Abstract
The effect of ageing on hyperthermia-induced changes in cardiac function is unknown. This study tested the hypothesis that hyperthermia-induced changes in left ventricular systolic and diastolic function are attenuated in older adults when compared with young adults. Eight older (71 ± 5 years old) and eight young adults (29 ± 5 years old), matched for sex, physical activity and body mass index, underwent whole-body passive hyperthermia. Mean arterial pressure (Finometer Pro), heart rate, forearm vascular conductance (venous occlusion plethysmography) and echocardiographic indices of diastolic and systolic function were measured during a normothermic supine period and again after an increase in internal temperature of ~1.0 °C. Hyperthermia decreased mean arterial pressure and left ventricular end-diastolic volumes and increased heart rate to a similar extent in both groups (P > 0.05). Ageing did not alter the magnitude of hyperthermia-induced changes in indices of systolic (lateral mitral annular S′ velocity) or diastolic function (lateral mitral annular E′ velocity, peak early diastolic filling and isovolumic relaxation time; P > 0.05). However, with hyperthermia the global longitudinal systolic strain increased in the older group, but was unchanged in the young group (P = 0.03). Also, older adults were unable to augment late diastolic ventricular filling [i.e. E/A ratio and A/(A + E) ratio] during hyperthermia, unlike the young (P <0.05). These findings indicate that older adults depend on a greater systolic contribution (global longitudinal systolic strain) to meet hyperthermic demand and that the atrial contribution to diastolic filling was not further augmented in older adults when compared with young adults.
Introduction
In recent decades, heat waves have significantly impacted population mortality and morbidity around the globe. Elderly populations are among the most vulnerable to heat waves and associated elevated temperatures, particularly with respect to respiratory and cardiovascular mortality (Åström et al. 2011). Indeed, elevated environmental temperatures increase the risk of cardiovascular mortality and morbidity (Basu & Samet, 2002; Åström et al. 2011; Tian et al. 2013) primarily due to increased cardiovascular strain, which is particularly prevalent among the elderly, who have limited adaptive responses.
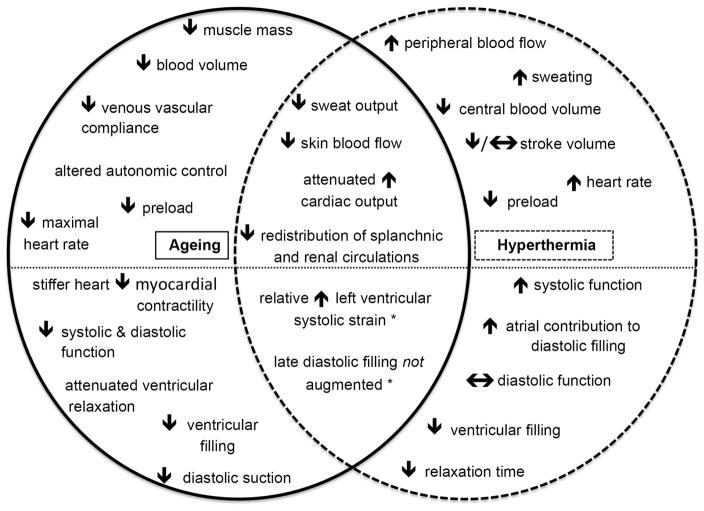
With normal healthy ageing, even in the absence of comorbidities, the heart undergoes profound changes, including alterations in ventricular filling (mitral inflow E/A ratio), global relaxation (isovolumetric relaxation time, IVRT), dynamic longitudinal wall relaxation (myocardial tissue Doppler velocities) and diastolic suction (propagation velocity of early mitral inflow), as shown via Doppler measures of cardiac function (Bryg et al. 1987; Prasad et al. 2007; Fig. 1). Healthy ageing also alters the cardiovascular mechanisms that underlie human thermoregulatory control. During hyperthermia, older individuals typically respond with attenuated sweat gland output (Kenney & Fowler, 1988), decreased skin blood flow (Armstrong & Kenney, 1993; Kenney et al. 1997), reduced increases in cardiac output (Minson et al. 1998) and less redistribution of blood flow from combined splanchnic and renal circulations (Minson et al. 1998, 1999) when compared with young adults (Fig. 1). Attenuated increases in cardiac output and smaller decreases in splanchnic and renal blood flows are associated with attenuated increases in skin blood flow during hyperthermia in older adults (Minson et al. 1998), while diminished increases in cardiac output have been attributed to the inability to maintain stroke volume (Minson et al. 1998). However, the effect of ageing on hyperthermia-induced changes in indices of cardiac function is not known.
Figure 1. Schematic overview of age-related and hyperthermia-induced changes to the cardiovascular system.
Note that below the dotted line are echocardiographic responses. Novel findings from the present study are denoted with an asterisk (*).
Whole-body passive hyperthermia increases left ventricular and left atrial systolic function, while left ventricular diastolic function is maintained despite hyperthermia-related decreases in preload (Brothers et al. 2009). Added to this, increased left ventricular untwisting rates during hyperthermia facilitate left ventricular filling (Nelson et al. 2010; Stöhr et al. 2011), presumably contributing to the maintenance (Crandall et al. 2008) or attenuated reductions (Wilson et al. 2009; Nelson et al. 2010) in left ventricular end-diastolic volume during hyperthermia. Thus, stroke volume is typically maintained during whole-body passive hyperthermia in young adults, despite reductions in central blood volume (Crandall et al. 2008) and cardiac filling pressures (Rowell et al. 1969; Wilson et al. 2007; Fig. 1). Age-related reductions in stroke volume during whole-body passive hyperthermia may be due to attenuated left ventricular systolic function and/or impairment in diastolic function during hyperthermia.
The aim of the present study, therefore, was to test the hypothesis that hyperthermia-induced changes in left ventricular systolic and diastolic function are attenuated in older adults when compared with young adults.
Methods
Eight healthy older (71 ± 5 years old, mean ± SD; four men and four women) and young adults (29 ± 5 years old; four men and four women), matched for sex, physical activity (using the International Physical Activity Questionnaire) and body mass index, participated in this study. Their physical characteristics are shown in Table 1. All subjects had no history of cardiovascular, cerebrovascular or respiratory disease, were free from taking cardiovascular acting medications and were non-smokers. All subjects refrained from alcohol, caffeine and exercise for 24 h before the study. Written informed consent was obtained before participation in this study, which was approved by the University of Texas Southwestern Medical Center and Presbyterian Hospital of Dallas and complied with the Declaration of Helsinki.
Table 1.
Subject characteristics
| Characteristic | Young subjects
|
Older subjects
|
||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| Age (years) | 29 ± 5 | 22–36 | 71 ± 5* | 65–78 |
| Body mass index (kg m−2) | 25.1 ± 3.3 | 20.1–30.3 | 24.6 ± 2.6 | 20.5–28.1 |
| Mass (kg) | 77.9 ± 13.1 | 53.4–97.8 | 72.0 ± 10.9 | 56.4–86.0 |
| Height (m) | 1.76 ± 0.11 | 1.63–1.91 | 1.71 ± 0.12 | 1.56–1.88 |
| Body surface area (m2) | 1.94 ± 0.21 | 1.57–2.27 | 1.84 ± 0.20 | 1.55–2.08 |
| Estimated physical activity (METS) | 2299 ± 1514 | 99–4080 | 1755 ± 1313 | 698–4392 |
Significantly different from young group, P < 0.05.
Abbreviation: METS, metabolic equivalents.
Instrumentation
At the beginning of the experimental day, subjects voided their bladder before nude body mass was recorded. Urine specific gravity was measured using a digital refractometer (Atago, Tokyo, Japan). Subjects were then dressed in a long-sleeved and -legged tube-lined perfusion suit (Med-Eng, Ottawa, ON, Canada) enabling the control of skin temperature and body core temperature (Tc) via the temperature of the water perfusing the suit. Body core temperature was measured using a telemetry temperature pill swallowed ~2 h before the onset of data collection (HQ Inc., Palmetto, FL, USA). Whole-body mean skin temperature (Tsk) was measured from the electrical average of six thermocouples (Taylor et al. 1989) fixed to the skin with porous adhesive tape. Beat-to-beat arterial blood pressure was measured and reconstructed to the brachial artery via finger cuff photoplethysmography (Finometer Pro, FMS, Amsterdam, The Netherlands; or NexFin HD, BMEYE BV, Amsterdam, The Netherlands). Cardiac output and stroke volume were measured using the Innocor foreign gas rebreathing system containing a gas mixture of 0.1% sulfur hexaflouride (blood-insoluble gas), 0.5% nitrous oxide (blood-soluble gas) and 28% oxygen in nitrogen gas (Innovision A/S, Odense, Denmark). Heart rate (HR) was collected from an electrocardiogram signal (Agilent, Munich, Germany) interfaced with a cardiotachometer (CWE, Ardmore, PA, USA). Age-predicted maximal heart rates (HRmax) were calculated using the equation HRmax = 220 - age. Changes in forearm blood flow were measured on the left arm six times (three times per minute) using venous occlusion plethysmography during both normothermic and hyperthermic conditions (Wilkinson & Webb, 2001). Forearm vascular conductance (FVC) was calculated as the ratio of forearm blood flow to mean arterial pressure.
Echocardiography
Echocardiographic images were obtained using commercially available ultrasound equipment (iE33; Philips Ultrasound, Bothell, WA, USA). All examinations were performed during supine resting conditions, with the subject in the left lateral decubitus position. All images were obtained by an experienced sonographer, stored on the iE33 hard drive and were later exported for offline analysis by the sonographer using commercially available software (Xcelera cardiovascular image management system; Philips Ultrasound).
Tissue Doppler imaging
Measurements of septal and lateral mitral annular early diastolic (E′), late diastolic (A′) and systolic velocities (S′) were obtained to provide indices of left ventricular diastolic function (Prasad et al. 2007), left atrial systolic function (Nagueh et al. 2001; Pela et al. 2004) and left ventricular systolic function (Shimizu et al. 1998), respectively, via standard tissue Doppler imaging techniques, as previously described (Prasad et al. 2007; Brothers et al. 2009). Tissue Doppler measurements were obtained from the apical four-chamber view with a 2.0 mm sample volume positioned on the septal and lateral mitral annulus (Prasad et al. 2007). In one young and two older subjects, the Doppler image became fused during hyperthermia; therefore, the data illustrated for early (E ′) and late diastolic velocities (A′) and diastolic function are representative of a total of 13 subjects.
Mitral inflow velocities
Mitral inflow velocities were assessed from the apical four-chamber view using pulsed wave Doppler, with a sample volume of 2.0 mm positioned over the mitral valve leaflet tips. Measurements were obtained of peak inflow velocity during the early phase of left ventricular relaxation (E wave), which provides an index of early left ventricular diastolic function (Labovitz & Pearson, 1987; Hatle, 1993; Cohen et al. 1996), and during left atrial contraction (A wave), which provides an index of late diastolic filling (Cohen et al. 1996). These values were subsequently used to quantify diastolic function as the E/A ratio (Labovitz & Pearson, 1987; Hatle, 1993). Left atrial systolic function was calculated as the relative atrial contribution (late diastolic filling) to left ventricular diastolic filling using the following formula: [A/(E + A)] ×100 (Brothers et al. 2009). As a result of difficulties in obtaining mitral inflow velocities in one young and two older subjects owing to fusion of the E and A wave complexes at high HR during hyperthermia, the reported data are from a total of 13 subjects.
Global longitudinal systolic strain (GLSS)
Peak GLSS was obtained from an apical four-chamber grey-scale image optimized for speckle tracking analysis (Qlab v 9.0; Philips Medical Systems; Leitman et al. 2004). After tracing the left ventricular endocardial border, automated tracking analysis of myocardial speckles was performed and visually inspected to ensure adequate tracking. In the four-chamber view, the myocardium was divided into seven segments starting from the basal inferoseptum through the basal anterolateral wall. If segment tracking was not satisfactory, the endocardial border was redrawn. No segments were excluded from analysis; rather, if multiple segments tracked suboptimally due to poor image quality, data from that subject were not included in analysis. Owing to poor image quality in two older subjects during hyperthermia, the data illustrated for this variable are representative of six older subjects.
Diastolic suction and relaxation
Isovolumetric relaxation time represents the time interval between aortic outflow during systole and the opening of the mitral valve during diastole and thus is commonly used as an index of left ventricular relaxation (Prasad et al. 2007). The IVRT was determined using a five-chamber apical view with the sample volume set at 4.0 mm and was measured as the time between aortic valve closure and mitral valve opening.
Colour M-mode images of left ventricular inflow were obtained with the sampling area positioned to extend from midatrium to the apex, directly through the mitral valve orifice. The scale was reduced sufficiently to display clear aliasing within the early diastolic portion of the mitral inflow. The resulting mitral inflow spatiotemporal velocity profile pattern was used to derive the early propagation velocity (Vp) of mitral inflow as the slope of the early diastolic aliasing velocity. This technique has been described previously (Rivas-Gotz et al. 2003; Prasad et al. 2007).
Left ventricular end-diastolic volume (LVEDV)
Two-dimensional echography was used to measure LV volumes via manual tracing of the endocardial border in the apical four-chamber images. The LVEDV was indexed to body surface area (EDVI). Due to imaging difficulties in one older subject, the data illustrated for this variable are representative of seven older subjects.
Protocol
Following instrumentation, subjects rested in a supine position for at least 30 min while normothermic water (34 °C) circulated through the suit. After this 30 min period, thermal and haemodynamic baseline measures were obtained for 5 min. Forearm blood flow measurements were then obtained, followed by echocardiographic assessment of the aforementioned variables. Subjects were then passively heated by circulating ~49 °C water through the suit until Tc increased by ~1.0 °C. The water temperature of the suit was then lowered to ~46 °C to limit further increase in body temperature. Thermal, haemodynamic, forearm blood flow and echocardiographic measures where then repeated, as described for normothermia.
Data analysis
Thermal and haemodynamic data were acquired continuously at 50 Hz throughout the experiment (Biopac, Santa Barbara, CA, USA). Data from a 4 min baseline period were averaged and comparisons made between groups and thermal conditions. Average measurements obtained from four consecutive cardiac cycles for each echocardiographic parameter were compared between groups and thermal conditions. A two-way mixed-model ANOVA with one between-subject (age) and one within-subject factor (thermal condition) was used to identify differences in thermal, haemodynamic and echocardiographic measures between the young and older subjects. Bonferroni post hoc tests were used to evaluate pairwise differences if a significant interactive effect between the main variables (age ×temperature) was detected. Student’s unpaired t test was used to compare urine specific gravity and the magnitude of the reduction in body mass by hyperthermia between groups. All values are reported as means ± SD. Values of P < 0.05 were considered statistically significant.
Results
Thermal and haemodynamic measures
Before whole-body passive hyperthermia, subjects’ body mass (older 72.0 ± 10.9 kg versus young 77.9 ± 13.1 kg) and urine specific gravity (older 1.013 ± 0.005 versus young 1.009 ± 0.007) were similar between older and young adults (age P > 0.05; Table 1). Body core and skin temperatures were likewise similar between groups at normothermic baseline (Table 2). Mean arterial pressure was elevated in the older group (age P = 0.02), while HR and FVC were similar (Table 2).
Table 2.
Thermal and haemodynamic measures in young and older adults during normothermic and hyperthermic conditions
| Parameter | Young subjects
|
Older subjects
|
||
|---|---|---|---|---|
| Normothermia | Hyperthermia | Normothermia | Hyperthermia | |
| Tc (°C)* | 37.1 ± 0.2 | 38.2 ± 0.1 | 36.9 ± 0.1 | 38.0 ± 0.1 |
| Tsk (°C)* | 33.8 ± 0.3 | 38.4 ± 0.6 | 33.8 ± 0.5 | 38.4 ± 0.6 |
| MAP (mmHg)*† | 83 ± 7 | 69 ± 7 | 93 ± 9 | 77 ± 9 |
| Cardiac output (l min−1)*† | 6.2 ± 1.2 | 8.1 ± 1.0 | 3.9 ± 1.1 | 5.4 ± 1.5 |
| Stroke volume (ml)*† | 101 ± 26 | 87 ± 17 | 60 ± 17 | 60 ± 17 |
| HR (beats min−1)* | 59 ± 9 | 94 ± 13 | 67 ± 8 | 93 ± 12 |
| % HRmax (%)*† | 31 ± 4 | 49 ± 6 | 45 ± 5 | 63 ± 7 |
| FVC (ml min 100−1 mmHg)* | 2.5 ± 1.4 | 17.2 ± 5.6 | 2.9 ± 0.7 | 11.5 ± 4‡ |
Abbreviations: FVC, forearm vascular conductance; HR, heart rate; % HRmax, percentage of predicted maximal heart rate; MAP, mean arterial pressure; Tc, body core temperature; and Tsk, mean skin temperature.
Significant main effect of thermal condition, P < 0.05.
Significant main effect of age, P < 0.05.
Significant post hoc pairwise comparison between age groups within thermal condition, P < 0.05.
Whole-body passive hyperthermia resulted in similar increases in Tc (older 1.0± 0.1 °C; young 1.1 ± 0.1 °C; P < 0.01 from normothermia) and mean skin temperature between groups (Table 2). Hyperthermia decreased mean arterial pressure and increased cardiac output and HR to a similar extent between groups (age ×temperature interaction P > 0.05; Table 2). There was also an attenuated increase in FVC in the older adults during hyperthermia (post hoc P < 0.01, age ×temperature interaction P < 0.02; Table 2). Using age-predicted HRmax, the HR of the older adults during hyperthermia was 63 ± 7% of maximum, whereas this value was 49 ± 6% of maximum for the young adults (age P < 0.01). Following whole-body passive hyperthermia, young adults had a greater reduction in body mass (1.2 ± 0.4 kg; 1.6 ± 0.4% body mass; age P = 0.04) relative to the older adults (0.6 ± 0.3 kg; 0.8 ± 0.5% body mass). There was no difference between age groups for the passive heating time to increase Tc by 1.0 °C (older 47 ± 10 min versus young 51 ± 4 min; P > 0.05) or the total time for which subjects were heat stressed (i.e. heating time plus hyperthermic measurement time; older 75 ± 5 min versus young 72 ± 12 min; P > 0.05).
Echocardiographic measures
Left ventricular systolic function
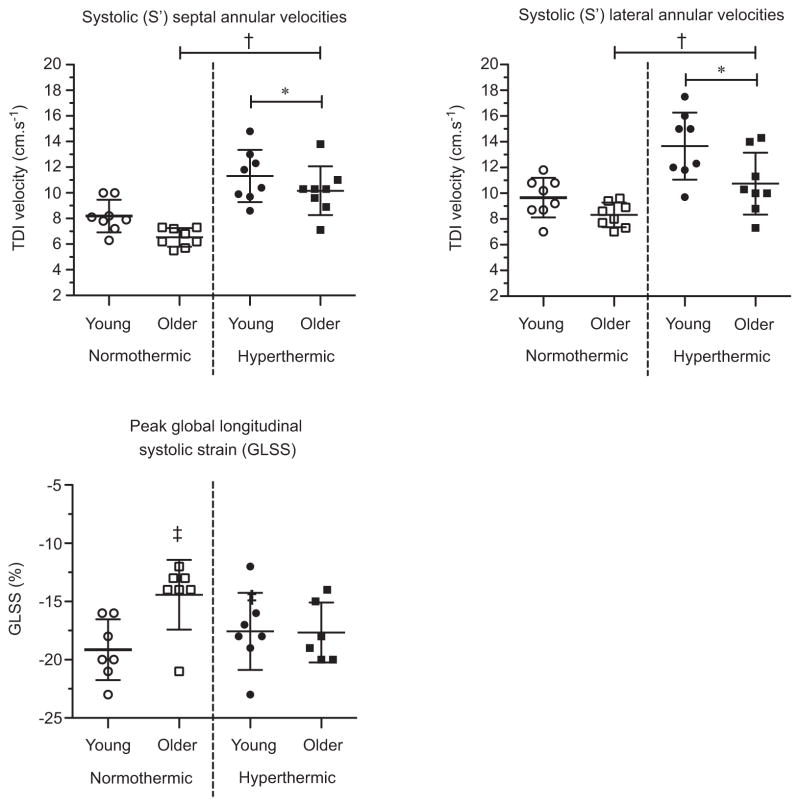
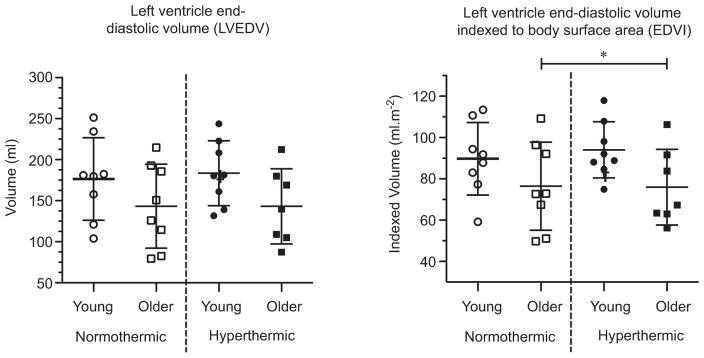
Systolic mitral annular velocities were lower in the older adults (septal S′ and lateral S′: age P = 0.03 and P < 0.01, respectively; Fig. 2). Hyperthermia increased systolic mitral annular velocities in both age groups (temperature P < 0.01; age ×temperature interaction P > 0.05 for both septal S′ and lateral S′). The GLSS was higher in older adults at normothermic baseline (post hoc P = 0.01), but with hyperthermia these age-related differences disappeared (age ×temperature interaction P = 0.03; Fig. 2). The LVEDV showed no age-related or hyperthermia-related effects (age P > 0.05; temperature P > 0.05; Fig. 3). The EDVI was lower in older adults, with neither age group showing any hyperthermia-related changes (age P = 0.05; temperature P > 0.05; Fig. 3).
Figure 2. Systolic annular velocities (S′) and peak global longitudinal systolic strain in young and older adults in normothermic and hyperthermic conditions.
Individual and group-averaged echocardiographic data. Hyperthermia increased global longitudinal systolic strain (GLSS) in the older adults while remaining unchanged in the young, indicating an increased reliance on systolic mechanisms in the older adults. *Significant main effect of thermal condition, P < 0.05. †Significant main effect of age, P < 0.05. ‡Significant post hoc pairwise comparison between age groups within thermal condition, P < 0.05.
Figure 3. Left ventricular end-diastolic volumes [absolute (in millilitres), left; and relative to body surface area (in millilitres per square metre), right] in young and older adults in normothermic and hyperthermic conditions.
Individual and group-averaged echocardiographic data. Left ventricular end-diastolic volumes were unchanged by hyperthermia in both the young and older adults. *Significant main effect of age, P < 0.05.
Early diastolic filling and function
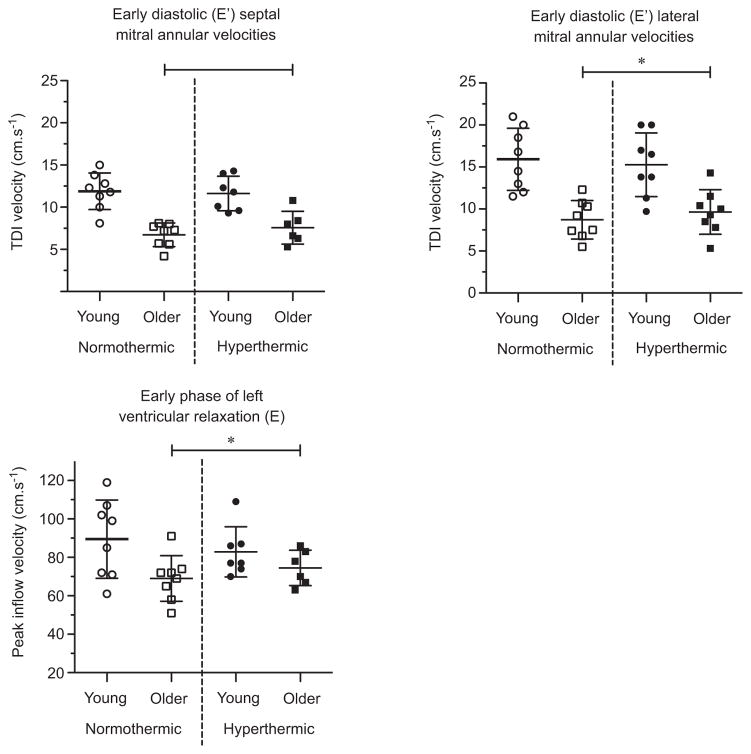
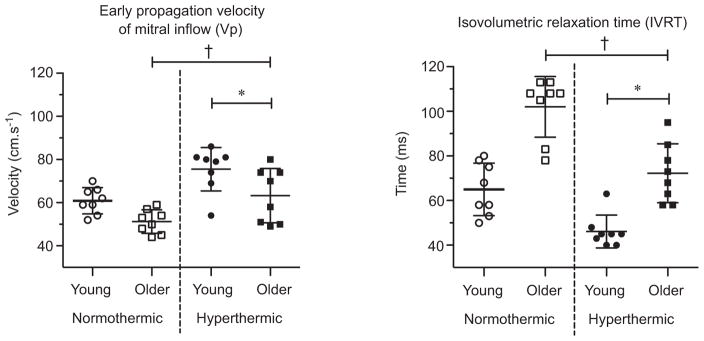
Early diastolic mitral annular velocities and mitral E inflow were lower in older adults (septal E′, lateral E′ and E: age P < 0.01, P < 0.01 and P = 0.04, respectively; Fig. 4). Hyperthermia had no effect on early diastolic filling in either group (temperature P > 0.05; age ×temperature interaction P > 0.05). The Vp was slower and IVRT longer in older adults (Vp and IVRT: age P < 0.01; Fig. 5). With hyperthermia, Vp increased and IVRT was shortened to a similar extent in both age groups (Vp and IVRT: temperature P < 0.01; age ×temperature interaction P > 0.05).
Figure 4. Indices of early diastolic function in young and older adults in normothermic and hyperthermic conditions.
Individual and group-averaged echocardiographic data of early diastole mitral annular velocities (E′) and mitral inflow velocity (E) during early diastolic filling. Hyperthermia did not change septal or lateral mitral annular velocities or peak mitral inflow velocity during early diastolic filling in young or older adults. *Significant main effect of age, P < 0.05. TDI, Tissue Doppler imaging.
Figure 5. Early propagation velocity of mitral inflow (Vp) and isovolumetric relaxation time (IVRT) in young and older adults in normothermic and hyperthermic conditions.
Individual and group-averaged echocardiographic data. The IVRT shortened and Vp increased during hyperthermia in both young and older adults, indicating an increased rate of relaxation and suction for the left ventricle. *Significant main effect of thermal condition, P < 0.05. †Significant main effect of age, P < 0.05.
Atrial function and late diastolic ventricular filling
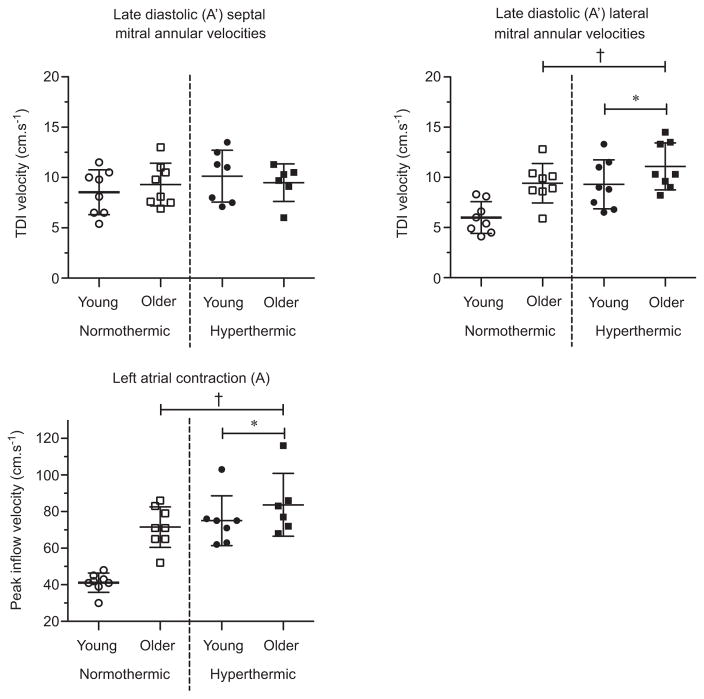
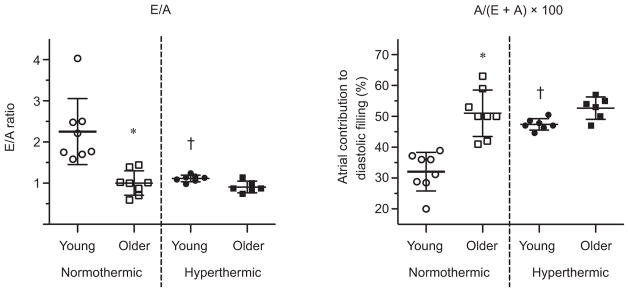
Lateral A′ mitral annular velocities and mitral A inflow were higher in the older adults (age P ~0.01 for all; Fig. 6). Hyperthermia caused similar increases in lateral A′ mitral annular velocities and mitral A inflow between groups (temperature P < 0.01; age ×temperature interaction P > 0.05). Septal A′ mitral annular velocities showed no age-related or hyperthermia-related effects (age P > 0.05; temperature P > 0.05). The E/A ratio was lower in older adults at normothermic baseline (post hoc P < 0.01; age ×temperature interaction P = 0.02; Fig. 7). Hyperthermia had no effect on older adults, but decreased the E/A ratio in young adults (post hoc P < 0.01), removing the age-related differences present at normothermia. The atrial contribution to diastolic filling was lower in the young adults at normothermia (post hoc P < 0.01; age ×temperature interaction P = 0.02) and increased in hyperthermic conditions (post hoc P < 0.01; Fig. 7), whereas the older adults showed no hyperthermia-related change (post hoc P > 0.05).
Figure 6. Indices of late diastolic ventricular filling in young and older adults in normothermic and hyperthermic conditions.
Individual and group-averaged echocardiographic data of mitral annular velocities during late (A′) diastole and peak mitral inflow velocity during atrial contraction (A). Hyperthermia increased lateral A′ mitral inflow velocity and augmented A in both young and older adults, indicating an increased left atrial contribution to late diastolic filling. *Significant main effect of thermal condition, P < 0.05. †Significant main effect of age, P < 0.05.
Figure 7. The ratio of early and late mitral inflow velocities during diastolic filling (E/A ratio; left) and the contribution of atrial contraction to diastolic filling (right) in young and older adults in normothermic and hyperthermic conditions.
Individual and group-averaged echocardiographic data. *Significant post hoc pairwise comparison between age groups within thermal condition, P < 0.05. †Significant post hoc pairwise comparison between thermal condition within age groups, P < 0.05.
Discussion
The novel findings from this study are as follows: (i) hyperthermia-induced changes in indices of gross systolic (i.e. mitral annular tissue S′ velocity) and early diastolic filling and function (i.e. lateral E′, E and IVRT) are generally unaffected by ageing; (ii) however, relative to normothermia, older adults depend on an increase in left ventricular systolic strain (GLSS) to meet hyperthermic demand, matching younger hyperthermic GLSS values; and (iii) older adults did not further augment late diastolic ventricular filling [i.e. E/A ratio and A/(A + E) ratio] during hyperthermia, unlike the young adults.
Haemodynamic responses to hyperthermia
In the present study, the cardiovascular demand with hyperthermia was similar between older and young subjects, given that the absolute increase in cardiac output was not different between age groups (1.5 versus 1.9 l min−1 in older and young subjects, respectively). Corresponding to this, LVEDV and EDVI (factors contributing to stroke volume) were unchanged by hyperthermia in both the older and young adults (Fig. 3). Furthermore, age-related differences were apparent only in EDVI, but these differences were not exacerbated with hyperthermia. We have previously (Wilson et al. 2009), though not consistently (Crandall et al. 2008), shown a small reduction (~11%) in LVEDV and EDVI with hyperthermia in young adults, as have others (Nelson et al. 2010; Stöhr et al. 2011). These differences are probably due to the sensitivity of LVEDV measures as well as the use of different measurement techniques [Doppler ultrasound (Wilson et al. 2009; Stöhr et al. 2011) and cardiac magnetic resonance imaging (Nelson et al. 2010) versus multiple-gated acquisition (Crandall et al. 2008)]. It is also worth noting that a moderate hyperthermic stimulus was used in the present study (+1.0 °C increase in body core temperature), which may have contributed to the lack of a reduction in LVEDV and EDVI (Stöhr et al. 2011). It is well established that ageing alters the cardiovascular mechanisms that underlie human thermoregulatory control. In the present study, an attenuated increase in FVC and a smaller reduction in body mass following hyperthermia is indicative of typical age-related changes in skin blood flow and sweat rate to the hyperthermic challenge (Kenney & Fowler, 1988; Armstrong & Kenney, 1993; Kenney et al. 1997; Minson et al. 1998). Such changes are consistent with smaller redistributions of blood from the central to the peripheral circulations during hyperthermia (Minson et al. 1998, 1999) and/or the assertion that inotropic responses are attenuated in healthy older individuals during hyperthermia (Minson et al. 1998; Fig. 1). Taken together, these findings indicate that an equivalent hyperthermic stimulus between older and young adults (i.e. same elevation in Tc) elicits the same absolute increase in cardiac output, although cardiac output was lower in older adults when evaluated across both thermal conditions. In contrast to the latter observation, Minson et al. (1998) reported attenuated increases in cardiac output during heat stress in older relative to younger subjects. The reasons for this apparent discrepancy are not clear but may be related to the activity level of the older subjects, with the present subjects being fairly active.
Hyperthermia increases the inotropic and chronotropic modulation of the heart (Frey & Kenney, 1979). As previously reported (Minson et al. 1998), older and young adults showed a similar chronotropic response to hyperthermia, with HR increasing from ~63 to ~94 beats min−1. However, it is important to consider that in older adults a HR of 94 beats min−1 represented a higher percentage of their HRmax (~14% greater than young adults based on age-predicted HRmax). Thus, a similar hyperthermic state evoked a greater reliance on their chronotropic reserve and perhaps a larger relative myocardial strain in the older adults. This is probably the result of typical age-related changes in chronotropic responsiveness to β-adrenergic stimulation (Seals et al. 1994). Perhaps due to this response, older adults when hyperthermic have less cardiac reserve to manage further increases in cardiovascular strain that occurs with, for instance, more severe hyperthermia, dehydration and/or physical activity. Despite this greater relative chronotropic strain, the hyperthermia-induced increases in indices of systolic and diastolic function were generally similar between groups. It may be that hyperthermia evoked a greater sympathetic stimulation in the older adults (above typical normothermic age-related elevations in sympathetic activation) because of typical age-related diminished β-adrenergic responsiveness (Seals et al. 1994). However, to date the age-related differences in sympathetic activation during hyperthermia have not been investigated.
Echocardiographic responses to hyperthermia
Despite a similar hyperthermic cardiovascular demand, hyperthermia increased GLSS (an index of left ventricular strain and contractility; Reisner et al. 2004) only in the older group, while GLSS was essentially unchanged in the young group (Figs 1 and 2). Thus, during hyperthermia, typical age-related attenuations in left ventricular function (Arbab-Zadeh et al. 2004; Chahal et al. 2010) were no longer apparent. These findings indicate that during moderate hyperthermia, older adults have an increased reliance on systolic mechanisms to maintain LVEDV (i.e. increasing GLSS during hyperthermia when young adults showed no change). Healthy ageing did not alter the magnitude of hyperthermia-induced increases in systolic function in the present study (Fig. 2), which has consistently been shown to improve during hyperthermia in young adults (Tei et al. 1995; Crandall et al. 2008; Brothers et al. 2009; Nelson et al. 2010; Stöhr et al. 2011). However, this is the first study to identify that systolic function can be augmented in aged individuals during hyperthermia (though older adults still had an age-appropriate overall decrease in systolic function when compared with the young; Chahal et al. 2010). Interestingly, systolic contractile reserve in the elderly did not appear to be impaired by hyperthermia because it increased to a proportionally similar degree, although general age-related differences remained.
The velocity of blood flow entering the ventricle during the late phase of diastolic filling (A) is dependent on left atrial contraction and left ventricular compliance (Cohen et al. 1996). In the present study, hyperthermia increased A′ mitral annular (albeit lateral A′ only) and inflow velocities in both the older and the young adults (Fig. 6). This increase in late diastolic filling seems indicative of an increased left atrial contribution to late diastolic filling, as previously demonstrated during whole-body passive hyperthermia in young individuals (Brothers et al. 2009; Nelson et al. 2010). In the present study, the E/A ratio decreased with hyperthermia in the young adults (Figs 1 and 6). This reduction was mediated by an increase in A velocity and signifies an increase reliance on late diastolic filling during hyperthermia in this age group. This finding is further supported by the relative contribution of A to left ventricular filling during diastole (i.e. A/(E + A) ×100). Older adults did not augment this response during hyperthermia, unlike the young, probably due to an already high reliance on atrial filling at baseline. These findings indicate a possible limitation of the atria to contribute further to diastolic filling during whole-body passive hyperthermia in the elderly.
The IVRT shortened in hyperthermic conditions in both older and young adults (Fig. 5), consistent with prior, but not all, findings in young adults (Brothers et al. 2009; Nelson et al. 2010; Stöhr et al. 2011). Isovolumetric relaxation time is a measure of ventricular relaxation determined by the following factors: (i) the pressure gradient between the left atrium and left ventricle, with a decrease in preload (e.g. as would occur during lower body negative pressure) causing a lengthening in IVRT (Prasad et al. 2007); and (ii) lusitropic properties inherent to the myocardium (e.g. as dictated via catecholamine sensitivity). We observed a shortening of IVRT with hyperthermia in both groups despite preload reductions (Prasad et al. 2007), indicating that in both older and young adults, hyperthermia increased the rate of left ventricular relaxation prior to the opening of the mitral valve. This more rapid myocardial relaxation could be due to inotropic and lusitropic effects of increased sympathetic activity and circulating catecholamines that also contribute to the maintenance of stroke volume during hyperthermia-related decreases in left ventricular pressure (Rowell, 1986). Like IVRT, Vp is strongly influenced by preload as well as myocardial suction during early diastole (Prasad et al. 2007). Hyperthermia increased Vp in both older and young adults (Fig. 5). Thus, the capacity of the left ventricle to create suction was improved during hyperthermia, regardless of age. Importantly, hyperthermia-related increases in Vp observed herein and left ventricular untwisting (Nelson et al. 2010) indicate that hyperthermic inotropic effects increased left ventricular suction, compensating for potential reductions in left ventricular filling.
In the present study, early left ventricular diastolic flow was unchanged by hyperthermia in both older and young adults (Fig. 4). Likewise, other studies have shown no change in these indices of diastolic filling during hyperthermia (Brothers et al. 2009; Nelson et al. 2010). It has been postulated that unchanged diastolic filling/flow in the face of hyperthermia-related reductions in ventricular filling pressures and central blood volume (i.e. preload) suggest improved diastolic function (i.e. more rapid ventricular relaxation and/or increased suction) during hyperthermia, maintaining early left ventricular flow despite reductions in filling pressures (Brothers et al. 2009; Nelson et al. 2010; Stöhr et al. 2011). Therefore, it seems reasonable to suggest that such improvements are apparent in healthy older adults given that hyperthermia-related reductions in preload, which are similar between older and young individuals (Minson et al. 1998), did not exacerbate age-related differences in diastolic function. Nevertheless, overall age-related impairments in diastolic function remained, and thus, diastolic function and filling remains impaired in healthy older adults during hyperthermia.
Age-related changes in echocardiographic responses
During normothermia, the older adults showed an overall decrease in systolic function (as indicated by lower global left ventricular longitudinal strain and systolic myocardial tissue velocities), similar to that reported in healthy aged individuals (Dalen et al. 2010) and typical of subclinical age-related reductions in cardiac contractility (Yamakado et al. 1997; Onose et al. 1999). Characteristic age-related decreases in diastolic function (as indicated by mitral inflow velocity) and Vp (Prasad et al. 2007) as well as prolongation of IVRT (Prasad et al. 2007) were also apparent in the older adults (Onose et al. 1999; Prasad et al. 2007). Such age-related changes indicate impaired left ventricular relaxation, recoil and filling in the older adults (Labovitz & Pearson, 1987; Hatle, 1993; Cohen et al. 1996; Prasad et al. 2007).
Limitations
For one younger and two older participants, the high HR during hyperthermia caused fusion of the E and A wave complexes. Thus, measures incorporating these values are reported for a total of 13 subjects. Conceivably, some of our results might have been different with a larger sample size. That said, power analysis indicated a power of 0.80–1.00 for variables with 13 total subjects (note that septal E′, septal A′ and GLSS were the only variables where power ranged between 0.80 and 0.95). Consequently, the present study is not statistically underpowered. However, to address in part this problem of a low sample size, individual data are presented throughout.
In the present study, cardiac output and stroke volume were measured non-invasively using the Innocor rebreathing system. This system has been validated against the direct Fick and thermodilution estimates of cardiac output during exercise (Agostoni et al. 2005). However, the lack of validation studies using this rebreathing system during whole-body passive hyperthermia suggests that such estimates of cardiac output and stroke volume should be treated with caution.
Implications
The present study demonstrates that healthy older adults meet hyperthermia-related increases in cardiac demand via increased reliance on both systolic mechanisms (GLSS) and their cardiac reserve, whereas young adults rely primarily on increasing late diastolic filling. Furthermore, healthy older adults appear unable to augment the atrial contribution to diastolic filling further during hyperthermia, indicating a myocardial limitation to hyperthermia in older populations. Though speculative, age-related differences in cardiac responses to hyperthermia may partly explain an increased mortality risk in the elderly during extremely hot days, particularly in those with existing co-morbidities (Medina-Ramón et al. 2006; Åström et al. 2011).
Conclusions
These data indicate that during hyperthermia, systolic and diastolic function can be appropriately augmented to meet cardiac demand in healthy older adults, although overall age-related impairments remain. One exception was late diastolic ventricular filling [i.e. E/A ratio and A/(A + E) ratio], which in the older adults was not further augmented during hyperthermia, unlike their young counterparts. Therefore, to meet cardiac demand healthy older adults appear to depend on a greater systolic contribution (increasing GLSS during hyperthermia when young adults showed no change) and proportion of their cardiac reserve.
New Findings.
What is the central question of this study?
The effect of ageing on hyperthermia-induced changes in cardiac function is unknown.
What is the main finding and its importance?
Using echocardiography, we show that during hyperthermia the systolic and diastolic function can be appropriately augmented to meet cardiac demand in healthy older adults, although overall age-related impairments remain. One exception was late diastolic ventricular filling [i.e. E/A ratio and A/(A + E) ratio], which in the older adults was not further augmented during hyperthermia, unlike their young counterparts. To meet cardiac demand, therefore, healthy older adults appear to depend on an increased left ventricular systolic strain and proportion of their cardiac reserve.
Acknowledgments
Funding
This study was supported by the National Institutes of Health – National Heart, Lung, and Blood Institute under award number NIH: HL61388. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We would like to thank the subjects for participating in our study. We would also like to thank Jena Kern, RN and Naomi Kennedy, RN for their technical assistance.
Footnotes
Competing interests
None declared.
Author contributions
All authors contributed to the conception and design of the experiment; collection, analysis and interpretation of data; and writing of the manuscript. All authors read and approved the final manuscript. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
References
- Agostoni P, Cattadori G, Apostolo A, Contini M, Palermo P, Marenzi G, Wasserman K. Noninvasive measurement of cardiac output during exercise by inert gas rebreathing technique: a new tool for heart failure evaluation. J Am Coll Cardiol. 2005;46:1779–1781. doi: 10.1016/j.jacc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- Armstrong CG, Kenney WL. Effects of age and acclimation on responses to passive heat exposure. J Appl Physiol. 1993;75:2162–2167. doi: 10.1152/jappl.1993.75.5.2162. [DOI] [PubMed] [Google Scholar]
- Åström DO, Forsberg B, Rocklöv J. Heat wave impact on morbidity and mortality in the elderly population: a review of recent studies. Maturitas. 2011;69:99–105. doi: 10.1016/j.maturitas.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Basu R, Samet JM. Relation between elevated ambient temperature and mortality: a review of the epidemiologic evidence. Epidemiol Rev. 2002;24:190–202. doi: 10.1093/epirev/mxf007. [DOI] [PubMed] [Google Scholar]
- Brothers RM, Bhella PS, Shibata S, Wingo JE, Levine BD, Crandall CG. Cardiac systolic and diastolic function during whole body heat stress. Am J Physiol Heart Circ Physiol. 2009;296:H1150–1156. doi: 10.1152/ajpheart.01069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryg RJ, Williams GA, Labovitz AJ. Effect of aging on left ventricular diastolic filling in normal subjects. Am J Cardiol. 1987;59:971–974. doi: 10.1016/0002-9149(87)91136-2. [DOI] [PubMed] [Google Scholar]
- Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Normative reference values for the tissue Doppler imaging parameters of left ventricular function: a population-based study. Eur J Echocardiogr. 2010;11:51–56. doi: 10.1093/ejechocard/jep164. [DOI] [PubMed] [Google Scholar]
- Cohen GI, Pietrolungo JF, Thomas JD, Klein AL. A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol. 1996;27:1753–1760. doi: 10.1016/0735-1097(96)00088-5. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol. 2008;586:293–301. doi: 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalen H, Thorstensen A, Aase SA, Ingul CB, Torp H, Vatten LJ, Stoylen A. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr. 2010;11:176–183. doi: 10.1093/ejechocard/jep194. [DOI] [PubMed] [Google Scholar]
- Frey MAB, Kenney RA. Cardiac response to whole-body heating. Aviat Space Environ Med. 1979;50:387–389. [PubMed] [Google Scholar]
- Hatle L. Doppler echocardiographic evaluation of diastolic function in hypertensive cardiomyopathies. Eur Heart J. 1993;14(Suppl J):88–94. [PubMed] [Google Scholar]
- Kenney WL, Fowler SR. Methylcholine-activated eccrine sweat gland density and output as a function of age. J Appl Physiol. 1988;65:1082–1086. doi: 10.1152/jappl.1988.65.3.1082. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol Heart Circ Physiol. 1997;41:H1609–H1614. doi: 10.1152/ajpheart.1997.272.4.H1609. [DOI] [PubMed] [Google Scholar]
- Labovitz AJ, Pearson AC. Evaluation of left ventricular diastolic function: clinical relevance and recent Doppler echocardiographic insights. Am Heart J. 1987;114:836–851. doi: 10.1016/0002-8703(87)90795-2. [DOI] [PubMed] [Google Scholar]
- Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain–a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Medina-Ramón M, Zanobetti A, Cavanagh DP, Schwartz J. Extreme temperatures and mortality: assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ Health Perspect. 2006;114:1331–1336. doi: 10.1289/ehp.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol Regul Integr Comp Physiol. 1999;276:R203–R212. doi: 10.1152/ajpregu.1999.276.1.r203. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Sun H, Kopelen HA, Middleton KJ, Khoury DS. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol. 2001;37:278–285. doi: 10.1016/s0735-1097(00)01056-1. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Haykowsky MJ, Petersen SR, DeLorey DS, Cheng-Baron J, Thompson RB. Increased left ventricular twist, untwisting rates, and suction maintain global diastolic function during passive heat stress in humans. Am J Physiol Heart Circ Physiol. 2010;298:H930–H937. doi: 10.1152/ajpheart.00987.2009. [DOI] [PubMed] [Google Scholar]
- Onose Y, Oki T, Mishiro Y, Yamada H, Abe M, Manabe K, Kageji Y, Tabata T, Wakatsuki T, Ito S. Influence of aging on systolic left ventricular wall motion velocities along the long and short axes in clinically normal patients determined by pulsed tissue Doppler imaging. J Am Soc Echocardiogr. 1999;12:921–926. doi: 10.1016/s0894-7317(99)70144-6. [DOI] [PubMed] [Google Scholar]
- Pela G, Regolisti G, Coghi P, Cabassi A, Basile A, Cavatorta A, Manca C, Borghetti A. Effects of the reduction of preload on left and right ventricular myocardial velocities analyzed by Doppler tissue echocardiography in healthy subjects. Eur J Echocardiogr. 2004;5:262–271. doi: 10.1016/j.euje.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol. 2007;99:1629–1636. doi: 10.1016/j.amjcard.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630–633. doi: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol. 2003;91:780–784. doi: 10.1016/s0002-9149(02)03433-1. [DOI] [PubMed] [Google Scholar]
- Rowell L. Human Circulation: Regulation During Physical Stress. Oxford University Press; New York: 1986. [Google Scholar]
- Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969;27:673–680. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- Seals DR, Taylor JA, Ng AV, Esler MD. Exercise and aging: autonomic control of the circulation. Med Sci Sports Exerc. 1994;26:568–576. [PubMed] [Google Scholar]
- Shimizu Y, Uematsu M, Shimizu H, Nakamura K, Yamagishi M, Miyatake K. Peak negative myocardial velocity gradient in early diastole as a noninvasive indicator of left ventricular diastolic function: comparison with transmitral flow velocity indices. J Am Coll Cardiol. 1998;32:1418–1425. doi: 10.1016/s0735-1097(98)00394-5. [DOI] [PubMed] [Google Scholar]
- Stöhr EJ, González-Alonso J, Pearson J, Low DA, Ali L, Barker H, Shave R. Effects of graded heat stress on global left ventricular function and twist mechanics at rest and during exercise in healthy humans. Exp Physiol. 2011;96:114–124. doi: 10.1113/expphysiol.2010.055137. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, Tanaka N. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation. 1995;91:2582–2590. doi: 10.1161/01.cir.91.10.2582. [DOI] [PubMed] [Google Scholar]
- Tian Z, Li S, Zhang J, Guo Y. The characteristic of heat wave effects on coronary heart disease mortality in Beijing, China: a time series study. PLoS ONE. 2013;8:e77321. doi: 10.1371/journal.pone.0077321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52:631–646. doi: 10.1046/j.1365-2125.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Brothers RM, Tollund C, Dawson E, Nissen P, Yoshiga C, Jons C, Secher N, Crandall CG. Effect of thermal stress on Frank–Starling relations in humans. J Physiol. 2009;587:3383–3392. doi: 10.1113/jphysiol.2009.170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol. 2007;585:279–285. doi: 10.1113/jphysiol.2007.137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakado T, Takagi E, Okubo S, Imanaka-Yoshida K, Tarumi T, Nakamura M, Nakano T. Effects of aging on left ventricular relaxation in humans. Analysis of left ventricular isovolumic pressure decay. Circulation. 1997;95:917–923. doi: 10.1161/01.cir.95.4.917. [DOI] [PubMed] [Google Scholar]