Abstract
Background
High dose ionizing radiation can induce ovarian cancer, but the effect of low dose radiation on the development of ovarian cancer has not been extensively studied. We evaluated the effect of low dose radiation and total background radiation, and the radiation delivered to the ovaries during the treatment of rectosigmoid cancer and breast cancer on ovarian cancer incidence.
Materials and Methods
Background radiation measurements are from Assessment of Variations in Radiation Exposure in the United States, 2011. Ovarian cancer incidence data are from the Centers for Disease Control and Prevention. Standardized incidence ratios (SIR) of ovarian cancer following breast cancer and rectosigmoid cancer are from Surveillance, Epidemiology, and End Results (SEER) data. Obesity data by US state are from the Centers for Disease Control and Prevention. Mean ages of US state populations are from the United States Census Bureau.
Results
We calculated standardized incidence ratios (SIR) from Surveillance, Epidemiology, and End Results (SEER) data, which reveal that in 194,042 cases of breast cancer treated with beam radiation, there were 796 cases of ovarian cancer by 120+ months of treatment (0.41%); in 283, 875 cases of breast cancer not treated with radiation, there were 1,531 cases of ovarian cancer by 120+ months (0.54%). The difference in ovarian cancer incidence in the two groups was significant (p < 0.001, two tailed Fisher exact test). The small dose of scattered ovarian radiation (about 3.09 cGy) from beam radiation to the breast appears to have reduced the risk of ovarian cancer by 24%. In 13,099 cases of rectal or rectosigmoid junction cancer treated with beam radiation in the SEER data, there were 20 cases of ovarian cancer by 120+ months of treatment (0.15%). In 33,305 cases of rectal or rectosigmoid junction cancer not treated with radiation, there were 91 cases of ovarian cancer by 120+ months (0.27%). The difference in ovarian cancer incidence in the two groups was significant (p = 0.017, two tailed Fisher exact test). In other words, the beam radiation to rectum and rectosigmoid that also reached the ovaries reduced the risk of ovarian cancer by 44%. In addition, there was a significant inverse relationship between ovarian cancer in white women and radon background radiation (r = − 0.465. p = 0.002) and total background radiation (r = − 0.456, p = 0.002). Because increasing age and obesity are risk factors for ovarian cancer, multivariate linear regression was performed. The inverse relationship between ovarian cancer incidence and radon background was significant (β = − 0.463, p = 0.002) but unrelated to age (β = − 0.080, p = 0.570) or obesity (β = − 0.180, p = 0.208).
Conclusions
The reduction of ovarian cancer risk following low dose radiation may be the result of radiation hormesis. Hormesis is a favorable biological response to low toxin exposure. A pollutant or toxin demonstrating hormesis has the opposite effect in small doses as in large doses. In the case of radiation, large doses are carcinogenic. However, lower overall cancer rates are found in U.S. states with high impact radiation. Moreover, there is reduced lung cancer incidence in high radiation background US states where nuclear weapons testing was done. Women at increased risk of ovarian cancer have two choices. They may be closely followed (surveillance) or undergo immediate prophylactic bilateral salpingo-oophorectomy. However, the efficacy of surveillance is questionable. Bilateral salpingo-oophorectomy is considered preferable, although it carries the risk of surgical complications. The data analysis above suggests that low-dose pelvic irradiation might be a good third choice to reduce ovarian cancer risk. Further studies would be worthwhile to establish the lowest optimum radiation dose.
Keywords: Ovarian cancer, hormesis, radiation, background, radon
Introduction
High dose ionizing radiation can induce ovarian tumors in mice (Upton et al., 1960). Nuclear workers appear to be at increased risk of ovarian cancer (Greene et al., 2003). Boice and Miller found deaths from ovarian cancer in atomic bomb survivors exposed to 2.237 SV (sieverts or 223.7 cGy), which they felt could be attributed to radiation (Boice and Miller, 1999).
But the effect of low dose radiation on the development of ovarian cancer has not been extensively studied. We now report that low dose radon and total background radiation, and the radiation delivered to the ovaries during the treatment of rectosigmoid cancer and breast cancer, are associated with reduced ovarian cancer incidence. This reduction may be the result of radiation hormesis.
Materials and Methods
Background radiation measurements are from Assessment of Variations in Radiation Exposure in the United States (Mauro and Briggs, 2005), which was commissioned by the U.S. Environmental Protection Agency, Office of Radiation and Indoor Air. The measurements come from information compiled and developed into a database on the nationwide variations in annual radiation exposures due to various sources of radiation in the environment. These sources include terrestrial radiation, cosmic radiation, indoor radon, internal emitters, nuclear weapons testing fallout, diagnostic medical procedures, and consumer products.
2011 Ovarian cancer incidence data are from the United States Cancer Statistics (USCS) Cancer Types Grouped by State and Region (Centers for Disease Control and Prevention, 2015).
Standardized incidence ratios (SIR) of ovarian cancer following rectosigmoid cancer and breast cancer are from Surveillance, Epidemiology, and End Results (SEER) data (Hayat et al., 2007). The SEER data include stage of cancer at the time of diagnosis, as well as follow-up of all patients for survival times, patient demographics, primary tumor site, histology, and first course of treatment. Each SEER registry identifies cancer cases through records from hospitals, private laboratories, radiotherapy units, nursing homes, and other health service units in the registry’s defined geographic area, as well as death certificates on which cancer is listed as a cause of death.
Obesity data by US state are from the Centers for Disease Control and Prevention (Centers for Disease Control and Prevention, 2015). Mean ages of US state populations are from the 2010 US Census (United States Census Bureau, 2015).
Results
Standardized incidence ratios (SIR) from Surveillance, Epidemiology, and End Results (SEER) data (Hayat et al., 2007) reveal that in 194,042 cases of breast cancer treated with beam radiation, there were 796 cases of ovarian cancer by 120+ months of treatment (0.41%). In 283,875 cases of breast cancer not treated with radiation, there were 1,531 cases of ovarian cancer by 120+ months (0.54%). The difference in ovarian cancer incidence in the two groups was significant (p<0.001, two tailed Fisher exact test). In other words, the small dose of ovarian radiation scatter from beam radiation to the breast (calculated to be 3.09 cGy (Berris et al., 2013) reduced the risk of ovarian cancer by 24%.
In 13,099 cases of rectal or rectosigmoid junction cancer treated with beam radiation in the SEER data, there were 20 cases of ovarian cancer by 120+ months of treatment (0.15%). In 33,305 cases of rectal or rectosigmoid junction cancer not treated with radiation, there were 91 cases of ovarian cancer by 120+ months (0.27%). The difference in ovarian cancer incidence in the two groups was significant (p = 0.017, two tailed Fisher exact test). In other words, the beam radiation to rectum and rectosigmoid that also reached the ovaries reduced the risk of ovarian cancer by 44%.
There was a significant inverse relationship between ovarian cancer in white women and radon background radiation (r = − 0.465. p = 0.002, Figure 1) and total background radiation (r = − 0.456, p = 0.002). Because increasing age and obesity are risk factors for ovarian cancer (Booth et al., 1989), multivariate linear regression was performed. The inverse relationship between ovarian cancer incidence and radon background was significant (β = − 0.463, p = 0.002) but unrelated to age (β = − 0.080, p = 0.570) or obesity (β = − 0.180, p = 0.208).
Figure 1. Ovarian Cancer Incidences in White Women (cases per 100,000) versus Radon Background Dose in 50 US States and the District of Columbia.
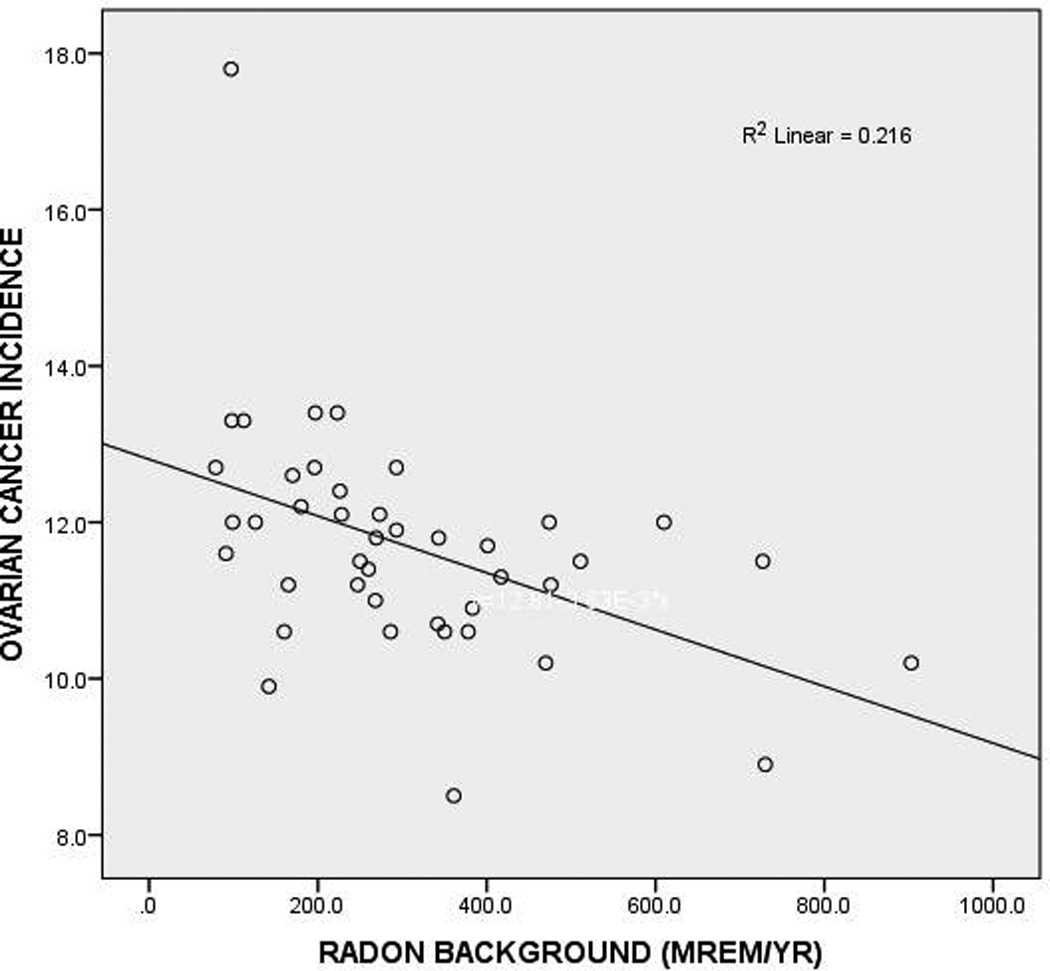
The correlation is significant (p = 0.002). The prominent outlier is Alaska, with 17.8 cases of ovarian cancer per 100,000 population
Discussion
Hormesis is a favorable biological response to low toxin exposure (Calabrese, 2014). A pollutant or toxin demonstrating hormesis has the opposite effect in small doses as in large doses (Mattson, 2008). In the case of radiation, large doses are carcinogenic. However, Frigerio et al found lower overall cancer rates in U.S. states with high impact radiation (Frigerio et al., 1973). Moreover, there is reduced lung cancer incidence in high radiation background US states where nuclear weapons testing was done (Lehrer and Rosenzweig, 2014).
Tomasetti and Vogelstein propose that variation in cancer risk among tissues can be explained by the number of stem cell divisions (Tomasetti and Vogelstein, 2015). For example, colorectal tissue containing many rapidly dividing stem cells is much more cancer-prone than bone tissue, which contains few rapidly dividing stem cells. Random mutations arising during DNA replication in normal, non-cancerous stem cells could account for 65% of the variation of cancer rates in different tissues.
The low dose radiation we describe here might be capable of reducing cancer risk by preferentially eliminating non-cancerous stem cells in which mutations have taken place, since DNA mutations increase radiation sensitivity (Hall and Giaccia, 2006). However, when radiation dose is substantially increased, cancer risk would increase because of malignant transformation of non-stem cells and subsequent tumor development. This mechanism might be one explanation the phenomenon of hormesis, especially as it pertains to radiation (Scott, 2014).
Berris et al have calculated organ doses from breast cancer radiotherapy (Berris et al., 2013). They report that for a treatment course delivering 50.4 Gy to the breast tumor site with a field size of 10 × 16 cm, the ovaries would receive 3.09 cGy. In effect, a dose as small as 3.09 cGy could reduce ovarian cancer risk by 24%.
A weakness in our analysis, presented above, is possible confounding by the ecological fallacy (or ecological inference fallacy), a logical fallacy in the interpretation of statistical data where inferences about the nature of individuals are derived from inference for the group to which those individuals belong (Schwartz, 1994). In this case, inferences about individuals’ radon and total background exposures are being drawn from the characteristics of US states where they reside, rather than from the individuals themselves. Also, another problem with correlational studies is that two variables may be correlated, even if there is no causal link between them, if each is correlated with another variable.
The SEER database does not provide information on chemotherapy and limits our analysis. The chemotherapies used for breast and rectal cancers show some activity in ovarian cancer, and chemotherapy may affect the incidence of clinically diagnosed cases of ovarian cancer.
As noted above, there was a higher incidence of ovarian cancer in the breast cancer group that did not receive radiation therapy. This group likely underwent total mastectomy, rather than breast conserving surgery, for DCIS or stage I or II disease; thus the lack of indication for radiation therapy. Those women who elect to undergo total mastectomy may potentially be more likely to be a BRCA mutation carrier, in which case they would be at higher risk of ovarian cancer, consistent with the results. Information on BRCA or family history is not provided in the SEER database, so this potential confounder cannot be evaluated by our study design.
Women with rectosigmoid cancer who do not undergo radiation therapy are more likely to have early stage disease, which is treated by surgery. At higher stages, neoadjuvant chemotherapy and radiation are given, usually followed by surgery. Women may elect to undergo hysterectomy and/or bilateral salpingo-oophorectomy at the time of colorectal surgery, which would obviously lower the risk of ovarian cancer. If premenopausal, women may desire to retain their ovaries. Postmenopausal women or women who have undergone premature ovarian failure in the setting of neoadjuvant chemotherapy and radiation therapy may have their ovaries removed. The SEER database only has information regarding the surgery pertinent to colon cancer therapy, so it is difficult to evaluate this potential confounder in our study design.
Women at increased risk of ovarian cancer now have prevention choices. They may be closely followed (surveillance) or undergo immediate prophylactic bilateral salpingo-oophorectomy (Meeuwissen et al., 2005). However, the efficacy of surveillance is questionable (Woodward et al., 2007).
Bilateral salpingo-oophorectomy is considered preferable. But It results in hormone deprivation and has the possibility of surgical complications; salpingectomy alone might be almost as effective (Poole et al., 2015).
Oral contraceptives may be used to prevent ovarian cancer in women at high risk (Walker et al., 2015). Moreover, dietary factors may be preventive. There is considerable interest in the potential protective effects of soy products and gingseng. The phytoestrogens, such as genistein and daidzein in soy, can bind to estrogen receptors and therefore interfere with the action of estrogen itself, a well-established risk factor for ovarian cancer, as well as breast and endometrial cancers. Although not all results are consistent, there is good evidence for protective influence of soy products against all three of these cancers (Kim, 2008).
Our data analysis above suggests that low-dose pelvic irradiation might be another choice. Further studies would be worthwhile to establish the lowest optimum radiation dose to reduce ovarian cancer risk in high risk women.
References
- Berris T, Mazonakis M, Stratakis J, et al. Calculation of organ doses from breast cancer radiotherapy: a Monte Carlo study. J Appl Clin Med Phys. 2013;14:4029. doi: 10.1120/jacmp.v14i1.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice JD, Jr, Miller RW. Childhood and adult cancer after intrauterine exposure to ionizing radiation. Teratol. 1999;59:227–233. doi: 10.1002/(SICI)1096-9926(199904)59:4<227::AID-TERA7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Booth M, Beral V, Smith P. Risk factors for ovarian cancer: a case-control study. Br J Cancer. 1989;60:592–598. doi: 10.1038/bjc.1989.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis: from mainstream to therapy. J Cell Commun Signal. 2014;8:289–291. doi: 10.1007/s12079-014-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Overweight and Obesity. 2015 Ref Type: Online Source. [Google Scholar]
- Centers for Disease Control and Prevention. United States Cancer Statistics (USCS) Cancer Types Grouped by State and Region. National Program of Cancer Registries (NPCR) [1-14-2015];2015 Ref Type: Electronic Citation. [Google Scholar]
- Frigerio NA, Eckerman KF, Stowe RS. Carcinogenic hazard from low-level, low-rate radiation, Part I. Rep. Argonne, IL: Argonne Natl Lab; 1973. [Google Scholar]
- Greene T, Latowsky G, Silver K. Cancer and Workers Exposed to Ionizing Radiation. Boston: Center for Environmental Health Studies; 2003. Ovarian Cancer and Exposure to Ionizing Radiation; pp. 79–83. Ref Type: Serial (Book,Monograph) [Google Scholar]
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. USA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- Kim J. Protective effects of Asian dietary items on cancers - soy and ginseng. Asian Pac J Cancer Prev. 2008;9:543–548. [PubMed] [Google Scholar]
- Lehrer S, Rosenzweig KE. Lung cancer hormesis in high impact states where nuclear testing occurred. Clin Lung Cancer. 2014;16:152–155. doi: 10.1016/j.cllc.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro J, Briggs NM. In: Assessment of Variations in Radiation Exposure in the United States. Czyscinski K, editor. Washington, D.C.: U.S. Environmental Protection Agency Office of Radiation and Indoor Air; 2005. [7-15-2005]. pp. 1–35. Ref Type: Serial (Book,Monograph) [Google Scholar]
- Meeuwissen PA, Seynaeve C, Brekelmans CT, et al. Outcome of surveillance and prophylactic salpingo-oophorectomy in asymptomatic women at high risk for ovarian cancer. Gynecol Oncol. 2005;97:476–482. doi: 10.1016/j.ygyno.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Poole EM, Rice MS, Crum CP, Tworoger SS. Salpingectomy as a potential ovarian cancer risk-reducing procedure. J National Cancer Ins. 2015;107 doi: 10.1093/jnci/dju490. [DOI] [PubMed] [Google Scholar]
- Schwartz S. The fallacy of the ecological fallacy: the potential misuse of a concept and the consequences. Am J Public Health. 1994;84:819–824. doi: 10.2105/ajph.84.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR. Radiation-hormesis phenotypes, the related mechanisms and implications for disease prevention and therapy. J Cell Commun Signal. 2014;8:341–352. doi: 10.1007/s12079-014-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. Census - census.gov. 2015. 2010 Ref Type: Online Source.
- Upton AC, Odell TT, Jr, Sniffen EP. Influence of age at time of irradiation on induction of leukemia and ovarian tumors in RF mice. Proc Soc Exp Biol Med. 1960;104:769–772. doi: 10.3181/00379727-104-25982. [DOI] [PubMed] [Google Scholar]
- Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121:2108–2120. doi: 10.1002/cncr.29321. [DOI] [PubMed] [Google Scholar]
- Woodward ER, Sleightholme HV, Considine AM, et al. Annual surveillance by CA125 and transvaginal ultrasound for ovarian cancer in both high-risk and population risk women is ineffective. BJOG. 2007;114:1500–1509. doi: 10.1111/j.1471-0528.2007.01499.x. [DOI] [PubMed] [Google Scholar]


