Abstract
A target presented on a background of dynamic noise disappears from awareness after a few seconds of maintained peripheral viewing. Whereas the effects of bottom-up factors in such filling-in are well documented, the roles of different top-down functions remain relatively unexplored. Here, we investigated the roles of attention and working memory (WM) by manipulating load in concurrent tasks while participants reported filling-in of a peripheral target. In Experiment 1, increasing perceptual load reduced the probability of filling-in and increased the latency of its occurrence. In Experiment 2, increasing WM load shortened the time before filling-in occurred – the opposite effect to increasing perceptual load. These results demonstrate that different top-down functions may have dissociable effects on filling-in.
Keywords: filling-in, perceptual completion, artificial scotoma, attention, working memory, load theory, perceptual load, working memory load
Introduction
A peripheral figure on a background of dynamic noise seems to disappear after a few seconds of maintained peripheral viewing, seemingly filled-in by the dynamic background (Ramachandran and Gregory, 1991). This creates an artificial scotoma, where the physically present figure – the target – is no longer consciously perceived. Filling-in modulates signals in retinotopic visual cortex (De Weerd et al., 1995;Weil et al., 2008), suggesting a low-level phenomenon driven primarily by bottom-up sensory factors; and bottom-up factors like eccentricity, boundary length (De Weerd et al., 1998) and luminance (Welchman and Harris, 2001) affect the time it takes (latency) for filling-in to occur.
In contrast, the roles of various top-down factors in filling-in remain relatively unexplored. Directing spatial attention to the target increases the probability of filling-in (De Weerd et al., 2006), supporting theoretical accounts viewing filling-in as an active process, rather than as a result of passive ignoring that leads to loss of neural representation (Dennett, 1991). But directing spatial attention to a specific location over others is not the only top-down manipulation that can modulate perception. The roles of different top-down functions can be elucidated by manipulating them independently.
Here, we independently manipulated the load placed on two different top-down functions to investigate their roles in the perception of an artificial scotoma. We manipulated either perceptual (Experiment 1) or working memory (WM) load (Experiment 2), and examined how these affected the probability and latency of filling-in. Similar manipulations of perceptual (Rees et al., 1997;Schwartz et al., 2005;Bahrami et al., 2008;van Boxtel et al., 2010) and WM load (de Fockert et al., 2001;Lavie et al., 2004;Dalton et al., 2009a;Dalton et al., 2009b) have so far been used mostly to investigate processing of task-irrelevant stimuli, and are grounded in a theoretical framework known as the load theory of selective attention and cognitive control (Lavie, 2005;Lavie et al., 2004).
Load theory proposes that increasing the load of a perceptual task will consume processing capacity, reducing resources available to process stimuli irrelevant to that task. Both behavioral (Lavie and de Fockert, 2003;Bahrami et al., 2008) and neuroimaging studies (Schwartz et al., 2005;Bahrami et al., 2007) have consistently shown that increasing perceptual load reduces processing of stimuli outside the focus of attention. Load may thus determine the way attention is distributed spatially, but increased perceptual load does not simply entail a spatial narrowing of perceptual capacity: although increasing load at fixation reduces perception in peripheral locations (Lavie, 1995;Schwartz et al., 2005), increasing load in a peripheral ring-shaped area impairs processing of fixated stimuli (Beck and Lavie, 2005;Carmel et al., 2007) as well as stimuli further out from the attended area (Macdonald and Lavie, 2008).
If filling-in is an active process requiring attentional resources, then reducing the availability of such resources for processing the target and background, by increasing the perceptual load of a concurrent task at fixation, should lead to a lower probability of filling-in and delay its onset. (Note that with such a manipulation, the comparison is not between filling-in at attended versus unattended locations, as both the attended location and the location of the filled-in stimulus are kept constant. Rather, the manipulation modulates the extent to which processing resources are exhausted by a concurrent task; cf (De Weerd et al., 2006)). If, however, filling-in is a form of passive ignoring (Dennett, 1991), opposite effects should occur, because reduced attentional resources should exacerbate loss of representation.
Load theory contrasts perceptual and WM load, proposing that WM is part of the executive control mechanism that maintains processing priorities when different stimuli compete for perceptual resources. Hence, exhausting the capacity of WM reduces the ability to maintain prioritization of current behavioral goals, leading to more (rather than less, as with perceptual load) processing of task-irrelevant stimuli. This prediction has been supported by studies demonstrating increased interference from irrelevant distractors under high WM load (Lavie et al., 2004;de Fockert et al., 2001). Filling-in involves no distractors, but we hypothesized that since the background competes with the target, increasing WM load in a concurrent task may disrupt the differential allocation of processing resources to the target compared to the background. This would be similar to decreasing the relative contrast between target and background, which leads to faster filling-in (Spillman and De Weerd, 2003;Sturzel and Spillmann, 2001), and we would thus expect increased WM load to lead to a shorter latency and increased probability of filling-in. Alternatively, if WM served only to divert attention from the filling-in task, increasing WM load would, like perceptual load, delay and reduce the probability of filling-in.
Importantly, by comparing the effects of two different concurrent tasks, we controlled for any general effects that performing a concurrent task may have on filling-in (Macdonald and Lavie, 2008).
Methods
Participants
Twelve volunteers participated in experiment 1 (mean age 27.8 years, range 19-37 years, 5 female) and ten participated in experiment 2 (mean age 26.6 years, range 21-32 years, 4 female). All had normal or corrected-to-normal vision and gave written informed consent to participate in the study, which was approved by the local ethics committee.
Stimuli
Visual stimuli were presented on a CRT display (21” Sony GDM-F520; 800x600 resolution; 60Hz refresh rate), in a darkened room. A chin rest ensured a fixed viewing distance of 57cm. Stimulus display and response collection were controlled by Matlab 6.5.1 (Mathworks Inc.) using the COGENT 2000 toolbox (www.vislab.ucl.ac.uk/Cogent2000/index.html). In experiment 2 participants wore headphones.
Stimuli consisted of full-field random dynamic achromatic noise (33º x 25º; mean luminance 23.3cd/m2) and a flickering peripheral target. To generate random dynamic noise, we created 30 arrays of 200x200 pixels. The arrays were presented in random order at the screen refresh rate (60Hz). The target was a small flickering achromatic square (1.12º x 1.12º) superimposed on the background in the lower left visual field at 9.43º eccentricity (8º across, 5º down) flickering between black (luminance 0.51cd/m2) and white (luminance 80.9cd/m2) at a rate of 7.5Hz. The target was presented in the lower half of the visual field, where filling-in is more robust (Mendola et al., 2006), and flickered to avoid stimulus-contingent after-effects.
Perceptual filling-in procedure
On each trial, participants were presented with dynamic noise. After 3s, a flickering target appeared in the periphery. Participants were instructed to fixate centrally and to indicate the appearance of the peripheral figure using three different keys on a standard PC keyboard. When the figure first appeared they were required to press one key. If the figure disappeared (through filling-in), participants pressed a second key. Participants indicated any re-appearance of the figure by a third key-press. These key-presses were used to define the reaction times (RTs) to the appearance of the target at the beginning of each trial, and the latency of filling-in. The probability of filling-in was defined as the proportion of trials during which the target was filled-in for at least 1000ms. Participants were instructed to be conservative in their responses and only report filling-in once the target had completely disappeared.
Trials lasted 15 seconds and were followed by a 500ms interval during which a grey screen (luminance 21.8cd/m2) was presented. Trial blocks comprised 30 trials and participants received quantitative feedback at the end of every block consisting of the percentage of trials where they had reported filling-in for longer than one second. Participants completed 4 blocks in experiment 1 and 2 blocks in experiment 2. They were encouraged to blink between trials and during breaks between blocks, but were not told to abstain from blinking during the rest of the experiment. All participants received training prior to testing, to ensure they could experience filling-in and assign consistent responses to the different perceptual states.
Eye movement recording and analysis
During testing, eye position and pupil diameter were continuously sampled at 250Hz using a high-speed video eye tracker (Cambridge Research Systems Ltd). Blinks and periods of signal loss were removed from the data. Deviations away from fixation of greater than 10 degrees were considered as artefacts and removed. Eye position (defined as the mean deviation of the eye from fixation) and eye movements (defined as the number of saccades greater than 1.3 deg per trial) were then computed for each participant under each load (low/high) and visibility condition (peripheral target visible/filled-in). Repeated-measures ANOVAs established whether these measures differed between the experimental conditions.
Experiment 1 - Perceptual Load
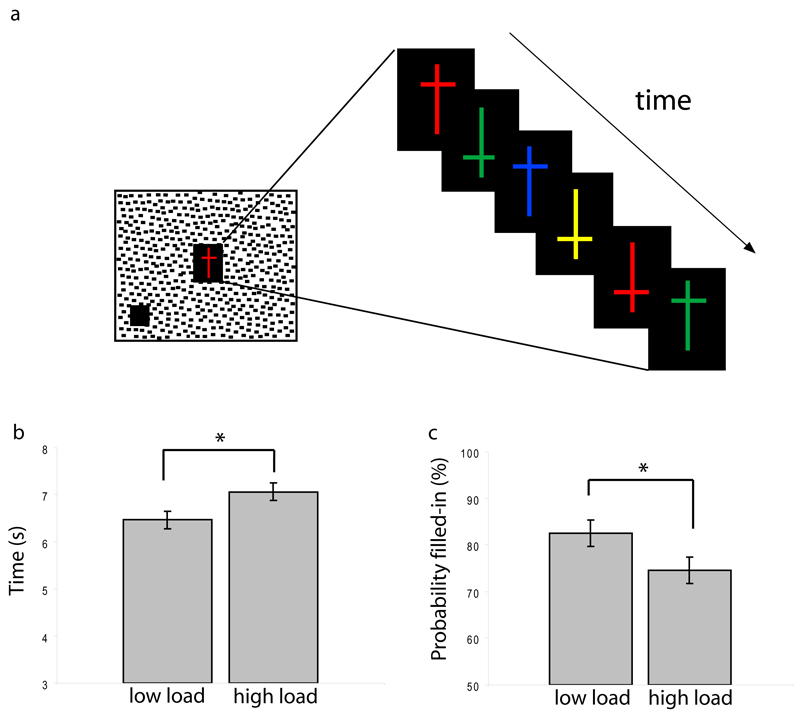
While participants monitored the appearance and filling-in of the peripheral figure, they concurrently performed a perceptual task previously shown to effectively manipulate perceptual load (Schwartz et al., 2005;Bahrami et al., 2008;van Boxtel et al., 2010). This task required a serial visual search on a stream of targets presented at fixation. Crosses spanning 0.52º (vertical line) by 0.22º (horizontal line) were presented at fixation on a rectangular black background (1.3º x 1º). Crosses could appear in any of six colors (red, green, yellow, blue, cyan and purple) and two orientations (upright or inverted – the horizontal line of the cross was placed 0.25º above or below the centre of the vertical line; Figure 1a). Each cross was displayed for 250ms, followed by a blank period of 500ms before the appearance of the next cross. For identical stimulus parameters, participants performed either a low perceptual load feature search (responding to red crosses among other cross colors), or a high perceptual load conjunction search (responding to either upright yellow or inverted green crosses, but not the opposite conjunctions). Participants responded with a button-press whenever a cross target was detected. Half of the participants used their left hand to respond to the fixation task and their right hand to report filling-in; this was switched for the other participants.
Figure 1. Experiment 1: Effect of perceptual load on filling-in.
(a) Procedure. Participants reported the appearance and subsequent filling-in of a peripheral achromatic figure, using key-presses. The figure flickered between black and white. At the same time, participants viewed a series of central crosses and responded to the appearance of cross targets defined either by their color (red crosses, low perceptual load) or color and orientation (upright yellow or inverted green crosses, high perceptual load). (b,c) Results. Increasing perceptual load increased the latency of filling-in (b), and reduced its probability (c). Data are averaged across twelve participants. Error bars represent 1 standard error of the mean difference. * p<.05, 2-tailed t-test.
Note that load theory (Lavie, 2005) defines an increase in perceptual load as an increase in either the number of stimuli or the number of stimulus features that have to be processed in order to detect a target. The present task entailed maintaining a more complex target template in memory under high load, but by the above definition this imposed a higher level of perceptual load because it increased the amount of processing required to decide whether each stimulus seen at fixation was a target. In contrast, the WM load manipulation of Experiment 2 (described below) increased load on WM by increasing the number of entirely task-irrelevant items that had to be kept while performing the task.
Experiment 2 - WM Load
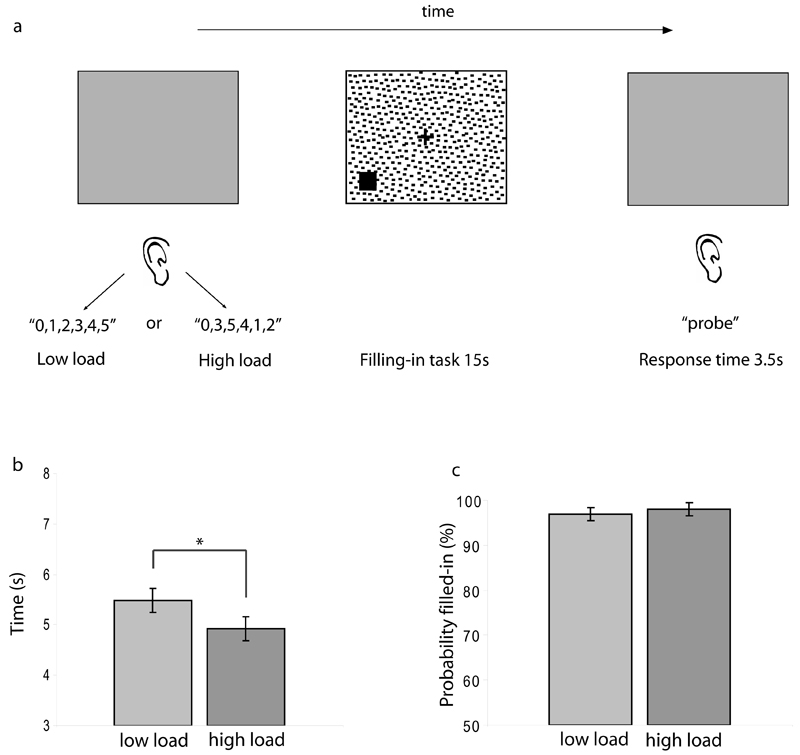
Each trial began with presentation of a grey screen with a central red fixation cross. Participants listened to a sequence of five numbers (1 to 5), always preceded by “zero”, presented through headphones. The number sequence was presented in either ascending order (low WM load) or in random order (high WM load). This was followed by the 15-second filling-in task, which was identical to that in experiment 1. Unlike experiment 1, there was no task at fixation; participants fixated on a small static red cross (0.4o x 0.4o; Figure 2a). At the end of the filling-in task, participants were presented with a grey screen and heard the word ‘probe’ followed by a digit chosen randomly from the original memory set. They then had 3.5 seconds to report the digit that followed the probe in the original memory set by pressing the appropriate key on the keyboard’s number pad. The last digit in the memory set was never the probe and all sets began with “0” to ensure that all five digits between 1 and 5 were used as responses in both conditions. A new trial began at the end of the response period. This WM load manipulation has been used in previous studies (de Fockert et al., 2001;Dalton et al., 2009a;Dalton et al., 2009b), and has the advantage of avoiding a dissimilar set-size confound, which would arise if the alternative method of manipulating the number of digits held in WM had been used. Although the low-load condition imposes relatively low demands on WM, participants were still required to remember which type of trial they were performing. Thus the dual task and response requirements were fully matched across both WM load conditions.
Figure 2. Experiment 2: Effect of WM load on filling-in.
(a) Procedure Participants viewed a grey screen while hearing a sequence of 5 numbers, always preceded by ‘0’, presented in either ascending (low WM load) or random order (high WM load). This was followed by the filling-in task (Methods and Figure 1). Participants then viewed another grey screen and heard the word ‘probe’, followed by a number from the original memory set. They had to report the digit that followed the probe number in the original memory set. (b,c) Results. The effect of high (versus low) WM load on filling-in was in the opposite direction to that of perceptual load: Latency of filling-in was reduced under high WM load (b), but the probability of filling-in was not affected by WM load (c; note, however, that it was already near ceiling under low load). Data are averaged across ten participants. Error bars represent 1 standard error of the mean difference. * p<.05, 2-tailed t-test.
Results
Experiment 1
Perceptual load
The perceptual load manipulation was effective: Mean RTs to target crosses were faster under low (483ms) compared to high perceptual load (568ms; t(11)=7.7, SEM=11.1ms , p<.0005). Mean detection of central targets was better under low (93%) compared to the high load (88%; t(11)=3.2, SEM=1.4%, p=.009), ruling out a speed-accuracy trade-off.
Effect of perceptual load on filling-in
The mean probability of filling-in was reduced under high (75%) compared to low perceptual load (83%; t(11)=2.8, SEM=2.8%, p=.017; Fig. 1b); and mean latency of filling-in was greater under high (7066ms) than under low perceptual load (6468ms; t(11)= 3.1, SEM=191.8ms, p=.01; Fig 1c). Increasing perceptual load therefore reduced the ability to initiate and maintain filling-in.
Mean RTs to the physical appearance of the target were slower under high (1006ms) than under low perceptual load (909ms; t(11)=3.4, SEM=28.2ms, p=.006), but the magnitude of the difference between the time to filling-in under high versus low load was far greater (598ms) than for noting the appearance of the target (96ms). This suggests that although high load does increase RTs to a target, its effect on filling-in cannot be attributed to reaction time alone.
Eye position data
A repeated-measures ANOVA on mean eye deviation from fixation showed a main effect of visibility (target visible or filled-in; F(1,11)=5.57, MSE=0.12 p=.038), indicating that participants’ eyes tended to deviate more from fixation when the target was visible. (Note that this difference in deviation was extremely small – less than 0.3º (Supplementary Fig 1a) – and does not amount to a break from fixation.) There was, however, no main effect of perceptual load (low or high; F(1,11)=0.013, MSE=0.009, ns), and no interaction between visibility and load (F(1,11)=0.98, MSE=0.011, ns), ruling out the possibility that the effects of load on filling-in could be attributed to differences in deviation from fixation. (Supplementary Fig. 1a).
A repeated measures ANOVA on the number of saccades per trial showed a marginal main effect of visibility (F(1,11)=4.6, MSE=0.045, p=.055; Supplemental Fig. 1b ). There was again no significant effects for perceptual load (F(1,11)=0.013, MSE=0.51, ns) or the interaction between visibility and load (F(1,11)=3.58, MSE=0.38, ns).
Experiment 2
WM load
The WM load manipulation was effective: Mean RTs were faster under low (679ms) than under high WM load (1267ms; t(9)=5.6, SEM=106ms , p<.0005); and mean accuracy was better under low (99%) than under high WM load (82%; t(9)=3.2, SEM= 5.4%, p=.01), ruling out a speed-accuracy trade-off.
Effect of WM load on filling-in
The effect of WM load on the latency of filling-in was in the opposite direction to that of perceptual load (Fig 2b). Mean latency (time until filling-in) was shorter for high (4796ms) than for low WM load (5428ms; t(9)=2.36, SEM=237ms, p=.042). In contrast to the effect of perceptual load, there was no difference in the mean probability of filling-in between high (97%) and low WM load (98%; t(9) =.71, SEM=1.5%, ns). There were also no differences in mean RTs to the appearance of the target between high (787ms) and low WM load (1011ms; t(9)=.87, SEM=256ms, ns).
Eye position data
A repeated-measures ANOVA on mean eye deviation from fixation showed no main effects of visibility (target visible or filled-in; F(1,9)=0.069,MSE=0.13, ns) or load (low or high; F(1,9) =0.11,MSE=0.2, ns), and no interaction between visibility and load (F(1,9)=0.94,MSE=0.34, ns; Supplemental Fig. 1c). Similar results were found in an identical ANOVA on the number of saccades per trial (visibility: (F(1,9)=0.92, MSE=0.22, ns; load: (F(1,9) =0.04, MSE=0.56, ns; interaction: F(1,9)=3.9, MSE=0.05, ns; Supplemental Fig. 1d). Thus the effects of WM load on filling-in cannot be attributed to differences in fixation or eye movements.
We also compared the overall eye movements between the two tasks, by comparing the mean eye position and saccades per trial across all conditions, using an independent samples t-test for the perceptual load and WM load manipulation. We found no difference in the mean eye position between the perceptual load experiment (mean deviation from fixation 0.79 deg) and the working memory experiment (1.3 deg, t(11.6)=1.4, SED=0.37, ns) and no difference in the number of saccades per trial between the two experiments (1.0 saccades per trial in the perceptual load experiment, 1.1 in the WM experiment, t(18.4)=0.07, SED=0.71, ns).
Discussion
Manipulations of perceptual and WM load had opposite effects on filling-in of an artificial scotoma: Increasing concurrent perceptual load lowered the probability of filling-in and caused it to occur later, whereas increasing concurrent WM load caused filling-in to occur earlier. Increasing perceptual load and increasing working memory load thus have contrasting effects on the perceptual completion of artificial scotomas. These results are unlikely to be due to shifts of response criteria, as the effect of perceptual load on response times to physical events (target onset) was an order of magnitude smaller than the effect on filling-in (Experiment 1) and there was no effect of working memory load on such response times (Experiment 2). Rather, the results shed light on the different roles that perceptual and WM capacity play in filling-in.
Directing spatial attention to the target’s location has been previously shown to increase the probability of filling-in, compared to directing attention away from it (De Weerd et al., 2006). In Experiment 1 of the present study, participants were always attending to the target to be filled-in, but were concurrently performing a task at fixation that placed either greater (high load) or lesser (low load) demands on attentional resources. Thus, in this dual task setting, the attended locations (the filling-in target and fixation) were kept constant, but exhausting attentional resources made filling-in less likely and later than when more attentional resources were available. Unlike the study by De Weerd et al (2006), our task did not manipulate spatial attention, but instead manipulated the amount of attentional resources available for processing in a secondary task. Importantly, perceptual load is unlikely to simply lead to a narrower focus of attention, as increasing perceptual load in a ring-shaped area can reduce the processing of stimuli at fixation (Beck and Lavie, 2005;Carmel et al., 2007) and further out from the attended area (Macdonald and Lavie, 2008). Furthermore, unlike De Weerd et al (2006), who only found attentional effects on the probability (but not latency) of filling-in, we find effects of perceptual load on both probability and latency. Increasing perceptual load in the manipulation reported here might have exhausted attentional resources more completely than directing attention away from the target (De Weerd et al., 2006).
In this study, we made use of eye-tracking equipment capable of monitoring eye movements and gaze direction. We find that the effects of perceptual and WM load on filling-in observed in both experiments cannot be attributed to differences between low and high load in eye movements. In Experiment 1, there was an effect of target visibility on eye deviation away from fixation, consistent with previous findings that eye movements have an impact on perceptual filling-in of both artificial scotomas (Troncoso et al., 2008;Yokota and Yokota, 2010) and Troxler fading (Martinez-Conde et al., 2006). It is also known that microsaccades are affected by cognitive and attentional factors (Engbert and Kliegl, 2003;Hafed and Clark, 2002), suggesting that the effects of load manipulations could conceivably be mediated by such eye movements. However, the spatial resolution of our eye tracker precluded the accurate measurement of these very small amplitude fixational eye movements. Moreover, the effects of attention on microsaccades are complex, strongly influenced by task factors and thought not to be the cause of attentional shifts in the visual field (see Rolfs, 2009) for a comprehensive review). The complex dependence of microsaccade dynamics on task parameters make it unclear how such a common mechanism could mediate the opposing effects of WM and perceptual load on filling-in. It is not known whether the perceptual and WM manipulations studied here have any effects on the temporal or spatial dynamics of microsaccades or other fixational eye movements, so this may be a promising area for future study.
Increasing perceptual load decreased the probability of filling-in, but increasing WM load had no effect on this measure. However, the probability of filling-in was already at ceiling under low WM load, leaving no room for high WM load to have the opposite effect to perceptual load. To increase the power to detect a difference in probability of filling-in in the WM load task, the filling-in target could be made larger or less eccentric, making filling-in less likely. Nevertheless, in the present study we kept the filling-in stimulus parameters constant for the two manipulations to be able to contrast their effects.
Overall, filling-in tended to be more likely and occurred with a shorter latency for the WM task relative to the perceptual task, regardless of the load imposed. This may be because the perceptual task competed more directly with the filling-in task than the WM task did, as both filling-in and the perceptual task required perceptual judgements. It is also possible the perceptual task was more demanding than the working memory task, and that this may have reduced filling-in. However, there is no good quantitative metric for what constitutes a ‘unit’ of load across different tasks. Therefore, we cannot conclusively attribute the difference in filling-in across experiments to differences in the load levels of the different tasks. In general, it is not currently possible to define absolute levels of load, only relative ones: within any given manipulation, load can be increased or decreased. The magnitude of load increases in the two manipulations of this study are thus not directly comparable. Nonetheless, the results show conclusively that the effect of increasing perceptual load on filling-in was in the opposite direction to that of increasing WM load.
The role of attention in modulating perceptual completion in humans has only rarely been studied in other forms of filling-in. A study of color fading during fixation (Lou, 1999) showed that attended discs were more likely to fade than unattended discs, and a recent study of motion induced blindness (Scholvinck and Rees, 2009) similarly showed that attended targets are more likely to disappear and that increasing perceptual load is associated with reduced periods of invisibility. Further work is needed to clarify to what extent these other forms of perceptual completion share common feedback mechanisms with the filling-in of artificial scotomas investigated here.
The effects of perceptual load on the latency and probability of filling-in are consistent with the predictions of load theory (Lavie, 2005), and extend the theory’s scope to the phenomenon of filling-in. If filling-in is an active process requiring top-down control, then increasing perceptual load reduces the processing capacity available for the peripheral target, making filling-in slower and less probable.
The effect of WM load is to disrupt the differential allocation of processing resources to the target compared to the background. This would be similar to decreasing the relative contrast between target and background, which leads to faster filling-in (Spillman and De Weerd, 2003;Sturzel and Spillmann, 2001), and we thus expected increased WM load to lead to more (i.e., shorter latency and increased probability) filling-in.
Load theory also proposed that WM maintains stimulus-processing priorities. In situations where different stimuli compete for processing resources, increasing WM load thus has an opposite effect to increasing perceptual load, impairing such prioritization (Lavie et al., 2004;Lavie, 2005). In the context of filling-in, this is consistent with the prediction that depleting WM capacity would disrupt the differential allocation of perceptual processing resources to the target compared to the background, leading to a reduction in the perceived contrast between the target and background.
Previous studies have shown that filling-in is enhanced by a dynamic, compared to a noisy but static, background, and by a static compared to a uniform background (Spillmann and Kurtenbach, 1992). Moreover, filling-in is also enhanced for reduced luminance, motion and contrast differences between the target and background (Welchman and Harris, 2001;Spillman and De Weerd, 2003;Sturzel and Spillmann, 2001) and impaired for more salient red targets, compared to green targets (De Weerd et al., 2006). Therefore, we suggest that WM load may impact filling-in by modulating the relative contrast or salience of the background and target.
Alternatively, increasing WM load may increase arousal, which might enhance filling-in. (Note, however, that there is no reason to assume WM load has this effect but perceptual load does not; nonetheless, contrasting the effects of manipulations of arousal and WM load on filling-in may be a fruitful avenue for further enquiry.)
Conclusion
We have shown that the effects of manipulating two different types of load in concurrent perceptual and WM tasks have opposite effects on the latency of filling-in of an artificial scotoma. This provides new evidence for an interaction between top-down functions and the processes of filling-in, supporting a view of filling-in as an active process.
Supplementary Material
Supplementary Figure 1. Eye movement analysis: (a) Mean eye deviation away from central fixation for each condition in the perceptual load task. (b) Number of saccades per trial in the perceptual load task. (c) Mean eye deviation away from fixation for each condition in the working memory task. (d) Number of saccades per trial in the working memory task. Error bars represent 1 standard error of the mean. Saccades were defined as greater than 1.3 deg from fixation.
Acknowledgements
This work was supported by the Medical Research Council (RW), the International Brain Research Foundation (DC), and the Wellcome Trust (GR). We thank Bahador Bahrami and Elaine Anderson for helpful discussions on the manuscript.
Footnotes
Commercial relationships: none
Reference List
- Bahrami B, Carmel D, Walsh V, Rees G, Lavie N. Unconscious orientation processing depends on perceptual load. J Vis. 2008;8:12–10. doi: 10.1167/8.3.12. [DOI] [PubMed] [Google Scholar]
- Bahrami B, Lavie N, Rees G. Attentional load modulates responses of human primary visual cortex to invisible stimuli. Curr Biol. 2007;17:509–513. doi: 10.1016/j.cub.2007.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DM, Lavie N. Look here but ignore what you see: effects of distractors at fixation. J Exp Psychol Hum Percept Perform. 2005;31:592–607. doi: 10.1037/0096-1523.31.3.592. [DOI] [PubMed] [Google Scholar]
- Carmel D, Saker P, Rees G, Lavie N. Perceptual load modulates conscious flicker perception. J Vis. 2007;7:14–13. doi: 10.1167/7.14.14. [DOI] [PubMed] [Google Scholar]
- Dalton P, Lavie N, Spence C. The role of working memory in tactile selective attention. Q J Exp Psychol (Colchester ) 2009a;62:635–644. doi: 10.1080/17470210802483503. [DOI] [PubMed] [Google Scholar]
- Dalton P, Santangelo V, Spence C. The role of working memory in auditory selective attention. Q J Exp Psychol (Colchester ) 2009b;62:2126–2132. doi: 10.1080/17470210903023646. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- De Weerd P, Desimone R, Ungerleider LG. Perceptual filling-in: a parametric study. Vision Res. 1998;38:2721–2734. doi: 10.1016/s0042-6989(97)00432-x. [DOI] [PubMed] [Google Scholar]
- De Weerd P, Gattass R, Desimone R, Ungerleider LG. Responses of cells in monkey visual cortex during perceptual filling-in of an artificial scotoma. Nature. 1995;377:731–734. doi: 10.1038/377731a0. [DOI] [PubMed] [Google Scholar]
- De Weerd P, Smith E, Greenberg P. Effects of Selective Attention on Perceptual Filling-in. J Cogn Neurosci. 2006;18:335–347. doi: 10.1162/089892906775990561. [DOI] [PubMed] [Google Scholar]
- Dennett D. Consciousness explained. Boston: Little, Brown and Company; 1991. [Google Scholar]
- Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Res. 2003;43:1035–1045. doi: 10.1016/s0042-6989(03)00084-1. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Clark JJ. Microsaccades as an overt measure of covert attention shifts. Vision Res. 2002;42:2533–2545. doi: 10.1016/s0042-6989(02)00263-8. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends Cogn Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N, de Fockert JW. Contrasting effects of sensory limits and capacity limits in visual selective attention. Percept Psychophys. 2003;65:202–212. doi: 10.3758/bf03194795. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. J Exp Psychol Gen. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lou L. Selective peripheral fading: evidence for inhibitory sensory effect of attention. Perception. 1999;28:519–526. doi: 10.1068/p2816. [DOI] [PubMed] [Google Scholar]
- Macdonald JS, Lavie N. Load induced blindness. J Exp Psychol Hum Percept Perform. 2008;34:1078–1091. doi: 10.1037/0096-1523.34.5.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Troncoso XG, Dyar TA. Microsaccades counteract visual fading during fixation. Neuron. 2006;49:297–305. doi: 10.1016/j.neuron.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Mendola JD, Conner IP, Sharma S, Bahekar A, Lemieux S. fMRI Measures of Perceptual Filling-in in the Human Visual Cortex. J Cogn Neurosci. 2006;18:363–375. doi: 10.1162/089892906775990624. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Gregory RL. Perceptual filling in of artificially induced scotomas in human vision. Nature. 1991;350:699–702. doi: 10.1038/350699a0. [DOI] [PubMed] [Google Scholar]
- Rees G, Frith CD, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278:1616–1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Rolfs M. Microsaccades: small steps on a long way. Vision Res. 2009;49:2415–2441. doi: 10.1016/j.visres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Rees G. Attentional influences on the dynamics of motion-induced blindness. J Vis. 2009;9:38–39. doi: 10.1167/9.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Attentional load and sensory competition in human vision: modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cereb Cortex. 2005;15:770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- Spillman L, De Weerd P. Mechanisms of surface completion: Perceptual filling-in of texture. In: Pessoa L, De Weerd P, editors. Filling-in: From perceptual completion to cortical reorganisation. New York: Oxford University Press; 2003. pp. 81–105. [Google Scholar]
- Spillmann L, Kurtenbach A. Dynamic noise backgrounds facilitate target fading. Vision Res. 1992;32:1941–1946. doi: 10.1016/0042-6989(92)90053-l. [DOI] [PubMed] [Google Scholar]
- Sturzel F, Spillmann L. Texture fading correlates with stimulus salience. Vision Res. 2001;41:2969–2977. doi: 10.1016/s0042-6989(01)00172-9. [DOI] [PubMed] [Google Scholar]
- Troncoso XG, Macknik SL, Martinez-Conde S. Microsaccades counteract perceptual filling-in. J Vis. 2008;8:15–19. doi: 10.1167/8.14.15. [DOI] [PubMed] [Google Scholar]
- van Boxtel JJ, Tsuchiya N, Koch C. Opposing effects of attention and consciousness on afterimages. Proc Natl Acad Sci U S A. 2010;107:8883–8888. doi: 10.1073/pnas.0913292107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil RS, Watkins S, Rees G. Neural correlates of perceptual completion of an artificial scotoma in human visual cortex measured using functional MRI. Neuroimage. 2008;42:1519–1528. doi: 10.1016/j.neuroimage.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Welchman AE, Harris JM. Filling-in the details on perceptual fading. Vision Res. 2001;41:2107–2117. doi: 10.1016/s0042-6989(01)00087-6. [DOI] [PubMed] [Google Scholar]
- Yokota M, Yokota Y. Eye movement inhibits the facilitation of perceptual filling-in. Conf Proc IEEE Eng Med Biol Soc 2010. 2010:6629–6632. doi: 10.1109/IEMBS.2010.5627140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.