory effect we observed on the trMCF cell line compared to MCF-10F and bsMCF suggests that DHA and its oxidized derivatives may be beneficial in treating cancer in early stages or possibly breast cancer prevention.
This study supports the hypothesis that treatment of DHA and its metabolites inhibits breast cancer cell proliferation. DHA produced an antiproliferative effect on multiple cell lines in our model; however, the effect was more pronounced when cells received 4-OXO-DHA treatment. Additionally, 4-OXO-DHA treatment preferentially affected triple-negative breast cancer cells. The larger inhibition of cell proliferation produced by this metabolite suggests that metabolites of DHA may be more beneficial in cancer treatment than DHA. The largest inhibition of cell proliferation was produced by 4-OXO-DHA (100 µM). Since DHA is metabolized into 4-OH-DHA and likely to 4-OXO-DHA, these metabolites may be the potential compounds that are in fact inhibiting cell proliferation. Thus, understanding the metabolism of DHA and how the metabolites react with the cell is essential in understanding the anticancer effect produced by DHA.
Acknowledgments
This project was supported by Komen Foundation grant KG081632, NCI core grant CA06927, and an appropriation from the Commonwealth of Pennsylvania. We also acknowledge the use of the tissue culture facility at Fox Chase Cancer Center
Contributor Information
Thomas J. Pogash, Email: Thomas.Pogash@fccc.edu, Breast Cancer Research Laboratory, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
Karam El-Bayoumy, Department of Biochemistry and Molecular Biology, Pennsylvania State University College of Medicine, Hershey Medical Center, Hershey, PA, USA.
Shantu Amin, Department of Pharmacology, Pennsylvania State University College of Medicine, Hershey Medical Center, Hershey, PA, USA.
Krishne Gowda, Department of Pharmacology, Pennsylvania State University College of Medicine, Hershey Medical Center, Hershey, PA, USA.
Ricardo López de Cicco, Breast Cancer Research Laboratory, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
Maria Barton, Breast Cancer Research Laboratory, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
Yanrong Su, Breast Cancer Research Laboratory, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
Irma H. Russo, Breast Cancer Research Laboratory, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA
Julie A. Himmelberger, Natural Science Department, DeSales University, Center Valley, PA, USA
Michael Slifker, Department of Biostatistics and Bioinformatics, Fox Chase Cancer Center, Philadelphia, PA, USA.
Andrea Manni, Department of Medicine, Pennsylvania State University College of Medicine, Hershey Medical Center, Hershey, PA, USA.
Jose Russo, Breast Cancer Research Laboratory, Fox Chase Cancer Center, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
References
- Anti M, Marra G, Armelao F, Bartoli GM, Ficarelli R, Percesepe A, De Vitis I, Maria G, Sofo L, Rapaccini GL, et al. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology. 1992;103:883–891. doi: 10.1016/0016-5085(92)90021-p. [DOI] [PubMed] [Google Scholar]
- Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, Thomas MJ, Thornburg T, Kulik G, Smith A, Edwards IJ, D’Agostino R, Zhang H, Wu H, Kang JX, Chen YQ. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117:1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckaert V, Ulmann L, Mimouni V, Antol J, Brancquart L, Chenais B. Docosahexaenoic acid intake decreases proliferation, increases apoptosis and decreases the invasive potential of the human breast carcinoma cell line MDA-MB-231. Int J Oncol. 2010;36:737–742. doi: 10.3892/ijo_00000549. [DOI] [PubMed] [Google Scholar]
- Carroll KK, Braden LM. Dietary fat and mammary carcinogenesis. Nutr Cancer. 1984;6:254–259. doi: 10.1080/01635588509513831. [DOI] [PubMed] [Google Scholar]
- Chajes V, Hulten K, Van Kappel AL, Winkvist A, Kaaks R, Hallmans G, Lenner P, Riboli E. Fatty-acid composition in serum phospholipids and risk of breast cancer: an incident case-control study in Sweden. Int J Cancer. 1999;83:585–590. doi: 10.1002/(sici)1097-0215(19991126)83:5<585::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr Opin Gastroenterol. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- Gago-Dominguez M, Yuan JM, Sun CL, Lee HP, Yu MC. Opposing effects of dietary n-3 and n-6 fatty acids on mammary carcinogenesis: the Singapore Chinese Health Study. Br J Cancer. 2003;89:1686–1692. doi: 10.1038/sj.bjc.6601340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MD, Hunter DJ, Colditz GA, Stampfer MJ, Hankinson SE, Speizer FE, Rosner B, Willett WC. Association of dietary intake of fat and fatty acids with risk of breast cancer. JAMA. 1999;281:914–920. doi: 10.1001/jama.281.10.914. [DOI] [PubMed] [Google Scholar]
- Huang Y, Fernandez SV, Goodwin S, Russo PA, Russo IH, Sutter TR, Russo J. Epithelial to mesenchymal transition in human breast epithelial cells transformed by 17beta-estradiol. Cancer Res. 2007;67:11147–11157. doi: 10.1158/0008-5472.CAN-07-1371. [DOI] [PubMed] [Google Scholar]
- Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, Nagy L, Yamamoto K, Schwabe JW. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol. 2008;15:924–931. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Murota I, Yoshikai K, Yamada S, Yamamoto K. Synthesis of docosahexaenoic acid derivatives designed as novel PPARgamma agonists and antidiabetic agents. Bioorg Med Chem. 2006;14:98–108. doi: 10.1016/j.bmc.2005.07.074. [DOI] [PubMed] [Google Scholar]
- Kaizer L, Boyd NF, Kriukov V, Tritchler D. Fish consumption and breast cancer risk: an ecological study. Nutr Cancer. 1989;12:61–68. doi: 10.1080/01635588909514002. [DOI] [PubMed] [Google Scholar]
- Kang KS, Wang P, Yamabe N, Fukui M, Jay T, Zhu BT. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS One. 2010;5:e10296. doi: 10.1371/journal.pone.0010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Sandler DP, Galanko J, Martin C, Sandler RS. Intake of polyunsaturated fatty acids and distal large bowel cancer risk in whites and African Americans. Am J Epidemiol. 2010;171:969–979. doi: 10.1093/aje/kwq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- Liu YE, Pu W, Wang J, Kang JX, Shi YE. Activation of Stat5 and induction of a pregnancy-like mammary gland differentiation by eicosapentaenoic and docosapentaenoic omega-3 fatty acids. FEBS J. 2007;274:3351–3362. doi: 10.1111/j.1742-4658.2007.05869.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murphy RA, Mourtzakis M, Mazurak VC. n-3 polyunsaturated fatty acids: the potential role for supplementation in cancer. Curr Opin Clin Nutr Metab Care. 2012;15:246–251. doi: 10.1097/MCO.0b013e328351c32f. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Minami M, Yagasaki R, Kinoshita K, Earashi M, Kitagawa H, Taniya T, Miyazaki I. Chemoprevention of DMBA-induced mammary carcinogenesis in rats by low-dose EPA and DHA. Br J Cancer. 1997;75:348–353. doi: 10.1038/bjc.1997.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Veigas JM, Williams PJ, Fernandes G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Res Treat. 2013;141:341–352. doi: 10.1007/s10549-013-2703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravacci GR, Brentani MM, Tortelli T, Jr, Torrinhas RS, Saldanha T, Torres EA, Waitzberg DL. Lipid raft disruption by docosahexaenoic acid induces apoptosis in transformed human mammary luminal epithelial cells harboring HER-2 overexpression. J Nutr Biochem. 2013;24:505–515. doi: 10.1016/j.jnutbio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Rovito D, Giordano C, Vizza D, Plastina P, Barone I, Casaburi I, Lanzino M, DeAmicis F, Sisci D, Mauro L, Aquila S, Catalano S, Bonofiglio D, Ando S. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARgamma activation in MCF-7 breast cancer cells. J Cell Physiol. 2013;228:1314–1322. doi: 10.1002/jcp.24288. [DOI] [PubMed] [Google Scholar]
- Russo J, Balogh GA, Chen J, Fernandez SV, Fernbaugh R, Heulings R, Mailo DA, Moral R, Russo PA, Sheriff F, Vanegas JE, Wang R, Russo IH. The concept of stem cell in the mammary gland and its implication in morphogenesis, cancer and prevention. Front Biosci. 2006a;11:151–172. doi: 10.2741/1788. [DOI] [PubMed] [Google Scholar]
- Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH. 17-Beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J. 2006b;20:1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol. 2003;87:1–25. doi: 10.1016/s0960-0760(03)00390-x. [DOI] [PubMed] [Google Scholar]
- Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, Carughi A, Perelman D, Kanaoka Y, Sangiovanni JP, Gronert K, Smith LE. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3:69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137:548–553. doi: 10.1093/jn/137.3.548. [DOI] [PubMed] [Google Scholar]
- Signori C, DuBrock C, Richie JP, Prokopczyk B, Demers LM, Hamilton C, Hartman TJ, Liao J, El-Bayoumy K, Manni A. Administration of omega-3 fatty acids and Raloxifene to women at high risk of breast cancer: interim feasibility and biomarkers analysis from a clinical trial. Eur J Clin Nutr. 2012;66:878–884. doi: 10.1038/ejcn.2012.60. [DOI] [PubMed] [Google Scholar]
- Signori C, El-Bayoumy K, Russo J, Thompson HJ, Richie JP, Hartman TJ, Manni A. Chemoprevention of breast cancer by fish oil in preclinical models: trials and tribulations. Cancer Res. 2011;71:6091–6096. doi: 10.1158/0008-5472.CAN-11-0977. [DOI] [PubMed] [Google Scholar]
- Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- Stripp C, Overvad K, Christensen J, Thomsen BL, Olsen A, Moller S, Tjonneland A. Fish intake is positively associated with breast cancer incidence rate. J Nutr. 2003;133:3664–3669. doi: 10.1093/jn/133.11.3664. [DOI] [PubMed] [Google Scholar]
- Sun H, Berquin IM, Owens RT, O’Flaherty JT, Edwards IJ. Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008;68:2912–2919. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatten LJ, Solvoll K, Loken EB. Frequency of meat and fish intake and risk of breast cancer in a prospective study of 14,500 Norwegian women. Int J Cancer. 1990;46:12–15. doi: 10.1002/ijc.2910460105. [DOI] [PubMed] [Google Scholar]
- Yee LD, Lester JL, Cole RM, Richardson JR, Hsu JC, Li Y, Lehman A, Belury MA, Clinton SK. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr. 2010;91:1185–1194. doi: 10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuri T, Danbara N, Tsujita-Kyutoku M, Fukunaga K, Takada H, Inoue Y, Hada T, Tsubura A. Dietary docosahexaenoic acid suppresses N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats more effectively than eicosapentaenoic acid. Nutr Cancer. 2003;45:211–217. doi: 10.1207/S15327914NC4502_11. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou L, Shi W, Song N, Yu K, Gu Y. A mechanism underlying the effects of polyunsaturated fatty acids on breast cancer. Int J Mol Med. 2012;30:487–494. doi: 10.3892/ijmm.2012.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]