Abstract
White matter abnormalities are implicated in major depressive disorder (MDD). As omega-3 polyunsaturated fatty acids (PUFAs) are low in MDD and affect myelination, we hypothesized that PUFA supplementation may alleviate depression through improving white matter integrity. Acutely depressed MDD patients (n=16) and healthy volunteers (HV, n=12) had 25-direction diffusion tensor imaging before and after 6 weeks of fish oil supplementation. Plasma phospholipid omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and omega-6 PUFA arachidonic acid (AA) levels were determined before and after supplementation using high-throughput extraction and gas chromatography and expressed as a percentage of total phospholipids (PUFA%). Fractional anisotropy (FA) was computed using a least-squares-fit diffusion tensor with non-linear optimization. Regression analyses were performed with changes in PUFA levels or Hamilton Depression Rating Scale scores as predictors, voxel-wise difference maps of FA as outcome, covariates age and sex, with family-wise correction for multiple comparisons. Increases in plasma phospholipid DHA% (but not EPA% or AA%) after fish oil predicted increases in FA in MDD but not HV, in a cluster including genu and body of the corpus callosum, and anterior corona radiata and cingulum (cluster-level p<0.001, peak t-score=8.10, p=0.002). There was a trend for greater change in FA in MDD responders over nonresponders (t=−1.874, df=13.56, p=0.08). Decreased depression severity predicted increased FA in left corticospinal tract and superior longitudinal fasciculus (cluster-level p<0.001, peak t-score=5.04, p=0.0001). Increased FA correlated with increased DHA% and decreased depression severity after fish oil supplementation suggests therapeutic effects of omega-3 PUFAs may be related to improvements in white matter integrity.
Keywords: Omega-3, DTI, PUFA, major depressive disorder, docosahexaenoic acid, fractional anisotropy
Graphical Abstract
Introduction
Major Depressive Disorder (MDD) is one of the top five causes of disability worldwide (1), with a lifetime prevalence of approximately 10 – 18% (2, 3). The cause of MDD is not known, although aberrant neurocircuitry and factors affecting brain health are active areas of research. Linking a causal mechanism to a treatment may help improve prognosis.
Abnormalities in white matter observed in MDD include hyperintensities seen on structural magnetic resonance imaging (MRI) (4, 5) and reduced myelin integrity as measured using magnetization transfer imaging (6). Similarly, post-mortem histopathologic studies have found altered deep white matter staining in MDD (7–9). White matter abnormalities could lead to diminished functional connections between brain regions and thereby contribute to depression symptomatology.
Microstructural changes of white matter within neural networks can be detected using diffusion tensor imaging (DTI) to quantify fractional anisotropy (FA), a measure of the directionality of water diffusion (10, 11). Healthy white matter generally has high anisotropy, because water movement in myelinated nerve fibers is primarily in the direction of the axon fiber bundles (11).
Abnormalities in FA of prefrontal (12–14), temporal (13, 15), and parietal (14) cortex and in anterior cingulate (16) are reported in MDD compared with healthy volunteers (HV). One large DTI study (n=132) that found differences between MDD and HV in regions including splenium, genu and body of the corpus callosum, superior longitudinal fasciculus, and anterior corona radiata, also found a negative correlation between depression severity and white matter integrity (17). In elderly depressed patients, FA impairment is associated with executive dysfunction (18). First-episode, medication-naïve (19–21) adults demonstrate similar deficits in frontal and parietal white matter, which negatively correlate with severity of the depressive symptoms (14). Adolescent MDD patients likewise exhibit white matter abnormalities and low FA in subgenual anterior cingulate cortex and amygdala (22).
One determinant of white matter health is the balance of lipids in the brain. For example, polyunsaturated fatty acids (PUFAs), key components of phospholipids in cell membranes, comprise 35% of lipids in the brain (23) and are critical for nervous system development and functioning (24–28). Highly unsaturated long-chain PUFAs arachidonic acid (AA, 20:4n-6) and docosahexaenoic acid (DHA, 22:6n-3), are the major constituents of brain PUFAs, and have been implicated in psychiatric illness, including major depression (29), bipolar disorder (30, 31) and suicide risk (32–34). Eicosapentaenoic acid (EPA, 20:5n-3), although present in considerably lower quantities in brain as a result of its rapid β-oxidation and metabolism (35–37), also is reported to have specific effects related to neuropsychiatric conditions (38–43).
Given that both reduced white matter integrity and lower omega-3 PUFAs are seen in MDD, we hypothesized that supplementation with omega-3 PUFAs would cause increased FA in MDD greater than HV, and that increased FA would correlate with improvement in depression symptoms. We used DTI in a prospective study to test effects of fish oil supplementation for 6 weeks on white matter integrity in MDD compared with HV, and to generate brain maps of correlations of FA with 1) plasma phospholipid PUFAs and 2) depression severity.
Methods and Materials
Sample
This study was approved by the Institutional Review Board of the New York State Psychiatric Institute in accordance with the latest version of the Declaration of Helsinki. After the procedures were fully explained, all subjects (n =28) gave written informed consent to participate in this research study, which included a positron emission tomography (PET) scan component (not discussed here). At study entry, 16 depressed adults, ages 22–50, met DSM-IV criteria (44) for a current major depressive episode in context of major depressive disorder (MDD) without any history of psychosis, and no drug or alcohol abuse within the past 2 months or drug or alcohol dependence (except nicotine) within the past 6 months, based on the Structured Clinical Interview for DSM-IV (45). Patients were not actively suicidal, had not received electroconvulsive therapy within the past 6 months, and presented with scores between 16 and 25, inclusive, on the 17-item Hamilton Depression Rating Scale (HDRS) (46, 47) at study entry. MDD participants were permitted to be on a single antidepressant or were medication-free and had no history of antipsychotic medications or mood stabilizers within 6 weeks; no washouts were performed. HV (n=12) had no history of Axis I or Axis II illness. Participants in both groups did not have active medical illness based on history, physical examination and laboratory tests, and did not report more than occasional use of non-steroidal anti-inflammatory drugs (NSAIDs) or other medications known to interfere with the arachidonic acid pathway, including no use of omega-3 supplements within 3 months. Females were premenopausal. All participants were assessed for self-reported handedness.
PUFA supplementation
After the initial assessments and DTI scans, all participants received dietary supplementation daily with gelcaps containing a highly-purified, commercially available mixture of fatty acids from fish oil derived from anchovies, sardines and mackerel (OmegaLife-3, Unicity International, Inc., Orem, UT) for approximately six weeks. Participants took 4 gelcaps/day amounting to 4 g of total fish oil/d, including EPA, 1.6 g/d; DHA, 0.8 g; 0.8 mg saturated fat; inactive ingredients gelatin and glycerin; d-alpha tocopheryl 20 IU for stability; and orange oil to increase palatability. These doses and the EPA/(EPA+DHA) ratio of 67% EPA were consistent with those found effective in placebo-controlled, randomized clinical trials of fish oil supplementation as a treatment of depression (41). The six-week supplementation period was chosen in order to mitigate potential attrition over time, since it was important to obtain an additional scan at the end of the treatment; and taking into account several clinical trials that demonstrated separation from placebo as early as three (48) or four (49, 50) weeks.
PUFA purification
Plasma from fasting blood samples was obtained within 3 weeks of the DTI scan and shipped on dry ice to the Nathan S. Kline Institute for Psychiatric Research (Orangeburg, NY) for biochemical analysis.
Plasma phospholipid PUFA levels were determined using a modified version of the rapid, high-throughput protocol of Glaser et al (51). Briefly, plasma proteins were precipitated in cold methanol, glycerophospholipid fatty acids were selectively esterified with sodium methoxide and acidified, and fatty acid methyl esters (FAMEs) were extracted in hexane. Separation and quantitation of FAMEs were accomplished via gas chromatography with flame ionization detection as described previously (52), and individual PUFA species are reported as a percentage of total plasma phospholipid PUFAs.
Image acquisition
MRI images were acquired on a 3.0T Signa Advantage system (GE Healthcare, Waukesha, WI, USA). Anatomical T1-3D images were acquired with the following parameters: echo time (TE) = 2.8 ms, repetition time (TR) = 7.1 ms, field of view (FOV) 256×256 mm2, matrix size=256×256, slice thickness=1 mm (voxel size 1×1×1 mm3), number of slices=178, with an acquisition time of 5 minutes. Diffusion images were acquired using a single-shot EPI (echo planar imaging) sequence. Scan parameters were as follows: TR = 14000 ms, TE = 82 ms, flip angle 90 degrees, slice thickness=3 mm, Number of Excitation for signal averaging (NEX) = 1, FOV (field of view) = 240×240 mm2, voxel dimensions = 0.95×0.95×3 mm, acquisition matrix=256×256, b value = 1000 s/mm2, and 25 collinear directions with 5 non-weighted images. DTI scan time was approximately 11 minutes.
Image processing
Each DTI image underwent a series of quality assurance tests for common artifacts, including ghost, ring, slice-wise intensity, venetian blind, and gradient-wise motion artifacts (53). Diffusion images were corrected for distortion induced by gradient coils and simple head motion using the eddy current correction routine from the FMRIB’s Diffusion Toolbox (FSL, http://fsl.fmrib.ox.ac.uk/fsl/fsl-4.1.9/fdt/) with default settings. Following this, Camino (http://web4.cs.ucl.ac.uk/research/medic/camino/pmwiki/-pmwiki.php) (54) was used to estimate FA, computing the least-squares-fit diffusion tensor with non-linear optimization using a Levenburg-Marquardt algorithm, constrained to be positive by fitting its Cholesky decomposition. The individual FA maps were aligned into the up-sampled version (91×109×91 voxel, 2×2×2 mm3/voxel) of the common FMRIB58 FA template (www.fmrib.ox.ac.uk/fsl/data/FMRIB58_FA) using FSL’s FMRIB Nonlinear Image Registration Tool (FNIRT) (55, 56).
Global Tractography
We performed global tractography using findings from the FA analysis as a seed, in order to visualize regions of gray matter subserved by the white matter region where the change in FA (ΔFA) correlated positively with change in DHA% (ΔDHA%) among MDD but not HV. DTI was obtained after performing Insight Segmentation and Registration Toolkit (ITK, National Library of Medicine, http://www.itk.org)-based tensor reconstruction (57) on the preprocessed diffusion weighted images. The eddy-corrected diffusion weighted images were processed through MITK-Diffusion (58), which implements the Gibbs Tracking Algorithm (59), a global tractography method that reconstructs all brain fibers simultaneously while searching for a global optimum (56), and has outranked other tractography algorithms (60). The subset of tracts that passed through the chosen seed were extracted, and brain regions connected via the extracted tracts were identified based on individual brain atlases derived from running Freesurfer’s surface-based reconstruction pipeline (http://surfer.nmr.mgh.harvard.edu) on the T1-weighted anatomical image.
Statistical Analyses
Sample
For demographic and clinical characterizations, MDD and HV groups were compared with respect to sex, age, race, body mass index (BMI), and income, and also with regard to plasma phospholipid PUFA concentrations before and after fish oil supplementation, and percentage change over the course of supplementation. Improvement in depression severity after supplementation was assessed with a paired t-test in HDRS scores within the MDD group. Within the depressed group, clinical responders were defined as having achieved at least a 50% improvement in HDRS scores over the course of supplementation. Analyses were performed using IBM SPSS Statistics (version 23, Armonk, NY).
Fractional Anisotropy
For voxel-based analysis, an inclusion mask for white matter was created using the FA standard template by thresholding, which excluded all voxels with FA values > 0.2. Analysis was performed using Statistical Parametric Mapping (SPM8, v4290) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) with Matlab (version 7.14, Mathworks, Natick, Massachusetts) on a 64 bit iMac with OS X 10.7.5. For these analyses, all images were smoothed with an isotropic Gaussian kernel with full width half maxima (FWHM) = 2 mm. This relatively small FWHM was chosen as it adequately removed conspicuous noise without introducing any apparent partial volume effect. The Gaussian kernel was utilized as the FA images had a signal-to-noise ratio (SNR) greater than 2.0, a level at which the noise distribution is more nearly approximated by Gaussian than Rician distribution (61).
A 2-way repeated measures ANCOVA was performed with the independent factors of diagnosis (2 levels, MDD vs. HV), and time point (2 levels: pre and post supplementation), dependent factor of FA, and age as a covariate.
For pre-post correlation analysis, pre-supplementation FA maps were subtracted from post-supplementation FA maps, and these voxel-wise difference maps were submitted to separate multiple regression analyses with post-supplementation minus pre-supplementation DHA%, AA%, or EPA% levels as predictor variables, covarying for age and sex. The PUFA change scores (ΔPUFAs) were tested and found to be normally distributed, so log-transformation was not required. For voxel-wise analyses, uncorrected p<0.01 at voxel level and cluster-level p<0.05 corrected for multiple comparisons with family-wise error (FWE), were used to determine statistical significance. Results were not further corrected for the multiple comparisons due to testing for three different PUFAs.
Additionally, separate regression analyses were performed in the MDD group with either pre-treatment HDRS as predictor, and pre-treatment FA as the outcome measure; or pre- post-supplementation change in HDRS (ΔHDRS) scores as predictor, and pre- to post-treatment ΔFA as the outcome measure. Age and sex were covariates of no interest using the same statistical thresholds as in the analyses with PUFAs as predictors. Post-hoc analyses compared responders to nonresponders with respect to ΔFA in the region of maximal correlation between ΔPUFAs and ΔFA.
Another post-hoc analysis quantified the observed brain regions that were common to ΔFA correlating with ΔHDRS, and ΔFA correlating with ΔDHA%. This was achieved by taking the intersection between the ΔFA by ΔHDRS and the ΔFA by ΔDHA% regression analyses. For this exploratory analysis, a less conservative a priori statistical threshold was set as uncorrected p<0.05 at voxel level and FWE-corrected p<0.05 at cluster level.
Additional exploratory analyses are found in the Supplemental Material, namely an assessment of radial and axial diffusivity, and a mediation analysis testing whether ΔFA mediated the association between ΔDHA% and ΔHamilton Depression scores after treatment.
Results
Sample
As detailed in Table 1, MDD and HV groups did not differ with respect to sex, age, or other demographic characteristics examined, although the MDD group trended toward a higher percentage of white participants. Participants were adults ages 22–50. Depressed participants had not taken psychotropic medications for at least 14 days prior to PET studies with the exception of three participants (one on sertraline, one on duloxetine, and one on mirtazapine plus clonazepam). MDD participants were mildly-moderately depressed at the time of the first PET scan (mean HDRS score = 17.8 ± 3.85 SD). Three MDD participants had a history of suicide attempt. Following fish oil supplementation, five participants were determined to be clinical responders, defined as ≥ 50% reduction in HDRS scores after PUFA supplementation. None of the responders were taking any antidepressant medications.
Table 1.
Demographic and clinical characters of the research participants, including plasma phospholipid concentrations before and after fish oil supplementation.
| Characteristic | MDD (n=16) |
HV (n=12) |
Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | df |
p- value |
|||||||
| Sex (% male) | 31.3% | 41.7% | 0.324 | 1 | 0.569 | ||||
| Race (% white) | 68.8% | 33.3% | 3.458 | 1 | 0.063 | ||||
| aHandedness (% right) | 80.0% | 91.7% | 0.605 | ||||||
| aTobacco use (% smokers) | 12.5% | 0% | 0.492 | ||||||
| mean (SD) | mean (SD) | t-score | df |
p- value |
|||||
| Age (yrs) | 34.4 (8.2) | 30.9 (7.9) | 1.117 | 26 | 0.274 | ||||
| BMI (kg*m−2) | 25.1 (3.4) | 25.7 (6.4) | −0.330 | 26 | 0.744 | ||||
| Education (yrs) | 15.4 (2.2) | 15.0 (1.8) | −0.710 | 26 | 0.484 | ||||
|
mean (SD) |
median | IQR |
mean (SD) |
median | IQR | z-score |
p- value |
||
| bIncome (US $1,000/yr) | 41.1 (38.8) |
26 | 52.7 | 23.2 (13.2) |
19 | 16.7 | −0.612 | 0.540 | |
|
Plasma phospholipid PUFAs (w/w%) |
mean (SD) | mean (SD) | t-score | df |
p- value |
||||
| Pre-supplementation | |||||||||
| DHA% | 3.26 (0.81) | 2.92 (0.53) | 1.282 | 26 | 0.211 | ||||
| EPA% | 0.72 (0.30) | 0.73 (0.26) | −0.248 | 26 | 0.806 | ||||
| AA% | 11.82 (2.39) | 12.0 (1.62) | −0.228 | 26 | 0.821 | ||||
| Post-supplementation | |||||||||
| DHA% | 4.90 (0.97) | 4.28 (1.23) | 1.510 | 26 | 0.143 | ||||
| EPA% | 3.02 (1.86) | 3.44 (2.43) | −0.522 | 26 | 0.606 | ||||
| AA% | 10.36 (2.09) | 10.52 (1.42) | −0.222 | 26 | 0.826 | ||||
| Percentage change | |||||||||
| ΔDHA% | 159.81 (56.51) | 148.38 (42.50) | 0.586 | 26 | 0.563 | ||||
| ΔEPA% | 488.47 (322.61) | 553.82 (549.86) | −0.395 | 26 | 0.696 | ||||
| ΔAA% | 88.85 (15.99) | 88.66 (14.50) | 0.033 | 26 | 0.974 | ||||
| FA (range:0–1) | mean (SD) | mean (SD) | t-score | df |
p- value |
||||
| Pre-supplementation | 0.36 (0.02) | 0.38 (0.01) | −1.994 | 26 | 0.057 | ||||
| Post-supplementation | 0.36 (0.03) | 0.38 (0.01) | −1.522 | 26 | 0.140 | ||||
Fisher’s exact test (2-sided).
Mann-Whitney test.
Abbreviations: AA%, DHA%, EPA%, arachidonic acid, docosahexaenoic acid, eicosapentaenoic acid, respectively, as a percentage of plasma phospholipids; df,degrees of freedom; FA, fractional anisotropy; HV, healthy volunteers; IQR, Inter-quartile range; MDD, major depressive disorder; PUFAs, polyunsaturated fatty acids; SD, standard deviation.
Supplementation caused significant increases in DHA% and EPA%, and decreases in AA%, that were comparable in magnitude in both MDD and HV groups (see Table 1). No group differences were observed in plasma phospholipid concentrations of PUFA before or after supplementation, nor did the magnitude of ΔPUFA differ between groups. MDD group depression levels were of moderate severity before PUFA supplementation. Depressed participants improved significantly with supplementation (pre-supplementation HDRS mean score 17.8 ± 3.9; post-supplementation mean score 11.5 ± 5.9; t-score=3.981, df=15, p=0.001); and the 31% who were responders had higher final DHA% levels than nonresponders (t=2.414, df=14, p=0.03).
Fractional Anisotropy
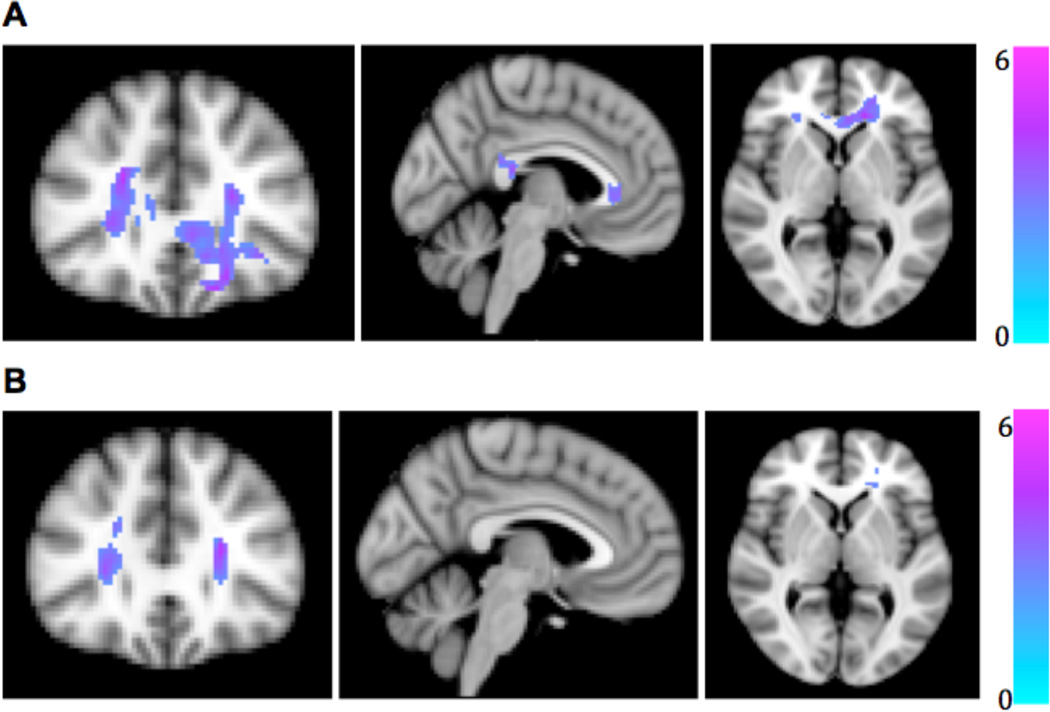
Group differences were seen in regional FA at the applied statistical thresholds after correction for age and sex, at both pre-supplementation (peak voxel, MNI −18, 38, −18, peak-level t-score = 5.97, observed cluster size = 1041 voxels, p<0.001) and post-supplementation (peak voxel, MNI 24, 26,12, peak-level t-score =3.90, observed cluster size = 263 voxels, p=0.026) timepoints (Figure 1). FA in MDD was lower than in HV in genu and splenium of corpus callosum, anterior corona radiata bilaterally, and right superior longitudinal fasciculus before supplementation (Figure 1A). After supplementation, however, the regions of group difference were reduced in extent, such that only anterior corona radiata still showed lower FA in MDD than HV (Figure 1B), suggesting a possible mitigation of abnormal FA by omega-3 PUFA treatment. There were no within-group differences between pre- and post-supplementation total FA in either MDD or HV (Table 1).
Figure 1.
Lower fractional anisotropy (FA) in patients with major depressive disorder compared to healthy volunteers (A) before and (B) after fish oil supplementation for six weeks. Affected regions are displayed on an MNI T1 template with corresponding t-score color bar.
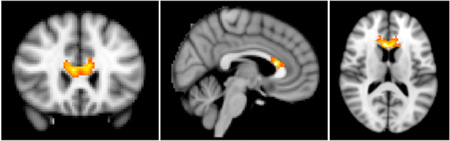
In regression models, for the depressed group, plasma phospholipid DHA% positively correlated with FA in the body of the corpus callosum prior to supplementation (peak voxel, MNI −10, −42, 24, peak-level t-score=5.27, observed cluster size = 679 voxels, cluster-level p<0.001; Figure 2A). After supplementation, the increase in plasma phospholipid DHA% correlated with increase in FA in MDD more anteriorly in a region encompassing genu and body of corpus callosum, and anterior corona radiata and cingulum bilaterally (peak voxel, MNI 4,20,12, peak-level t-score = 5.97, observed cluster size = 525 voxels, cluster-level p<0.001; Figure 2B).
Figure 2.
Correlations between fractional anisotropy (FA) and plasma phospholipid docosahexaenoic acid as a percentage of total plasma phospholipid PUFAs (DHA%) in patients with major depressive disorder. A. FA positively correlates with DHA% before fish oil supplementation. B. Change in FA positively correlates with change in DHA after fish oil supplementation. Affected regions are displayed on MNI T1 template with corresponding t-score color bar.
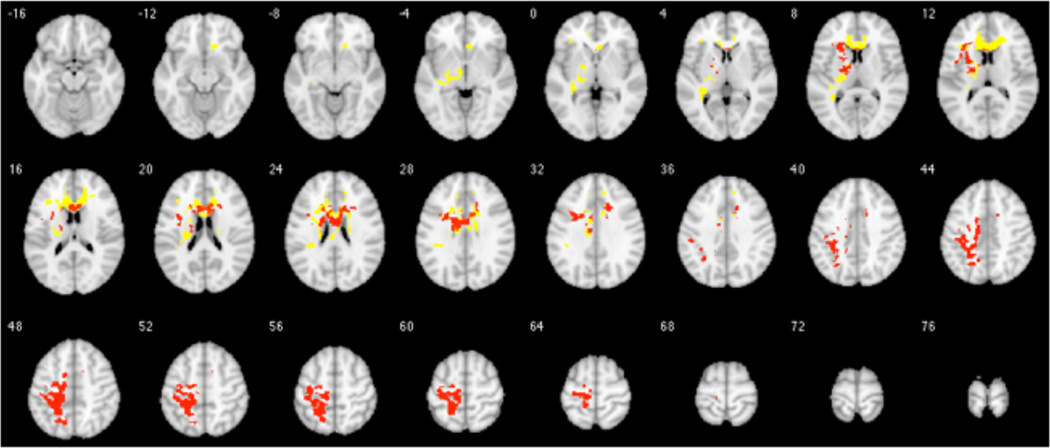
In the depressed group, there were no correlations between baseline or post-supplementation HDRS scores and FA at those timepoints. However, ΔHDRS scores correlated positively with ΔFA in left corticospinal tract and longitudinal fasciculus (peak voxel, MNI −34, −26, 62, peak-level t-score=5.04, observed cluster size = 517 voxels, p<0.001). This region overlapped with 17 percent of voxels from the cluster in which ΔDHA% correlated with ΔFA (Figure 4).
Figure 4.
Intersection between parametric brain maps of change in fractional anisotropy (ΔFA) correlated with change in plasma phospholipid docosahexaenoic acid as a percentage of total plasma phospholipid PUFAs (ΔDHA%) (yellow) and with change in depression severity scores (ΔHDRS) (red), in the MDD group. Depression severity is measured with the 17-item Hamilton Depression Rating Scale Using xjView toolbox (http://www.alivelearn.net/xjview), SPM-derived t-score maps are superimposed on a series of transaxial slices [4 mm apart] of a coregistered anatomical MRI template. For this exploratory analysis, a less conservative statistical threshold was set a priori as uncorrected p<0.05 at voxel level and FWE-corrected p<0.05 at cluster level.
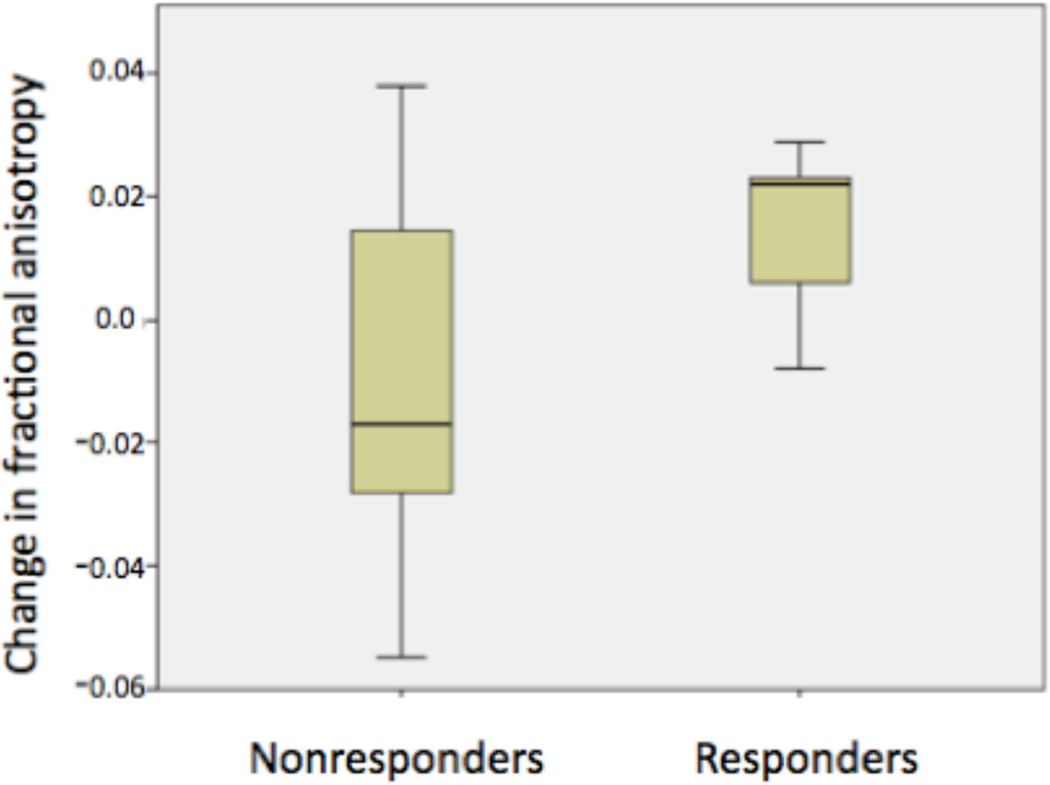
Post-hoc analyses comparing MDD responders to nonresponders in the identified ΔDHA% − ΔFA correlation region found that 80% of responders (4/5) showed an increase in FA after supplementation, compared with only 45% (5/11) of non-responders (Figure 5). The group difference between MDD responders and nonresponders was at a trend level in this small sample (t=−1.874, df=13.56, p=0.08).
Figure 5.
Comparison of change in fractional anisotropy (ΔFA) between MDD clinical responders and nonresponders, in the region in which change in fractional anisotropy (ΔFA) correlated positively with change in plasma phospholipid docosahexaenoic acid as a percentage of total plasma phospholipid PUFAs (ΔDHA%). (t=−1.874, df=13.56, p=0.08)
No correlations were seen between increased DHA% and ΔFA in the HV group. Neither AA% nor EPA% levels predicted changes in FA in either group.
Whole Brain Tractography
In the voxel cluster where increased DHA% correlated positively with increased FA in MDD (including portions of corpus callosum, anterior corona radiata, and cingulum) as a seed region, white matter tracts passing through the seed mapped bilaterally to cortical regions comprising rostral middle frontal and superior frontal gyri (Figure 3).
Figure 3.
Global tractography using seed regions in which change in fractional anisotropy correlated positively with change in plasma phospholipid docosahexaenoic acid as a percentage of total plasma phospholipid PUFAs (DHA%) for major depressive disorder. Tractography results from one representative healthy volunteer are shown here, superimposed on the same individual’s structural MRI. Red - left to right; blue – inferior to superior; green – anterior to posterior.
Discussion
This is the first reported DTI study of PUFA supplementation effects on white matter in MDD. We found that white matter deficits in MDD, relative to HV, improved after six weeks of fish oil supplementation, as defined by increased FA in a single voxel cluster. Moreover, although changes were seen in plasma phospholipid levels of all three PUFAs, only the DHA% increases correlated with brain FA increases, and only in the MDD group, with a trend toward greatest FA increases in clinical responders. We also observed that the brain region in which improved depression correlated with increased FA overlapped with the region in which increased DHA% correlated with increased FA.
The pre-supplementation MDD deficits in FA relative to the HV group were not simply a function of lower baseline omega-3 PUFA levels in MDD, since although previous studies comparing MDD to HV found that depressed patients have lower omega-3 PUFA concentrations (29), in this sample MDD and HV had comparable levels prior to supplementation. This may have been due to the modest level of depression severity in this particular sample, as some studies have reported an inverse association between depression severity and plasma (62) or erythrocyte phospholipid (63) levels of EPA. Additionally, our sample had a low percentage of suicide attempters (2%), and suicide attempt history has been linked to lower EPA and DHA levels (32).
Although age-related decreases in FA have been reported [Salami et al., 2012], this potential confound was addressed by including age as a covariate in the analyses. Moreover, concern about age-related effects was mitigated by the fact that there were no participants over 50 yrs old.
Supplementation with fish oil was associated with a moderate antidepressant effect, as about one third of the patients were responders. Given the correlation observed between changes in FA and improvement in depression severity in the responder group, we could speculate that in a subset of depressed patients, clinical response to omega-3 PUFAs might depend on the degree of increase in FA. However, the lack of complete congruity between parametric maps of ΔFA correlating with ΔDHA% and ΔFA correlating with ΔHDRS suggests that other factors contribute. It is also possible that improved white matter integrity may relate to cognitive subdomains of depression symptoms not captured by the HDRS.
Neuroanatomically, our findings of abnormal FA in corpus callosum, anterior radiata and superior longitudinal fasciculus in major depression comport closely with previous results of Cole et al. (17). These structures mediate three different dimensions of communication within the brain: interhemispheric (corpus callosum), cortical-cortical (superior longitudinal fasciculus) and cortical-brainstem (anterior radiata). Structural changes in corpus callosum have been repeatedly implicated in depressive illness (64–68), and lower FA has been reported in MDD in the superior longitudinal fasciculus (14, 21), a long association pathway running from parietal lobe to premotor and prefrontal cortices, including the dorsolateral prefrontal cortex. The relatively greater size of these three white matter tracts may confer a higher statistical power to detect FA changes there even in small samples. Future, larger studies might have the power to quantify more nuanced associations with respect to FA in finer white matter tracts.
The gray matter regions subserved by these tracts, as mapped out by whole brain tractography, also are consistent with our previous positron emission tomography (PET) findings in a separate sample of MDD, in which plasma phospholipid levels of DHA%, but not EPA%, correlated negatively with relative regional uptake of glucose (rCMRglu) in cingulate, middle frontal, inferior frontal, and superior frontal gyri (69).
Among PUFAs tested, only increases in DHA% correlated with increases in FA in the MDD group, consistent with DHA’s role as the predominant omega-3 species in brain. Rat studies indicate that although EPA and DHA enter the brain at similar rates, most of the EPA is rapidly β-oxidized, and is recycled into brain phospholipids to a much lower extent than DHA, resulting in a much higher DHA concentration in brain (37).
Counterintuitively, however, in clinical trials EPA appears to have greater therapeutic value than DHA, for acute treatment of major depression (39, 41, 42). Suggested explanations for EPA effects in depression have included its peripheral anti-inflammatory effects (70, 71), actions of EPA metabolites (72), or direct effects on cerebral capillaries (73). However, in order to be consistent with both hypotheses that EPA is the active antidepressant agent and that brain DHA has effects on depression through increasing FA, we would need to postulate that increased peripheral EPA facilitates plasma DHA entry into brain in a manner superior to directly providing DHA supplements. This has not been proven, although it is true that most dietary EPA is taken up by the liver, where one fate is conversion to bioactive DHA (73). Alternatively, the explanation may lie in the relative proportions of unesterified DHA and EPA, not measured here, as unesterified PUFAs cross the blood-brain barrier most readily (74, 75).
Our ability to discern PUFA-related structural brain changes over a 6-week period is temporally consistent with another study (76), in bipolar disorder, in which omega-3 PUFA treatment of 4 weeks’ duration resulted in MRI changes in brain water proton transverse relaxation times (T2), that, like DTI (77), reflect myelin content and changes in water environments (78).
Studies in other psychiatric populations have found links between PUFA levels and white matter integrity. For example, total PUFA concentration correlated with FA in the bilateral uncinate fasciculus of young adult males with a recent-onset psychotic disorder (79). In a later study by the same group (80), lower total PUFA concentrations in men with early-phase psychosis correlated with lower FA in the corpus callosum, and bilateral parietal, occipital, temporal, and frontal white matter tracts. In contrast to our findings in MDD, in the group of psychotic males lower concentrations of arachidonic acid (AA), nervonic acid (24:1n-9), and docosapentaenoic acid (22:5n-3), but not DHA, directly correlated with lower FA (80).
The importance of PUFA status to brain function may be due in part to the effects of PUFA composition on myelin. Studies in rats find that lower omega-3 PUFA intake causes abnormalities of myelin (81), and that omega-3 PUFA administration stimulates expression of myelin proteins (82). Experimental traumatic brain injury studies in rodents support this link between PUFAs and myelination. Following spinal cord injury, white matter damage is prevented by injection of DHA; progressive protective effects are induced over a 6-week period, including increased synaptic formation and repair and reduced myelin damage (83). In addition, dietary supplementation with EPA and DHA prior to impact acceleration brain injury reduces the number of axons positive for beta amyloid precursor protein (APP), a marker of brain injury, at 30 days post-injury, to amounts comparable to those in uninjured rodents (84). Furthermore, a PUFA-enriched diet prevents post-injury loss of myelin, preserving the integrity of the myelin sheath, and maintaining the nerve fiber conductivity (85). In a different paradigm, maternal omega-3 fatty acid supplementation protects the neonatal rat brain from white matter injury due to lipopolysaccharide exposure (86).
Evidence from human populations also indicates a relationship between PUFA and myelination. In elderly people, dietary intake of fish with higher EPA and DHA concentrations was prospectively linked over a five-year interval to fewer sub-clinical infarcts and fewer white matter abnormalities on MRI (87), and plasma DHA levels were inversely associated with white matter hyperintensity volumes (a marker of white matter damage) and cognitive impairments, although inexplicably, depression weakened the association (88). In a small (n=16) prospective open intervention study of multiple sclerosis patients, dietary advice and omega-3 PUFA supplementation plus vitamins resulted in higher plasma omega-3 PUFA levels and a lower rate of exacerbations and decreased disability over a two-year period (89). EPA administration also has been found to reduce brain atrophy over 6–9 months in one case of a treatment-resistant depressed patient (90) and in a small placebo-controlled study in patients with advanced Huntington’s disease (91).
Limitations
Our findings should be interpreted cautiously in view of the small sample size of this study, although this is mitigated to some extent by its within-subject, prospective study design. The prospective nature of the study suggests that white matter changes are due to fish oil supplementation; however, we note that due to the lack of placebo group, we cannot rule out some other factor at work. Some group differences in PUFA and FA may have been undetected due to low statistical power. We were not able to parse out possible effects of medications taken by 3 patients in this small sample. The range of participants’ ages is relatively narrow, so these results may not apply to older or younger populations. PUFA determinations were not made on the day of the DTI, which may add noise to the data. Dietary absorption might be affected by different formulations of omega-3 PUFAs, e.g. the bioavailability of triglyceride-associated PUFAs predominant in fish oil is reportedly lower than phosphoglyceride-associated PUFAs in as in krill oil (92). However, krill meal, which also contains DHA and EPA bound to phospholipids, has similar bioavailability to fish oil, suggesting the triglyceride-phospholipid difference may not be the main arbiter of absorption (92). Free (unesterified) fatty acids also have been suggested to have superior bioavailability (93) but are a target for oxidation that may result in breakdown and in gastrointestinal side-effects (94). Other factors influencing bioavailability include food consumed with the supplements, matrix effects (e.g. capsule composition) and galenic formulation (oils vs. emulsion) (94). The relative importance of these factors for delivery of PUFAs into brain is unknown, as the primary circulatory carriers, lipoproteins and albumin, transport omega-3 PUFAs derived from both triglycerides and phospholipids [reviewed in (95)]. There is an inexact correspondence between plasma and brain concentrations, due to unmeasured effects of the blood-brain barrier. Different results might be obtained if PUFA status were assessed with different measures, such as plasma or erythrocyte levels, unesterified state, or percentage of total omega-3 PUFAs.
Conclusions
Our observations replicate previous findings that corpus callosum and anterior corona radiata are regions of vulnerability in MDD (17), and suggest that omega-3 PUFA supplements have restorative effects on white matter integrity that may relate to antidepressant efficacy in some patients. Additional, larger placebo-controlled studies are needed to replicate these findings and test whether omega-3 PUFA-induced enhancement of white matter integrity can cause improvements in specific depression symptoms such as cognitive deficits.
Supplementary Material
Highlights.
First DTI study examining the effects of fatty acids on white matter in major depression
White matter deficits in depression improved after 6 wks of fish oil supplementation
Docosahexaenoate and fractional anisotropy changes positively correlate in depression
Acknowledgments
Drs. Mann and Oquendo receive royalties for commercial use of the C-SSRS from the Research foundation for Mental Hygiene. Dr. Mann received past unrelated grants from Novartis and GSK. Dr. Oquendo received unrestricted educational grants and/or lecture fees from Astra-Zeneca, Bristol-Meyers Squibb, Eli Lilly, Janssen, Otsuka, Pfizer, Sanofi-Aventis, and Shire as well as financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to the current manuscript. In addition, Dr. Oquendo’s family owns stock in Bristol Myers Squibb.
Role of funding source
This work was funded by K-08 MH079033 (PI:Sublette) and R01 MH48514 (PI:Oquendo). Omega-3 PUFA supplements were donated by Unicity, International, Inc. The funding sources had no involvement in the study design, data collection and analysis, or interpretation of results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These data were presented previously as a poster at the 2014 Society for Biological Psychiatry Conference.
Dr. Dongrong Xu performed a much-appreciated critical reading of the manuscript.
Conflict of Interest
Other authors have no conflicts of interest to report.
Contributors
Mr. Chhetry and Ms. Hezghia contributed equally. Mr. Chhetry performed the image analyses and wrote the methods and results sections. Ms. Hezghia performed the literature search and wrote the first draft. Dr. Miller oversaw the image analysis and participated in interpretation of results. Dr. Lee was the statistician. Mr. Rubin-Falcone performed portions of the image analysis and wrote portions of the methods text. Mr. Cooper performed the biochemical analyses. Research participants were assessed through Dr. Oquendo's comprehensive assessment protocol, and Drs. Oquendo and Mann participated in interpretation of results. Dr. Sublette was the PI on the imaging protocol and oversaw project implementation, data analysis and interpretation. All authors participated in writing or critically reading, and editing of the manuscript.
References
- 1.Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. The British journal of psychiatry : the journal of mental science. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 2.Williams DR, Gonzalez HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305–315. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA : the journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 4.Coffey CE, Figiel GS, Djang WT, Weiner RD. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- 5.Coffey CE, Wilkinson WE, Weiner RD, Parashos IA, Djang WT, Webb MC, et al. Quantitative cerebral anatomy in depression. A controlled magnetic resonance imaging study. Arch Gen Psychiatry. 1993;50:7–16. doi: 10.1001/archpsyc.1993.01820130009002. [DOI] [PubMed] [Google Scholar]
- 6.Gunning-Dixon FM, Hoptman MJ, Lim KO, Murphy CF, Klimstra S, Latoussakis V, et al. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2008;16:255–262. doi: 10.1097/JGP.0b013e3181602a66. [DOI] [PubMed] [Google Scholar]
- 7.Thomas AJ, O'Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 8.Thomas AJ, O'Brien JT, Barber R, McMeekin W, Perry R. A neuropathological study of periventricular white matter hyperintensities in major depression. J Affect Disord. 2003;76:49–54. doi: 10.1016/s0165-0327(02)00064-2. [DOI] [PubMed] [Google Scholar]
- 9.Regenold WT, Phatak P, Marano CM, Gearhart L, Viens CH, Hisley KC. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Res. 2007;151:179–188. doi: 10.1016/j.psychres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in biomedicine. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 11.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. Journal of magnetic resonance imaging : JMRI. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 12.Shimony JS, Sheline YI, D'Angelo G, Epstein AA, Benzinger TL, Mintun MA, et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66:245–252. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Q, Huang X, Hong N, Yu X. White matter microstructural abnormalities in late-life depression. International psychogeriatrics / IPA. 2007;19:757–766. doi: 10.1017/S1041610207004875. [DOI] [PubMed] [Google Scholar]
- 14.Zou K, Huang X, Li T, Gong Q, Li Z, Ou-yang L, et al. Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:525–530. [PMC free article] [PubMed] [Google Scholar]
- 15.Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, et al. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77:120–122. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 17.Cole J, Chaddock CA, Farmer AE, Aitchison KJ, Simmons A, McGuffin P, et al. White matter abnormalities and illness severity in major depressive disorder. Br J Psychiatry. 2012;201:33–39. doi: 10.1192/bjp.bp.111.100594. [DOI] [PubMed] [Google Scholar]
- 18.Murphy CF, Gunning-Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, et al. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma N, Li L, Shu N, Liu J, Gong G, He Z, et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry. 2007;164:823–826. doi: 10.1176/ajp.2007.164.5.823. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Ma N, Li Z, Tan L, Liu J, Gong G, et al. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. 2007;1168:124–128. doi: 10.1016/j.brainres.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 21.Wu F, Tang Y, Xu K, Kong L, Sun W, Wang F, et al. Whiter matter abnormalities in medication-naive subjects with a single short-duration episode of major depressive disorder. Psychiatry Res. 2011;191:80–83. doi: 10.1016/j.pscychresns.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49:173–183. e171. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benatti P, Peluso G, Nicolai R, Calvani M. Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J Am Coll Nutr. 2004;23:281–302. doi: 10.1080/07315724.2004.10719371. [DOI] [PubMed] [Google Scholar]
- 24.Luchtman DW, Song C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies. Neuropharmacology. 2013;64:550–565. doi: 10.1016/j.neuropharm.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res. 2001;40:1–94. doi: 10.1016/s0163-7827(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 26.Gerster H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res. 1998;68:159–173. [PubMed] [Google Scholar]
- 27.Singh M. Essential fatty acids, DHA and human brain. Indian J Pediatr. 2005;72:239–242. [PubMed] [Google Scholar]
- 28.Spector AA. Essentiality of fatty acids. Lipids. 1999;34(Suppl):S1–S3. doi: 10.1007/BF02562220. [DOI] [PubMed] [Google Scholar]
- 29.Lin PY, Huang SY, Su KP. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Rapoport SI. Lithium and the Other Mood Stabilizers Effective in Bipolar Disorder Target the Rat Brain Arachidonic Acid Cascade. ACS chemical neuroscience. 2014 doi: 10.1021/cn500058v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sublette ME, Russ MJ, Smith GS. Evidence for a role of the arachidonic acid cascade in affective disorders: a review. Bipolar Disord. 2004;6:95–105. doi: 10.1046/j.1399-5618.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 32.Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, et al. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Lewis MD, Hibbeln JR, Johnson JE, Lin YH, Hyun DY, Loewke JD. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. J Clin Psychiatry. 2011 doi: 10.4088/JCP.11m06879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 35.Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins, leukotrienes, and essential fatty acids. 2009;80:157–163. doi: 10.1016/j.plefa.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Chen CT, Liu Z, Bazinet RP. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: an intracerebroventricular study. Journal of neurochemistry. 2011;116:363–373. doi: 10.1111/j.1471-4159.2010.07116.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen CT, Bazinet RP. beta-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. PLEFA. 2014 doi: 10.1016/j.plefa.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Beier AM, Lauritzen L, Galfalvy HC, Cooper TB, Oquendo MA, Mann JJ, et al. Low Plasma Eicosapentaenoic Acid Levels are Associated with Elevated Trait Aggression and Impulsivity in Major Depressive Disorder with a History of Comorbid Substance Use Disorder. J Psychiatr Res. 2014 doi: 10.1016/j.jpsychires.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2009;28:525–542. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- 40.Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17:1144–1149. doi: 10.1038/mp.2012.25. [DOI] [PubMed] [Google Scholar]
- 41.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011 doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin PY, Mischoulon D, Freeman MP, Matsuoka Y, Hibbeln J, Belmaker RH, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry. 2012;17:1161–1163. doi: 10.1038/mp.2012.111. author reply 1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21. doi: 10.1186/1476-511X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSMIV) Washington, D.C.: 1994. [Google Scholar]
- 45.First M, Williams J, Spitzer R, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington D.C.: American Psychiatric Publishing, Inc.; 1997. [Google Scholar]
- 46.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nemets B, Stahl Z, Belmaker R. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry. 2002;159:477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 49.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 50.Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13:267–271. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 51.Glaser C, Demmelmair H, Koletzko B. High-throughput analysis of fatty acid composition of plasma glycerophospholipids. J Lipid Res. 2010;51:216–221. doi: 10.1194/jlr.D000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sublette ME, Segal-Isaacson CJ, Cooper TB, Fekri S, Vanegas N, Galfalvy HC, et al. Validation of a food frequency questionnaire to assess intake of n-3 polyunsaturated fatty acids in subjects with and without major depressive disorder. J Am Diet Assoc. 2011;111:117–123. e111–e112. doi: 10.1016/j.jada.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Z, Wang Y, Gerig G, Gouttard S, Tao R, Fletcher T, et al. Quality Control of Diffusion Weighted Images. Proceedings - Society of Photo-Optical Instrumentation Engineers. 2010:7628. doi: 10.1117/12.844748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cook PA, Bai Y, Nedjati-Gilani S, Seunarine KK, Hall MG, Parker GJ, et al. 14th Scientific Meeting of the International Society for Magnetic Resonance. Seattle, WA, USA: 2006. Camino: Open-Source Diffusion-MRI Reconstruction and Processing. [Google Scholar]
- 55.Andersson JLR, Jenkinson M, Smith S. FMRIB technical reports. Oxford, United Kingdom: FMRIB Centre; 2007. Non-linear optimisation. [Google Scholar]
- 56.Andersson JLR, Jenkinson M, Smith S. FMRIB technical reports. FMRIB Centre; 2007. Non-linear registration, aka spatial normalisation. [Google Scholar]
- 57.Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Med Image Anal. 2002;6:93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- 58.Fritzsche KH, Neher PF, Reicht I, van Bruggen T, Goch C, Reisert M, et al. MITK diffusion imaging. Methods Inf Med. 2012;51:441–448. doi: 10.3414/ME11-02-0031. [DOI] [PubMed] [Google Scholar]
- 59.Reisert M, Mader I, Anastasopoulos C, Weigel M, Schnell S, Kiselev V. Global fiber reconstruction becomes practical. Neuroimage. 2011;54:955–962. doi: 10.1016/j.neuroimage.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 60.Fillard P, Descoteaux M, Goh A, Gouttard S, Jeurissen B, Malcolm J, et al. Quantitative evaluation of 10 tractography algorithms on a realistic diffusion MR phantom. Neuroimage. 2011;56:220–234. doi: 10.1016/j.neuroimage.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 61.Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magn Reson Med. 1995;34:910–914. doi: 10.1002/mrm.1910340618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Féart C, Peuchant E, Letenneur L, Samieri C, Montagnier D, Fourrier-Reglat A, et al. Plasma eicosapentaenoic acid is inversely associated with severity of depressive symptomatology in the elderly: data from the Bordeaux sample of the Three-City Study. Am J Clin Nutr. 2008;87:1156–1162. doi: 10.1093/ajcn/87.5.1156. [DOI] [PubMed] [Google Scholar]
- 63.Adams P, Lawson S, Sanigorski A, Sinclair A. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31:S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 64.Benedetti F, Yeh PH, Bellani M, Radaelli D, Nicoletti MA, Poletti S, et al. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol Psychiatry. 2011;69:309–317. doi: 10.1016/j.biopsych.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 65.Cyprien F, Courtet P, Malafosse A, Maller J, Meslin C, Bonafe A, et al. Suicidal behavior is associated with reduced corpus callosum area. Biol Psychiatry. 2011;70:320–326. doi: 10.1016/j.biopsych.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 66.Walterfang M, Yucel M, Barton S, Reutens DC, Wood AG, Chen J, et al. Corpus callosum size and shape in individuals with current and past depression. J Affect Disord. 2009;115:411–420. doi: 10.1016/j.jad.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Walterfang M, Malhi GS, Wood AG, Reutens DC, Chen J, Barton S, et al. Corpus callosum size and shape in established bipolar affective disorder. Aust N Z J Psychiatry. 2009;43:838–845. doi: 10.1080/00048670903107534. [DOI] [PubMed] [Google Scholar]
- 68.Lyoo IK, Kwon JS, Lee SJ, Han MH, Chang CG, Seo CS, et al. Decrease in genu of the corpus callosum in medication-naive, early-onset dysthymia and depressive personality disorder. Biol Psychiatry. 2002;52:1134–1143. doi: 10.1016/s0006-3223(02)01436-1. [DOI] [PubMed] [Google Scholar]
- 69.Sublette ME, Milak MS, Hibbeln JR, Freed PJ, Oquendo MA, Malone KM, et al. Plasma polyunsaturated fatty acids and regional cerebral glucose metabolism in major depression. PLEFA. 2009;80:57–64. doi: 10.1016/j.plefa.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhattacharya A, Sun D, Rahman M, Fernandes G. Different ratios of eicosapentaenoic and docosahexaenoic omega-3 fatty acids in commercial fish oils differentially alter pro-inflammatory cytokines in peritoneal macrophages from C57BL/6 female mice. J Nutr Biochem. 2007;18:23–30. doi: 10.1016/j.jnutbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y, Joshi-Barve S, Barve S, Chen LH. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23:71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]
- 72.Brooks JD, Milne GL, Yin H, Sanchez SC, Porter NA, Morrow JD. Formation of highly reactive cyclopentenone isoprostane compounds (A3/J3-isoprostanes) in vivo from eicosapentaenoic acid. J Biol Chem. 2008;283:12043–12055. doi: 10.1074/jbc.M800122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Igarashi M, Chang L, Ma K, Rapoport SI. Kinetics of eicosapentaenoic acid in brain, heart and liver of conscious rats fed a high n-3 PUFA containing diet. PLEFA. 2013;89:403–412. doi: 10.1016/j.plefa.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ouellet M, Emond V, Chen CT, Julien C, Bourasset F, Oddo S, et al. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: An in situ cerebral perfusion study. Neurochem Int. 2009;55:476–482. doi: 10.1016/j.neuint.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 75.Purdon D, Arai T, Rapoport S. No evidence for direct incorporation of esterified palmitic acid from plasma into brain lipids of awake adult rat. J Lipid Res. 1997;38:526–530. [PubMed] [Google Scholar]
- 76.Hirashima F, Parow AM, Stoll AL, Demopulos CM, Damico KE, Rohan ML, et al. Omega-3 fatty acid treatment and T(2) whole brain relaxation times in bipolar disorder. Am J Psychiatry. 2004;161:1922–1924. doi: 10.1176/ajp.161.10.1922. [DOI] [PubMed] [Google Scholar]
- 77.Sakuma H, Nomura Y, Takeda K, Tagami T, Nakagawa T, Tamagawa Y, et al. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion-weighted MR imaging. Radiology. 1991;180:229–233. doi: 10.1148/radiology.180.1.2052700. [DOI] [PubMed] [Google Scholar]
- 78.Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DK, Paty DW. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med. 1997;37:34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- 79.Peters BD, Duran M, Vlieger EJ, Majoie CB, den Heeten GJ, Linszen DH, et al. Polyunsaturated fatty acids and brain white matter anisotropy in recent-onset schizophrenia: a preliminary study. PLEFA. 2009;81:61–63. doi: 10.1016/j.plefa.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Peters BD, Machielsen MW, Hoen WP, Caan MW, Malhotra AK, Szeszko PR, et al. Polyunsaturated fatty acid concentration predicts myelin integrity in early-phase psychosis. Schizophr Bull. 2013;39:830–838. doi: 10.1093/schbul/sbs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trapp BD, Bernsohn J. Essential fatty acid deficiency and CNS myelin. Biochemical and morphological observations. J Neurol Sci. 1978;37:249–266. doi: 10.1016/0022-510x(78)90207-1. [DOI] [PubMed] [Google Scholar]
- 82.Salvati S, Natali F, Attorri L, Di Benedetto R, Leonardi F, Di Biase A, et al. Eicosapentaenoic acid stimulates the expression of myelin proteins in rat brain. J Neurosci Res. 2008;86:776–784. doi: 10.1002/jnr.21537. [DOI] [PubMed] [Google Scholar]
- 83.Ward RE, Huang W, Curran OE, Priestley JV, Michael-Titus AT. Docosahexaenoic acid prevents white matter damage after spinal cord injury. J Neurotrauma. 2010;27:1769–1780. doi: 10.1089/neu.2010.1348. [DOI] [PubMed] [Google Scholar]
- 84.Mills JD, Hadley K, Bailes JE. Dietary supplementation with the omega-3 fatty acid docosahexaenoic acid in traumatic brain injury. Neurosurgery. 2011;68:474–481. doi: 10.1227/NEU.0b013e3181ff692b. discussion 481. [DOI] [PubMed] [Google Scholar]
- 85.Pu H, Guo Y, Zhang W, Huang L, Wang G, Liou AK, et al. Omega-3 polyunsaturated fatty acid supplementation improves neurologic recovery and attenuates white matter injury after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1474–1484. doi: 10.1038/jcbfm.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tuzun F, Kumral A, Dilek M, Ozbal S, Ergur B, Yesilirmak DC, et al. Maternal omega-3 fatty acid supplementation protects against lipopolysaccharide-induced white matter injury in the neonatal rat brain. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25:849–854. doi: 10.3109/14767058.2011.587917. [DOI] [PubMed] [Google Scholar]
- 87.Virtanen JK, Siscovick DS, Longstreth WT, Jr, Kuller LH, Mozaffarian D. Fish consumption and risk of subclinical brain abnormalities on MRI in older adults. Neurology. 2008;71:439–446. doi: 10.1212/01.wnl.0000324414.12665.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bowman GL, Silbert LC, Howieson D, Dodge HH, Traber MG, Frei B, et al. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology. 2012;78:241–249. doi: 10.1212/WNL.0b013e3182436598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nordvik I, Myhr KM, Nyland H, Bjerve KS. Effect of dietary advice and n-3 supplementation in newly diagnosed MS patients. Acta Neurol Scand. 2000;102:143–149. doi: 10.1034/j.1600-0404.2000.102003143.x. [DOI] [PubMed] [Google Scholar]
- 90.Puri B, Counsell S, Hamilton G, Richardson A, Horrobin D. Eicosapentaenoic acid in treatment-resistant depression associated with symptom remission, structural brain changes and reduced neuronal phospholipid turnover. Int J Clin Pract. 2001;55:560–563. [PubMed] [Google Scholar]
- 91.Puri BK, Bydder GM, Counsell SJ, Corridan BJ, Richardson AJ, Hajnal JV, et al. MRI and neuropsychological improvement in Huntington disease following ethyl-EPA treatment. Neuroreport. 2002;13:123–126. doi: 10.1097/00001756-200201210-00029. [DOI] [PubMed] [Google Scholar]
- 92.Kohler A, Sarkkinen E, Tapola N, Niskanen T, Bruheim I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects--a randomized, single-dose, cross-over trial. Lipids Health Dis. 2015;14:19. doi: 10.1186/s12944-015-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davidson MH, Johnson J, Rooney MW, Kyle ML, Kling DF. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: the ECLIPSE (Epanova((R)) compared to Lovaza((R)) in a pharmacokinetic single-dose evaluation) study. Journal of clinical lipidology. 2012;6:573–584. doi: 10.1016/j.jacl.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 94.Schuchardt JP, Hahn A. Bioavailability of long-chain omega-3 fatty acids. PLEFA. 2013;89:1–8. doi: 10.1016/j.plefa.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 95.Liu JJ, Green P, John Mann J, Rapoport SI, Sublette ME. Pathways of polyunsaturated fatty acid utilization: Implications for brain function in neuropsychiatric health and disease. Brain Res. 2014 doi: 10.1016/j.brainres.2014.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.