Abstract
RAR-related orphan receptor γt (ROR-γt) directs differentiation of pro-inflammatory T helper 17 (TH17) cells and is a potential therapeutic target in chronic autoimmune and inflammatory diseases1–3. However, ROR-γt-dependent group 3 innate lymphoid cells (ILC3s) provide essential immunity and tissue protection in the intestine4–11, suggesting that targeting ROR-γt could also result in impaired host defense to infection or enhanced tissue damage. Here, we demonstrate that transient chemical inhibition of ROR-γt in mice selectively reduces cytokine production from TH17 cells but not ILC3s in the context of intestinal infection with Citrobacter rodentium, resulting in preserved innate immunity. Transient genetic deletion of ROR-γt in mature ILC3s also did not impair cytokine responses in the steady state or during infection. Finally, pharmacologic inhibition of ROR-γt provided therapeutic benefit in mouse models of intestinal inflammation, and reduced the frequencies of TH17 cells but not ILC3s isolated from primary intestinal samples of individuals with inflammatory bowel disease (IBD). Collectively, these results reveal differential requirements for ROR-γt in the maintenance of TH17 cell versus ILC3 responses, and suggest that transient inhibition of ROR-γt is a safe and effective therapeutic approach during intestinal inflammation.
While therapeutic targeting of the TH17 cell pathway is effective in several autoimmune diseases12–14, in Crohn’s disease, blockade of IL-17A is ineffective and in some cases was associated with worsened disease or increased opportunistic infections15–17. This is consistent with mouse models demonstrating protective functions for IL-17A and the often co-expressed cytokine IL-22 in the intestine18–23, which is in part mediated by ROR-γt+ ILC34–11. Small-molecular weight inhibitors of ROR-γt are an alternative therapeutic approach, with several identified and found to exhibit efficacy in models of autoimmunity24–28. However, the effect of targeting ROR-γt in intestinal immune cells remains unexplored.
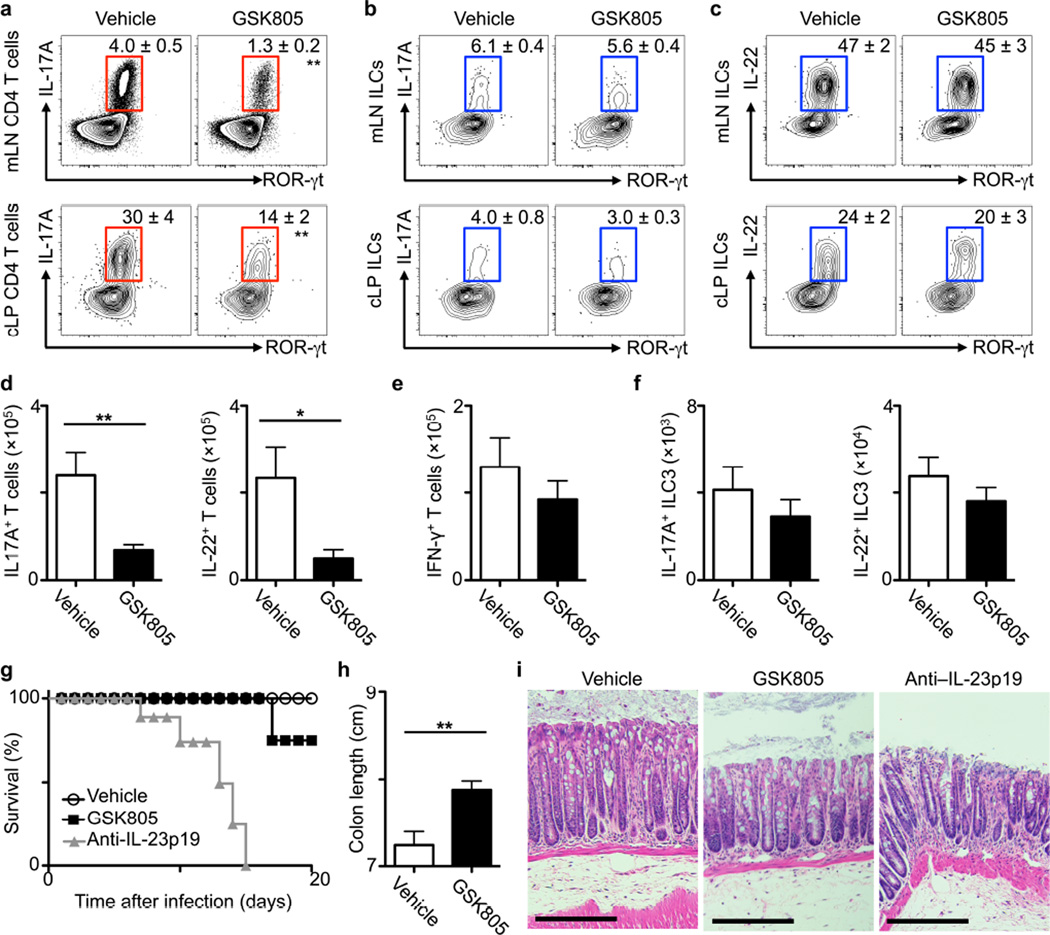
To investigate the impact of ROR-γt inhibitors in the intestine, C57BL/6 mice were infected with the enteric pathogen Citrobacter rodentium, which elicits ROR-γt+ TH17 cell and ILC3 responses6,7,29,30, and treated with a vehicle control or GSK805, a orally-available inhibitor of ROR-γt-mediated transcription24. Relative to vehicle control, oral GSK805 treatment reduced the frequency and total number of IL-17A- and IL-22-producing TH17 cells, but not IFN-γ+ TH1 cells, in the colon lamina propria (cLP) and mesenteric lymph node (mLN; Fig. 1a, d and e). In contrast, GSK805 treatment did not alter the percentage or total numbers of IL-17A- or IL-22-producing ILC3 in the colon or mLN (Fig. 1b, c and f). IL-22 production from ILC3s and T cells mediates protective immunity to C. rodentium at early versus late stages of infection, respectively6,7,29. Consistent with this, GSK805-treated mice had a comparable burden of C. rodentium early during infection, but increased levels at later stages as compared to control treated mice (Supplementary Fig. 1a). Preserved innate immunity allowed for 75% of mice to survive at day 20 post infection, in contrast to anti-IL-23 monoclonal antibody administration which resulted in 100% mortality (Fig. 1g), due to inhibition of ILC3 and TH17 cell responses7,23,30. Colon length was increased (Fig. 1h) and histologic signs of inflammation and tissue damage were reduced (Fig. 1i) in GSK805-treated mice relative to controls. In response to a systemic infection with the opportunistic pathogen Candida albicans, vehicle control and GSK805-treated mice had comparable IL-17A+ ILC3 responses in the spleen, fungal burdens, and a lack of inflammation in the kidney, as opposed to Il17a−/− mice which exhibited significantly increased fungal burdens and kidney inflammation (*P < 0.05, Supplementary Fig. 1b–d).
Figure 1. Transient chemical inhibition of ROR-γt selectively reduces TH17 responses but not ILC3s during infection.
(a–i) C57BL/6 mice were infected with C. rodentium and treated daily with vehicle control or GSK805 (10 mg/kg) starting on day –1 and continued for the duration of the experiment (a) Frequency of IL-17A-producing TH17 cells and (b) IL-17A- or (c) IL-22-producing ILC3 at day 10 post infection. (d) Total numbers of IL-17A+ and IL-22+ TH17 cells, (e) IFN-γ+ T cells and (f) IL-17A+ and IL-22+ ILC3 in the colon at day 10 post infection. (g) Survival curve of infected mice. (h) Colon length and (i) Hematoxylin and eosin stained histologic sections of the distal colon at day 10 post infection, representative of n = 5. Scale bar, 200 μm. All data are representative of five individual mice (n = 5) and was replicated in two independent experiments. Data shown are mean ± SEM (error bars). Statistics compare treatment versus control groups using Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
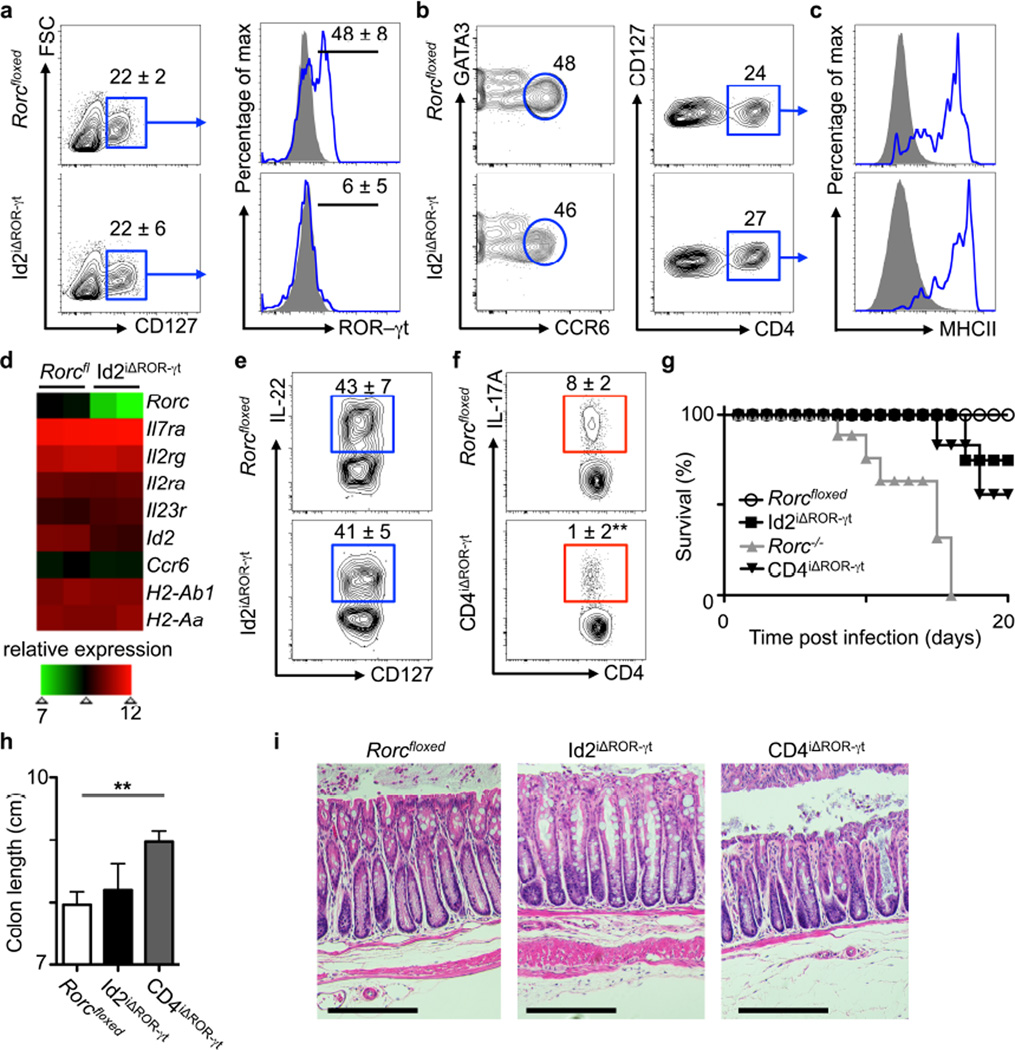
To further define the requirements for ROR-γt in the maintenance of ILC3 responses, we generated mice where Rorc can be temporally deleted in mature ILC3. This was accomplished by crossing Rorcfloxed mice and Id2creERT2 mice, which targets cre recombinase activity to ILCs following tamoxifen administration (Supplementary Fig. 2a), to generate Id2iΔROR-γt mice. After 2 weeks of tamoxifen administration, comparable frequencies of total ILCs (Lineage−(Lin−)CD127+) were observed in the mLN of control and Id2iΔROR-γt mice, with efficient deletion of ROR-γt protein (Fig. 2a). Despite a loss of ROR-γt, analyses of the total ILCs revealed comparable frequencies of cells with an ILC3-like phenotype, including those expressing CCR6, CD4 and MHCII in the mLN of control and Id2iΔROR-γt mice (Fig. 2b, c). Futher, microarray analyses of sort purified CCR6+ ILCs (gated as Lin–CD127+CCR6+) revealed a decrease in Rorc transcripts from tamoxifen administered Id2iΔROR-γt mice relative to Rorcfloxed mice, but sustained expression of other genes associated with ILC3 function (Fig. 2d). To determine how long ILC3-like cells could persist in the absence of Rorc, 6 week old Id2iΔROR-γt mice were continuously administered tamoxifen for 12 weeks. At this time point, cells with an ILC3-like phenotype could be observed in the mLN, with modest changes in absolute cell numbers and surface markers (Supplementary Fig. 3). In contrast, an ILC3-like population was not maintained in the small intestine, but rather an increase in the numbers of Lin−CD127+NKp46+ ILC1-like cells was evident (Supplementary Fig. 4). These results suggest that loss of ROR-γt may cause an expansion of ex-ILC3 or ILC1 in the small intestine31–33, or a loss of pro-inflammatory ILC334,35. Consistent with a loss in pro-inflammatory ILC3, a decrease in IFN-γ+ ILCs was observed in the small intestine, while no change was observed in the mLN (Supplementary Fig. 4). Despite changes in the intestine, comparable frequencies and numbers of ILCs lacking detectable ROR-γt expression but able to produce both IL-17A and IL-22 were maintained in the mLN following 12 weeks of continuous tamoxifen administration (Supplementary Fig. 4).
Figure 2. Transient genetic deletion of ROR-γt impairs TH17 cells but not ILC3s during homeostasis and intestinal infection.
(a–g) Analyses of ILC3s in Rorcfloxed control and Id2iΔROR-γt mice following daily treatment with tamoxifen for two weeks. (a) Gating for ILCs and subsequent analysis for ROR-γt. Lin–CD127+ cells from the mLN were analyzed for (b) CCR6 and (c) CD4 protein, as well as sequential gating for MHCII protein. (d) Relative expression of ILC3-associated genes from mLN sort purified CCR6+ ILCs was assessed by genome-wide microarrays. (e–h) Mice were infected with C. rodentium and treated daily with tamoxifen starting on day –1. (e) Identification of IL-22-producing ILC3 and (f) IL-17-producing TH17 cells at day 10 post infection. (i) Survival curve of infected mice. (g) Colon length and (h) Hematoxylin and eosin stained histologic sections of the distal colon at day 10 post infection, representative of n = 5. Scale bar, 200 μm. All data are representative of five individual mice (n = 5) and was replicated in two independent experiments. Data shown are mean ± SEM (error bars). Statistics compare treatment versus control groups using Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001
Consistent with a preservation of ILC3 function, tamoxifen-administered Rorcfloxed control and Id2iΔROR-γt mice exhibited comparable IL-17A+ ILC3 responses in the spleen, CFU and a lack of inflammation in the kidney following infection with C. albicans (in contrast to Il17a−/− mice, Supplementary Fig. 2b, c). Furthermore, Rorcfloxed and Id2iΔROR-γt mice exhibited comparable IL-22+ ILC3 responses in the mLN following C. rodentium infection (Fig. 2e). To compare these results to transient genetic deletion of ROR-γt in CD4+ T cells, Rorcfloxed mice were crossed to CD4creERT2 mice, to generate CD4iΔROR-γt mice. IL-17A production was reduced in CD4+ T cells in the mLN of tamoxifen-administered and Citrobacter rodentium infected CD4iΔROR-γt mice relative to littermate controls (Fig. 2f), demonstrating differential requirements for ROR-γt in ILC3 versus TH17 cells. Further, 75% of Id2iΔROR-γt mice survived at day 20 post infection with C. rodentium, in comparison to mice with a genomic deletion in ROR-γt (Rorc-−/− mice) and thus impaired ILC3 and TH17 development, which exhibited 100% mortality (Fig. 2g). CD4iΔROR-γt mice were partially susceptible at later stages post-infection (Fig 2g), yet exhibited increased colon length (Fig. 2h) and reduced histologic signs of intestinal inflammation (Fig. 2i) relative to control and Id2iΔROR-γt mice, suggesting that while TH17 cells are important for immunity at late stages of infection, they also are primary mediators of intestinal damage and inflammation.
Previous studies reported a low turnover rate of ILC336, and pulse-chase experiments with tamoxifen administration in Id2iΔROR-γt mice revealed differing half-lives of ILC3 in the mLN and intestine (Supplementary Fig. 5). Further, in all tissues assessed, the markers of proliferation were higher in TH17 cells than ILC3 at steady state (Supplementary Fig. 6). However, this did not account for the differential requirements of sustained ROR-γt expression, as treatment of mice with IL-2/anti-IL-2 complexes induced ILC3 proliferation (Supplementary Fig. 7) and equally expanded a population of the RORγt– ILC3 in the mLN of tamoxifen treated Id2iΔROR-γt mice that produced comparable levels of IL-17A and IL-22 relative to RORγt+ ILC3 in control mice (Supplementary Fig. 8).
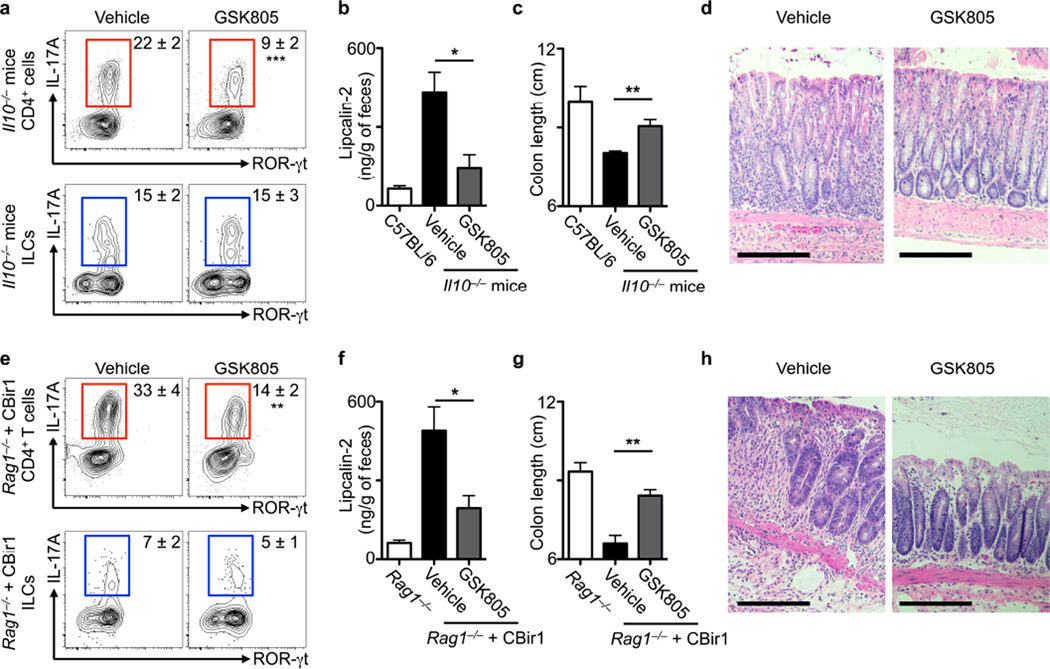
To experimentally address whether chemical inhibition of ROR-γt can provide therapeutic benefit in the context of intestinal inflammation, Il10−/− mice were aged to 10 weeks (a time point at which spontaneous colonic inflammation is observed), and orally administered either vehicle control or GSK805 at a dose 10 mg/kg/day for two weeks. GSK805 administration selectively reduced the frequency of IL-17A+ TH17 cells in the colonic tissues, while no effect on the frequency of IL-17A+ ILC3 was observed (Fig. 3a). Furthermore, GSK805 treatment reduced elevated amounts of fecal lipocalin-2 (Fig. 3b), prevented shortening of the colon (Fig. 3c) and reduced histologic signs of inflammation in the colon (Fig. 3d) of Il10−/− mice relative to controls. In a second model of intestinal inflammation, CBir1 transgenic CD4+ T cells, which recognize an immunodominant and commensal bacteria-derived antigen in Crohn’s disease37, were transferred to Rag1−/− mice. At two weeks post transfer, intestinal inflammation was observed and then mice were orally administered either vehicle control or GSK805 at a dose 10 mg/kg/day for two weeks. GSK805 treatment significantly reduced the frequency of colonic CBir1-specific TH17 cells, but not IL-17A+ ILC3 (**P < 0.01, Fig. 3e). Further, GSK805 treatment reduced fecal lipcalin-2, colonic shortening and histologic signs of inflammation in the colon of Rag1−/− mice receiving a CBir1 T cell transfer, relative to control mice (Fig. 3f–h). Together, these results demonstrate that transient inhibition of ROR-γt provides therapeutic benefit in the context of two mouse models of intestinal inflammation.
Figure 3. Transient inhibition of ROR-γt selectively limits TH17 cell responses and reduces intestinal inflammation.
(a–d) Ten-week-old Il10−/− mice were treated with vehicle control or GSK805 (10 mg/kg) for two weeks. (a) Frequency of IL-17A-producing TH17 cells (red) or ILC3 (blue). Amount of fecal lipocalin-2 (b), colon length (c), and Hematoxylin and eosin stained histologic sections of the distal colon (d). Scale bar, 200 μm, representative of n = 5. (e–h) Adoptive transfer of CBir1 TCR transgenic T cells into Rag1−/− mice, followed 2–3 weeks later by daily treatment with either vehicle control or GSK805 (10 mg/kg) for 2 weeks. (e) Analyses of IL-17A-producing TH17 cells (top) or ILC3 (bottom), (f) amount of fecal lipocalin-2, (g) colon length, and (h) Hematoxylin and eosin stained histologic sections of the distal colon. Scale bar, 200 μm, representative of n = 5. All data are representative of five individual mice (n = 5) and was replicated in two independent experiments. Data shown are mean ± SEM (error bars). Statistics compare treatment versus control groups using Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001
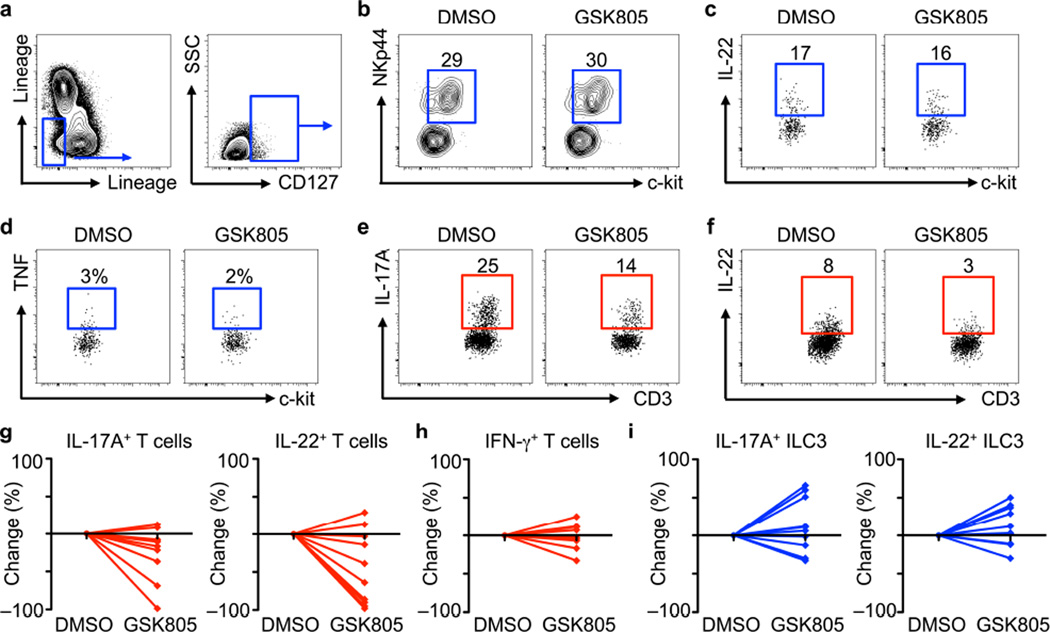
To interrogate how transient inhibition of ROR-γt modulates human TH17 cell and ILC3 responses, colonic resection tissues from pediatric individuals with Crohn’s disease were digested and single cell suspensions were cultured in vitro for 12 hours in the presence of DMSO control or GSK805. ILC3s were identified by gating on Lin− cells and examining for CD127+ populations (Fig. 4a) followed by co-expression of NKp44 and c-kit. Incubation with GSK805 relative to DMSO control did not alter ILC3 frequencies (Fig. 4b), production of IL-22 (Fig. 4c), or production of the pro-inflammatory cytokine TNF (Fig. 4d). In contrast, in vitro culture with GSK805 resulted in a decrease in the frequency of CD4+ T cells that produce IL-17A (Fig. 4e) and IL-22 (Fig. 4f) relative to DMSO. The decrease in IL-17A+ and IL-22+ TH17 cells following culture in the presence of GSK805 was observed in cell suspensions from 8 out of 10 pediatric individuals with Crohn’s disease (Fig. 4g), did not influence IFN-γ+ T cells (Fig. 4h), and resulted in comparable or increased frequencies of IL-17A+ and IL-22+ ILC3 (Fig. 4i). Similar data were also obtained when another ROR-γt inhibitor, Digoxin25, was utilized in vitro (Supplementary Fig. 9). Collectively, these results demonstrate that similar to mice, transient in vitro inhibition of ROR-γt in cells isolated from individuals with IBD selectively limits TH17 cells, with limited influence on ILC3s.
Figure 4. Transient inhibition of ROR-γt selectively reduces TH17 cells in intestinal tissue from pediatric individuals with Crohn’s disease.
(a–i) Intestinal resection tissue from pediatric individuals with Crohn’s disease was cultured with DMSO control or GSK805 (0.5 μM) for 12 hours. (a) Identification of Lin–IL-7Rα+ ILCs. (b) Percentage of NKp44+c-kit+ ILC3s. (c) Production of IL-22 and (d) TNF by gated c-kit+ ILC3s. (e) Expression of IL-17A and (f) IL-22 by CD3+ TH17 cells. (g) Fold change in the percentage of IL-17A+ or IL-22+ TH17 cells, (h) IFN-γ+ TH1 cells and (i) IL-17A+ or IL-22+ ILC3 following culture with GSK805 relative to DMSO. All data are representative or shown for n = 10 samples from pediatric individuals with Crohn’s disease.
Subsets of ILCs and CD4+ T helper cells exhibit remarkably similar transcriptional profiles and effector cytokine responses, yet can promote differential functions in the context of immunity, inflammation and tissue repair1–3,11,38. Therefore, therapeutically targeting conserved pathways in the context of chronic inflammatory diseases has the potential to influence both arms of the immune system, and in addition to limiting a pathologic response, could inappropriately target beneficial responses that mediate protective immunity or tissue repair. This may account for off-target consequences of biologic therapies in patients with IBD that broadly inhibit cytokine-cytokine receptor interactions, such as the anti-IL-17 clinical trials that resulted in increased infections or worse disease15–17,39. Here, we uncover an unexpected cellular mechanism by which transient inhibition of the transcription factor ROR-γt allows for selective targeting of adaptive TH17 cells without influencing protective ILC3 responses in both mice and in vitro cultures with human tissues samples. These data provoke a challenge to our understanding of the modular effects of lineage-specifying transcription factors in the maintenance and function of innate versus adaptive lymphocytes, and identify ROR-γt inhibition as an attractive future therapeutic approach to treat IBD and other chronic inflammatory diseases.
Online methods
Mice
C57BL/6, CD4creERT2 (ref. 40), Id2creERT2 (ref. 41), Rorc−/−, Il10−/−, ROSAmT/mG, Rag1−/−, and Rorcfloxed (ref. 24) mice were originally obtained from the Jackson Laboratory (Bar Harbor, ME) and bred and maintained at Weill Cornell Medical College, (New York, NY, USA) or the University of Birmingham (Birmingham, UK). CBir1 mice and Il17a−/− mice were previously described37,42. Mice used were a mix of male and female animals, aged 6–10 weeks at the start of the experiment. For experiments at the University of Birmingham, animals were used in accordance with Home Office guidelines at the University of Birmingham. For experiments performed at Weill Cornell, all protocols were approved by the Weill Cornell Institutional Animal Care and Use Committee (IACUC), and all experiments were performed according to the guidelines of the Weill Cornell IACUC. To generate Id2iΔROR-γt and CD4iΔROR-γt mice, Id2creERT2 and CD4creERT2 mice were crossed to Rorcfloxed mice respectively. Where noted, experimental and control mice were maintained on Tamoxifen-containing diet (TAM400, Harlan Laboratories) for at least 10–14 days prior to analysis or infection, or were gavaged daily with 10 mg/kg of tamoxifen in corn oil (Sigma). GSK805 (C23H18Cl2F3NO4S) was previously identified by a Fluorescence Resonance Energy Transfer (FRET) assay that examined for inhibition of the ROR-γt ligand-binding domain and cofactor peptide SRC1 and compared to other nuclear receptors24. Inhibition of ROR-γ was not tested. Efficacy and potency was previously demonstrated, and RNAseq data revealed comparable transcriptional changes to that observed in Rorc gene deletion24. In this study, GSK805 was provided from EMD Milipore (53136) at a purity ≥ 95% by HPLC. Mice received 10 mg/kg/day of GSK805 dissolved in DMSO and provided by oral gavage in corn oil, or DMSO alone in corn oil as a vehicle control. For BrdU incorporation, mice were given 1.5 mg BrdU i.p. and analyzed after 24 hours. Mice were injected i.p. for 3 consecutive days with IL-2/anti-IL2 complexes generated by mixing recombinant protein and antibody for 30 minutes at 37 °C prior to injection. Per injection, each mouse received 1 µg IL-2 (Peprotech) and 5 µg anti-IL-2 monoclonal antibody (clone JES6-1A12, eBioscience), and control mice received PBS. No animals were excluded from the analyses.
Citrobacter rodentium infection
Citrobacter rodentium (formerly Citrobacter freundii, biotype 4280) strain DBS100 was prepared by selecting a single colony and culturing in LB broth overnight as previously described7. Mice were inoculated with approximately 1 × 108 CFU in 200–250 μL broth via oral gavage. Quantification of fecal CFU was determined by homogenization in sterile PBS and serial dilutions on MacConkey’s agar.
Candida albicans infection
Candida albicans infections were performed as previously described43. In brief, 104–105 CFU of Candida albicans strain SC5314 were administered to mice by i.v. injection and analyzed three days later.
Mouse models of intestinal inflammation
To induce intestinal inflammation, Il10−/− mice were aged to 10 weeks in our animal facilities, followed by 2 weeks of GSK805 treatment as described above. The CBir1 T cell transfer model of intestinal inflammation was previously described44. In brief, 2 × 106 CD3+CD4+ T cells were sort purified as from spleens of CBir1 transgenic mice and adoptively transferred to Rag1−/− recipients by i.v. injection. This protocol induces substantial colonic inflammation at 3 to 4 weeks44, at which point mice were then treated for 2 weeks with GSK805 as described above.
Flow cytometry
Lymph nodes were teased using fine forceps and digested for 25 minutes in RPMI containing with 250 µg/ml Collagenase-Dispase (Roche) and 25 µg/ml DNase I. Digested tissue was then crushed through a 70 μm nylon mesh. In some experiments, LNs were only crushed through a 70 μm nylon mesh. Antibody staining was performed at 4 °C for 30 minutes. Antibodies raised against the following mouse antigens were used: B220 (clone RA3-6B2, eBioscience), CCR6 (clone 29-2L17, Biolegend), CD3 (clone 145-2C11, eBioscience), CD4 (clone GK1.5, eBioscience), CD45.1 (clone A20, eBioscience), CD45.2 (clone 104, eBioscience), CD5 (clone 53-7.3, eBioscience), CD11b (clone M1/70, eBioscience), CD11c (clone N418, eBioscience), GATA-3 (TWAJ, eBioscience), MHCII (clone AF6-120.1, eBioscience), IL-7Rα (clone A7R34, eBioscience), IL-17A (clone TC11-18H10.1, eBioscience), IL-22 (clone 1H8PWSR, eBioscience), Ki-67 (clone SolA15, eBioscience), NKp46 (clone 29A1.4, eBioscience), ROR-γt (clone B2D, eBioscience), T-bet (clone eBio4B10, eBioscience). All antibodies were used at a 1:200 dilution. The lineage dump channel contained antibodies against B220, CD3, CD5, NK1.1, CD11b and CD11c all in one cocktail, or divided into two channels. Intracellular staining was done using the FoxP3 fixation and permeabilization kit (eBioscience) according to manufacturer’s instructions. Incorporation of BrdU was detected using FITC BrdU Flow Kit (BD) according to manufacturer’s instructions. Addition of Spherotech Accucount blank particles was used to calculate absolute cell numbers. Flow cytometry was performed on a Fortessa analyzer using FACSDiva6.2 software (BD), with data subsequently analyzed with FlowJo software (Tree Star). For cytokine production, cells were cultured ex vivo in the presence of either mouse recombinant IL-1β (14-8012), IL-2 (14-8021), IL-6 (14-8061) and IL-23 (14-8231) at a concentration of 20 ng/mL for 2 hours (all obtained from eBioscience). Cells were then stimulated with 50 ng/mL PMA, 750 ng/mL Ionomycin and 10 μg/mL Brefeldin A (all obtained from Sigma-Aldrich) for 4 hours.
Microarray analysis
For microarray analysis, Lin–CCR6+CD90.2+ cells were sorted from the mLN of Rorcfloxed or Id2iΔROR-γt mice were lysed directly in TRIzol. RNA was isolated, amplified, reverse-transcribed to cDNA and hybridized to an Affymetrix GeneChip (Mouse Gene 1.0ST), or obtained via the GEO database and normalized as previously described (41). All data sets were deposited in the GEO database and are available at ascension code GSE76201.
Histological sections
Distal colons were fixed with 4% PFA and embedded in paraffin, and 5 μm sections were cut and stained with Hematoxylin and eosin. Whole kidneys were fixed with 4% PFA and embedded in paraffin, and 5 μm sections were cut, stained with Periodic acid–Schiff and counterstained with Hematoxylin.
Human tissue and flow cytometry
Deidentified intestinal resection tissues from the colon of pediatric individuals with Crohn’s disease were obtained from the Children’s Hospital of Philadelphia (CHOP) following an CHOP Institutional Review Board (IRB) approved protocol (14-010981) and informed consent of participating individuals. Tissues were processed by first incubating in 1 mM EDTA, 1 mM DTT and 5% FCS (all obtained from Fisher Scientific) for 30 minutes at 37 °C with shaking to remove intestinal epithelial cells. Supernatants were then discarded and the remaining tissues were incubated in 0.5 mg/mL collagenase/dispase and 20 μg/mL DNase I for 20–30 minutes at 37°C with shaking to obtain the lamina propria fraction. Any remaining tissues were also included following mechanical dissociation and filtering through a 70 μm cell strainer. All cells were then viably cryopreserved at 150 °C in 90% FBS and 10% DMSO for future side-by-side analyses. Following thawing, cells were re-stimulated ex vivo with human recombinant IL-1β, IL-2, IL-6, IL-7 and IL-23 (20 ng/mL, eBioscience) for 8 hours in the presence of DMSO control, 0.5 µM GSK805 (EMD Milipore) or 5 μM digoxin (Sigma-Aldrich). Cells were then stimulated with 50 ng/mL PMA, 750 ng/mL Ionomycin and 10 μg/mL Brefeldin A (all obtained from Sigma-Aldrich) for 4 hours. Cells were then stained with antibodies for the following markers: anti-CD3 (clone UCHT1, eBioscience), anti-CD4 (clone OKT-4, eBioscience), anti-CD5 (clone UCHT2, eBioscience), anti-CD11b (clone M1/7B, eBioscience), anti-CD11c (clone 3.9, eBioscience), anti-CD14 (clone 61D3, eBioscience), anti-CD19 (clone 2H7, eBioscience), anti-CD127 (clone A019D5, Biolegend), anti-FcεR1 (clone AER-37, eBioscience), anti-CD117/c-kit (clone 104D2, eBioscience), anti-NKp44 (clone Z231, BC Cytometry). For intracellular staining, cells were fixed and permeabilized using a commercially available kit (eBioscience) and staining with anti-IL-17A (clone eBio64DEC17, eBioscience), anti-IL-22 (clone 22URTI, eBioscience) or anti-IFN-γ (clone 4S.B3, eBioscience). All antibodies were used at a 1:200 dilution. Lineage cocktails are defined in Figure 4 as the x-axis: CD19, CD11b, CD11c, and the y-axis: CD3, CD5, CD14, FcεR1.
Statistics
Data were analysed using GraphPad Prism (version 5.01). Student‘s t test or Mann-Whitney were used to determine significance which was set at P < 0.05. We did not use statistical methods to predetermine sample size, there was no randomization designed in the experiments, and the studies were not blinded. Samples sizes were estimated based upon sample availability and previous experimental studies of the intestinal immune system8,9,45,46. No exclusion criteria were utilized in these studies.
Supplementary Material
Acknowledgments
Members of the Sonnenberg and Withers laboratories are thanked for discussions and critical reading of the manuscript. We also thank Dr. Charles Elson (University of Alabama at Birmingham) for kindly providing CBir1 transgenic mice and valuable expertise, as well as Dr. Tobias Hohl (Memorial Sloan Kettering Cancer Center) for experimental expertise. Research in the Sonnenberg laboratory is supported by the National Institutes of Health (DP5OD012116, R56AI114724 and R01AI123368 to G.F.S), the National Institute of Allergy and Infectious Diseases (NIAID) Mucosal Immunology Studies Team (MIST) Scholar Award in Mucosal Immunity (to G.F.S.), and the Institute for Translational Medicine and Therapeutics Transdisciplinary Program in Translational Medicine and Therapeutics (UL1-RR024134 from the US National Center for Research Resources to G.F.S.) and the Crohn’s and Colitis Foundation of America (#297365 to M.R.H.). Research in the Withers laboratory is supported by a Wellcome Trust Research Career Development Fellowship to D. Withers.
Footnotes
Accession codes
Author Contributions
D.R.W., G.F.S. designed and performed experiments, analyzed data, and wrote the paper; M.R.H., X.W., E.C.M., E.E.H., E.E.D., C.L.M., V.B-W., M.V. designed and performed experiments; J.K. and R.N.B. provided critical reagents and invaluable expertise.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 2.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberl G, Littman DR. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches. Immunol Rev. 2003;195:81–90. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 5.Sawa S, et al. RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 6.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hepworth MR, et al. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science. 2015 doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hepworth MR, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortha A, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papp KA, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N. Engl. J. Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 13.Leonardi C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N. Engl. J. Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 14.Genovese MC, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 15.Hueber W, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Targan SR, et al. Mo2083 A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Tolerability, and Efficacy of AMG 827 in Subjects With Moderate to Severe Crohn's Disease. Gastroenterology. 143:e26. [Google Scholar]
- 17.Colombel JF, Sendid B, Jouault T, Poulain D. Secukinumab failure in Crohn's disease: the yeast connection? Gut. 2013;62:800–801. doi: 10.1136/gutjnl-2012-304154. [DOI] [PubMed] [Google Scholar]
- 18.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor W, Jr, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 21.Ishigame H, et al. Differential roles of interleukin-17A and-17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 24.Xiao S, et al. Small-molecule RORgammat antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity. 2014;40:477–489. doi: 10.1016/j.immuni.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh JR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solt LA, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soroosh P, et al. Oxysterols are agonist ligands of RORgammat and drive Th17 cell differentiation. Proc Natl Acad Sci U S A. 2014;111:12163–12168. doi: 10.1073/pnas.1322807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huh JR, Littman DR. Small molecule inhibitors of RORgammat: targeting Th17 cells and other applications. Eur J Immunol. 2012;42:2232–2237. doi: 10.1002/eji.201242740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basu R, et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 31.Klose CS, et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 32.Vonarbourg C, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernink JH, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 34.Buonocore S, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song C, et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med. 2015 doi: 10.1084/jem.20151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawa S, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 37.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 39.Kaser A. Not all monoclonals are created equal - lessons from failed drug trials in Crohn's disease. Best Pract Res Clin Gastroenterol. 2014;28:437–449. doi: 10.1016/j.bpg.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Aghajani K, Keerthivasan S, Yu Y, Gounari F. Generation of CD4CreERT(2)) transgenic mice to study development of peripheral CD4-T-cells. Genesis. 2012;50:908–913. doi: 10.1002/dvg.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 43.Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 2014;40:117–127. doi: 10.1016/j.immuni.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonnenberg GF, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackley EC, et al. CCR7-dependent trafficking of RORgamma(+) ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun. 2015;6:5862. doi: 10.1038/ncomms6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.