Abstract
Patient: Female, 40
Final Diagnosis: Gout
Symptoms: Joint pain
Medication: —
Clinical Procedure: Dual energy Computed tomography
Specialty: Rheumatology
Objective:
Rare co-existance of disease or pathology
Background:
Gout is characterized by deposition of uric acid crystals (monosodium urate) in tissues and fluids. This can cause acute inflammatory arthritis. The 2015 ACR/EULAR criteria for the diagnosis of gout include dual energy computed tomography (DECT)-demonstrated monosodium urate crystals as a new criterion. DECT is a spectral decomposition that permits recognition of different types of tissues based on their characteristic energy-dependent photon attenuation. A positive scan is defined as the presence of urate at articular or periarticular sites.
Case Report:
We describe a 51-year-old woman known to have anorexia nervosa. During our clinical examination, we detected plenty of tophi on both hands, but no swollen joints. The diagnosis of gout was made by visualizing crystals in a biopsy from a tophus. The first line of treatment was allopurinol, the second line was rasburicase, and the current treatment is febuxostat 80 mg/day, allopurinol 300 mg twice a day, and colchicine 0.5 mg twice a day. The patient has unchanged arthralgia and the size and number of tophi remain the same as before treatment in spite of active treatment for 3 years. Previously the patient had problems with adherence, but now she claims that she follows the proposed treatment. The last plasma urate (P-urate) was 0.57 mmol/L. Following two years of treatment, DECT of hands visualized monosodium urate crystal deposits in the tophi, as seen on the clinical photos, but also crystals in relation to the tendons and soft tissue.
Conclusions:
DECT is an imaging modality useful to assess urate crystal deposits at diagnosis of gout and could be considered during treatment evaluation. Lack of adherence to treatment should be considered when P-urate values vary significantly and when DECT scans over years persistently visualize monosodium urate crystals.
MeSH Keywords: Absorptiometry, Photon; Anorexia Nervosa; Gout
Background
Gout is the most common inflammatory arthritis among men and postmenopausal women with a prevalence between 2% and 4% [1]. Women represent 5–20% of patients with gout [2]. The prevalence of hyperuricemia (a precondition of gout) is more than 21% [3]. Gout is characterized by deposition of uric acid crystals (monosodium urate) in tissues and synovial fluids. This can cause an acute inflammatory arthritis characterized by sudden, severe attacks of pain, redness, and inflammation in joints. Gout initially affects only one joint at a time, but over time, it can become chronic and could affect several joints. Gout is one of the most painful forms of arthritis and is the cause of disability for many [4,5]. Tophaceous gout is a terminal manifestation of gout [6]. Tophaceous gout in a patient with anorexia nervosa is very seldom described in the literature [2].
Microscopic examination by polarized compensated light microscopy of the joint fluid or of tophi is the gold standard for verifying the diagnosis [7]. The 2015 ACR/EULAR criteria for the diagnosis of gout include dual energy computed tomography (DECT)-demonstrated monosodium urate crystals as a criterion. If monosodium urate crystals are not found in synovial fluids or tophi, the diagnosis of gout can be based on the entry criterion followed by a scoring system. The entry criterion is at least one characteristic attack of gout in a peripheral joint or bursa as mentioned above. The scoring system includes three major domains: clinical features, laboratory measurements, and imaging evidence. A score ≥8 is diagnostic for gout. Detection of monosodium urate crystals with DECT yields 4 points. Four extra points can be added if gout-related joint damage can be visualized [5,7].
DECT has spurred interest as a noninvasive examination for detection of, for example, urinary stone composition, hepatic fatty infiltration, and several cancers [8–10]. A DECT scanner uses simultaneously a regular X-ray and a second X-ray with lower energy, typically a setting of 80 kVp and 140 kVp, which gives more possibilities than standard computed tomography (CT) [5,11]. The greatest benefit of DECT is spectral decomposition that permits recognition of different types of tissues, based on their characteristic energy-dependent photon attenuation [5,8–10].
In previous studies, DECT imaging has shown to be a useful noninvasive tool to confirm the presence of monosodium urate crystals within joints, tophi, and tendons [3]. A positive scan is defined as the presence of urate at articular or periarticular sites.
Case Report
The patient is a 51-year-old Caucasian woman known to have had anorexia nervosa since her teenage years [12]. For several years, she was followed at the Department of Nutrition Disorders. Four years before our first contact with the patient, a tentative diagnosis of gout was made by her private practitioner. He visualized needle-shaped crystals in a biopsy from a skin lesion at a toe tip. Before admission, she was treated with allopurinol in varying doses, but probably with varying adherence.
She was sent to the hospital’s diagnostic unit because of weight loss, which was found to be associated with her anorexia (down to 38.5 kg), and arthralgia. Malignant disease was ruled out, and the patient was referred to the Department of Rheumatology.
Clinical examination demonstrated plenty of tophi on both hands (Figure 1), but no swollen joints. The patient could elaborate on her medical history and tell that small hard nodules localized in the skin of the fingers often broke and subsequently flowed with toothpaste-like material.
Figure 1.
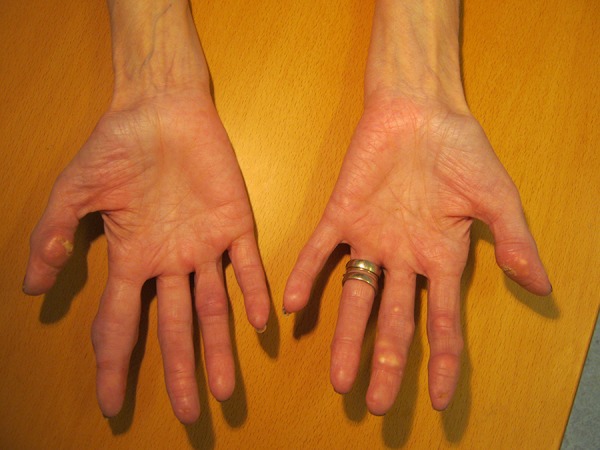
Clinical photos of the patient’s hands after two years of treatment.
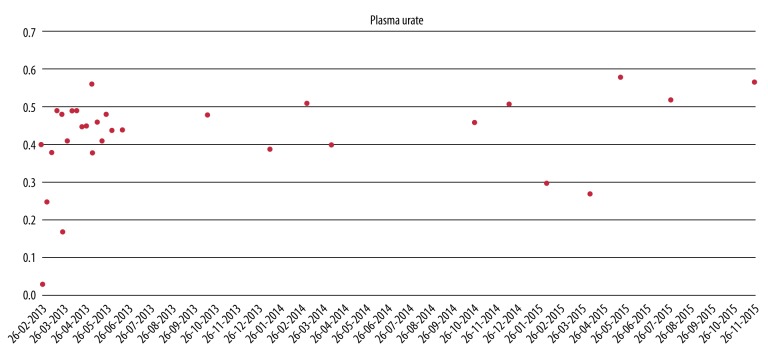
Plasma urate (P-urate) was normal, 0.37 mmol/L, at first visit (Figure 2). Estimated glomerular filtration rate (GFR) was 60 mL/min/1.73 m2, and C-reactive protein (CRP) was <10 mg/L. The patient was rheumatoid factor and cyclic citrullinated peptide antibody negative. Biochemistry was as follows: serum urate varied considerably during the following three years from 0.03 to 0.59 (0.16–0.40), as well as albumin from 16 to 33 g/L (36–48 g/L), potassium from 1.8 to 5.6 mmol/L (3.5–4.4 mmol/L), and magnesium from 0.49 to 0.71 mmol/L (0.71–0.94 mmol/L). Microscopic examination of material aspirated from a tophus confirmed the gout diagnosis.
Figure 2.
Plasma urate changes over time with different treatments. Second-line treatment with rasburicase from February until May 2013. Treatment with allopurinol and colchicine from May until October 2014. Third-line treatment with febuxostat since October 2014.
X-ray of the patient’s feet showed considerable arthritis; hands were normal without arthritis.
CT scan of thorax and abdomen showed no signs of malignant disease, cholecystectomia facta, pancreatitis sequela, or arteriosclerosis aortae.
Dual-energy x-ray absorptiometry (DEXA) scan results were as follows: T score Columna –4.2, collum femoris –3.7. A DECT HD 750 General Electric CT scanner was used to perform an initial scan of the hands and feet, and after 1 and 2 years of treatment visualized nonsignificant changes in the amount of monosodium urate crystal deposits in the tophi seen on the clinical photos, but also crystals in relation to the tendons and soft tissue of the hands. The localization of the color coding corresponded to the localization of the tophis seen on the clinical picture. By measuring the DECT z-values in different crystals, we obtained a mean value of 7.31±0.21. In comparison, the calculated z-value for the urate crystals was 6.95. Comparing the results from the DECT scans, we saw no obvious differences before and after two years of treatment (Figures 3, 4).
Figure 3.
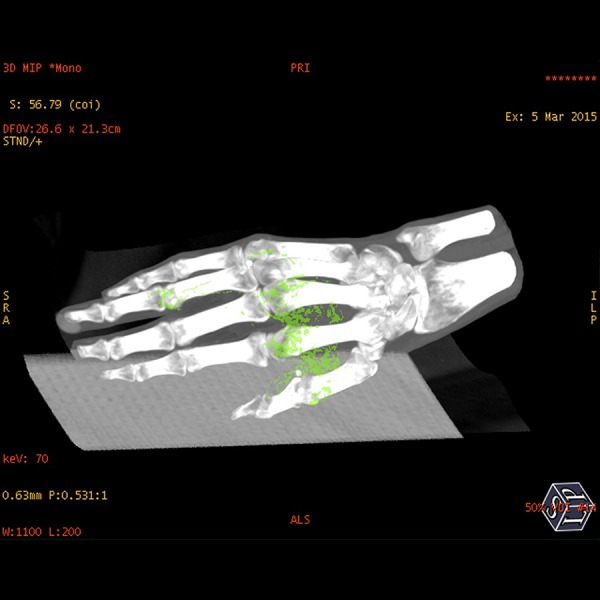
DECT scan after two years of treatment.
Figure 4.
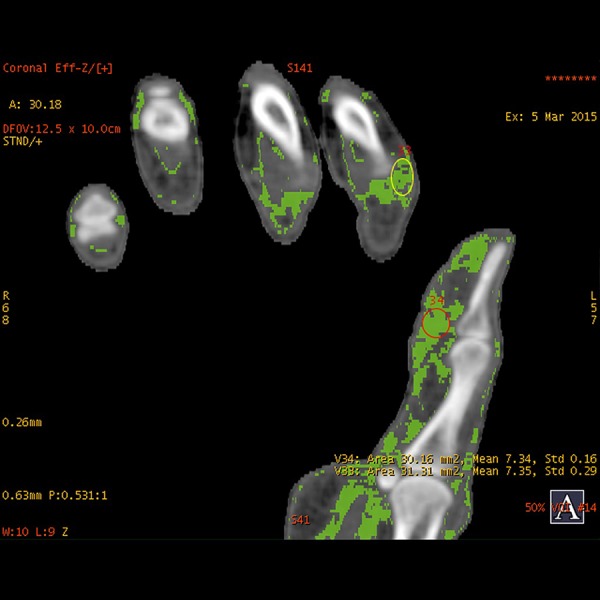
DECT scan after two years of treatment.
The patient restarted treatment with allopurinol without sufficient effect; P-urate increased to 0.48 mmol/L. The second line of treatment was rasburicase, a recombinant urate oxidase.
The patient was given rasburicase 7.5 mg intravenously, first with a dose a day for 2 days and then once a week. She was treated with rasburicase for 3 months.
The patient developed intolerance to rasburicase, and the treatment was discontinued. After the first two treatments with rasburicase, the P-urate fell to 0.03 mmol/L, but after one month it was back to 0.48 mmol/L.
A second attempt was made to treat the patient with allopurinol 500 mg/day and colchicine 0.5 mg/day. The treatment was still not sufficient to keep the P-urate at the target level of 0.30 mmol/L and to prevent the arthralgia.
Since October 2014, the patient has been treated with febuxostat 80 mg/day, a xanthine oxidase inhibiter, combined with allopurinol 300 mg twice a day and colchicine 0.5 mg twice a day. Blood samples initially showed a decline in P-urate, the lowest level down to 0.27 mmol/L, but lately levels have increased to more than 0.57 mmol/L
The patient complains that after three years of treatment her arthralgia is unchanged. It is estimated that the numbers and sizes of tophi are unchanged as well. From older files we noticed that the patient had problems with adherence, but she claims that she now follows the proposed treatment.
Discussion
Treatment of patients with anorexia nervosa can be very difficult, as this case illustrates, and adherence might be problematic [13]. P-urate was normal initially in our patient. DECT was used to visualize monosodium urate crystals in soft tissues, joints, and tendons. The relative importance of DECT is yet unknown, but it can differentiate between pyrophosphate crystals and monosodium urate crystals [14], and it is a useful tool in evaluating diagnosis and the extent of diseases in patients who present with arthralgia and/or arthritis symptoms. DECT can thereby be an especially important tool in the detection of subclinical disease [5]. To our knowledge, this is the first time monosodium urate crystals have been visualized by DECT in a patient diagnosed with anorexia nervosa.
In our case DECT was valuable in the detection of monosodium urate deposition. At this point, the greatest challenge regarding DECT and its use in the diagnosis and monitoring of gout treatment is the fact that DECT is very user dependent. The patient in this case study did not have arthritis but arthralgia, and for the purpose of considering differential diagnoses in this patient, DECT was valuable [14].
For differential diagnosis purposes, malignancy had to be ruled out, especially because of simultaneous weight loss, as in this case. Other differential diagnoses could be small cutaneous abscesses, tendinitis, or pyrophosphate arthritis.
The z-values in our visual tophi were comparable to urate crystal composition. Despite the absence of gout attack/flares and no improvement in arthralgia, the diagnosis of gout was supported by DECT.
As concluded in an article by Hu et al. [11], the results of a DECT scan should be interpreted carefully because of false findings. Predefined setting in DECT increases the risk for false- positive and false-negative results. More studies have to be made for better determination of the parameters.
Bongartz et al. [15] concluded that DECT has a high specificity and sensitivity for diagnosis of gout, particularly when it is not possible to detect needle-shaped crystals with microscopy and thereby confirm the diagnosis. However, they found reduced sensitivity for early-onset gout.
In evaluating the treatment response of tophaceous gout, we suggest that besides counting the number of tophi and repeating the DECT scan, clinicians use the newly introduced patient-reported outcome measure of tophus burden: The Tophus Impact Questionnaire [16].
Conclusions
DECT is an imaging modality useful to assess urate crystal deposits at diagnosis of gout and could have a place during treatment evaluation.
Lack of adherence to treatment should be considered when P-urate values vary significantly and monosodium urate crystals are visualized by DECT scan, especially if the scans do not change significantly after years of treatment. Getting a sufficient treatment response in patients with anorexia nervosa and gout might be very difficult.
Acknowledgments
Petur Weihe and Jess Lambrechtsen are thanked for their inspiration to do this work.
Footnotes
Conflict of interest
The authors hereby declare that there is no conflict of interest between them and any third party.
References:
- 1.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125(7):679–87.e1. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 2.Kita K, Kawabuchi MKN, Nagagaito H, Koura T, Yasmashiro S. Tophaceous gout in anorexia nervosa. General Medicine. 2013;1:61–63. [Google Scholar]
- 3.Mikuls TR, Farrar JT, Bilker WB, et al. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis. 2005;64(2):267–72. doi: 10.1136/ard.2004.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor WJ, Grainger R. Chapter 9 – Clinical features of gout. In: Terkeltaub R, editor. Gout & Other Crystal Arthropathies [Internet] Philadelphia: W.B. Saunders; 2012. pp. 105–20. [cited 2015 Nov 18] Available from: http://www.sciencedirect.com/science/article/pii/B9781437728644100090. [Google Scholar]
- 5.Fritz J, Henes JC, Fuld MK, et al. Dual-energy computed tomography of the knee, ankle, and foot: noninvasive diagnosis of gout and quantification of monosodium urate in tendons and ligaments. Semin Musculoskelet Radiol. 2016;20(1):130–36. doi: 10.1055/s-0036-1579709. [DOI] [PubMed] [Google Scholar]
- 6.Chhana A, Dalbeth N. The gouty tophus: A review. Curr Rheumatol Rep. 2015;17(3):19. doi: 10.1007/s11926-014-0492-x. [DOI] [PubMed] [Google Scholar]
- 7.Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74:1789–98. doi: 10.1136/annrheumdis-2015-208237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boellaard TN, Henneman OD, Streekstra GJ, et al. The feasibility of colorectal cancer detection using dual-energy computed tomography with iodine mapping. Clin Radiol. 2013;68(8):799–806. doi: 10.1016/j.crad.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Hur J, Kim YJ, et al. Additional value of dual-energy CT to differentiate between benign and malignant mediastinal tumors: An initial experience. Eur J Radiol. 2013;82(11):2043–49. doi: 10.1016/j.ejrad.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 10.Shi JW, Dai HZ, Shen L, Xu DF. Dual-energy CT: Clinical application in differentiating an adrenal adenoma from a metastasis. Acta Radiol. 2014;55(4):505–12. doi: 10.1177/0284185113501660. [DOI] [PubMed] [Google Scholar]
- 11.Hu HJ, Liao MY, Xu LY. Clinical utility of dual-energy CT for gout diagnosis. Clin Imaging. 2015;39(5):880–85. doi: 10.1016/j.clinimag.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Weihe JP, Morrillon MB, Lambrechtsen J, Hansen IMJ. Dual energy CT (DECT) imaging of tophi and monosodium urate deposits in a patient with longstanding Anorexia nervosa. Scan J Rheumatol. 2014;9(43):73–74. [Google Scholar]
- 13.Towell DB, Woodford S, Reid S, et al. Compliance and outcome in treatment–resistant anorexia and bulimia: A retrospective study. Brittish Clin Psycho. 2001;40:189–95. doi: 10.1348/014466501163634. [DOI] [PubMed] [Google Scholar]
- 14.Ward IM, Scott JN, Mansfield LT, Battafarano DF. Dual-energy computed tomography demonstrating destructive calcium pyrophosphate deposition disease of the distal radioulnar joint mimicking tophaceous gout. J Clin Rheumatol. 2015;21(6):314–17. doi: 10.1097/RHU.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 15.Bongartz T, Glazebrook KN, Kavros SJ, et al. Dual-energy CT for the diagnosis of gout: an accuracy and diagnostic yield study. Ann Rheum Dis. 2015;74(6):1072–77. doi: 10.1136/annrheumdis-2013-205095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aati O, Taylor WJ, Siegert RJ, et al. Development of a patient-reported outcome measure of tophus burden: The Tophus Impact Questionnaire (TIQ-20) Ann Rheum Dis. 2015;74:2144–50. doi: 10.1136/annrheumdis-2014-205671. [DOI] [PubMed] [Google Scholar]