Abstract
Aims
Retinoids (vitamin A (retinol), and structurally related molecules) possess metabolic modulating properties, prompting new interest in their role in the treatment of diabetes and fatty liver disease, but little is known about the effects of specific retinoic acid receptor (RAR) agonists in these diseases.
Materials and Methods
Synthetic agonists for retinoic acid receptor RARβ2 were administered to wild type (wt) mice in a model of high fat diet (HFD)-induced type 2 diabetes (T2D) and to ob/ob and db/db mice (genetic models of obesity-associated T2D).
Results
We demonstrate that administration of synthetic agonists for the retinoic acid receptor RARβ2 to either wild type (wt) mice in a model of high fat diet (HFD)-induced type 2 diabetes (T2D) or to ob/ob and db/db mice (genetic models of obesity-associated T2D) reduces hyperglycemia, peripheral insulin resistance, and body weight. Furthermore, RARβ2 agonists dramatically reduce steatosis, lipid peroxidation, and oxidative stress in the liver, pancreas, and kidneys of obese, diabetic mice. RARβ2 agonists also lower levels of mRNAs involved in lipogenesis, such as SREBP1 and FASN (fatty acid synthase), and increase mRNAs that mediate mitochondrial fatty acid β-oxidation, such as CPT1α, in these organs. RARβ2 agonists lower triglyceride levels in these organs, and in muscle.
Conclusions
Collectively, our data show that orally active, rapidly acting, high affinity pharmacological agonists for RARβ2 improve the diabetic phenotype while reducing lipid levels in key insulin target tissues. We suggest that RARβ2 agonists should be useful drugs for T2D therapy and for treatment of hepatic steatosis.
INTRODUCTION
The term retinoids defines a family of synthetic and natural compounds that exhibits structural similarities to retinol (vitamin A, [VA]) and its most biologically active metabolite, all-transretinoic acid (RA) (1, 2). Retinoids play essential roles during fetal development, including a role in pancreatic and insulin producing β-cell specification (3, 4). Retinoids act primarily through direct binding to one or more of the three retinoic acid receptors, RARα, RARβ, and RARγ (2). RA, an endogenous agonist for all three RARs, can favorably regulate pathways relevant to some metabolic disorders, including diabetes and obesity (5, 6). However, little is known about metabolism modulating properties of RAR-isotype specific synthetic retinoids.
We previously reported a key role for RARβ in the differentiation of embryonic stem cells (ESCs) to insulin positive endocrine cells (7), and for VA in maintaining normoglycemia in adult mice (8). These data raise the fundamental question of whether synthetic agonists for RARβ possess anti-diabetic properties. Using both high fat diet (HFD)-induced obesity and genetically obese mouse models of insulin resistance (IR), we demonstrate that the highly selective, potent RARβ2 agonists AC261066 and AC55649 (9) can mitigate obesity driven-ectopic lipid accumulation in liver, kidney, pancreas, and muscle through modulation of genes central to lipogenesis and mitochondrial free fatty acid (FFA) oxidation. Administration of RARβ2 agonists reversed the diabetic phenotype marked by hyperglycemia, IR, and expansion of pancreatic β-cell mass without evidence of toxicity or unwanted weight gain. We show for the first time that high affinity, synthetic RARβ2 agonists mitigate diabetes and fatty liver disease.
MATERIALS AND METHODS
Mice, Diet, and Drug Treatments
Genetically obese mice studies: Lep-ob (ob/ob stock #000632) and Lepr-db (db/db, (stock #000697) mice (Jackson Labs, Bar Harbor, Maine), that spontaneously develop obesity and severe insulin resistance by 4-5 weeks of age (10, 11), were used for RARβ2 agonist studies (SI Materials and Methods). Chronic and Short-term High Fat Dietary obesity Studies: Wild type (wt) C57/BL6 male mice were maintained on either a standard laboratory chow (Con) diet (# 5053, Pico Diet, St. Louis, MO) or a high fat diet (HFD) with 45% kcals from fat, (#58125, Test Diet, St. Louis, MO) for RARβ2 agonist studies (SI Materials and Methods).
Metabolic Measurements
Glucose tolerance testing (GTT) and insulin tolerance testing (ITT) were performed as described (8) (SI Materials and Methods).
Liver Histology
Liver samples were scored for evidence of steatosis, steatohepatitis, and fibrosis by the Department of Surgical Pathology at New York-Presbyterian Hospital/Weill Cornell Medical College using established criteria (12) (SI Materials and Methods).
Insulin Measurements
Pancreatic and serum insulin levels were measured in lysates from pancreatic tissues and serum using an ultrasensitive Insulin ELISA Kit (Alpco, Salem, NH) per the manufacturers’ instructions as described (8) (SI Materials and Methods).
Measurement of β-Cell Mass and Islet Area Distribution
Pancreatic endocrine cell mass and islet size distributions were determined using direct morphometry coupled with double immunofluorescence labeling (8) (SI Materials and Methods).
RNA Isolation and Quantitative RT-PCR (Q-PCR)
Total RNA was isolated from tissue and converted to complementary DNA (cDNA), which, using gene specific primers (SI Table 1), was used to measure transcripts in multiple tissues, as described (8) (SI Materials and Methods).
Tissue Triglyceride Analysis
Total tissue lipids were extracted using the Folch method (13) and analyzed with a commercial triglyceride reagent kit (Waco Diagnostics) (SI Materials and Methods).
4-Hydroxynoneal (4-HNE) and Translocase of outer mitochondrial membrane 20 (Tom20) Immunohistochemistry
4-HNE and Tom20 tissue staining was performed as described (14) (SI Materials and Methods).
Statistics
All statistical analyses were performed using GraphPad Prism 6.0 statistical software (GraphPad Software, San Diego, CA) as previously described(8) (SI Materials and Methods).
RESULTS
Effects of RARβ2 Agonists on Body Weight, Glucose Intolerance, and Insulin Resistance (IR) in Genetic Models of Diabetes
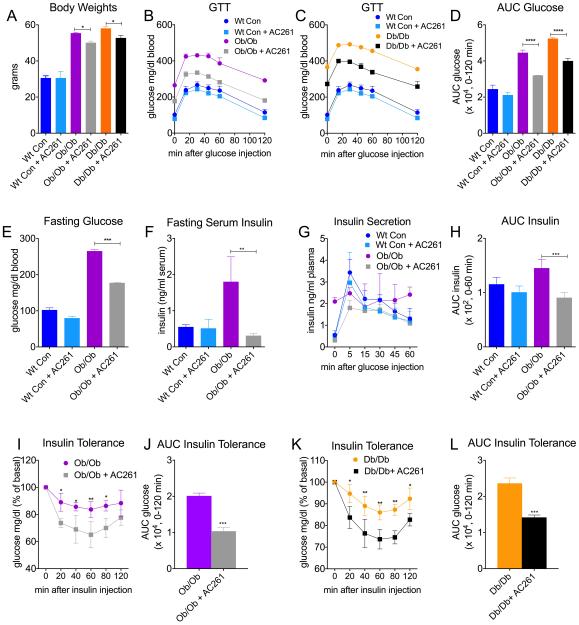
We examined the effects of 8 weeks of treatment with the RARβ2 agonist AC261066, given in the water, on body weight, glucose intolerance, and insulin resistance (IR), using two frequently employed, spontaneous genetic models of obesity and IR, ob/ob and db/db mice (10, 11). Compared to chow-fed wt control mice, body weights (BW) were increased by almost 2-fold in chow-fed ob/ob and db/db mice (Fig. 1A, p < 0.05). Treatment with the RARβ2 agonist AC261066 for 8 weeks had no effect on BW in chow-fed wt mice (Fig 1A). After 4-5 weeks of treatment with AC261066, BWs of ob/ob and db/db mice were decreasing compared to untreated ob/ob and db/db controls (Fig S1A, S1B), and by week 8 of treatment, the decrease was 10-11% (Fig. 1A, p < 0.05). ob/ob and db/db mice spontaneously develop severe glucose intolerance and IR by 5-6 weeks of age (10, 11). Eight weeks of AC261066 treatment dramatically attenuated glucose excursions, area under the curve (AUC) glucose, and fasting glucose levels in both ob/ob and db/db mice (Fig. 1B-E). The glucose lowering effect of AC261066 occurred within 24 hours of administration (Fig. S1C), and was sustained throughout the 8 weeks of administration in both ob/ob and db/db mice (S1D, S1E). AC261066 had no effect on food intake (Fig. S1F). AC261066 decreased fasting serum insulin levels (Fig. 1F), glucose-stimulated insulin secretion (GSIS), and (AUC) insulin (Fig. 1G, H) in ob/ob mice, but had no effect on fasting insulin levels (Fig. 1F), or GSIS in normoglycemic, wt mice (Fig 1G,H). Consistent with the reductions in fasting insulin levels (Fig 1F) and improved insulin excursions (Fig 1G), peripheral insulin sensitivity was markedly improved in both ob/ob and db/db mice treated with AC261066 for 8 weeks (Fig 1I-L).
Figure. 1. Retinoic Acid Receptor β (RARβ) Agonists Diminish Body Weight Increases, Glucose Intolerance, and Insulin Resistance in Genetic Models of Diabetes.
A) Body weights of wild type (wt) chow-fed C57/Bl6, and genetically obese ob/ob and db/db male mice that were fed either a standard chow diet (con, 13% Kcal/fat), or a con diet plus the RARβ2 agonist AC261066 (AC261) in their drinking water for 8 weeks. (Wt Con, n=4), (Wt Con + AC261, n=3), (ob/ob, con, n=3), (ob/ob, con + AC261, n=3), (db/db, con, n=3), (db/db, con + AC261, n=3). B-D) Glucose tolerance tests (GTT) and Area Under the Curve Glucose (AUC) from wt and ob/ob and db/db mice described in A. (B and C: Wt Con (dark blue line) and Wt Con + AC261 (light blue line) groups are repeated in GTT experiments in Fig 1B and 1C). E) Fasting glucose levels in wt and ob/ob mice described in A. F) Fasting insulin, G) insulin secretion, and H) AUC insulin of ob/ob mice subjected to ITT. I-L) Insulin tolerance testing (ITT) and ITT AUC glucose levels of ob/ob and db/db mice described in A. Errors bars represent ± SEM. *=p < 0.05, **=p < 0.01, ***=p < 0.001, ****=p < 0.0001.
Effects of RARβ2 Agonists On Glucose Intolerance and Insulin Resistance in a High Fat Diet Model of Obesity and Insulin Resistance
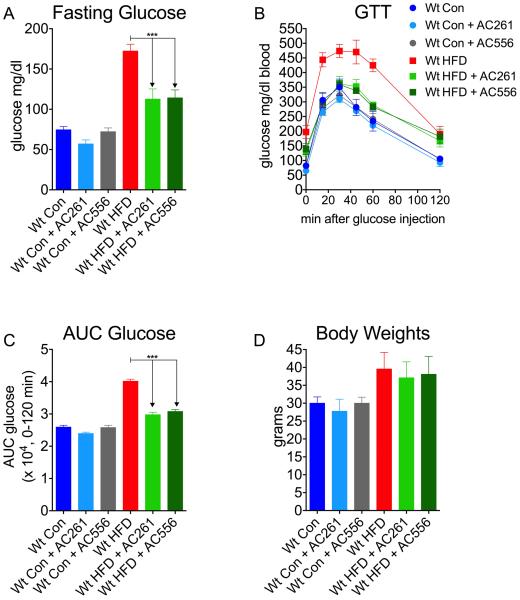
We next measured the effects of either AC261066 or a second RARβ2 agonist with a different chemical structure, AC55649, in a high fat diet (HFD, 45% Kcal/fat) model of T2D in wt mice. We performed GTT on chow-fed and HFD-fed wt mice and found that, similar to our observations in genetically obese mice, 16 weeks of treatment with either AC261066 or AC55649 markedly improved fasting hyperglycemia, glucose excursions, and AUC glucose (Fig. 2A, B, C). 16 weeks of treatments with AC261066 or AC55649 had not effect on BW in HFD-fed wt mice (Fig. 2D).
Figure. 2. Retinoic Acid Receptor β2 Agonist Reduces High Fat Diet Induced Glucose Intolerance in Diabetes.
A) Fasting glucose levels of wt C57/Bl6 male mice after 16 weeks of being fed either: a standard control chow (13% Kcal/fat) diet (Wt Con, n=4); a high fat (45%Kcal/fat) diet (HFD, n=5); or Con chow plus HFD with the RARβ2 agonist, AC261066 (Wt Con + AC261, n=3, Wt HFD + AC261, n=4) or another RARβ2 agonist, AC55649 (Wt Con + AC556, n=3, Wt HFD + AC556, n=4), in their drinking water. B) Glucose tolerance tests (GTT) and C) Area under the curve (AUC) glucose from mice described in A. D) Body weights of mice described in A. Errors bars represent ± SEM. ***=p < 0.0001.
Acute Administration of RARβ2 Agonist Reverses Hyperglycemia and Insulin Resistance in a High Fat Diet Model of T2D
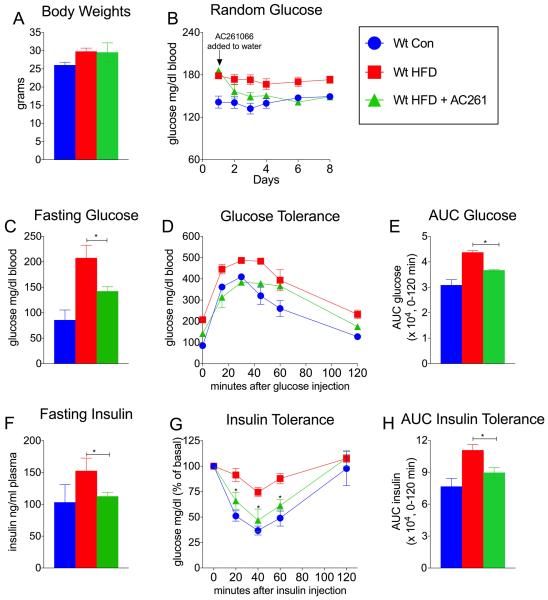
We investigated the more acute effects of AC261066 treatment on hyperglycemia and insulin resistance in HFD-fed wt mice. AC261066 was administered in the drinking water for seven days to wt mice fed a HFD for 3 months that, compared to wt-chow fed mice, had increased BW (Fig. 3A). Short-term treatments with AC261066 had no effect on BW, water, or food intake (Fig. 3A, Fig.S2A, S2B).
Figure. 3. Acute Administration of RARβ Agonist Reduces Glucose Intolerance and Insulin Resistance in a High Fat Model of Diabetes After an Extended Exposure to a High Fat Diet.
A) Body weights of wild type (wt) C57/Bl6 con and wt-HFD-fed male mice after 3 months of being fed either: standard chow (13% Kcal/fat) diet (Wt Con, n=4); or a high fat (45%Kcal/fat) diet (HFD, n=8); followed by 8-day treatment with the RARβ2 agonist, AC261066, in wt-HFD fed mice (Wt HFD + AC261, n=4). B) Random glucose of mice described in A. C) Fasting glucose, D) Glucose tolerance test (GTT), and E) Area Under the Curve Glucose (AUC) glucose of mice described in A. F) Fasting insulin, G) Insulin tolerance tests (ITT) and, H) AUC insulin of mice of mice described in A. Errors bars represent ± SEM. *=p < 0.05.
Within 24 hrs of administration AC261066 markedly reduced random glucose levels (Fig. 3B), and maintained lower random glucose in HFD-fed wt mice for the length of the treatment (Fig. 3B). After seven days we performed metabolic testing and found that HFD-fed wt mice treated with AC261066 had reduced fasting glucose (Fig 3C), improved glucose excursions, reduced AUC glucose (Fig 3D, E), reduced fasting insulin levels (Fig 3F), and greater insulin sensitivity (Fig. 3G, H) compared to HFD-fed mice.
RARβ2 Agonist Reduces the Numbers of Large Pancreatic Islets and Pancreatic Insulin Content in High Fat Diet and Genetic Models of Obesity and Diabetes
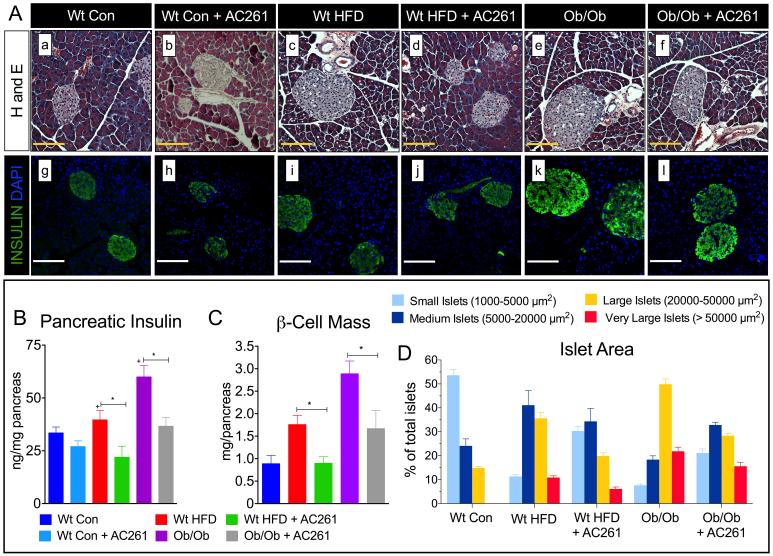
In response to increasing peripheral insulin demands, individuals with T2D show marked expansion of β-cell mass, islet area, and compensatory pancreatic insulin secretion (15). We measured pancreatic cell mass, insulin content, and islet size distribution to determine if RARβ2 agonists affected the pancreatic endocrine compensatory response. Compared to chow-fed wt mice, pancreatic sections from HFD-fed wt mice and chow-fed ob/ob mice showed increased numbers of larger islets (Fig. 4A [a, vs. c, e]), more insulin positive β-cells (Fig. 4A [g, vs. i, k]), increases in pancreatic insulin levels (Fig. 4B) and increased β-cell mass (Fig. 4C). We analyzed islet size distributions and found that, consistent with the increases in pancreatic islet sizes and insulin content, HFD-fed wt and genetically obese, chow-fed ob/ob mice showed 66% to 75% increases in the numbers of larger islets (20000-50000 μm2) and a 50-80% reduction in small (5000-1000 μm2) and medium size islets (5000-20000 μm2) compared to chow fed wt mice (Fig. 4D). We also observed very large islets (> 50000 μm2) in both HFD-fed wt mice and chow-fed ob/ob mice, but not in chow-fed wt mice (wt con, Fig. 4D). HFD-fed wt and chow-fed ob/ob mice treated with the RARβ2 agonist AC261066 exhibited 50% reductions in the numbers of larger islets and 40%-62% reductions in very large islets compared to HFD-fed wt and chow-fed ob/ob mice (Fig. 4C). We also detected 44%-68% increases in the percentages of small to medium sized islets in AC261066 treated mice (Fig 4D). Thus, AC261066 treatments shifted the islet size distributions in HFD-wt and chow-fed ob/ob mice, reducing the percentages of larger islets and increasing the percentages of medium to small islets.
Figure. 4. RARβ2 Agonist AC261066 Decreases the Number of Large Pancreatic Islets and Pancreatic Insulin Content in High Fat and Genetic Models of Diabetes.
A) Representative images of Hematoxylin and Eosin [a-f] and insulin stained (green) [g-l] islets in Wt and ob/ob mice fed experimental diets as described in Fig. 1A and Fig. 2A, respectively. Magnification 400X, Scale Bars =100 μm. B) Pancreatic insulin content (ng/μg of pancreatic protein) in Wt Con and ob/ob mice fed experimental diets with and without the RARβ2 agonist as described in Fig. 1A and Fig 1E, respectively. C). Pancreatic β-Cell mass (mg/pancreas) in Wt Con, Wt HFD, and chow-fed ob/ob mice with and without the RARβ2 agonist as described in Fig. 1A and Fig. 2A, respectively. Errors bars represent ± SEM. *=p < 0.05, += p < 0.05 vs. Wt con (blue bars). D) Relative percentages of very large islets: (area > 50,000 μm2), large islets: (area = 20,000-50,000 μm2) medium islets: (area =5,000-20,000 μm2) and small islets: (area=1,000-5000 μm2) in Wt and ob/ob mice fed HFD and chow diets, respectively, with and without the RARβ2 agonist, as described in Fig. 1A and Fig 2A, respectively. Errors bars represent ± SEM. += p < 0.05 vs. Wt con (blue bars), *=p < 0.05 vs. Wt HFD-fed wt mice, #=p < 0.05 vs. chow-fed ob/ob mice.
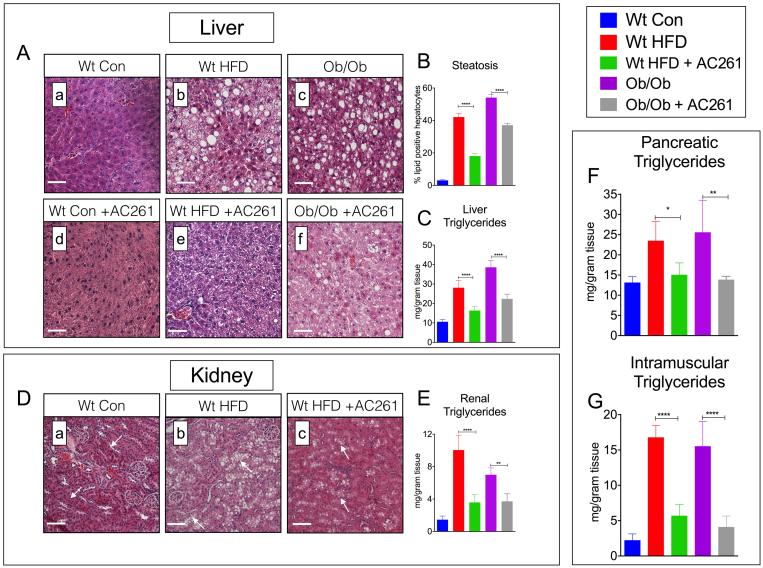
RARβ2 Agonist Reduces Hepatic Steatosis and Ectopic Triglycerides (TGs) in the Kidneys, Pancreas, and Muscle in Dietary and Genetic Models of Diabetes
Ectopic accumulation of TGs and free fatty acids (FFAs) contributes to the pathogenesis of T2D (16-18). We performed histological examinations and measurements of tissue lipids in HFD-fed wt and genetically obese mice. Compared to chow-fed wt, HFD-fed wt and chow-fed ob/ob mice displayed marked increases in hepatic steatosis (Fig. 5A[a, b, c], B); however, compared to untreated obese mice, development of steatosis was attenuated in livers of AC261066 treated HFD-fed wt by 51% (Fig. 5B, p < 0.001) and by 31% in ob/ob (Fig. 5B, p < 0.001). In agreement with our lipid histology analyses, total hepatic TG content was increased in HFD-fed wt and ob/ob mice (Fig. 5C, p < 0.001, vs. chow-fed wt), but mitigated by ~42% in AC261066 treated HFD-fed wt and ob/ob mice (Fig. 5C, Fig. 5B, p < 0.001, vs. untreated, HFD-fed wt and chow-fed ob/ob, respectively). An in-depth histopathology analysis found no evidence of liver injury marked by steatohepatitis (inflammation) or fibrosis (e.g. collagen deposition) in livers from wt-HFD fed or AC261066 treated, HFD-fed wt (Fig. S3).
Figure. 5. RARβ2 Agonist AC261066 Reduces the Accumulation of Liver, Pancreas, Kidney, Muscle and Adipose Triglycerides in Dietary and Genetic Models of Diabetes.
A) Representative images of Hematoxylin and Eosin stained liver sections (a-f), from wt and ob/ob mice fed either HFD or chow, respectively, +/− AC261066 as in Fig. 1A and Fig. 2A. Magnification 200X, Scale Bars =50 μm. B) Percent hepatic steatosis and C) hepatic triglycerides (mg/gram of tissue) from mice described in A. D) Representative images of Hematoxylin and Eosin stained kidney sections (a-c), from Wt mice fed a HFD +/− AC261066 for 4 months, as in Fig. 2A. Magnification 200X, Scale Bars =50 μm. E-G) Kidney, pancreatic, and muscle (gastrocnemius) triglycerides (mg/gram of tissue) from mice described in 2A. Errors bars represent ± SEM. * = p <0.05, ** = p < 0.01, **** = p < 0.0001.
We analyzed lipid histology in kidney sections and also found dramatic increases in lipid accumulation in HFD-fed wt mice (Fig. 5D[a,b]). The renal lipid accumulation in HFD-fed wt was almost exclusively in the proximal tubules (Fig. 5D[a,b], white arrows). Measurements of total renal TG content confirmed increased renal TG levels in HFD-wt, as well as in ob/ob mice (Fig. 5E). We found a marked reduction in lipid vacuoles in the proximal tubules of AC261066 treated, HFD-fed wt (Fig. 5D[b, c], white arrows), a 64% reduction in renal TG content in HFD-wt (Fig. 5E), and a 39% reduction in renal TGs in ob/ob mice (Fig. 5E). We also measured total TG content in the pancreas and muscle and found that, consistent with our liver and kidney analyses, total pancreatic and muscle TGs were increased in HFD-fed wt and in chow-fed ob/ob mice (Fig. 5F,G, p < 0.001, vs. chow-fed wt), but were 35%-45% lower in pancreas and 66%-73% lower in muscle of AC261066 treated, HFD-fed wt and chow-fed ob/ob mice (Fig. 5F,G).
RARβ2 Agonist AC261066 Alters Expression of Genes Involved in Lipogenesis and Mitochondrial Oxidation of Lipids
We next assessed the mechanism by which AC261066 functions by measuring the expression of key genes involved in de novo lipogenesis and fatty acid β-oxidation in multiple tissues. Compared to chow-fed wt, livers from HFD-fed wt and chow-fed ob/ob mice showed increases in transcripts of genes involved in de novo lipogenesis, including peroxisome proliferator-activated receptor gamma (PPAR-γ), fatty acid-binding protein 4 (FABP4), sterol regulatory element-binding transcription factor 1 (SREBP1), fatty acid synthase (FASN), acetyl-CoA carboxylase (ACC1), stearoyl-CoA desaturase (SCD), and diglyceride acyltransferase (DGAT1) (Fig. S4A). We also detected increases in pancreatic mRNA levels of SREBP1 and ACC1, and kidney mRNA levels of SREBP1 and FASN in HFD-fed wt and chow-fed ob/ob mice, respectively (Fig. S4C, D); these increases were eliminated or attenuated by AC261066 treatment (Fig. S4C, D).
We then measured genes involved in the mitochondrial oxidation of fatty acids and associated with IR and T2D (19, 20). Our Q-PCR analysis showed that, compared to chow-fed wt mice, transcripts of genes involved in lipid β-oxidation in livers, pancreata, and kidneys of HFD-fed wt and chow-fed ob/ob mice, including peroxisome proliferator-activated receptor-α (PPARα), carnitine palmitoyltransferase-α (CPT1-α), carnitine palmitoyltransferase 2 (CPT2), malonyl-CoA decarboxylase (MCD), and acetyl-CoA acetyltransferase 1 (ACAT1), were decreased (Fig. S4B). However, compared to HFD-fed wt and ob/ob without AC261066, transcripts of these genes were increased in livers of AC261066 treated, HFD-fed and chow-fed ob/ob mice (Fig. S4B). We also detected increased transcript levels of PPARα, CPT1-α, CPT2, MCD, and PDK1 in the pancreata, and PPARα and CPT1-α in the kidneys of AC261066 treated HFD-fed wt and chow-fed ob/ob mice (Fig. S4C, D). Thus, the RARβ2 agonist AC261066 regulates the expression of genes involved in both lipogenesis and oxidative fatty acid metabolism. These results indicate that the lipid lowering properties of RARβ2 agonists involve transcriptional regulation of genes involved in both lipogenesis and fatty acid oxidation.
RARβ2 Agonist AC261066 Increases Mitochondrial Marker Tom20 in Livers of Diabetic Mice
Given the increased transcript levels of genes involved in mitochondrial oxidation of FFAs in AC261066 treated, HFD-fed mice, we measured hepatic protein expression of translocase of outer mitochondrial membrane 20 (Tom20), a marker of mitochondrial oxidative phosphorylation (21), and found that compared to chow-fed wt, livers of wt HFD-fed mice showed reductions in Tom20 immunopositive areas, whereas livers of HFD-fed mice treated with AC261066 for 16 weeks did not (Fig. S5). These data, combined with our gene expression analyses (Fig. S4), further support our conclusion that AC261066 treatments result in changes in pathways central to mitochondrial fatty acid oxidation and oxidative phosphorylation.
RARβ2 Agonist AC261066 Reduces Oxidative Stress In Liver, Pancreas, and Kidney
Increased peroxidation of FFAs promotes cellular oxidative stress and exacerbates the pathogenesis of T2D (22). The lipid peroxidation byproduct, 4-hydroxy-2-nonenal (4-HNE), is a reliable marker of oxidative stress, and therefore we examined 4-HNE in the liver, pancreas, and kidneys. Compared to chow-fed wt mice, the liver, pancreas, and kidneys of HFD-fed wt mice showed increased 4-HNE levels (Fig. S6, liver [a,b], pancreas [d,e], kidney [g,h]), and these increases were attenuated by AC261066 (Fig.S6, liver [b,c], pancreas[e,f], kidney[h,i]).
Discussion
There is a growing interest in the metabolism-modulating properties of retinoids (5), but, to date, synthetic, RAR-selective retinoids have not been tested in mice as anti-diabetic drugs. Our metabolic studies demonstrate that the specific RARβ2 agonists AC261066 and AC55649 dramatically improve hyperglycemia and peripheral IR in both HFD-driven and genetic obesity-driven models of IR (Fig. 1B-D, I-L; 2A-C, 3B-H). Given our previous findings that VA deficiency can decrease β-cell mass and insulin secretion (8), we were particularly interested in the effects of RARβ2 agonists on the pancreatic endocrine profiles (e.g. β-cell mass, insulin content, and GSIS). RARβ2 agonists did not correct hyperglycemia by stimulating basal insulin secretion or GSIS (Fig. 1F,G), as we did not observe an increase in GSIS, but rather an ~83% decrease in basal insulin and GSIS in AC261066 treated, ob/ob mice (Fig. 1F,G). Unlike the insulin secretagogue class of oral hypoglycemic drugs, such as sulfonylureas, incretins, and DPP-4 inhibitors, that stimulate pancreatic insulin independent of metabolic profile (23), AC261066 had no effect on fasting insulin levels or GSIS in non-diabetic chow fed wt mice (Fig 1F, G), furthering supporting that AC261066 does not restore euglycemia by enhancing insulin secretion.
RARβ2 agonists greatly reduce the classic islet expansion and compensatory insulin response typical of early stage IR and T2D (24) (Fig 4A). AC261066 reduced pancreatic insulin levels, β-cell mass, and percentages of very large (> 50000 μm2), and large (20000-50000 μm2) islets in HFD wt and chow-fed ob/ob mice (Fig. 4B-D). The diminished insulin secretion and β-cell mass sizes in AC261066 treated mice are consistent with the observations that conventional weight loss, weight loss from bariatric surgery, and/or intensive therapy with thiazolidinediones (TZDs) in individuals with T2D reduce GSIS and improve peripheral insulin responses, leading to reduced but more functional β-cell mass (25, 26).
We found that the transcriptional expression profiles of key, lipid-metabolizing genes, such as SREBP1 (lipogenesis), PPARα, and CPT1α (mitochondrial fatty acid β-oxidation), in HFD-fed and chow-fed ob/ob mice are consistent with previous reports in rodents and humans with obesity, IR, and T2D (Fig. S4A-D) (27-29). We show here that AC261066 treatment lowers mRNA levels of lipogenic genes, such as SREBP1 and FASN (Fig. S4A, C, D), and increases mRNA levels of key mediators of mitochondrial fatty acid β-oxidation, such as PPARα and CPT1α, in the liver, pancreas, and kidneys compared to the levels in obese mice (Fig. S4B-D). Our gene expression studies in AC261066 treated obese mice reflect findings in normoglycemic mice that short treatments with RA at very high doses (100 mg/kg BW) promote lipid catabolism through modulation of lipogenic and β-oxidation genes (30-34).
Our data with RARβ2 selective agonists demonstrate that in addition to liver, activation of RARβ2 by AC261066 results in reductions in TG levels in many tissues (Fig. 5). From a drug development perspective, our findings highlight the benefits of highly selective agonists for RARβ2, as the doses of the endogenous, pan-RAR agonist RA used to achieve similar reductions of tissue TGs in rodents (30, 32, 33) would likely have side-effects in humans (35). RA is an endogenous agonist for all three RARs (2), which have broad tissue expression patterns in mammals (2). The dose of ~5.4 mg/kg BW of AC261066 was well tolerated by mice (e.g. no observed adverse effects), and close to FDA approved doses of retinoids used for oral RA treatment of acute promyelocytic leukemia in humans (36).
A previous study reported that RA mitigates insulin resistance through leptin signaling pathways (37). However, our experiments demonstrate that the glucose lowering and insulin sensitizing of RARβ2 agonists are unlikely to be mediated through leptin or leptin independent pathways, as leptin receptor knockout db/db mice had equally marked improvements in glucose tolerance (Fig. 1C,D) and insulin sensitivity (Fig. 1K,L) compared to HFD-fed (Fig. 2B, 3E) and ob/ob mice (Fig. 1B,D, I, J). Our data show that AC261066 does not activate PPARγ or elevate PPARγ mRNA; in fact, Q-PCR studies of liver show that AC261066 decreased transcripts of both a PPARγ responsive gene, FABP4, and FASN, a gene activated by PPARγ agonists in liver (38, 39). Moreover, compared to TZDs, which are PPARγ agonists known to cause unwanted weight gain in humans (40), long-term treatments with RARβ2 agonists did not lead to weight gain, but resulted in approximately a 10% decrease in BW in chow-fed ob/ob and db/db mice (Fig. 1A), and had not effect on BW in HFD-fed wt mice (Fig 2D).
We did detect increases in liver mRNA levels of PPARα and PPARα-regulated genes involved in β-oxidation of fatty acids, suggesting that AC261066 can activate PPARα signaling pathways; however, compared to the highly selective PPARα agonist fenofibrate, which improves steatosis and renal lipotoxicity in rodents (41, 42) but fails to show anti-diabetic properties in both animal and large-scale human studies (43, 44), RARβ2 agonists greatly reduce hyperglycemia and insulin resistance. Thus, our data suggest that the anti-diabetic and anti-steatosis effects of AC261066 and AC55649 are not primarily mediated through PPARα but rather through RARβ itself. Furthermore, both AC261066 and AC55649 show more than 100-fold selectivity for RARβ2 over RARα, RARγ and RXRα, RXRβ and RXRγ (9). Thus, we suggest that the strikingly similar reductions in tissue TGs (Fig. 5A-G), decreased expression of lipogenic genes, and increased expression of fatty acid β-oxidation genes in AC261066 treated obese mice (Fig. S4A-D) by RARβ2 agonists result from a RARβ2-driven metabolic program that increases mitochondrial lipid catabolism rather than from a broader activation of other hormone nuclear receptor signaling, such as signaling by PPARγ, PPARα, and/or other RARs.
The lipotoxicity model proposes that in states of obesity ectopic accumulation of FFAs and oxidative stress from increased FFA peroxidation in key insulin mediating and responsive organs contributes to the pathogenesis of IR and T2D (16-18, 22, 45). Therefore, given that RARβ2-agonist treated HFD-fed wt mice showed marked reductions in tissue TGs and oxidative stress (Fig 5A-G, Fig S6), some of the glucose lowering and insulin sensitizing effects of RARβ2 agonists likely result from an increase in lipid metabolism and a decrease in oxidative stress.
Retinoids promote mitochondrial lipid and energy metabolism (34) and among the RARs, RARβ can promote hepatic lipid catabolism (46). Given a strong clinical interest in developing novel pharmacological agents that can mitigate both hyperglycemia and consequences of NAFLD and tissue TG accumulation, our data demonstrate that RARβ2 agonists possess both of these properties, and, thus, should be further developed as novel anti-diabetic and anti-NAFLD drugs.
Supplementary Material
Acknowledgments
We thank Dr. John Wagner, Professor of Cell and Developmental Biology at WCMC, for critically reading this manuscript.
Financial Support. This research was supported by Weill Cornell funds and by R01CA043796 to LJG. ST was supported by NCI TGCA062948 during a portion of this research.
Footnotes
Author contributions. S.E.T. performed experiments, analyzed data, wrote the manuscript, reviewed/edited manuscript. XH.T. performed experiments, wrote manuscript, reviewed/edited manuscript. J.J. performed experiments researched data, reviewed/edited manuscript. L.J.G. performed experiments, wrote manuscript, reviewed/edited manuscript. Dr. Lorraine Gudas is the guarantor of this work and, as such, has full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Conflict of Interest: Weill Cornell has filed patents on some of the intellectual property in this manuscript and these were licensed to Sveikatal, Inc. LJG and XHT are founders and have financial interests in Sveikatal, Inc. There are no conflicts of interest for JJ or S.E.T associated with this publication.
REFERENCES
- 1.D'Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients. 2011;3(1):63–103. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gudas LJ. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim Biophys Acta. 2012;1821(1):213–21. doi: 10.1016/j.bbalip.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martín M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, et al. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284(2):399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Oström M, Loffler KA, Edfalk S, Selander L, Dahl U, Ricordi C, et al. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS One. 2008;3(7):e2841. doi: 10.1371/journal.pone.0002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brun PJ, Yang KJ, Lee SA, Yuen JJ, Blaner WS. Retinoids: Potent regulators of metabolism. BioFactors. 2013;39(2):151–63. doi: 10.1002/biof.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz EJ, Reginato MJ, Shao D, Krakow SL, Lazar MA. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol Cell Biol. 1997;17(3):1552–61. doi: 10.1128/mcb.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez RJ, Benoit YD, Gudas LJ. Deletion of retinoic acid receptor β (RARβ) impairs pancreatic endocrine differentiation. Exp Cell Res. 2013;319(14):2196–204. doi: 10.1016/j.yexcr.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trasino SE, Benoit YD, Gudas LJ. Vitamin A Deficiency Causes Hyperglycemia and Loss of Pancreatic β-Cell Mass. J Biol Chem. 2015;290(3):1456–73. doi: 10.1074/jbc.M114.616763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund BW, Piu F, Gauthier NK, Eeg A, Currier E, Sherbukhin V, et al. Discovery of a potent, orally available, and isoform-selective retinoic acid beta2 receptor agonist. J Med Chem. 2005;48(24):7517–9. doi: 10.1021/jm050891r. [DOI] [PubMed] [Google Scholar]
- 10.Chua SC, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271(5251):994–6. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 11.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 12.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 14.Tang XH, Osei-Sarfo K, Urvalek AM, Zhang T, Scognamiglio T, Gudas LJ. Combination of bexarotene and the retinoid CD1530 reduces murine oral-cavity carcinogenesis induced by the carcinogen 4-nitroquinoline 1-oxide. Proc Natl Acad Sci U S A. 2014;111(24):8907–12. doi: 10.1073/pnas.1404828111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steil GM, Trivedi N, Jonas JC, Hasenkamp WM, Sharma A, Bonner-Weir S, et al. Adaptation of beta-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab. 2001;280(5):E788–96. doi: 10.1152/ajpendo.2001.280.5.E788. [DOI] [PubMed] [Google Scholar]
- 16.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87(2):507–20. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L. Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin J Am Soc Nephrol. 2010;5(12):2373–9. doi: 10.2215/CJN.08160910. [DOI] [PubMed] [Google Scholar]
- 18.Unger RH. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab. 2003;14(9):398–403. doi: 10.1016/j.tem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patti ME. Gene expression in humans with diabetes and prediabetes: what have we learned about diabetes pathophysiology? Curr Opin Clin Nutr Metab Care. 2004;7(4):383–90. doi: 10.1097/01.mco.0000134359.23288.72. [DOI] [PubMed] [Google Scholar]
- 21.Wurm CA, Neumann D, Lauterbach MA, Harke B, Egner A, Hell SW, et al. Nanoscale distribution of mitochondrial import receptor Tom20 is adjusted to cellular conditions and exhibits an inner-cellular gradient. Proc Natl Acad Sci U S A. 2011;108(33):13546–51. doi: 10.1073/pnas.1107553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raptis SA, Dimitriadis GD. Oral hypoglycemic agents: insulin secretagogues, alpha-glucosidase inhibitors and insulin sensitizers. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S265–87. doi: 10.1055/s-2001-18588. [DOI] [PubMed] [Google Scholar]
- 24.Liu YQ, Jetton TL, Leahy JL, beta-Cell adaptation to insulin resistance Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem. 2002;277(42):39163–8. doi: 10.1074/jbc.M207157200. [DOI] [PubMed] [Google Scholar]
- 25.Ferrannini E, Camastra S, Gastaldelli A, Maria Sironi A, Natali A, Muscelli E, et al. beta-cell function in obesity: effects of weight loss. Diabetes. 2004;53(Suppl 3):S26–33. doi: 10.2337/diabetes.53.suppl_3.s26. [DOI] [PubMed] [Google Scholar]
- 26.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2007;292(3):E871–83. doi: 10.1152/ajpendo.00551.2006. [DOI] [PubMed] [Google Scholar]
- 27.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274(42):30028–32. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 28.Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280(37):32317–25. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 29.Lenhard JM. Lipogenic enzymes as therapeutic targets for obesity and diabetes. Curr Pharm Des. 2011;17(4):325–31. doi: 10.2174/138161211795164185. [DOI] [PubMed] [Google Scholar]
- 30.Amengual J, Ribot J, Bonet ML, Palou A. Retinoic acid treatment enhances lipid oxidation and inhibits lipid biosynthesis capacities in the liver of mice. Cell Physiol Biochem. 2010;25(6):657–66. doi: 10.1159/000315085. [DOI] [PubMed] [Google Scholar]
- 31.Kang HW, Bhimidi GR, Odom DP, Brun PJ, Fernandez ML, McGrane MM. Altered lipid catabolism in the vitamin A deficient liver. Mol Cell Endocrinol. 2007;271(1-2):18–27. doi: 10.1016/j.mce.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Kim SC, Kim CK, Axe D, Cook A, Lee M, Li T, et al. All-trans-retinoic acid ameliorates hepatic steatosis in mice by a novel transcriptional cascade. Hepatology. 2014;59(5):1750–60. doi: 10.1002/hep.26699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amengual J, Ribot J, Bonet ML, Palou A. Retinoic acid treatment increases lipid oxidation capacity in skeletal muscle of mice. obesity (Silver Spring) 2008;16(3):585–91. doi: 10.1038/oby.2007.104. [DOI] [PubMed] [Google Scholar]
- 34.Bonet ML, Ribot J, Palou A. Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim Biophys Acta. 2012;1821(1):177–89. doi: 10.1016/j.bbalip.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Johnson EM. A risk assessment of topical tretinoin as a potential human developmental toxin based on animal and comparative human data. J Am Acad Dermatol. 1997;36(3 Pt 2):S86–90. doi: 10.1016/s0190-9622(97)70064-1. [DOI] [PubMed] [Google Scholar]
- 36.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Woods WG, et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100(13):4298–302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- 37.Tsuchiya H, Ikeda Y, Ebata Y, Kojima C, Katsuma R, Tsuruyama T, et al. Retinoids ameliorate insulin resistance in a leptin-dependent manner in mice. Hepatology. 2012;56(4):1319–30. doi: 10.1002/hep.25798. [DOI] [PubMed] [Google Scholar]
- 38.Rogue A, Spire C, Brun M, Claude N, Guillouzo A. Gene Expression Changes Induced by PPAR Gamma Agonists in Animal and Human Liver. PPAR Res. 2010;2010:325183. doi: 10.1155/2010/325183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin J, Li B, Davis ME, Suh Y, Lee K. Comparative analysis of fatty acid-binding protein 4 promoters: conservation of peroxisome proliferator-activated receptor binding sites. J Anim Sci. 2009;87(12):3923–34. doi: 10.2527/jas.2009-2124. [DOI] [PubMed] [Google Scholar]
- 40.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304(4):411–8. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 41.Kostapanos MS, Kei A, Elisaf MS. Current role of fenofibrate in the prevention and management of non-alcoholic fatty liver disease. World J Hepatol. 2013;5(9):470–8. doi: 10.4254/wjh.v5.i9.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong YA, Lim JH, Kim MY, Kim TW, Kim Y, Yang KS, et al. Fenofibrate improves renal lipotoxicity through activation of AMPK-PGC-1α in db/db mice. PLoS One. 2014;9(5):e96147. doi: 10.1371/journal.pone.0096147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu M, Montgomery MK, Fiveash CE, Osborne B, Cooney GJ, Bell-Anderson K, et al. PPARα-independent actions of omega-3 PUFAs contribute to their beneficial effects on adiposity and glucose homeostasis. Sci Rep. 2014;4:5538. doi: 10.1038/srep05538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bajaj M, Suraamornkul S, Hardies LJ, Glass L, Musi N, DeFronzo RA. Effects of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma agonists on glucose and lipid metabolism in patients with type 2 diabetes mellitus. Diabetologia. 2007;50(8):1723–31. doi: 10.1007/s00125-007-0698-9. [DOI] [PubMed] [Google Scholar]
- 45.Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–24. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Wong K, Walsh K, Gao B, Zang M. Retinoic acid receptor β stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole-body energy homeostasis in mice. J Biol Chem. 2013;288(15):10490–504. doi: 10.1074/jbc.M112.429852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.