Abstract
It is well established that heavy ethanol consumption interferes with the immune system and inflammatory processes, resulting in increased risk for infectious and chronic diseases. However, these processes have yet to be systematically studied in a dose and sex-dependent manner. In this study, we investigated the impact of chronic heavy ethanol consumption on gene expression using RNA-seq in peripheral blood mononuclear cells isolated from female rhesus macaques with daily consumption of 4% ethanol available 22hr/day for 12 months resulting in average ethanol consumption of 4.3 g/kg/day (considered heavy drinking). Differential gene expression analysis was performed using edgeR and gene enrichment analysis using MetaCore™. We identified 1106 differentially expressed genes, meeting the criterion of ≥ two-fold change and p-value ≤ 0.05 in expression (445 up- and 661 down-regulated). Pathway analysis of the 879 genes with characterized identifiers showed that the most enriched gene ontology processes were “response to wounding”, “blood coagulation”, “immune system process”, and “regulation of signaling”. Changes in gene expression were seen despite the lack of differences in the frequency of any major immune cell subtype between ethanol and controls, suggesting that heavy ethanol consumption modulates gene expression at the cellular level rather than altering the distribution of peripheral blood mononuclear cells. Collectively, these observations provide mechanisms to explain the higher incidence of infection, delay in wound healing, and increase in cardiovascular disease seen in subjects with Alcohol use disorder.
Introduction
Current statistics shows 75% of adult men and 63% of adult women regularly drink alcohol. Alcohol Use Disorder (AUD), defined as the weekly use of ≥15 drinks for men and ≥8 drinks for women (1 drink equals 15 g of ethanol), is estimated to affect 9.4% of male and 4.7% of female adult US population [1]. AUD leads to decreased barrier function [2], liver damage [3], cardiovascular disease [4, 5], poor vaccine response and increased susceptibility to bacterial and viral infections resulting in overall increased mortality [6, 7]. Compared to men, women appear at a higher risk of developing ethanol-related diseases such as coronary heart diseases and stroke across all levels of consumption [8, 9]. Although some studies suggest that this increased susceptibility may be explained by the higher blood ethanol levels achieved after drinking [10], the mechanisms underlying this increased sensitivity are poorly understood.
Currently, there are only a handful of studies that have systematically examined sex differences in the effects of ethanol on the immune and inflammatory responses and most of these studies have been conducted in rodent models. Clinical studies aimed at the systematic exploration of dose-dependent, sex-specific immune regulation in response to chronic ethanol exposure pose significant challenges such as obtaining accurate information about the ethanol dose/exposure history, the presence of confounding factors such as smoking, the use of recreational or illicit drugs, and nutritional deficits. Additionally, individuals who suffer from AUD tend not to participate in research studies [11].
In this study, we leveraged a nonhuman primate model of voluntary self-administration to define the impact of chronic heavy ethanol consumption on immune homeostasis in young adult female macaques [12]. In this model, macaques are trained to self-administer ethanol first using a schedule-induced polydipsia to establish ethanol drinking, then allowing the monkeys access to both 4% ethanol and water for 22 h/day [13]. The animals segregate into categorically heavy and non-heavy drinkers and these patterns remain stable for greater than 12 months [14]. Using this model, we previously demonstrated that chronic ethanol consumption leads to reduced growth factor production by peripheral blood mononuclear cells due to changes in microRNA and transcription factor expression [15]. We also recently showed that robust changes in innate immune gene expression in animals that routinely drank to intoxication underlie defects in vaccine responses [16]. As a follow up, in the current study, we used peripheral blood mononuclear cells (PBMC) samples from 6 female macaques, which were categorized as heavy drinkers and 3 controls to define the impact of chronic heavy ethanol exposure on immune cell numbers and overall gene expression changes. Additionally, to identify changes at the protein level, we measured plasma levels of cytokines, chemokines and growth factors in both groups of animals.
Our analysis indicates that, although there was no difference in the numbers of circulating white blood cells between the controls and the drinkers, there were significant changes in gene expression. Specifically, genes involved in blood coagulation and the development of heart disease were up-regulated, while genes involved in innate immunity and inflammation were dysregulated. Furthermore, we report down-regulation of a number of transcription factors important in regulating inflammatory gene expression, including STAT3, the protein levels of which we previously reported was suppressed with ethanol consumption [15]. Additionally, expression patterns of several histone genes and chromatin modifiers were differentially regulated by heavy drinking suggesting a global regulation of gene expression changes in PBMC upon chronic exposure to ethanol.
Results
Chronic heavy ethanol consumption does not alter circulating immune cell frequency
The 6 ethanol-consuming monkeys were classified as heavy drinkers, based on their average daily ethanol intake and resultant blood ethanol concentration (BEC). Average individual BEC ranged from 41.2 (mg%) to 96.4 (mg%) while the average daily ethanol consumption ranged from 3.29 to 5.17 g/kg (Table 1), which translate into human equivalents of 11 to 18 drinks per day. Hematological analysis showed no differences in circulating white blood cells between the ethanol-consuming and control animals (S1A Fig). Moreover, flow cytometry analysis did not reveal any differences in frequency of circulating CD4 T, CD8 T, CD20 B cells, dendritic cells and monocytes (S1B Fig). In contrast, plasma levels of IL4, IL7, IL8, MIP1β, and SDF1α were significantly increased, while VEGFD levels were significantly decreased in heavy drinkers, indicative of changes in cellular function (S1C Fig).
Table 1. Summary of drinking behavior of animals used in this study.
| Animal ID | Weight Pre-EtOH (kg) | Weight at Necropsy (kg) | Mean BEC (mg%) | Mean daily intake (g/kg/day) |
|---|---|---|---|---|
| FC1 | 4.46 | 6.80 | 0 | 0 |
| FC2 | 4.63 | 5.80 | 0 | 0 |
| FC3 | 4.36 | 4.85 | 0 | 0 |
| FH1 | 4.16 | 4.95 | 49.8 | 3.93 |
| FH2 | 4.64 | 6.30 | 41.2 | 3.29 |
| FH3 | 4.56 | 5.20 | 74.9 | 4.89 |
| FH4 | 4.34 | 4.60 | 60 | 4.02 |
| FH5 | 4.69 | 5.55 | 66.3 | 3.94 |
| FH6 | 4.03 | 4.85 | 96.4 | 5.17 |
Chronic heavy ethanol consumption results in significant changes in gene expression
RNASeq was used to compare the PBMC transcriptomes between ethanol-consuming and ethanol-naïve animals. PBMC library from one of the ethanol-consuming animals failed, which was removed from all subsequent analyses. Principal component analysis (S2A Fig) clearly distinguishes the transcriptional profiles of ethanol naïve and ethanol-consuming animals. Comparing the transcriptomes of the 3 controls and 5 heavy drinkers resulted in 1106 differentially expressed genes (DEGs) with an FDR-corrected p-value of 0.05 and a fold change (FC) ≥ 2 (Fig 1B), with 445 up- and 661 down-regulated DEGs.
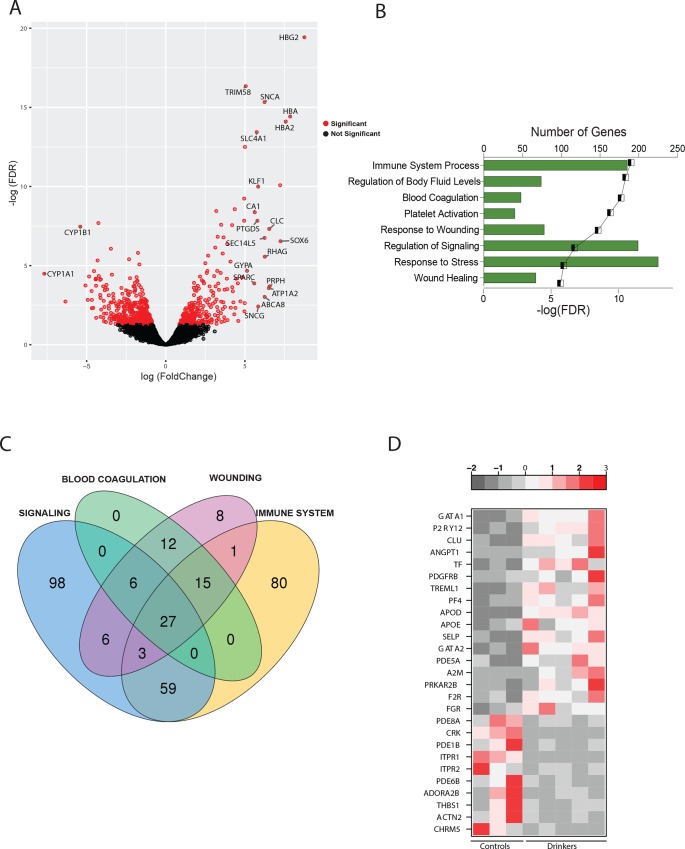
Fig 1. Chronic heavy ethanol consumption results in robust changes in gene expression within PBMC.
(A) Volcano plot of global gene expression changes with red specks denoting genes with significant fold changes in gene expression, with gene names annotated for those with fold change ≥ 32. (B) Bar graph depicting the 8 most significant Gene Ontology (GO) terms enriched among all differentially expressed genes (DEGs), (C) Venn diagram depicting the overlap of genes enriched for four major GO terms—Signaling, Blood Coagulation, Wounding and Immune System Process. (D) Heatmap of the 27 differentially expressed that belong to all four GO processes—red depicts higher expression and grey, lower expression.
Some of the most highly up-regulated DEGs are genes involved in different stages of heme synthesis, notably HBG2 (Hemoglobin γ2, FC = 433), HBB (β, FC = 332), HBA (α, FC = 232), HBA2 (α2, FC = 191), HBM (μ, FC = 40) as well as ALAS2 (5-aminolevulinate synthase, FC = 151) and AHSP (α hemoglobin stabilizing protein, FC = 19.6) (Fig 1A). The most highly repressed DEGs include CYP1A1 and CYP1B1 (Cytochrome P450 family 1 subfamily A and B polypeptide 1, FC = 203 and FC = 42.1 respectively), which are involved in the metabolism of toxins and carcinogens [17]; AHRR (Aryl hydrocarbon receptor repressor, FC = 37), which responds to xenobiotic stimulus [18]; and amino acid transporters such as SLC7A11 (Solute carrier family 7 member 11, FC = 25.7) and SLC24A1 (FC = 19) [19].
To understand the biological impact of these gene expression changes, we performed functional enrichment using MetaCore™. Since the software requires characterized gene identifiers for its analysis, this resulted in the removal of 227 DEGs. The remaining 879 DEGs enriched into several Gene Ontology (GO) processes notably ‘Immune System Process’, ‘Regulation of Signaling’, ‘Response to Wounding’ and ‘Blood Coagulation’ (Fig 1B). Since we observed a number of overlapping themes, we collapsed these terms into 4 major categories—Signaling (199 DEGs), Wounding (78 DEGs), Blood Coagulation (48 DEGs) and Immune System Process (185 DEGs).
A Venn diagram of the DEGs that mapped to these 4 GO terms revealed 27 common genes (Fig 1C). Of the 17 DEGs that were up-regulated (Fig 1D), several were involved in coagulation e.g. P2RY12 (Purinergic Receptor P2Y, G-protein Coupled 12, FC = 14.8), which plays a critical role in maintaining thrombus stability [20]; TREML1 (Triggering Receptor Expressed on Myeloid Cells like 1, FC = 9.1), which facilitates platelet aggregation [21]; and PF4 (Platelet Factor 4, FC = 8.8), involved in sealing blood clots [22]. Other genes in this list include growth factors such as ANGPT1 (Angiopoietin 1, FC = 12.4); adhesion proteins like SELP (P-Selectin, FC = 6.1) and CLU (Clusterin, FC = 12.5), and transcription factors GATA1 (GATA binding protein 1, FC = 23.4) and GATA2 (FC = 6).
Several of the 10 common DEGs that were down-regulated (Fig 2B) play crucial roles in signaling notably ADORA2B (Adenosine A2b Receptor, FC = 4.4), which regulates adenosine levels during T cell activation [23]; phosphodiesterases such as Phosphodiesterase 1B (PDE1B, FC = 2.2), PDE6B (FC = 3.3) and PDE8A (FC = 2); and THBS1 (Thrombospondin 1, FC = 4.9), which is critical for resolution of inflammation [24].
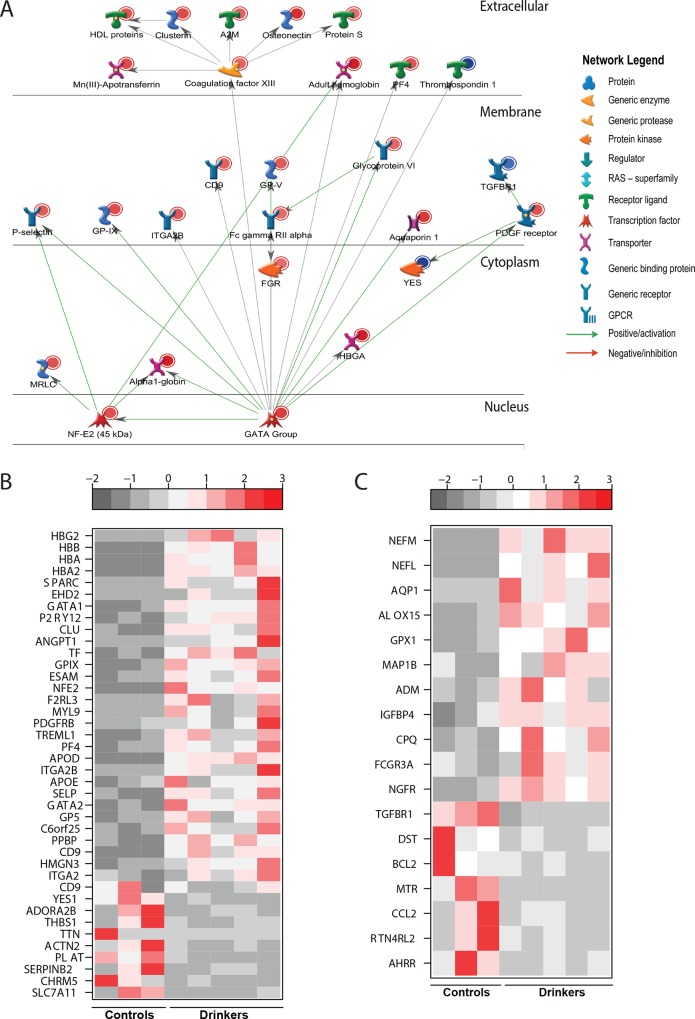
Fig 2. Chronic heavy ethanol consumption results in up-regulation of genes involved in blood coagulation and wound-healing.
(A) Network of DEGs with direct interactions that mapped to “Wound healing”. (B) Heatmap of the 40 DEGs with a fold-change ≥ four-fold (30 up-regulated and 10 down-regulated) involved in “Blood Coagulation”. (C) Heatmap of the 18 DEGs that mapped to “Wound healing” but did not map to “Blood Coagulation”.
Chronic heavy ethanol consumption results in up-regulation of genes involved in blood coagulation but decreases expression of genes critical for wound healing
The 48 DEGs that mapped to GO terms associated with coagulation also mapped to GO terms associated with wound repair. Overall, we noted an up-regulation of pro-clotting genes and down-regulation of anti-clotting genes (Fig 2A and 2B). Specifically, hemoglobin genes HBG2 (FC = 433), HBB (FC = 332), HBA2 (FC = 191), hemoglobin-stabilizing protein AHSP (FC = 19.6) and NFE2 (Nuclear Erythroid Factor 2, FC = 10.5) were highly up-regulated. In addition, coagulation factors such as F13A1 (Coagulation Factor XIII, FC = 3.4) and F2R (Coagulation Factor II receptor, FC = 2.8) were over-expressed. Moreover, we observed up-regulation of erythropoietic transcription factors GATA1 (FC = 23.4) and GATA2 (FC = 6), which regulate the switch from fetal to adult hemoglobin and activate the development of hematopoietic cell lineages [25]. Finally, expression of several platelet adhesion molecules also increased notably: SELP (P-Selectin, FC = 6.1) [26, 27], up-regulated in response to vascular injury; GP5 (Glycoprotein 5, FC = 5.9), which promotes platelet aggregation and adhesion to the site of vascular injury [28, 29]; and ITGA2 and ITGA2B (Integrin Alpha 2 and 2B, FC = 4.2 and FC = 7.4, respectively), which mediate adhesion of platelets and immune cells to the extracellular matrix [30]. In contrast, positive regulators of fibrinolysis were down-regulated such as the potent thrombin inhibitor SERPINB2 (Serpin Peptidase Inhibitor, Clade B member 2, FC = 7), and the plasminogen activator PLAT (Plasminogen activator tissue, FC = 2.7). Additionally, we observed increased expression of SPARC (FC = 48) and SPARCL1 (SPARC-like 1, FC = 19.3), which increase the production of matrix metalloproteinases and expression of growth factors at the site of injury [31].
The list of DEGs that mapped to wound healing but not blood coagulation is summarized in Fig 2C. The down-regulated genes include the monocyte chemoattractant CCL2 (Chemokine Ligand 2, FC = 11.8) [32] and the angiogenic factor TGFBR1 (Transforming growth factor receptors Beta 1, FC = 2.1) [33]. The up-regulated DEGs include several genes important for wound healing, notably AQP1 (Aquaporin 1, FC = 24), which promotes cell migration during wound healing events was increased [34]. ADM (Adrenomedullin, FC = 4.9), which promotes angiogenesis and remodeling; and GPX1 (Glutathione Peroxidase 1, FC = 10.8), an antioxidative enzyme that regulates wound healing [35].
Chronic heavy ethanol consumption results in dysregulation of genes involved in innate immunity and immune system development
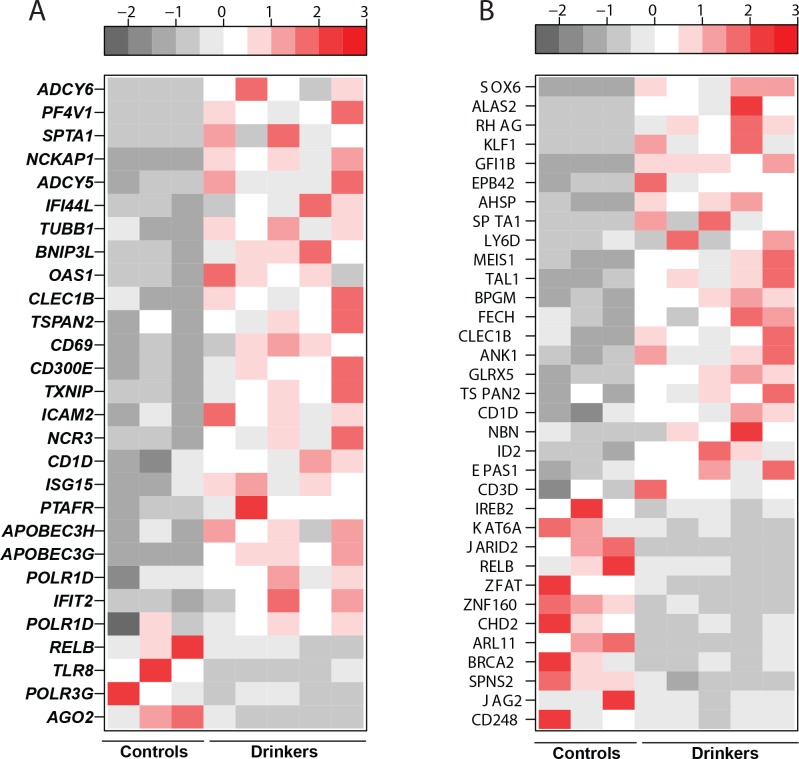
Of the 185 DEGs that mapped to Immune System Process, 46 DEGs also mapped to “Wound Healing” and 89 to “Signaling”. Additional bioinformatics analysis of the expression profiles of the 185 genes that mapped to ‘Immune System Process’ using the Immunological Genome Consortium database indicated that a significant number of these genes are highly expressed in innate immune cells–monocytes and dendritic cells (DCs). These results suggest that ethanol consumption exerts the biggest transcriptional impact on the innate immune system (S3A Fig). Indeed, the 80 DEGs that mapped exclusively to “Immune System Process” further enriched into GO processes: Defense Response/Innate Immune Response (28 DEGs) (Fig 3A), and Hematopoiesis/Immune System Development/Myeloid Cell Differentiation (34 DEGs) (Fig 3B).
Fig 3. Chronic heavy ethanol consumption results in dysregulation of genes involved in innate immunity and immune system development.
(A) Heatmap of DEGs that map to “Defense Response/Innate Immune Response” (B) Heatmap of DEGs that map to “Immune System Development”/“Myeloid Cell differentiation”.
Genes involved in defense response/innate immune response were largely up-regulated (Fig 3A). This list includes several interferon-induced genes including IFI44L (Interferon-Induced Protein 44 Like, FC = 5.7), IFIT2 (Interferon-Induced Proteins with Tetratricopeptide repeats 1B, FC = 2.2), as well as ISG15 (ISG15 Ubiquitin-Like Modifier, FC = 2.7). In addition, innate immune genes known for their role in antiviral defense, such as viral dsRNA sensor OAS1 (2’-5’ Oligoadenylate Synthetase 1, FC = 4.3) [36]; RNA editing enzymes APOBEC3G (FC = 2.3) and APOBEC3H (FC = 2.3) [37] were also up-regulated. Several receptors involved in host defense and cell activation were also up-regulated. This list includes CD300E (FC = 3.4), a receptor capable of activating innate immune responses in myeloid cells [38]; CD1D (FC = 2.8), a glycoprotein molecule responsible for selection of NKT cells [39]; and the adhesion molecule ICAM2 (FC = 2.9). Furthermore, several T and NK cell activation markers were over-expressed, notably CLEC1B (C-Type Lectin Domain Family 1, Member B, FC = 3.7); CD69 (FC = 3.4) [40, 41]; and, TSPAN2 (Tetraspanin 2, FC = 3.4) [42] (Fig 3A).
Some of the defense response genes that were under-expressed include viral single-stranded RNA sensor TLR8 (Toll Like Receptor 8, FC = 2.2) [43]; RELB (V-Rel Avian Reticuloendotheliosus Viral Oncogene Homolog B, FC = 2.2), a subunit of the NF-kB complex with critical role regulating the transition from innate to adaptive immunity [44], and AGO2 (Argonaute RISC Catalytic Component 2, FC = 3.3), an essential RNA binding protein that mediates remodeling of the microRNA repertoire [45].
Genes that mapped to “Hematopoiesis/Immune System Development/Myeloid Cell Differentiation” were also largely up-regulated. Most of these DEGs were primarily involved in erythroid function (Fig 3B), notably ALAS2 (Erythroid-specific delta aminolevulinate synthase 2, FC = 151), which catalyzes the first reaction in the heme biosynthetic pathway [46]; AHSP (Alpha Hemoglobin Stabilizing Protein, FC = 19.6), which plays a role in stabilizing free alpha hemoglobin [47]; and SPTA1 (Spectrin Alpha, FC = 18.4), responsible for maintaining the stability and flexibility of erythrocytes. A number of transcription factors involved in erythrocyte development were also up-regulated such as SOX6 (Sex Determining Region Y—box 6, FC = 152), KLF1 (Krueppel Like Factor 1, FC = 57.3), MEIS1 (MEIS Homeobox 1, FC = 9.2), GFI1B (Growth Factor Independent Transcriptional Repressor 1B, FC = 31.5), and EPB42 (Erythroid Membrane Protein Band 4.2, FC = 31) [48–52]. Among the genes down-regulated in this GO term were transcriptional repressors of immune system such as ZFAT (Zinc Finger Protein 406, FC = 2.4) [53, 54]; BRCA2 (Breast Cancer 2, Early Onset, FC = 3.2) [55], and ZNF160 (Zinc Finger Protein 160, FC = 2.5) [56].
Heavy ethanol consumption dysregulates genes involved in innate immune signaling
Additional enrichment analysis was performed on the 89 DEGs that mapped to both ‘Immune System Process’ and ‘Signaling’ using InnateDB [57] allowing us to identify enriched signaling pathways from a smaller pool of genes in an unbiased way. These 89 genes mapped to ‘Innate Immune System’ and ‘Apoptosis’ (Fig 4A and 4B). Specifically, we observed up-regulation of a number of genes encoding inflammatory molecules such as CCL23 (Chemokine C-C motif ligand 23, FC = 9.7), CCL4L1 (Chemokine C-C motif ligand 4-like, FC = 2.3) and CCL5 (FC = 3.7), which are all highly chemotactic for macrophage and NK-cell migration [58, 59]. Additional up-regulated genes of interest play a critical role in innate immune signaling, such as TLR2 (Toll-like Receptor 2, FC = 2.7); LY86 (Lymphocyte Antigen 86, FC = 4.1); MD2 (Lymphocyte Antigen 96, FC = 3.2); and RIPK2 (Receptor Interacting Protein Kinase 2, FC = 2.4), critical for NOD mediated activation of NF-κB and pro-inflammatory cytokine production [60].
Fig 4.
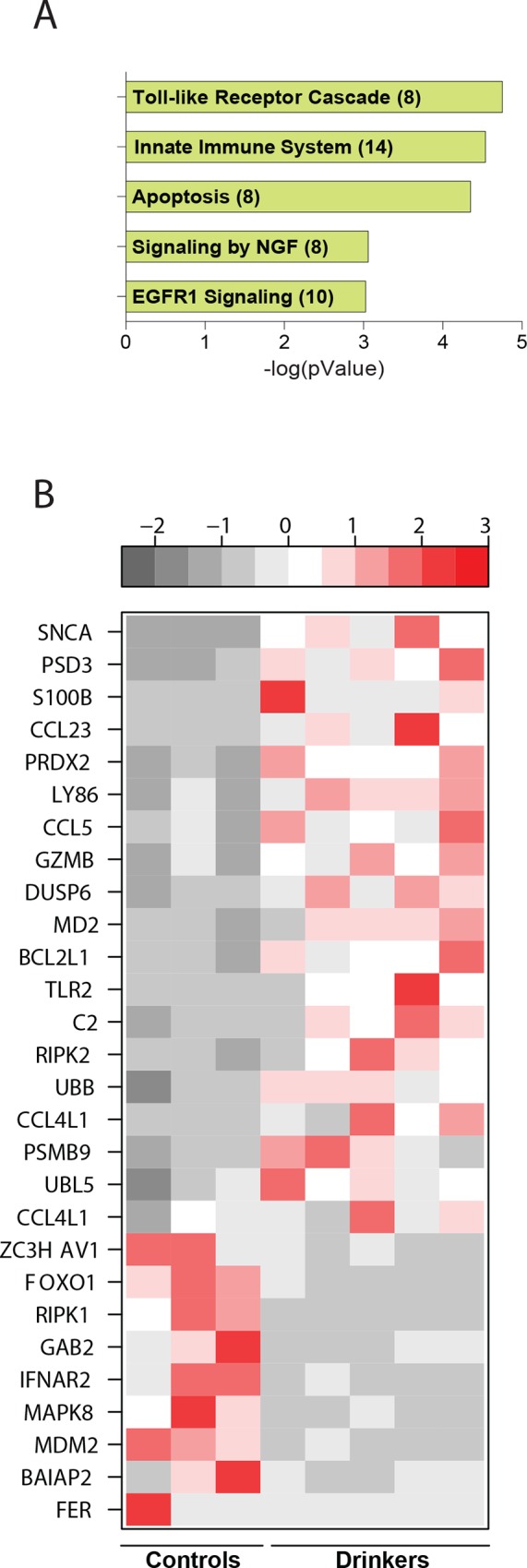
Chronic heavy ethanol consumption results in dysregulation of genes involved in innate immune signaling (A) Bar graph of 5 significant terms enriched among 89 genes mapping to “Immune System Process” and “Signaling” as predicted by InnateDB with the number of genes mapping to each term included in parenthesis (B) Heatmap of DEGs that map to “Innate Immune System” and “Apoptosis”.
Interestingly, a number of genes belonging to the Ubiquitin proteosome pathway were dysregulated in heavy drinkers. UBB (Ubiquitin B, FC = 2.3), involved in targeting proteins for proteolytic degradation by the proteasome [61]; UBL5 (Ubiquitin-like protein 5, FC = 2.1) and PSMB9 (Proteasome Subunit Beta 9, FC = 2.2) were up-regulated, whereas MDM2 (Murine double Minute -2, FC = 2.5), a ubiquitin ligase [62], was down-regulated.
Furthermore, a number of genes with roles in regulating innate anti-viral immunity were dysregulated. For example, PRDX2 (Peroxiredoxin, FC = 5.3), an anti-oxidant enzyme that contributes to antiviral activity of T-cells was up-regulated, whereas ZC3HAV1 (Zinc Finger CCCH-Type, Antiviral 1, FC = 2), an anti-viral protein that mediates degradation of viral RNA [63] was down-regulated (Fig 4B).
Among the 98 genes that were exclusive to the GO term ‘Signaling’, InnateDB’s Network Analyst application revealed enriched STAT3 signaling (S4A Fig). STAT3 (Signal Transducer and activator of transcription 3, FC = 2.1), which activates gene transcription of pro-inflammatory molecules via NF-KB activation pathway was down-regulated in heavy drinkers. Interestingly, we observed mixed changes in gene expression of its targets. Targets that play a role in inflammation and immunity such as IL1R1 (Interleukin 1 Receptor, Type 1, FC =), CCL2 (Chemokine C-C Motif Ligand 2, FC =) and THBS1 (Thrombospondin 1, FC) were down-regulated. In contrast, targets that play a role in interferon signaling were all up-regulated such as OAS1 (2’-5’ Oligoadenylate Synthase 1, FC = 4.3), IFIT1 (Interferon-Induced Protein With Tetratricopeptide Repeats 1, FC = 5.9), IFIT1B (FC = 89), IFI44 (Interferon-Induced Protein 44, FC = 5.4), and IFI44L (FC = 5.7).
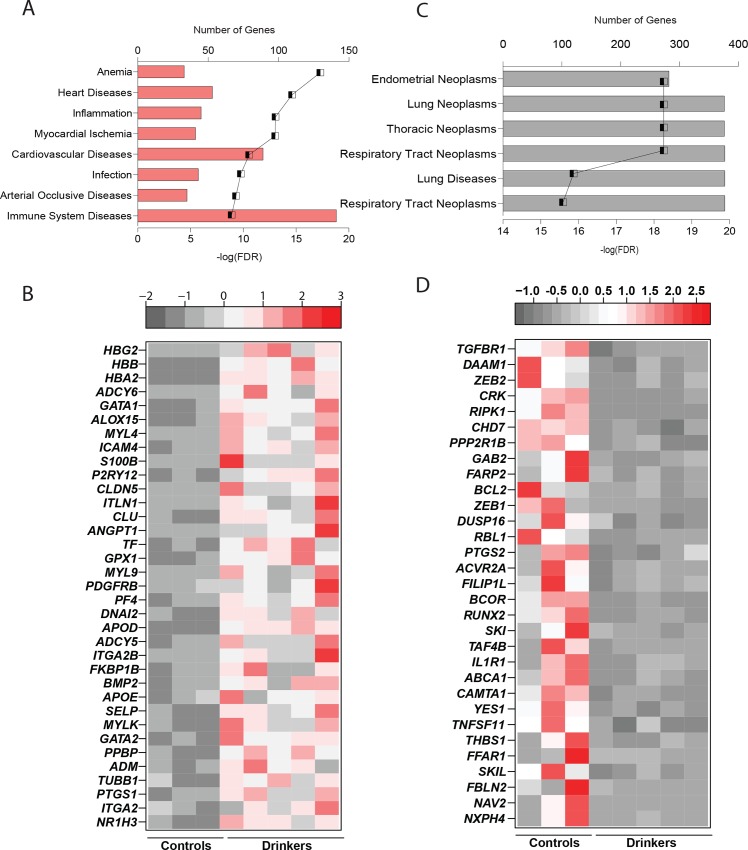
Chronic heavy ethanol consumption changes the expression of genes involved in heart diseases and cancer
To get a better understanding of the biological impact of the gene expression changes, we conducted disease enrichment analysis for up- and down-regulated genes separately. Up-regulated DEGs primarily mapped to Anemia, Myocardial ischemia, and heart diseases (Fig 5A). Indeed, several of the up-regulated DEGs have documented association with cardiovascular risk when expressed at high levels (Fig 5B). This includes enzymes like ALOX15 (Arachidonate-15-lipoxygenase, FC = 18.8) [64], GPX1 (Glutathione Peroxidase 1, FC = 10.8) [65], adhesion molecule SELP (P-Selectin, FC = 6.1) [27], and the apolipoprotein APOE (FC = 6.3) [66].
Fig 5. Chronic heavy ethanol consumption changes the expression of genes involved in heart diseases and cancer.
(A) Bar graph depicting 8 disease terms enriched among the up-regulated genes. The line graph in both figures represents negative log (FDR) of the enriched term. (B) Heatmap of up-regulated genes involved in cardiovascular diseases. (C) Bar graph depicting 8 disease terms enriched among the down-regulated genes. (D) Heatmap of the down-regulated genes involved in cancer.
Down-regulated genes enriched to several categories of cancer (Fig 5C). The 157 DEGs shared amongst these cancer terms contained transcription regulators such as CAMTA1 (Calmodulin Binding Transcription Activator 1, FC = 4) [67], RUNX2 (Runt-related transcription factor, FC = 3.5) [68] and ZEB1 (Zinc Finger E-Box Binding Homeobox 1, FC = 2.6); and proto-oncogenes like YES1 (FC = 4.4) [69] and SKI (FC = 3.6) [70](Fig 5D). Additionally, a number of signaling receptors also mapped to these GO terms including FFAR1 (Free fatty acid receptor 1, FC = 5.1) [71], TGFBR1 (Transforming Growth Factor, Beta Receptor 1, FC = 2) and TNFSF11 (Tumor necrosis factor receptor superfamily 11, FC = 4.6) [72]. Interestingly, several zinc finger genes that regulate cell cycle progression like ZC3H12C (FC = 6.2) [73], were also down-regulated in heavy drinkers.
Chronic heavy ethanol consumption results in changes in expression of epigenetic regulators
Our analysis revealed that a number of microRNAs were down-regulated in response to chronic ethanol consumption (Table 2). Of note, miR-27a (FC = 6.2), which regulates M2 macrophage polarization of human monocytes by targeting IL10 [74, 75], was down-regulated. Importantly, three of its targets were up-regulated in our analysis: PAQR9 (FC = 439) [76], NR2F6 (Nuclear Receptor Subfamily 2, Group F, Member 6, FC = 3.1) [77] and GATA2 (Phospholipase C-Like 2, FC = 2.3). Similarly, miR-24 (FC = 7.2) was under-expressed while several of its predicted targets were up-regulated: NEFM (Neurofilament Medium Polypeptide, FC = 345), BNIP3L (BCL2 Interacting Protein 3 Like, FC = 4.4). Finally, predicted targets of down-regulated miR-23a (FC = 6.7) such as CA2 (Carbonic anhydrase 2, FC = 6.2) and PTP4A2 (Protein Tyrosine Phosphate Type 4A, FC = 2.2) were also up-regulated. Interestingly, miR-23~27~24 clusters have been shown to regulate T-cell mediated immune homeostasis [78].
Table 2. Summary of down-regulated microRNAs and their up-regulated targets in drinkers.
| miRNA down-regulated in heavy drinkers | Fold Change | Gene targets up-regulated in heavy drinkers |
|---|---|---|
| miR-23a | 6.7 | PTP4A2, CA2, NCKAP1, XRCC2, GRTP1, MAP1B |
| miR-24 | 7.2 | NEFM, BNIP3L, STRADB, EHD2, WWRT1, XRCC2, AMOTL2, FKBP1B, HBQ1, C6orf25, GSTO2 |
| miR-27a | 6.2 | PAQR9, NR2F6, RGS2, RNF152, AMOTL2, GATA2, MAP1B |
| miR-663 | 18.7 | MYL9, NRGN, CCTN |
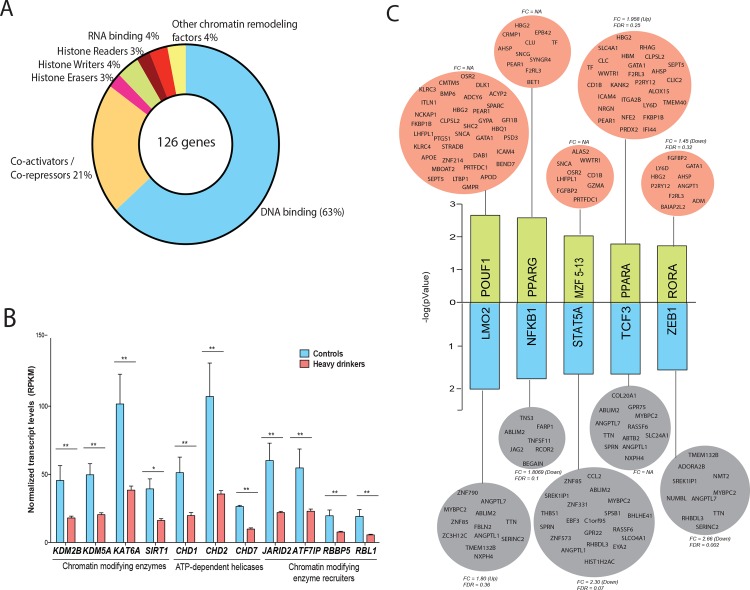
Initial gene enrichment analysis of all DEGs using MetaCore showed GO processes ‘Regulation of Transcription’ and ‘Regulation of Gene Expression’ as significantly down-regulated in heavy drinkers (S2C Fig). A significant number of these 128 genes were involved in chromatin reorganization, particularly histone erasers (HDAC9 (Histone deacetylase 9, FC = 2.8), histone writers PRDM8 (PR Domain Containing 8, FC = 2.8) and histone readers (CHD1 (Chromodomain Helicase DNA Binding Protein 1, FC = 2.1), and CHD2 (FC = 2.5)) (Fig 6A and 6B). Interestingly, these enzymes catalyze post-translational modifications primarily on histone H3 [79]. Furthermore, coregulators of these enzymes such as RBL1 (Retinoblastoma Like 1, FC = 2.9) were also down-regulated (Fig 6B). Additionally, a number of transcription factors that regulate cell proliferation, differentiation and transformation were down-regulated such as AFF3 (Lymphoid Nuclear Protein4, FC = 2.7), STAT3 (Signal Transducer and Activator of Transcription 3, FC = 2.1), HIVEP1 (Human Immunodeficiency Virus Type I Enhancer Binding Protein 1, FC = 2.3), FOXO1 (Forkhead Box O1, FC = 2.0), and FOSB (FBJ Murine Osteosarcoma Viral Oncogene Homolog B, FC = 2.3) [80–83].
Fig 6. Chronic heavy ethanol consumption results in changes in expression of epigenetic regulators.
(A) Functional profiles of the 128 down-regulated genes mapping to ‘Regulation of Gene Expression’ (B) Bar graph of expression levels (RPKM) of genes involved in chromatin remodeling (**—FDR of 5% and *—FDR of 10%). (C) Bar graph of 5 most significantly up- and down-regulated transcription factor networks. Green bars indicate up-regulated network and blue bars indicate down-regulated networks. Each bar is linked to a group of target genes (orange–up-regulated and grey down-regulated) that are differentially expressed in heavy drinkers.
Among the up-regulated transcription factors were MEIS1 (Meis Homeobox 1, FC = 9.2), which regulates hematopoeisis and megakaryocyte lineage development [84]; EPAS1 (Endothelial PAS Domain Protein 1, FC = 2.5), which regulates expression of oxygen-related genes [85]; NFE2 (Nuclear Factor Erythroid 2, FC = 10.5), which regulates immune functions, redox homeostasis and intracellular signaling in dendritic cells [86]; and, TXNIP (Thioredoxin Interacting Protein, FC = 3), an oxidative stress mediator required for the maturation of NK cells [87].
To identify dysregulated transcription factor networks, we performed transcription factor binding site (TFBS) analysis of all 1106 genes using InnateDB (Fig 6C). The results from this analysis indicate up-regulation of transcriptional networks regulated by POUF1, an activator of hormone genes [88]; PPARG (Peroxisome proliferator-activated receptor gamma) and PPARA, critical regulators of inflammatory response [89, 90]; MZF5-13 (Myeloid Zinc Finger 5–13), which plays a crucial role in negative regulation of proliferation of hematopoietic progenitors [91] and RORA (Retinoic-Acid-Receptor-Related Orphan Nuclear Receptor Alpha), a nuclear receptor required for natural helper cell development in all tissues[92].
The down-regulated transcriptional networks were regulated by NFKB1 (Nuclear Factor of Kappa Light Polypeptide Gene Enchancer in B-cells 1), a subunit of NFKB, whose activation has been shown to be inhibited following acute ethanol exposure in vitro resulting in reduced production of inflammatory cytokines [93, 94]. Additional suppressed transcriptional networks include STAT5A (Signal Transducer and Activator of Transcription 5A) [95]; TCF3 (Transcription Factor 3) and LMO2 (T-cell translocation protein 2), which are essential regulators of hematopoiesis and lymphopoiesis [96, 97]; and ZEB1 (Zinc Finger E-Box Binding Homeobox 1) and TCF8, which play a key role in cellular differentiation [98].
Discussion
The gender-specific impacts of alcohol consumption, including AUD are under-studied. In this study, we used RNA-Seq to characterize differences in gene expression in PBMC collected from female rhesus macaques after 12 months of chronic ethanol consumption using a model of voluntary ethanol self-administration that robustly simulates topographies of drinking in young adult humans [99]. Although there were no changes in frequencies of circulating white blood cells between the ethanol consuming and control animals, robust changes in gene expression were detected, suggestive of a robust effect of ethanol and its metabolites on regulation of gene expression within immune cells.
Among these gene expression changes, we saw a larger number of down-regulated (661 DEGs) compared to up-regulated genes (445 DEGS). Upon gene enrichment, a significant number of up-regulated genes mapped to blood coagulation. Notable up-regulated genes were involved in hemoglobin synthesis (HBB, HBA2, HBG2, AHSP, and NFE2), coagulation (F13A1, F2R, ITGA1, and ITGB5) and platelet adhesion (SELP and GP5). In contrast, genes known to inhibit coagulation (SERPINB2 and PLAT) were down-regulated. In addition, our findings suggest an increased shift of the hematopoiesis transcriptional machinery towards erythroid lineage. This includes transcription factors involved in various stages of erythrocyte development (GATA1, GATA2, KLF1, EPB42, TAL1, GFI1B, and MEIS1) as well as hematopoiesis specific enzymes and regulators (AHSP, ALAS2, ANK1 and BPGM). The imbalance in the expression of procoagulant, anticoagulant and fibrinolytic factors together with a shift towards erythrocyte development, suggests that chronic heavy ethanol consumption results in increased coagulation and an increased predisposition to cardiovascular diseases (CVD).
Indeed, additional functional enrichment analysis shows that up-regulated genes map to a number of CVD including anemia, myocardial ischemia, infarction and heart diseases. Recent studies have shown that APOE is a marker for CVD in women with high cholesterol and C-reactive protein levels but not in men [66]. Additionally ALOX15 and GPX1 were up-regulated in this study, which have been associated with higher risk of CVD [64, 65, 100–103]. These findings are consistent with clinical observations that heavy alcohol consumption is associated with alcoholic cardiomyopathy [104, 105]. Indeed, previous studies on the association of ethanol with hemolytic parameters such as the Framingham Offspring Study also reported that fibrinolytic potential decreases with increased ethanol consumption especially at doses greater than 7 drinks weekly and this could explain the increased risk of CVD in the drinking cohort [106]. Our observations are also in line with an increased incidence of thrombosis in beer-drinking men based on measurements of PLAT [107].
Heavy ethanol consumption interferes with several stages of wound healing including inflammation, angiogenesis, and restoration of extracellular matrix [108]. In support of these observations, several genes necessary for wound healing were down-regulated in our study including CCL2 and TGFBR1, which are involved in angiogenesis and remodeling of extracellular matrix. Additionally, we also saw up-regulation of anti-angiogenic factors like PF4, PF4V1, ANGPT1, SPARC, and SPARCL1. Furthemore, plasma levels of VEGFD were significantly reduced (S1C Fig), a finding that agrees with previous studies on ethanol-consuming male cynomolgus monkeys [109]. Thus, the altered expression profiles detected in our study supports the clinical observation of delayed wound repair as a result of chronic ethanol exposure.
We also observed up-regulation of a large number of genes involved in immune signaling and inflammation (BTK, CCL5, CCL4L1, CCL23, PF4V1), interferon stimulating genes (IFIT1, IFI44, IFI44L, IFIT1B, IFIT2, ISG12, and ISG15) and activation markers (CLEC1B, CLEC4G, CD69). Collectively, these observations suggest a dysregulation of the innate immune response where nonspecific inflammatory responses maybe enhanced but pathogen-specific responses maybe dampened. A large number of these DEGs are expressed by monocytes and DCs suggesting that ethanol exerts its biggest impact on innate immunity. This is corroborated by additional bioinformatics analysis using the native expression profiles of these genes in purified population of peripheral blood cells reported in ImmGen database [110] (S3A Fig).
In support of studies reporting increased susceptibility to infection with AUD [111–113], we report down-regulation of a number of genes important for host defense such as cytokines (IL1R1 and TNFSF11) [114], chemokines (CCL2), and receptors (TGFBR1 and TLR8). In contrast, we observed significant down-regulation of NFKB associated genes in PBMC with heavy alcohol consumption (TRAF3, RCOR2, JAG2, TNFSF11, and RELB). This dysregulated inflammatory landscape could be due to increased bacterial translocation seen with heavy ethanol consumption [2] and/or exosome signaling [115]. Moreover, important transcription factors required for activating immune responses (STAT3 and RELB) were down-regulated [116–118]. Interestingly, using the same model we have previously reported reduced levels of STAT3 mRNA and protein levels in PBMCs of a larger cohort of rhesus macaques. Additionally we have shown that these changes in mRNA and protein levels of STAT3 are mediated by increased expression of microRNAs miR-181 and miR-22 and resulted in ethanol dependent disruption of growth factors (VEGF, HGF) and inflammatory cytokine production (G-CSF) in PBMC following phorbol myristate acetate(PMA)/ ionomycin stimulation[15].
Our data support previously published reports of elevated levels of SNCA (Synuclein-Alpha, FC = 75.7) mRNA in the blood of rats, monkeys and humans with alcohol use disorder (AUD) [119–122]. Therefore, mRNA levels of SNCA may serve as a robust biomarker of AUD across species. Additionally, in accordance with a previous study on circulating plasma protein biomarkers associated with AUD in cynomolgus monkeys, we saw increased plasma levels of IL7 [109] (S1C Fig). Interestingly, in the PBMC, we saw down-regulation of IL7 (FC = 2.2, FDR = 0.08). This contradiction could be explained by the differences in tissue-specific gene expression profiles since IL7 is primarily secreted by the stromal cells of the thymus and bone marrow and not by circulating PBMC.
Our analysis also provides some insight into mechanisms of altered gene expression with chronic heavy ethanol consumption. We report down-regulation of miRNA molecules as well as changes in expression of chromatin modifying enzymes–writers and erasers. Several studies have provided increased evidence that miRNAs play a role in ethanol withdrawal [114], the etiology of alcoholism [123] and hepatotoxicity [124]. However, very few studies have investigated the impact of ethanol consumption on miRNA expression within immune cells. It was interesting to note that all the differentially expressed miRNAs detected my RNA-Seq were down-regulated. Of particular importance, miR-27a, which was significantly down-regulated, can regulate the M2 macrophage polarization of monocytes through regulation of ERK signaling and by the secretion of IL10 [75]. IL10 was up-regulated in our dataset, as were three predicted targets of this microRNA. Similarly, several targets of the down-regulated miR-24, (NEFM and BNIP3L) were up-regulated on heavy ethanol consumption.
We also report up-regulation of histone genes, but down-regulation of all histone modifiers (writers and erasers) including deacetylases (SIRT1, HDAC9), acetyl-transferases (KAT6A), methyltransferases (KMT2C), and demethylases (KDM5A, KDM2B, and JARID2). These results suggest that chronic heavy ethanol preferentially induces post-translational modifications on histone H3. Interestingly, brain chromatin remodeling of histone H3 and H4 have been attributed to facilitating ethanol withdrawal symptoms [125]. Furthermore, studies from alcohol exposed rat hepatocytes and livers of alcohol-fed rats indicate changes in acetylation of histone H3 [126, 127]. While it has been well established that epigenetic changes in histone H3 and H4 play a role in regulating inflammatory responses in immune cells following LPS stimulation [128], whether alcohol exposure affects epigenetic mechanisms to prolong expression of cytokines in immune cells is not yet known. Therefore, future studies will focus on uncovering mechanisms that regulate gene expression changes including methylation, expression changes in non-coding RNAs, and histone modifications in immune cells and how it correlates with gene expression changes at key inflammatory loci.
Materials and Methods
Animals and Sample Collection
This study was performed in strict accordance with the recommendations made in the Guide for Care and Use of Laboratory Animals of the National Institutes of Health, the Office of Animal Welfare and the United States Department of Agriculture. All animal work was approved by the ONPRC Institutional Animal Care and Use Committee. Nine female rhesus macaques (average age 4 years 2 months) were used in this study, with three animals serving as controls. We used schedule-induced polydipsia to establish self-administration of 4% (w/v) of ethanol in the remaining 6 female rhesus macaques. All animals were housed in quadrant cages (0.8 × 0.8 × 0.9 m) with constant temperature (20–22°C), humidity (65%) and a 11-h light cycle with visual, auditory and olfactory contact with other conspecifics. The barrier between monkeys housed side-by-side was removed for 2h/weekday and the monkeys shared the expanded housing cage. The animals had access to environmental enrichments like toys. For the purpose of this study, the animals were not sacrificed.
Briefly, monkeys were trained to operate a panel inserted as part of the sidewall in their housing cage and could obtain all fluids and food through this panel [14, 129]. Ethanol self-administration was established using an induction phase that lasted 4 months, with the dose of ethanol (0, 0.5, 1.0, 1.5 g/kg) increasing every 30th session. Following the induction phase, monkeys were allowed an “open-access” availability to fluids (4% w/v ethanol and water, n = 6 or water, n = 3) for 22 hrs/day with food available in 3 meals/day. The open access condition occurred daily for over 12 months and parameters of the daily ethanol intakes were used in the data analysis. The blood ethanol concentration (BEC) was measured using gas head-space chromatography (Hewlett-Packard 5890 Series II, Avondale, PA, USA; equipped with a headspace auto-sampler, flame ionization detector, and a Hewlett-Packard 3392A integrator)[12]. For gene expression analysis, unanesthetized blood samples were drawn from all 9 animals after 12 months of ethanol self-administration. Differentials were obtained from whole blood using a complete blood count machine (Hemavet; Drew Scientific Group, Waterbury, CT) calibrated for rhesus blood. PBMC were isolated by centrifugation over histopaque (Sigma, St Louis, MO) as per the manufacturer’s protocol. The PBMC were cryopreserved in Fetalplex™ Animal Serum Complex (Gemini Bio-Products, West Sacramento, CA)/DMSO until they could be analyzed as a batch.
Flow Cytometry
PBMC were surface-stained with antibodies against: (1) CD4 (eBioscience, San Diego, CA, USA), CD8β (Beckman Coulter, Brea, CA, USA), CD28 (BioLegend, San Diego, CA, USA) and CD95 (BioLegend) to delineate the naive (CD28+CD95−), central memory (CD28+CD95+) and effector memory (CD28−CD95+) T cell subsets; (2) CD20 (Beckman Coulter), IgD (Southern Biotech, Birmingham, AL, USA) and CD27 (BioLegend) to delineate naive (CD20+IgD+CD27−), marginal zone (MZ)-like (CD20+IgD+CD27+) and memory (CD20+IgD−CD27+) B cell subsets. (3) A second tube was stained with CD3 (BD Pharmingen, San Diego, CA, USA), CD20, HLA-DR (BioLegend), CD14 (BioLegend), CD123 (BioLegend) and CD11c (BioLegend) to delineate monocytes (CD3−CD20−CD14+HLA-DR+) and dendritic cells (DC, CD3−CD20−CD14−HLA-DR+). Dendritic cells were further defined into myeloid (mDC, CD123−CD11c+) and plasmacytoid (pDC, CD123+CD11c−). The samples were acquired using the LSRII instrument (Beckton Dickinson Company, San Jose, CA, USA) and data analyzed using FlowJo software (TreeStar, Ashland, OR, USA).
Cytokine, Chemokine and Growth Factor Analysis
The circulating cytokines were measured in the plasma using nonhuman primate Cytokine/Chemokine/GF (eBioscience, San Diego CA) 37-plex panel that measures IFNγ, IFNα, TNFα, IL1RA, IL1b, IL2, IL4, IL5, IL6, IL7, IL8, IL10, IL12p70, IL13, IL15, IL17A, IL18, IP10, IL23, sCD40L, SCF, MCP1, MIP1α, MIP1β, MIG, Eotaxin, ITAC, BLC, SDF1α, VEGFA, VEGFD, GCSF, GMCSF, BDNF, FGF2, NGFß and PDGFBB.
RNA Isolation and Library Preparation
Total RNA was isolated from PBMC using the mRNeasy kit (Qiagen, Valencia, CA), followed by polyA selection to enrich messenger (m)RNA. 1μg of polyA-enriched mRNA was fragmented followed by cDNA synthesis using random hexamers (New England Biolabs, Ipswich, MA). This was followed by end-repair, ligation of adapters and size selection using AMPure XP beads (Beckman Coulter Inc, Brea, CA) to isolate cDNA templates of 320 nucleotides, which were subsequently PCR amplified. Each library was indexed using a unique barcode for multiplexing and sequenced on the HiSeq2500 platform (Illumina, San Diego, CA) to yield single-end 100 bp sequences.
Bioinformatics Analysis
RNA-Seq data analysis was done in R using Bioconductor packages and using open source command line tools. Quality reports for the raw reads were generated using FASTQC. The reads were then aligned to the Macaca mulatta genome from Ensembl using splice aware short read aligner suite Bowtie2/TopHat2 [130, 131]. The transcript counts per gene were then summarized using the summarizeOverlaps function, which counts reads that align to exonic regions only. Since the libraries were non-stranded in nature, the reads were counted in a non-strand specific manner. The final files of RNA-Seq read samples have been submitted to NCBI (SRP064925).
Differential gene expression analysis was performed using GLM method from edgeR package [132] in Bioconductor. Differentially expressed genes (DEGs) were defined as those with a fold change ≥ 2 and a false discovery rate (FDR) of ≤ 5%. Functional enrichment of these genes was done using MetaCore™ (GeneGo™, Thomson Reuters, NY) to identify clusters of genes mapping to specific biological pathways or disease associations. To identify potential targets of microRNAs, TargetScan database [133] was scanned with the list of DEGs for possible associations based on computational prediction with a context ratio of at least 95%. ImmGen database was profiled to identify possible sources of transcriptional changes within the mixed population of cells [110]. Additional functional enrichment on select gene groups was performed using InnateDB Gene Ontology [134] and Transcription Factor Binding Site (TFBS) tool. Gene networks were constructed using NetworkAnalyst [135] identifying enriched gene networks in input genes.
Supporting Information
Cell counts and cytokine, chemokine, growth factor measurements (A) Cell counts from whole blood. (B) Percentage of major cell subsets within PBMC. (C) Average concentrations of cytokines and growth factors that differed between ethanol-consuming animals and controls.
(EPS)
(A) Principal Component Analysis and (B) Bar graph depicting the 8 most significant Gene Ontology (GO) terms enriched among up-regulated differentially expressed genes (DEGs), and (D) the down-regulated DEGs. The bar represents the number of DEGs mapping to each GO term while the line graph represents negative log (FDR) of the enriched term.
(EPS)
(A) Expression profile of genes mapping to ‘Immune System Process’ across several cell types as predicted by ImmGen’s MyGeneSet application. Genes are represented as individual rows while each column represents a study.
(EPS)
(A) Network image of the DEG that map to uniquely to ‘Signaling’ and show direct interactions.
(EPS)
Acknowledgments
We would like to thank Flora Engelmann for help with flow cytometry, Sumana Pasala for RNA extraction and library construction and Christina Nguyen for help generating figures.
Data Availability
All RNA-Seq fastq files are available for download from the NCBI SRA database (accession number SRA305670).
Funding Statement
This work was supported by NIAAA grant AA021947-02, U01 AA013510, and R24 AA109431.
References
- 1.SAMHSA. National Survey on Drug Use and Health (NSDUH). 2013.
- 2.Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42(5):349–61. 10.1016/j.alcohol.2008.03.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabo G, Mandrekar P. Focus on: Alcohol and the liver. Alcohol Res Health. 2010;33(1–2):87–96. [PMC free article] [PubMed] [Google Scholar]
- 4.Mukamal KJ, Rimm EB. Alcohol's effects on the risk for coronary heart disease. Alcohol Res Health. 2001;25(4):255–61. . [PMC free article] [PubMed] [Google Scholar]
- 5.Djousse L, Gaziano JM. Alcohol consumption and heart failure in hypertensive US male physicians. Am J Cardiol. 2008;102(5):593–7. 10.1016/j.amjcard.2008.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr T, Helms C, Grant K, Messaoudi I. Opposing effects of alcohol on the immune system. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:242–51. 10.1016/j.pnpbp.2015.09.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasala S, Barr T, Messaoudi I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. 2015;37(2):185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiner AP, Frank MB, Schwartz SM, Linenberger ML, Longstreth WT, Teramura G, et al. Coagulation factor XIII polymorphisms and the risk of myocardial infarction and ischaemic stroke in young women. Br J Haematol. 2002;116(2):376–82. . [DOI] [PubMed] [Google Scholar]
- 9.Dawson DA. Consumption indicators of alcohol dependence. Addiction. 1994;89(3):345–50. . [DOI] [PubMed] [Google Scholar]
- 10.Stote KS, Tracy RP, Taylor PR, Baer DJ. The effect of moderate alcohol consumption on biomarkers of inflammation and hemostatic factors in postmenopausal women. Eur J Clin Nutr. 2015. 10.1038/ejcn.2015.182 . [DOI] [PubMed] [Google Scholar]
- 11.Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med. 1988;319(5):267–73. 10.1056/NEJM198808043190503 . [DOI] [PubMed] [Google Scholar]
- 12.Jimenez VA, Helms CM, Cornea A, Meshul CK, Grant KA. An ultrastructural analysis of the effects of ethanol self-administration on the hypothalamic paraventricular nucleus in rhesus macaques. Front Cell Neurosci. 2015;9:260 10.3389/fncel.2015.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32(10):1824–38. 10.1111/j.1530-0277.2008.00765.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker EJ, Farro J, Gonzales S, Helms C, Grant KA. Chronic alcohol self-administration in monkeys shows long-term quantity/frequency categorical stability. Alcohol Clin Exp Res. 2014;38(11):2835–43. 10.1111/acer.12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asquith M, Pasala S, Engelmann F, Haberthur K, Meyer C, Park B, et al. Chronic ethanol consumption modulates growth factor release, mucosal cytokine production, and microRNA expression in nonhuman primates. Alcohol Clin Exp Res. 2014;38(4):980–93. 10.1111/acer.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barr T, Girke T, Sureshchandra S, Nguyen C, Grant K, Messaoudi I. Alcohol Consumption Modulates Host Defense in Rhesus Macaques by Altering Gene Expression in Circulating Leukocytes. J Immunol. 2015. 10.4049/jimmunol.1501527 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graeme IM, William TM, William FG, Burke MD. REGULATION, FUNCTION, AND TISSUE-SPECIFIC EXPRESSION OF CYTOCHROME P450 CYP1B1. Annu Rev Pharmacol Toxicol. 2001;41(1):297–316. 10.1146/annurev.pharmtox.41.1.297 [DOI] [PubMed] [Google Scholar]
- 18.Tigges J, Weighardt H, Wolff S, Götz C, Förster I, Kohne Z, et al. Aryl hydrocarbon receptor repressor (AhRR) function revisited: repression of CYP1 activity in human skin fibroblasts is not related to AhRR expression. J Invest Dermatol. 2013;133(1):87–96. 10.1038/jid.2012.259 [DOI] [PubMed] [Google Scholar]
- 19.He L, Vasiliou K, Nebert DW. Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genomics. 2009;3(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su X, Floyd DH, Hughes A, Xiang J, Schneider JG, Uluckan O, et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest. 2012;122(10):3579–92. 10.1172/JCI38576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Washington AV, Gibot S, Acevedo I, Gattis J, Quigley L, Feltz R, et al. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest. 2009;119(6):1489–501. 10.1172/JCI36175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amelot AA, Tagzirt M, Ducouret G, Kuen RL, Le Bonniec BF. Platelet factor 4 (CXCL4) seals blood clots by altering the structure of fibrin. J Biol Chem. 2007;282(1):710–20. 10.1074/jbc.M606650200 [DOI] [PubMed] [Google Scholar]
- 23.Mirabet M, Herrera C, Cordero OJ, Mallol J, Lluis C, Franco R. Expression of A2B adenosine receptors in human lymphocytes: their role in T cell activation. J Cell Sci. 1999;112 (Pt 4):491–502. [DOI] [PubMed] [Google Scholar]
- 24.Blanco-Mezquita JT, Hutcheon AEK, Zieske JD. Role of thrombospondin-1 in repair of penetrating corneal wounds. Invest Ophthalmol Vis Sci. 2013;54(9):6262–8. 10.1167/iovs.13-11710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki M, Shimizu R, Yamamoto M. Transcriptional regulation by GATA1 and GATA2 during erythropoiesis. Int J Hematol. 2011;93(2):150–5. 10.1007/s12185-011-0770-6 [DOI] [PubMed] [Google Scholar]
- 26.Verhaar MC, Beutler JJ, Gaillard CA, Koomans HA, Fijnheer R, Rabelink TJ. Progressive vascular damage in hypertension is associated with increased levels of circulating P-selectin. J Hypertens. 1998;16(1):45–50. [DOI] [PubMed] [Google Scholar]
- 27.Carter AM, Anagnostopoulou K, Mansfield MW, Grant PJ. Soluble P-selectin levels, P-selectin polymorphisms and cardiovascular disease. J Thromb Haemost. 2003;1(8):1718–23. [DOI] [PubMed] [Google Scholar]
- 28.Falati S, Edmead CE, Poole AW. Glycoprotein Ib-V-IX, a receptor for von Willebrand factor, couples physically and functionally to the Fc receptor gamma-chain, Fyn, and Lyn to activate human platelets. Blood. 1999;94(5):1648–56. [PubMed] [Google Scholar]
- 29.Li-Saw-Hee FL, Blann AD, Gurney D, Lip GY. Plasma von Willebrand factor, fibrinogen and soluble P-selectin levels in paroxysmal, persistent and permanent atrial fibrillation. Effects of cardioversion and return of left atrial function. Eur Heart J. 2001;22(18):1741–7. 10.1053/euhj.2000.2531 [DOI] [PubMed] [Google Scholar]
- 30.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120 Suppl 1:S5–9. 10.1016/j.thromres.2007.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107(9):1049–54. 10.1172/JCI12939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daly C, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation. 2003;10(3–4):247–57. 10.1038/sj.mn.7800190 [DOI] [PubMed] [Google Scholar]
- 33.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2(1):18–28. [PMC free article] [PubMed] [Google Scholar]
- 34.Mun GI, Jang SI, Boo YC. Laminar shear stress induces the expression of aquaporin 1 in endothelial cells involved in wound healing. Biochem Biophys Res Commun. 2013;430(2):554–9. 10.1016/j.bbrc.2012.11.114 [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Sng MK, Foo S, Chong HC, Lee WL, Tang MB, et al. Early controlled release of peroxisome proliferator-activated receptor beta/delta agonist GW501516 improves diabetic wound healing through redox modulation of wound microenvironment. J Control Release. 2015;197:138–47. 10.1016/j.jconrel.2014.11.001 . [DOI] [PubMed] [Google Scholar]
- 36.Sadler AJ, Williams BRG. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8(7):559–68. 10.1038/nri2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith HC, Bennett RP, Kizilyer A, McDougall WM, Prohaska KM. Functions and regulation of the APOBEC family of proteins. Semin Cell Dev Biol. 2012;23(3):258–68. 10.1016/j.semcdb.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brckalo T, Calzetti F, Perez-Cabezas B, Borras FE, Cassatella MA, Lopez-Botet M. Functional analysis of the CD300e receptor in human monocytes and myeloid dendritic cells. Eur J Immunol. 2010;40(3):722–32. 10.1002/eji.200939468 . [DOI] [PubMed] [Google Scholar]
- 39.Brutkiewicz RR. CD1d ligands: the good, the bad, and the ugly. J Immunol. 2006;177(2):769–75. . [DOI] [PubMed] [Google Scholar]
- 40.Borrego F, Robertson MJ, Ritz J, Peña J, Solana R. CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology. 1999;97(1):159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartel Y, Bauer B, Steinle A. Modulation of NK cell function by genetically coupled C-type lectin-like receptor/ligand pairs encoded in the human natural killer gene complex. Front Immunol. 2013;4:362 10.3389/fimmu.2013.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delaguillaumie A, Harriague J, Kohanna S, Bismuth G, Rubinstein E, Seigneuret M, et al. Tetraspanin CD82 controls the association of cholesterol-dependent microdomains with the actin cytoskeleton in T lymphocytes: relevance to co-stimulation. J Cell Sci. 2004;117(Pt 22):5269–82. 10.1242/jcs.01380 [DOI] [PubMed] [Google Scholar]
- 43.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–9. 10.1126/science.1093620 [DOI] [PubMed] [Google Scholar]
- 44.Xia Y, Pauza ME, Feng L, Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol. 1997;151(2):375–87. [PMC free article] [PubMed] [Google Scholar]
- 45.Bronevetsky Y, Villarino AV, Eisley CJ, Barbeau R, Barczak AJ, Heinz GA, et al. T cell activation induces proteasomal degradation of Argonaute and rapid remodeling of the microRNA repertoire. J Exp Med. 2013;210(2):417–32. 10.1084/jem.20111717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadlon TJ, Dell'Oso T, Surinya KH, May BK. Regulation of erythroid 5-aminolevulinate synthase expression during erythropoiesis. Int J Biochem Cell Biol. 1999;31(10):1153–67. [DOI] [PubMed] [Google Scholar]
- 47.Kihm AJ, Kong Y, Hong W, Russell JE, Rouda S, Adachi K, et al. An abundant erythroid protein that stabilizes free alpha-haemoglobin. Nature. 2002;417(6890):758–63. 10.1038/nature00803 [DOI] [PubMed] [Google Scholar]
- 48.Hagiwara N. Sox6, jack of all trades: a versatile regulatory protein in vertebrate development. Dev Dyn. 2011;240(6):1311–21. 10.1002/dvdy.22639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okada Y, Nagai R, Sato T, Matsuura E, Minami T, Morita I, et al. Homeodomain proteins MEIS1 and PBXs regulate the lineage-specific transcription of the platelet factor 4 gene. Blood. 2003;101(12):4748–56. 10.1182/blood-2002-02-0380 [DOI] [PubMed] [Google Scholar]
- 50.van der Meer LT, Jansen JH, van der Reijden BA. Gfi1 and Gfi1b: key regulators of hematopoiesis. Leukemia. 2010;24(11):1834–43. 10.1038/leu.2010.195 [DOI] [PubMed] [Google Scholar]
- 51.Tallack MR, Perkins AC. KLF1 directly coordinates almost all aspects of terminal erythroid differentiation. IUBMB Life. 2010;62(12):886–90. 10.1002/iub.404 [DOI] [PubMed] [Google Scholar]
- 52.An X, Schulz VP, Mohandas N, Gallagher PG. Human and murine erythropoiesis. Curr Opin Hematol. 2015;22(3):206–11. 10.1097/MOH.0000000000000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon HS, Scharer CD, Majumder P, Davis CW, Butler R, Zinzow-Kramer W, et al. ZBTB32 is an early repressor of the CIITA and MHC class II gene expression during B cell differentiation to plasma cells. J Immunol. 2012;189(5):2393–403. 10.4049/jimmunol.1103371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beaulieu AM, Zawislak CL, Nakayama T, Sun JC. The transcription factor Zbtb32 controls the proliferative burst of virus-specific natural killer cells responding to infection. Nat Immunol. 2014;15(6):546–53. 10.1038/ni.2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ezell SA, Tsichlis PN. Akt1, EMSY, BRCA2 and type I IFN signaling: a novel arm of the IFN response. Transcription. 2012;3(6):305–9. 10.4161/trns.21904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi K, Sugi Y, Hosono A, Kaminogawa S. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J Immunol. 2009;183(10):6522–9. 10.4049/jimmunol.0901271 [DOI] [PubMed] [Google Scholar]
- 57.Breuer K, Foroushani AK, Laird MR, Chen C, Sribnaia A, Lo R, et al. InnateDB: systems biology of innate immunity and beyond—recent updates and continuing curation. Nucleic Acids Res. 2013;41(Database issue):D1228–33. 10.1093/nar/gks1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. 2015;7(5). 10.1101/cshperspect.a016303 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. 10.1146/annurev-immunol-032713-120145 . [DOI] [PubMed] [Google Scholar]
- 60.Nachbur U, Stafford CA, Bankovacki A, Zhan Y, Lindqvist LM, Fiil BK, et al. A RIPK2 inhibitor delays NOD signalling events yet prevents inflammatory cytokine production. Nat Commun. 2015;6:6442 10.1038/ncomms7442 . [DOI] [PubMed] [Google Scholar]
- 61.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695(1–3):55–72. 10.1016/j.bbamcr.2004.09.019 [DOI] [PubMed] [Google Scholar]
- 62.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420(1):25–7. 10.1016/S0014-5793(97)01480-4 [DOI] [PubMed] [Google Scholar]
- 63.Mao R, Nie H, Cai D, Zhang J, Liu H, Yan R, et al. Inhibition of hepatitis B virus replication by the host zinc finger antiviral protein. PLoS Pathog. 2013;9(7):e1003494 10.1371/journal.ppat.1003494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kayama Y, Minamino T, Toko H, Sakamoto M, Shimizu I, Takahashi H, et al. Cardiac 12/15 lipoxygenase–induced inflammation is involved in heart failure. J Exp Med. 2009;206(7):1565–74. 10.1084/jem.20082596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faucher K, Rabinovitch-Chable H, Barrière G, Cook-Moreau J, Rigaud M. Overexpression of cytosolic glutathione peroxidase (GPX1) delays endothelial cell growth and increases resistance to toxic challenges. Biochimie. 2003;85(6):611–7. [DOI] [PubMed] [Google Scholar]
- 66.Corsetti JP, Gansevoort RT, Bakker SJL, Navis G, Sparks CE, Dullaart RPF. Apolipoprotein E predicts incident cardiovascular disease risk in women but not in men with concurrently high levels of high-density lipoprotein cholesterol and C-reactive protein. Metabolism. 2012;61(7):996–1002. 10.1016/j.metabol.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 67.Henrich K-O, Bauer T, Schulte J, Ehemann V, Deubzer H, Gogolin S, et al. CAMTA1, a 1p36 tumor suppressor candidate, inhibits growth and activates differentiation programs in neuroblastoma cells. Cancer Res. 2011;71(8):3142–51. 10.1158/0008-5472.CAN-10-3014 [DOI] [PubMed] [Google Scholar]
- 68.Lucero CMJ, Vega OA, Osorio MM, Tapia JC, Antonelli M, Stein GS, et al. The cancer-related transcription factor Runx2 modulates cell proliferation in human osteosarcoma cell lines. J Cell Physiol. 2013;228(4):714–23. 10.1002/jcp.24218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bilal E, Alexe G, Yao M, Cong L, Kulkarni A, Ginjala V, et al. Identification of the YES1 Kinase as a Therapeutic Target in Basal-Like Breast Cancers. Genes Cancer. 2010;1(10):1063–73. 10.1177/1947601910395583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deheuninck J, Luo K. Ski and SnoN, potent negative regulators of TGF-β signaling. Cell Res. 2008;19(1):47–57. 10.1038/cr.2008.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yonezawa T, Katoh K, Obara Y. Existence of GPR40 functioning in a human breast cancer cell line, MCF-7. Biochem Biophys Res Commun. 2004;314(3):805–9. [DOI] [PubMed] [Google Scholar]
- 72.Pise-Masison CA, Radonovich M, Dohoney K, Morris JC, O'Mahony D, Lee M-J, et al. Gene expression profiling of ATL patients: compilation of disease-related genes and evidence for TCF4 involvement in BIRC5 gene expression and cell viability. Blood. 2009;113(17):4016–26. 10.1182/blood-2008-08-175901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minagawa K, Katayama Y, Nishikawa S, Yamamoto K, Sada A, Okamura A, et al. Inhibition of G(1) to S phase progression by a novel zinc finger protein P58(TFL) at P-bodies. Mol Cancer Res. 2009;7(6):880–9. 10.1158/1541-7786.MCR-08-0511 [DOI] [PubMed] [Google Scholar]
- 74.Xie N, Cui H, Banerjee S, Tan Z, Salomao R, Fu M, et al. miR-27a regulates inflammatory response of macrophages by targeting IL-10. J Immunol. 2014;193(1):327–34. 10.4049/jimmunol.1400203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saha B, Bruneau JC, Kodys K, Szabo G. Alcohol-induced miR-27a regulates differentiation and M2 macrophage polarization of normal human monocytes. J Immunol. 2015;194(7):3079–87. 10.4049/jimmunol.1402190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith JL, Kupchak BR, Garitaonandia I, Hoang LK, Maina AS, Regalla LM, et al. Heterologous expression of human mPRalpha, mPRbeta and mPRgamma in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73(11):1160–73. 10.1016/j.steroids.2008.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hermann-Kleiter N, Baier G. Orphan nuclear receptor NR2F6 acts as an essential gatekeeper of Th17 CD4+ T cell effector functions. Cell Commun Signal. 2014;12:38 10.1186/1478-811X-12-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho S, Wu CJ, Yasuda T, Cruz LO, Khan AA, Lin LL, et al. miR-23 approximately 27 approximately 24 clusters control effector T cell differentiation and function. J Exp Med. 2016;213(2):235–49. 10.1084/jem.20150990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–95. 10.1038/cr.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7(1):41–51. 10.1038/nri1995 . [DOI] [PubMed] [Google Scholar]
- 81.Peng SL. Foxo in the immune system. Oncogene. 2008;27(16):2337–44. 10.1038/onc.2008.26 . [DOI] [PubMed] [Google Scholar]
- 82.Hinks A, Eyre S, Ke X, Barton A, Martin P, Flynn E, et al. Association of the AFF3 gene and IL2/IL21 gene region with juvenile idiopathic arthritis. Genes Immun. 2010;11(2):194–8. 10.1038/gene.2009.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gavala ML, Hill LM, Lenertz LY, Karta MR, Bertics PJ. Activation of the transcription factor FosB/activating protein-1 (AP-1) is a prominent downstream signal of the extracellular nucleotide receptor P2RX7 in monocytic and osteoblastic cells. J Biol Chem. 2010;285(44):34288–98. 10.1074/jbc.M110.142091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Argiropoulos B, Yung E, Humphries RK. Unraveling the crucial roles of Meis1 in leukemogenesis and normal hematopoiesis. Genes Dev. 2007;21(22):2845–9. 10.1101/gad.1619407 . [DOI] [PubMed] [Google Scholar]
- 85.Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18(18):2183–94. 10.1101/gad.1243304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aw Yeang HX, Hamdam JM, Al-Huseini LM, Sethu S, Djouhri L, Walsh J, et al. Loss of transcription factor nuclear factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2) leads to dysregulation of immune functions, redox homeostasis, and intracellular signaling in dendritic cells. J Biol Chem. 2012;287(13):10556–64. 10.1074/jbc.M111.322420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee KN, Kang HS, Jeon JH, Kim EM, Yoon SR, Song H, et al. VDUP1 is required for the development of natural killer cells. Immunity. 2005;22(2):195–208. 10.1016/j.immuni.2004.12.012 . [DOI] [PubMed] [Google Scholar]
- 88.Baugh JA, Chitnis S, Donnelly SC, Monteiro J, Lin X, Plant BJ, et al. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3(3):170–6. 10.1038/sj.gene.6363867 . [DOI] [PubMed] [Google Scholar]
- 89.Clark RB. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71(3):388–400. . [PubMed] [Google Scholar]
- 90.Szeles L, Torocsik D, Nagy L. PPARgamma in immunity and inflammation: cell types and diseases. Biochim Biophys Acta. 2007;1771(8):1014–30. 10.1016/j.bbalip.2007.02.005 . [DOI] [PubMed] [Google Scholar]
- 91.Gaboli M, Kotsi PA, Gurrieri C, Cattoretti G, Ronchetti S, Cordon-Cardo C, et al. Mzf1 controls cell proliferation and tumorigenesis. Genes Dev. 2001;15(13):1625–30. 10.1101/gad.902301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37(3):463–74. 10.1016/j.immuni.2012.06.012 . [DOI] [PubMed] [Google Scholar]
- 93.Mandrekar P, Catalano D, Szabo G. Alcohol-induced regulation of nuclear regulatory factor-kappa beta in human monocytes. Alcohol Clin Exp Res. 1997;21(6):988–94. . [PubMed] [Google Scholar]
- 94.Mandrekar P, Dolganiuc A, Bellerose G, Kodys K, Romics L, Nizamani R, et al. Acute alcohol inhibits the induction of nuclear regulatory factor kappa B activation through CD14/toll-like receptor 4, interleukin-1, and tumor necrosis factor receptors: a common mechanism independent of inhibitory kappa B alpha degradation? Alcohol Clin Exp Res. 2002;26(11):1609–14. 10.1097/01.ALC.0000036926.46632.57 . [DOI] [PubMed] [Google Scholar]
- 95.Grimley PM, Dong F, Rui H. Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 1999;10(2):131–57. . [DOI] [PubMed] [Google Scholar]
- 96.Nam CH, Rabbitts TH. The role of LMO2 in development and in T cell leukemia after chromosomal translocation or retroviral insertion. Mol Ther. 2006;13(1):15–25. 10.1016/j.ymthe.2005.09.010 . [DOI] [PubMed] [Google Scholar]
- 97.Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22(6):746–55. 10.1101/gad.1642408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sanchez-Tillo E, Lazaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29(24):3490–500. 10.1038/onc.2010.102 . [DOI] [PubMed] [Google Scholar]
- 99.Ashenhurst JR, Harden KP, Corbin WR, Fromme K. Trajectories of Binge Drinking and Personality Change Across Emerging Adulthood. Psychol Addict Behav. 2015. 10.1037/adb0000116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okazaki H, Igarashi M, Nishi M, Sekiya M, Tajima M, Takase S, et al. Identification of neutral cholesterol ester hydrolase, a key enzyme removing cholesterol from macrophages. J Biol Chem. 2008;283(48):33357–64. 10.1074/jbc.M802686200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bot I, Biessen EAL. Mast cells in atherosclerosis. Thromb Haemost. 2011;106(5):820–6. 10.1160/TH11-05-0291 [DOI] [PubMed] [Google Scholar]
- 102.Nair J, Shanker J, Jambunathan S, Arvind P, Kakkar VV. Expression analysis of leukotriene-inflammatory gene interaction network in patients with coronary artery disease. J Atheroscler Thromb. 2014;21(4):329–45. [DOI] [PubMed] [Google Scholar]
- 103.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103(47):17985–90. 10.1073/pnas.0605545103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Djousse L, Gaziano JM. Alcohol consumption and heart failure: a systematic review. Curr Atheroscler Rep. 2008;10(2):117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sesso HD, Cook NR, Buring JE, Manson JE, Gaziano JM. Alcohol consumption and the risk of hypertension in women and men. Hypertension. 2008;51(4):1080–7. 10.1161/HYPERTENSIONAHA.107.104968 . [DOI] [PubMed] [Google Scholar]
- 106.Mukamal KJ, Jadhav PP, D'Agostino RB, Massaro JM, Mittleman MA, Lipinska I, et al. Alcohol consumption and hemostatic factors: analysis of the Framingham Offspring cohort. Circulation. 2001;104(12):1367–73. . [DOI] [PubMed] [Google Scholar]
- 107.Dimmitt SB, Rakic V, Puddey IB, Baker R, Oostryck R, Adams MJ, et al. The effects of alcohol on coagulation and fibrinolytic factors: a controlled trial. Blood Coagul Fibrinolysis. 1998;9(1):39–45. . [DOI] [PubMed] [Google Scholar]
- 108.Tonnesen H, Nielsen PR, Lauritzen JB, Moller AM. Smoking and alcohol intervention before surgery: evidence for best practice. Br J Anaesth. 2009;102(3):297–306. 10.1093/bja/aen401 . [DOI] [PubMed] [Google Scholar]
- 109.Freeman WM, Salzberg AC, Gonzales SW, Grant KA, Vrana KE. Classification of alcohol abuse by plasma protein biomarkers. Biol Psychiatry. 2010;68(3):219–22. 10.1016/j.biopsych.2010.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shay T, Kang J. Immunological Genome Project and systems immunology. Trends Immunol. 2013;34(12):602–9. 10.1016/j.it.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruiz M, Ewig S, Marcos MA, Martinez JA, Arancibia F, Mensa J, et al. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160(2):397–405. 10.1164/ajrccm.160.2.9808045 . [DOI] [PubMed] [Google Scholar]
- 112.Harris DR, Gonin R, Alter HJ, Wright EC, Buskell ZJ, Hollinger FB, et al. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med. 2001;134(2):120–4. . [DOI] [PubMed] [Google Scholar]
- 113.Zisman DA, Strieter RM, Kunkel SL, Tsai WC, Wilkowski JM, Bucknell KA, et al. Ethanol feeding impairs innate immunity and alters the expression of Th1- and Th2-phenotype cytokines in murine Klebsiella pneumonia. Alcohol Clin Exp Res. 1998;22(3):621–7. . [DOI] [PubMed] [Google Scholar]
- 114.Guo Y, Sun X, Shibata K, Yamada H, Muta H, Podack ER, et al. CD30 is required for activation of a unique subset of interleukin-17A-producing gammadelta T cells in innate immunity against Mycobacterium bovis Bacillus Calmette-Guerin infection. Infect Immun. 2013;81(10):3923–34. 10.1128/IAI.00887-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Momen-Heravi F, Bala S, Kodys K, Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5:9991 10.1038/srep09991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651 10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. 10.1038/nrc2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheng F, Wang HW, Cuenca A, Huang M, Ghansah T, Brayer J, et al. A critical role for Stat3 signaling in immune tolerance. Immunity. 2003;19(3):425–36. . [DOI] [PubMed] [Google Scholar]
- 119.Liang T, Spence J, Liu L, Strother WN, Chang HW, Ellison JA, et al. alpha-Synuclein maps to a quantitative trait locus for alcohol preference and is differentially expressed in alcohol-preferring and -nonpreferring rats. Proc Natl Acad Sci U S A. 2003;100(8):4690–5. 10.1073/pnas.0737182100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bonsch D, Greifenberg V, Bayerlein K, Biermann T, Reulbach U, Hillemacher T, et al. Alpha-synuclein protein levels are increased in alcoholic patients and are linked to craving. Alcohol Clin Exp Res. 2005;29(5):763–5. . [DOI] [PubMed] [Google Scholar]
- 121.Bonsch D, Reulbach U, Bayerlein K, Hillemacher T, Kornhuber J, Bleich S. Elevated alpha synuclein mRNA levels are associated with craving in patients with alcoholism. Biol Psychiatry. 2004;56(12):984–6. 10.1016/j.biopsych.2004.09.016 . [DOI] [PubMed] [Google Scholar]
- 122.Walker SJ, Grant KA. Peripheral blood alpha-synuclein mRNA levels are elevated in cynomolgus monkeys that chronically self-administer ethanol. Alcohol. 2006;38(1):1–4. 10.1016/j.alcohol.2006.03.008 . [DOI] [PubMed] [Google Scholar]
- 123.Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59(2):274–87. 10.1016/j.neuron.2008.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32(2):355–64. 10.1111/j.1530-0277.2007.00584.x . [DOI] [PubMed] [Google Scholar]
- 125.Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28(14):3729–37. 10.1523/JNEUROSCI.5731-07.2008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Choudhury M, Park PH, Jackson D, Shukla SD. Evidence for the role of oxidative stress in the acetylation of histone H3 by ethanol in rat hepatocytes. Alcohol. 2010;44(6):531–40. 10.1016/j.alcohol.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shukla SD, Aroor AR, Restrepo R, Kharbanda KK, Ibdah JA. In Vivo Acute on Chronic Ethanol Effects in Liver: A Mouse Model Exhibiting Exacerbated Injury, Altered Metabolic and Epigenetic Responses. Biomolecules. 2015;5(4):3280–94. 10.3390/biom5043280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–8. 10.1038/nature05836 . [DOI] [PubMed] [Google Scholar]
- 129.Grant KA, Leng X, Green HL, Szeliga KT, Rogers LSM, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32(10):1824–38. 10.1111/j.1530-0277.2008.00765.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Friedman RC, Farh KK-H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. 10.1101/gr.082701.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lynn DJ, Winsor GL, Chan C, Richard N, Laird MR, Barsky A, et al. InnateDB: facilitating systems-level analyses of the mammalian innate immune response. Mol Syst Biol. 2008;4:218 10.1038/msb.2008.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xia J, Gill EE, Hancock RE. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat Protoc. 2015;10(6):823–44. 10.1038/nprot.2015.052 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell counts and cytokine, chemokine, growth factor measurements (A) Cell counts from whole blood. (B) Percentage of major cell subsets within PBMC. (C) Average concentrations of cytokines and growth factors that differed between ethanol-consuming animals and controls.
(EPS)
(A) Principal Component Analysis and (B) Bar graph depicting the 8 most significant Gene Ontology (GO) terms enriched among up-regulated differentially expressed genes (DEGs), and (D) the down-regulated DEGs. The bar represents the number of DEGs mapping to each GO term while the line graph represents negative log (FDR) of the enriched term.
(EPS)
(A) Expression profile of genes mapping to ‘Immune System Process’ across several cell types as predicted by ImmGen’s MyGeneSet application. Genes are represented as individual rows while each column represents a study.
(EPS)
(A) Network image of the DEG that map to uniquely to ‘Signaling’ and show direct interactions.
(EPS)
Data Availability Statement
All RNA-Seq fastq files are available for download from the NCBI SRA database (accession number SRA305670).