Abstract
Objectives
We aimed to selectively breed a spontaneous diabetic gerbil when a sub-line of inbred gerbil showed increased blood glucose levels was found recently. Then we investigated the characteristics including the serum insulin, triglyceride, cholesterol, leptin, adiponectin and explored the underlying molecular mechanism for the diabetic phenotype.
Methods
The spontaneous diabetic line of gerbils was selectively inbreed the sub-line of gerbil by monitoring blood glucose of each animal. The serum insulin, adiponectin, and leptin levels were tested using an ELISA kit. The expression levels of GLUT4, Akt, leptin, adiponectin, and calpain 10 (CAPN10) were tested by western blot and Quantitative Real-time PCR (qPCR) in liver, skeletal muscle, and white adipose.
Results
Our results show that the percentages of animals with FPG≥5.2 (mmol/l), PG2h≥6.8 (mmol/l) and both FPG≥5.2 and PG2h≥6.8 (mmol/l) were increased with the number of breeding generations from F0 (21.33%) to F6 (38.46%). These diabetic gerbils exhibited insulin resistance and leptin resistance as well as decreased adiponectin level in the serum. We also observed decreased expression of adiponectin and increased expression of leptin in the skeletal muscle, respectively.
Conclusions
These results indicate that we have primarily established a spontaneous diabetic gerbil line, and the diabetic phenotypes may have been accounted for by altered expression of leptin and adiponectin.
Introduction
Diabetes is a global public health issue and the number of people with diabetes is expected to increase by 42% (from 51 to 72 million) in industrialized countries between 1995 and 2025 and by 170% (from 84 to 228 million) in industrializing countries [1]. Diabetes also represents a major public health concern in China and the overall prevalence of diabetes was estimated to be 11.6% in Chinese adults [2]. Type 2 diabetes mellitus (T2DM) accounts for more than 90% of cases of diabetes [3]. The hallmark of T2DM is the development of insulin resistance [4], whereas type 1 diabetes is defined as insulin deficiency.
Animal models of T2DM are urgently needed in order to better understand the pathogenesis and potential therapeutic targets. The existing animal models of diabetes include those experimentally induced, spontaneous, and genetically modified mice, rats and minipigs [5, 6]. Experimental induced models are often established by streptozotocin treatment or high-fat diet feeding, which are time consuming. Furthermore, genetically modified models could only mimic limited features of T2DM in human. For example, db/db mice and ob/ob mice can only represent phenotypes of losing the single gene of leptin or its receptor [7]. Spontaneous model is very useful and valuable especially in studying genetic factors of diabetes; but such model is not readily available.
As a potential diabetic model, the Mongolian gerbils have been reported by Boquist L et al who found that some gerbils in their colony had relatively higher fasting blood glucose and obesity [8]. Unfortunately, the percentage of gerbils with high blood glucose is very low (3/42, 7.14%). During our study of inbreeding Mongolian gerbils, we found that a sub-line of inbred gerbils with a incidence of higher blood glucose (21.33%) much higher than what Boquist L et al reported. We went on to selectively inbreed this sub-line to establish a new gerbil model of diabetes. In present study, we reported the characterization of the diabetic phenotype in our diabetic gerbils.
T2DM has many etiological factors including environmental (diet and lifestyle) and genetic factors. There is an important connection between multiple genes and T2DM [9, 10]. Until now, many genes have been identified to associate with T2DM in genome-wide association studies (GWAS), such as transcription factor 7-like 2 (TCF7L2) gene, glucose transporter member 4 (GLUT4), and adiponectin [11, 12]. T2DM manifests itself with insulin resistance and defect in glucose utilization. In order to characterize the diabetic phenotypes in our spontaneous diabetic gerbils, we chose five candidate genes including GLUT4 and protein kinase B (Akt) which participate in glucose uptake[13]; leptin and adiponectin which are associated with insulin sensitivity [14, 15]; and calpain 10 (CAPN10) which was identified having associations with T2DM[16].
Methods
Ethics Statement
All experiments and animal procedures were strictly conducted in accordance with the Guidelines of Capital Medical University Animal Experiments and the Experimental Animals Management Committee. This study was approved by the Animal Experiments and Experimental Animal Welfare Committee of Capital Medical University (Permit Number: 2012-X-38).
Animals and Housing
The Mongolian gerbils were bred and maintained at the animal facility of the Capital Medical University under standard laboratory conditions (room temperature, 20–24°C; relative humidity, 50–70%; 12 h light/12 h dark cycle) with free access to food and water.
Selective Inbreeding Diabetic Gerbils
The experimental animals were placed in individual cages with one pair or one litter. When the animals were 3-month-old(S1 Fig), the fasting glucose (FPG) level as measured and 2h glucose tolerance test (PG2h) was performed using a blood glucose meter (SANNUO, China). All tests were repeated a week after. Then the male and female littermates were mated according to the criterion that both of them with the fasting glucose (FPG)≥5.2 (mmol/l)and 2h glucose tolerance (PG2h)≥6.8(mmol/l), as considering inbreeding depression, we set this standard FPG≥5.2 which was a little lower than Boquist L et al reported(8). When the breeding yielded offsprings, we tested the FPG and PG2h of the offsprings and continued to mate with the inbreeding method. When the animals were to 1–1.2 years old and had offsprings (S1 Fig), we euthanized the gerbils and tested a series of physiological and biochemical indexes, including insulin, leptin and adiponectin level in the serum. Tissues were harvested to pathological analysis. We repeated this process from F0 generation to F6 generation. The daily food intake of diabetic gerbils (n = 15)and control gerbils (n = 15) were tested for 8 days by weighing food every day for individual cage of every animal and calculated the average value reduction of food.
Selection of Animals and Tissue Preparation
When the animals were 1–1.2 years old and had offsprings, before being euthanized, the animals were tested for the FPG after 16h fasting; then the gerbils were anesthetized, whole blood samples were collected into anticoagulant tubes and non-anticoagulant tube from the orbital sinus. The serum in non-anticoagulant tube were separated and frozen at -80°C for ELISA tests. Plasma separated from anticoagulant tubes and serum were using for biochemical analsyis by Synchron cx5 (Beckman, USA) and MEK-7222K (NIHON KOHDEN, Japan). After blood collection, the gerbils were killed by giving an overdose of pentobarbital. Skeletal muscle, adipose tissue, liver, kidney and pancreas were collected. Each collected tissue was divided into three portions, one of which was fixed in formalin for histological analysis; two were stored at -80°C for Quantitative Real-time PCR (qPCR) and Western blot analysis.
Insulin, Glucose Tolerance Test and the Measurement of Insulin, Leptin and Adiponectin levels in Serum
Insulin tolerance test was performed by insulin (Novolin, China) intraperitoneal injection (0.75 U/kg) after 4h fasting. Glucose tolerance test was performed after 16 h fasting and gerbils were given glucose orally (2 g/kg). Then blood samples were collected from the tail tip at 0, 30, 60, and 120 min after glucose administration and were measured for blood glucose levels using a blood glucose meter (SANNUO, China). The serum levels of insulin, adiponectin and leptin were measured using 10 μl and 50 μl serum according to the instructions of the ELISA kits (Millipore, Germany abcam, USA abcam, USA). We used a microplate reader (BioTek, USA) reading the absorbance at a wavelength of 450 nm and calculate the value using the generated logistic curve-fit.
Histological Analysis
Skeletal muscle, adipose tissue, liver, kidney and pancreas were fixed on 4% paraformaldehyde for about 2 week. Then the five tissues were processed using routine histology procedures, paraffin embedding, and 2 μm-thick slices were cut and placed on glass slides. The paraffin sections were stained with hematoxylin and eosin (HE) and then examined microscopically. The individual(s) performing the histological examination were blinded for the animal information.
Rapid-amplification of cDNA Ends (RACE)
To obtain the full-length cDNA of 5 genes (GLUT4, Akt, adiponectin, leptin and CAPN10), total RNA was obtained from an outbred gerbil. The PCR primers (Table 1) were designed within the conservative fragments of the gerbil gene based the homology comparison. We used the full-length cDNA for the gene clone. Genes were cloned using a 5′ RACE System for Rapid Amplification of cDNA Ends kit (Invitrogen, USA) and a SMARTer™ RACE cDNA Amplification Kit (Clontech, USA) to receive gene’s 3′ as the protocols described. Fragment assembly was accomplished by using the DNAMAN 5.5 software (DNAMAN, USA). And DNA sequencing (Zhuandaoshengwu, China) and sequence analysis were performed by using the DNA Star v 7.1 software (DNA Star, USA).
Table 1. The primer sequences to amplified the conservative fragment in cloning the sequence of 5 candidate genes including GLUT4, Akt, Leptin, Adiponectin, and CAPN10 and the resulted lengths of conservative fragment.
| Name of Gene | GenBank accession number | Primer | Sequence | Product length (bp) | Total length (bp) |
|---|---|---|---|---|---|
| GLUT4 | KT377191 | Forward Reverse | CTCAGTGGTTGGGAAGGAAAAGG GCCCTAAGTATTCAAGTTCTG | 994 | 1486 |
| Akt | KT377189 | Forward Reverse | ATGARCGACGTRGCMATYGTGAA CCTCAGGCYGTSCCRCTGGC | 1344 | 2363 |
| Leptin | KT377190 | Forward Reverse |
CACCAAAACCCTCATCAAGAC AGAGHGARGCTTCCAGGAC |
329 | 634 |
| Adiponectin | KT377188 | Forward Reverse | CTTCTCTCCAGGAGTGCCATCTCTG TACTGGTCGTAGGTGAAGAGAAC | 344 | 1169 |
| CAPN10 | KT377192 | Forward Reverse | TTCCCSGCYTCRGASTCCTCGCT TTGSAGGGAAAGCARCTGTTGTT | 1038 | 2467 |
Quantitative Real-time PCR (qPCR)
Total RNA from muscle, adipose tissues, and liver from diabetic and control gerbil was extracted using the Trizol reagent (Tiangen, China), and cDNA was generated from 2 μg RNA in a 20μl reaction mixture according to the manufacturer’s protocols (Tiangen, China). Specific primers to each target gene were designed using Primer-BLAST (S1 Table). Real-time was performed using the CFX Real-Time PCR system (Bio-Rad, USA) in accordance with following protocols: pre-denaturation at 95°C for 15 min, 40 cycles of incubation at 95°C for 10 s, annealing and extension at 60°C for 35 s, and 71 cycles of melt curve analysis at 60°C for 10 s. Real-time quantitative amplification analysis was carried out by Bio-Rad CFX software (Bio-Rad, USA).
Western Blotting
The proteins were extracted from samples using the Proteins Extraction Kit (CWBIO, China) as following step: added in 500μl cold tissue protein extraction reagent buffer (CWBio, China) to tissues and the tissue debris was removed by centrifugation at l0 000 rpm and 4°C for 15 min. Then the total protein concentrations were determined using a BCA kit (CWBio, China). The proteins (30 μg) were separated by 12% or 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis at 160V. The separated proteins were transferred to 0.22 μm Nitrocellulose membranes (PALL, USA) at 0.2A for 2 h and incubated for 1 h at room temperature with 5% skim milk in Tris-buffered saline and Tween 20. The primary antibodies used include leptin (R&D, 1:1000 dilution), adiponectin (abcam, 1:1000 dilution), GLUT4 (Cell Signaling Technology, 1:1000), Akt (Cell Signaling Technology, 1:1000), CAPN10 (abcam, 1:1000 dilution) and GAPDH or beta-actin (Cell Signaling Technology, 1:1000). All antibodies were diluted in 0.5% skim milk solution. After overnight incubation with the primary antibody in 4°C, the membranes were washed and incubated with the horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG antibodies (Baltimore Pike, 1:5000) for 1 h at room temperature. The membranes were washed 3 times using Tris-buffered saline and Tween 20 once for 10 minutes. The signals were detected by ECL and quantified using Image Lab software (Bio-Rad, USA).
Statistical Analysis
All data were analyzed by student’s t-test. The differences were considered significant when the p value was less than 0.05. All results were expressed as means± S.D. of all independent experiments. Statistical analysis was carried out using the SPSS 16.0 (SPSS Inc., USA).
Results
Establishment of diabetic inbred gerbil line
After finding that the incidence of high blood glucose was relatively higher in a sub-line of inbreeding gerbils, we selectively bred the group by choosing animals with FPG≥5.2 and PG2h≥6.8 (mmol/l) when they were 3 months old and mating them with inbreeding method. We tested a total of 855 gerbils including 75, 115, 149, 189, 172, 116, and 39 gerbils from the F0, F1, F2, F3, F4, F5, and F6 generations, respectively. The blood glucose analysis results of FPG≥5.2 and PG2h≥6.8 (mmol/l) percentages from F0 to F6 are show in Table 2. We found that the percentage of animal with FPG≥5.2 (mmol/l) in F0, F1, F2, F3, F4, F5, and F6 were 41.33%, 58.26%, 48.99%, 56.61%, 50.58%, 59.48%, and 71.79%, respectively. The percentage of animal with PG2h≥6.8 (mmol/l) in F0, F1, F2, F3, F4, F5, and F6 were 38.67%, 40.00%, 28.19%, 37.57%, 27.33%, 31.90%, and 51.28%, respectively. The percentage of animals with both FPG≥5.2 and PG2h≥6.8 (mmol/l) in F0, F1, F2, F3, F4, F5, and F6 were 21.33%, 29.57%, 11.41%, 21.16%, 16.86%, 18.10%, and 38.46%, respectively. Our results showed that the incidence of animals with FPG≥5.2 (mmol/l), PG2h≥6.8 (mmol/l) and both FPG≥5.2 and PG2h≥6.8 (mmol/l) animals were increased with the number of breeding generations from F0 to F6 (Fig 1). The percentages of animals with FPG≥5.2 (mmol/l) was increased more quickly compared the other two indexes. Although the increase of percentage with both FPG≥5.2 and PG2h≥6.8 (mmol/l) were relatively slow (Fig 1), the trend was increasing and the percentage in F6 was 38.46%, which was far more than that in F0 (21.33%). Furthermore, more than 50% animals in F6 were complied with the standard (FPG≥5.2). These results indicated that the spontaneous diabetic gerbil line has been primarily established.
Table 2. The number of animals tested blood glucose, with FPG≥5.2, PG2h≥6.8 (mmol/l), and both FPG≥5.2 and PG2h≥6.8 (mmol/l).
The numbers in parentheses are the percentages of each number in each generation.
| generation | total animal number | FPG>5.2 | PG2h>6.8 | FPG>5.2 and PG2h>6.8 |
|---|---|---|---|---|
| D-F0 | 75 | 31(41.33%) | 29(38.67%) | 16(21.33%) |
| D-F1 | 115 | 67(58.26%) | 46(40.00%) | 34(29.57%) |
| D-F2 | 149 | 73(48.99%) | 42(28.19%) | 17(11.41%) |
| D-F3 | 189 | 107(56.61%) | 71(37.57%) | 40(21.16%) |
| D-F4 | 172 | 87(50.58%) | 47(27.33%) | 29(16.86%) |
| D-F5 | 116 | 69(59.48%) | 37(31.90%) | 21(18.10%) |
| D-F6 | 39 | 28(71.79%) | 20(51.28%) | 15(38.46%) |
Fig 1. The percentages of animals with FPG≥5.2, PG2h≥6.8 and both FPG≥5.2and PG2h≥6.8 (mmol/l).
Animals of FPG≥5.2, PG2h≥6.8 and both FPG≥5.2and PG2h≥6.8 were increased with the number of breeding generations from F0 to F6.
The changes in the serum levels of triglyceride, cholesterol, insulin, leptin and adiponectin
We randomly chose 20 gerbils (male n = 9, female n = 11) with high blood glucose as the experimental group, whose FPG were more than 5.2 mmol/l and PG2h were more than 6.8 mmol/l; and 20 general inbreeding gerbils (male n = 10, female n = 10) as control group. And the glucose tolerance of all these gerbils tested at their 3 months old showed glucose intolerance in the experimental group (Fig 2). The serum insulin, leptin and adiponectin levels were measured using ELISA kits and the serum triglyceride, cholesterol and fasting glucose levels were measured by using Synchron cx5. The results showed that fasting glucose, insulin, homoeostasis model assessment for insulin resistance (HOMA-IR), triglyceride, cholesterol and leptin levels in the experimental group were all significantly higher than those in the control group (Table 3). In contrast, the serum adiponectin level was significantly lower in experimental group (10.65 ug/ml) than that in control group (23.75 ug/ml) (Table 3). These data demonstrated that the diabetic inbred gerbils we selectively bred exhibited insulin and leptin resistance. The spontaneous diabetic gerbil group we established is T2DM model.
Fig 2. The glucose tolerance test between control and experiment group.
The glucose tolerance tested at their 3 months old showed glucose intolerance in the experimental group. Notes: “*” (p≤0.05), “**” (p≤ 0.01), and “***” (p≤0.001) showed significantly different between experimental and control animals.
Table 3. The FPG, insulin, triglyceride, cholesterol, leptin, adiponectin and HOMA-IR levels in serum from high blood glucose gerbil and control gerbil.
| Groups | FPG (mmol/l) | Insulin (μU/ml) | HOMA-IR1 | Triglyceride (mmol/l) | Cholesterol (mmol/l) | Leptin (pg/ml) | Adiponectin (μg/ml) |
|---|---|---|---|---|---|---|---|
| control | 4.54±0.94 | 13.61±8.03 | 4.37±2.98 | 0.52±0.33 | 2.52±0.33 | 288.29±146.25 | 23.75±4.64 |
| high | 9.02±2.40*** | 232.58±95.09*** | 70.17±31.32*** | 4.47±4.06*** | 5.87±2.44*** | 22080.41±7346.33*** | 10.65±3.93*** |
Notes: Values are means±S.E. from 20 control gerbils and 20 high blood glucose gerbils.
“*” (p≤0.05), “**” (p≤ 0.01), and “***” (p≤0.001) showed significantly different between experimental and control animals.
“1” HOMA-IR = fasting insulin (μU/ml)×fasting glucose(mmol/l)/22.5
The insulin tolerance test between control and experimental group
In order to further confirm insulin resistance in T2DM Mongolian gerbil, not type 1 diabetes mellitus (T1DM), we randomly chose 10 gerbils (male n = 5, female n = 5) with high blood glucose as the experimental group and 10 general inbreeding gerbils (male n = 5, female n = 5) as control group aged 1–1.2 years old. The result showed that compared with control group, experimental group exhibited decreased tolerance to insulin (Fig 3). It further confirmed that the spontaneous diabetic gerbil group we established is T2DM model.
Fig 3. The insulin tolerance test between control and experiment group.
The glucose tolerance tested at their 1–1.2 years old showed decreased tolerance to insulin in the experimental group. Notes: “*” (p≤0.05), “**” (p≤ 0.01), and “***” (p≤0.001) showed significantly different between experimental and control animals.
Pathological analysis of the diabetic target organs
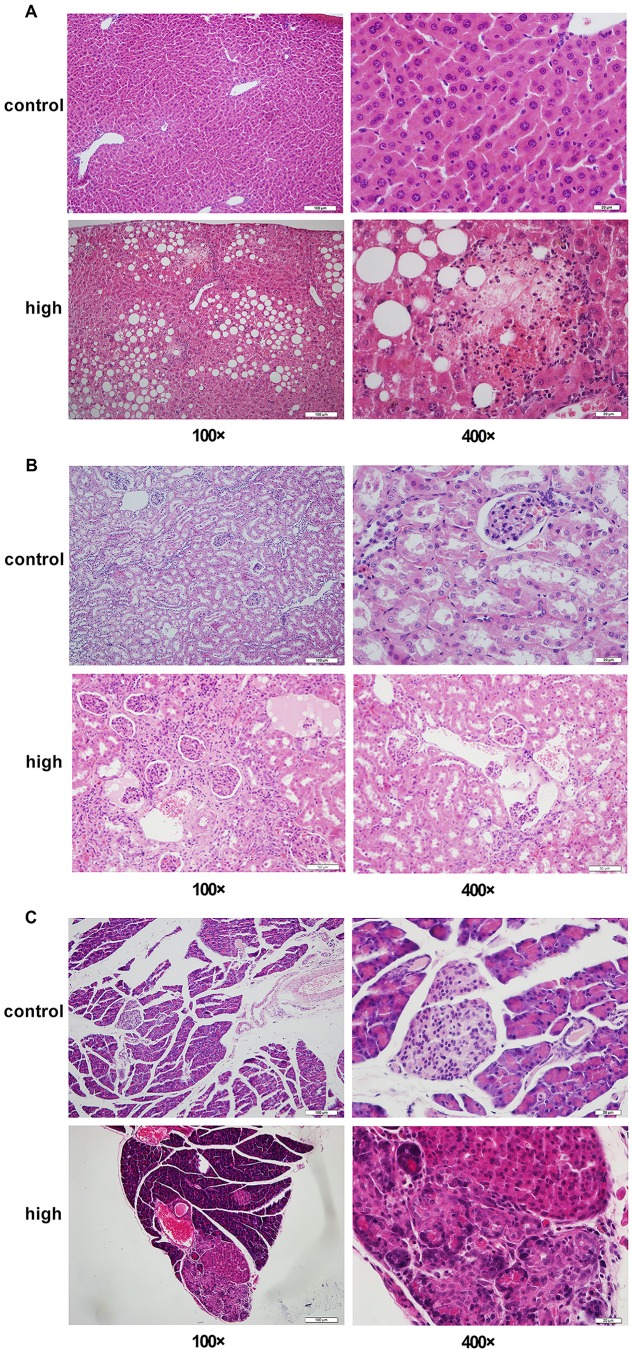
To investigate the target organs of this spontaneous diabetes model, we examined the histological changes of skeletal muscle, adipose tissue, liver, kidney and pancreas by HE stain in diabetic gerbils and the control gerbils. Our results showed that compared with the control gerbils, the diabetic gerbils had pathological changes in the liver, kidney and pancreas, but not in the skeletal muscle and adipose tissue. In the liver, we found marked hepatic steatosis and focal necrosis in the diabetic gerbils (Fig 4A). The injury in the diabetic kidney is glomerulus atrophy and tubular protein accumulation (Fig 4B). The pancreas of the diabetic gerbils showed nuclear pyrosis and cell necrosis leading to local necrosis (Fig 4C).
Fig 4. The pathological analysis of target organs between control group and experimental group using HE stain (100×, 400×): liver (A), kidney (B), and pancreas (C).
(A): in liver, we can find severe hepatic steatosis and focal necrosis in diabetic gerbils was the liver pathological changes.; (B): the kidney are glomerulus atrophy and the tubular can see protein substance, also some of the nucleus pycnosis, cataclastic, and solution in the end in diabetic gerbils; (C): the pancreas represent nuclear pyrosis and cell necrosis leading to local necrosis in diabetes gerbil. All of these three organs had histological changes between control and experimental group.
Molecular cloning and homology analysis of five candidate diabetic genes from gerbils
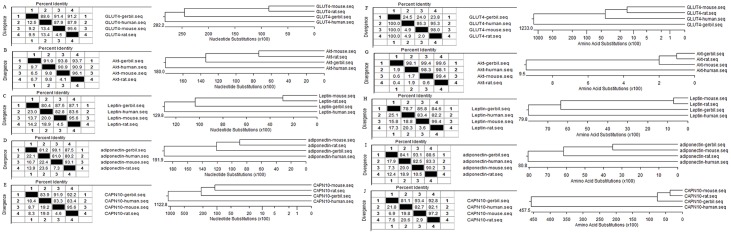
In order to understand the molecular mechanism for the diabetic phenotypes in gerbils, we chose to clone five candidate genes, including GLUT4, Akt, leptin, adiponectin, and CAPN10, by RACE (Table 1). The full-length sequences of these genes were submitted to GenBank and their accession numbers are shown in Table 1. We analyzed the sequences of the cDNAs of the gerbils and compared them with those of mice (Mus musculus), rats (Rattus norvegicus), and humans (Homo sapiens). In order to analyze their homology, we performed sequence distance analysis and drew the phylogenetic tree. We found that the sequences of the cDNAs of the 5 genes in gerbils were similar to the other mammalian species, while the Akt (91.0%, 90.9%, and 96.1% similarity in nucleic acid sequences with human, mouse, and rat) represented the greatest similarity among the 5 genes. GLUT4, leptin, adiponectin, and CAPN10 showed similar homology (Fig 5A–5E). Similar results were obtained when the amino acid sequences were analyzed, except that the human GLUT4 had the lowest similarity with gerbil (24.5%) (Fig 5F–5J).
Fig 5. Analysis CDS of the five genes with DNAStar.
We analyzed the sequences with the ORFs of Mus musculus and Rattus norvegicus, along with the human, and lined the sequence distances and drew the phylogenetic tree to analyze their homology and the expression level of the five genes. (A-E) shows the results of the GLUT4, Akt, leptin, adiponectin, and CAPN10 DNA sequence distances analysis and their phylogenetic tree; (F-J) shows the results of the GLUT4, Akt, leptin, adiponectin, and CAPN10 protein sequence distances analysis and their phylogenetic tree.
Analysis of the expression of candidate diabetic genes
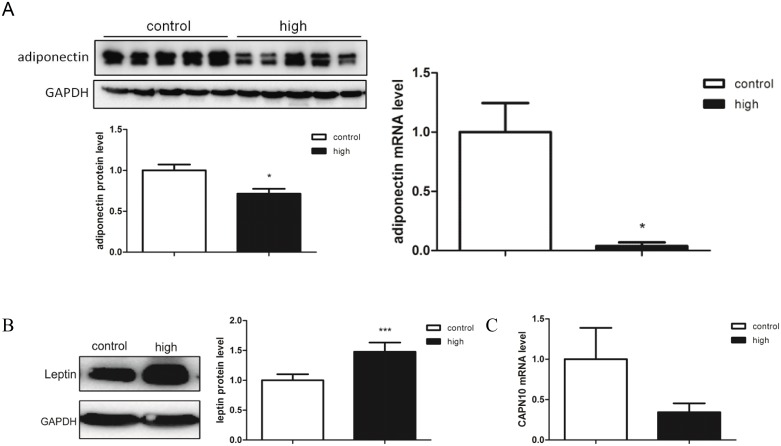
We performed qPCR and Western blot to measure the mRNA and protein expression of the above mentioned five candidate diabetic genes in the liver, skeletal muscle and white adipose. The results showed that the expression level of adiponectin was significant lower in the diabetic gerbils than control gerbils in the skeletal muscle at both mRNA and protein levels (Fig 6A). Leptin expression was significantly higher in the skeletal muscle of diabetic gerbils at the protein level (Fig 6B). CAPN10 showed a tendency of lower expression at the mRNA level in the skeletal muscle (Fig 6C), but the difference did not reach statistical significance. The expression of Akt was not affected in any of these tissues. These results suggested regulation of leptin and adiponectin may have contributed to the diabetic phenotype in our diabetic gerbils.
Fig 6. Expression level of the diabetic related genes between control and experimental gerbils with western blot and RT-qPCR.
(A) was the adiponectin expression changes at protein and mRNA level, both of them were significantly lower in high blood glucose group than that in control group; (B) was the letpin expression change at protein level was significantly higher in high blood glucose group than that in control group; (C) was the CAPN10 expression change at mRNA level, it had a tendency of lower expression in high blood glucose group. Notes: “*” (p≤0.05), “**” (p≤ 0.01), and “***” (p≤0.001) showed significantly different between experimental and control animals.
Discussion
Animal models of T2DM are essential for diabetes research. The existing T2DM models have their limitations. For example, the C57BL/6 mice are sensitive to high-fat diet induced T2DM, but it takes 3–4 months to induce typical diabetes [17]. Thus, more animal models are urgently needed to further investigate the mechanism of diabetes. As a spontaneous diabetic model, our diabetic gerbil could be used directly and time-saving of inducing with chemicals and diet. Mongolian gerbils were reported to be a potential model of diabetes. Nakama K. reported that gerbils can be established as a diabetic model after feeding on a diet containing tolbutamide. The tolbutamide-treated gerbils exhibited typical pathological diabetic changes in the pancreas. The increases in blood glucose, free fatty-acid and insulin levels in tolbutamide-treated gerbils were more dramatic than rat model of diabetes [18]. Nishigaki R. et al reported that streptozotocin treatment can also induce diabetes in gerbils [19]. However, these chemical-induced diabetic models have their limitations in understand the genetic basis of diabetes. While our diabetic gerbil is very useful and valuable especially in studying genetic factors of diabetes, for its exhibiting the features of T2DM—insulin resistance and multigenic disease. Psamomoys Obesus gerbil is another nutritionally induced diabetic animal model of which transition from native diet to laboratory rodent chow showed hyperinsulinemic and hyperglycemic with marked insulin resistance, and it’s a good model for studying insulin resistance and insulin signaling pathways in muscles, but its characteristics are not sustained [20–22]. While our inbreeding group is selected from generation to generation, its characteristics of insulin resistance, leptin resistance, low adiponectin level and the expression changes of the genes may sustain longer and be hereditary. Similar with other spontaneous diabetic models, our group also could comprehensively study the mechanism of T2DM as a complex polygenic disease.
In this study, we selectively bred a group of spontaneously hyperglycemic diabetic gerbils. After analyzing FPG≥5.2 and PG2h≥6.8 (mmol/l) percentages in every generation from F0 to F6, we found that the percentage of FPG≥5.2 (mmol/l) and PG 2h≥6.8 (mmol/l) is increasing from generation to generation. The results also indicated that as many as 31.58% animals in the F6 generation are diabetic and this incidence is much higher than what Boquist L et al reported [8].
Insulin resistance is the hallmark of T2DM. So we tested the insulin level and found the insulin levels in the diabetic gerbils were higher than the control animals. Our measurements of the serum leptin level and calculations of the insulin resistance index, as well as insulin tolerance test also suggested that our diabetic gerbils exhibited insulin resistance and leptin resistance. Leptin resistant is synonymous with obesity, as suggested by the observations made by Toshihiro Miura et al and Martin G. et al [23, 24]. In addition, in a cohort study of women, a large amount of T2DM could be attributed to obesity [25]. The examination of adiponectin level showed that it was significantly lower in the diabetic gerbils than the control gerbils. It was reported that reduced adiponectin level was associated with insulin resistance, obesity, and T2DM [26]. Therefore, all these data suggested that our diabetic gerbils are representative of T2DM.
The diabetic phenotypes of our diabetic gerbils were also supported by our histological results showing that diabetic histological changes were observed in the liver, kidney, and pancreas. T2DM is often accompanied by complications and there are more than 100 existed complications, such as kidney complications, neurological complications, and eye complications [27]. Liver is a metabolic organ and pancreas controls the production and secretion of insulin. The histological damages to the liver, kidney, and pancreas in our diabetic gerbils may have been secondary to the chronic diabetes.
To further understand the molecular basis of the diabetic phenotype, we measured the expression of some genes that are known to be associated with metabolic disorders. We chose and tested the expression levels of GLUT4, Akt, leptin, adiponectin, and CAPN10 as diabetic related genes. The results showed that adiponectin had reduced expression at both the mRNA and protein levels in the skeletal muscle; while leptin expression was increased at the protein level in the skeletal muscle. Liu Y. et al. had reported that muscle is a major target tissue for adiponectin, an adipokine that increases glucose uptake in the muscle [28]. It was also reported that adiponectin can stimulate glucose uptake and fatty-acid oxidation through the activation of AMPK in the skeletal muscle, thus, may lead to the increase of cholesterol in serum [29]. There were many reports suggesting that leptin regulates fatty acid oxidation through the AMPK pathway in the muscle [30]. Although further studies are needed, it is tempting for us to speculate that the spontaneous diabetic phenotypes may have been accounted for by altered expression of leptin and adiponectin. In the gene expression analysis, CAPN10 also exhibited tendency of lower expression at mRNA levels in the skeletal muscle of the diabetic gerbils. This result is consistent with previous reports that CAPN10 participated in glucose metabolism and had a decreased mRNA level in the muscle of T2DM animal models [31, 32]. The changes of expression level of three tested genes in the skeletal muscle also suggested that this tissue might be a key target tissue in this diabetic gerbil model. As for we tested daily food intake and found that there was no difference between dysglycemic gerbils and control gerbils (S2 Table), these genes may contribute more to the pathogenesis of diabetic gerbil.
In conclusion, we have established a spontaneous diabetic gerbil line and the regulation of leptin and adiponectin genes may be involved in the molecular mechanism of this T2DM model.
Supporting Information
(TIF)
(DOCX)
(DOCX)
Data Availability
All EST files are available from the GenBank database (accession number(s) KT377188-KT377192.
Funding Statement
This study was funded by the National Science Foundation of China Nos. 31272393 and 31572341 Xiaoyan Du, the website is http://www.nsfc.gov.cn/Portal0/default152.htm, Key Projects in the National Science & Technology Pillar Program (No. 2015BAI09B01) and the Beijing Natural Science Foundation (No. 7141002), the website is http://www.bjnsf.org.
References
- 1.Narayan KM, Gregg EW, Fagot-Campagna A, Engelgau MM, Vinicor F. Diabetes—a common, growing, serious, costly, and potentially preventable public health problem. Diabetes Res Clin Pract. 2000;50 Suppl 2:S77–84. . [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–59. 10.1001/jama.2013.168118 . [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Zhao P, Wan D, Zhou Q, Wang C, Shu G, et al. Antidiabetic Effect of Methanolic Extract from Berberis julianae Schneid. via Activation of AMP-Activated Protein Kinase in Type 2 Diabetic Mice. Evid Based Complement Alternat Med. 2014;2014:106206 10.1155/2014/106206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab. 2015;26(4):193–200. 10.1016/j.tem.2015.01.006 . [DOI] [PubMed] [Google Scholar]
- 5.Cefalu WT. Animal models of type 2 diabetes: clinical presentation and pathophysiological relevance to the human condition. ILAR J. 2006;47(3):186–98. . [DOI] [PubMed] [Google Scholar]
- 6.Bellinger DA, Merricks EP, Nichols TC. Swine models of type 2 diabetes mellitus: insulin resistance, glucose tolerance, and cardiovascular complications. ILAR J. 2006;47(3):243–58. . [DOI] [PubMed] [Google Scholar]
- 7.Henry ML, Davidson LB, Wilson JE, McKenna BK, Scott SA, McDonagh PF, et al. Whole blood aggregation and coagulation in db/db and ob/ob mouse models of type 2 diabetes. Blood Coagul Fibrinolysis. 2008;19(2):124–34. 10.1097/MBC.0b013e3282f41e56 . [DOI] [PubMed] [Google Scholar]
- 8.Boquist L. Obesity and pancreatic islet hyperplasia in the Mongolian gerbil. Diabetologia. 1972;8(4):274–82. . [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228–36. 10.1038/nrendo.2011.183 . [DOI] [PubMed] [Google Scholar]
- 10.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269–73. 10.5001/omj.2012.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prokopenko I, McCarthy MI, Lindgren CM. Type 2 diabetes: new genes, new understanding. Trends Genet. 2008;24(12):613–21. 10.1016/j.tig.2008.09.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segre AV, Wei N, Consortium D, Investigators M, Altshuler D, Florez JC. Pathways targeted by antidiabetes drugs are enriched for multiple genes associated with type 2 diabetes risk. Diabetes. 2015;64(4):1470–83. 10.2337/db14-0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govers R. Cellular regulation of glucose uptake by glucose transporter GLUT4. Adv Clin Chem. 2014;66:173–240. . [DOI] [PubMed] [Google Scholar]
- 14.Swellam M, Hamdy N. Association of nonalcoholic fatty liver disease with a single nucleotide polymorphism on the gene encoding leptin receptor. IUBMB Life. 2012;64(2):180–6. 10.1002/iub.597 . [DOI] [PubMed] [Google Scholar]
- 15.Siitonen N, Pulkkinen L, Lindstrom J, Kolehmainen M, Eriksson JG, Venojarvi M, et al. Association of ADIPOQ gene variants with body weight, type 2 diabetes and serum adiponectin concentrations: the Finnish Diabetes Prevention Study. BMC Med Genet. 2011;12:5 10.1186/1471-2350-12-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchiya T, Schwarz PE, Bosque-Plata LD, Geoffrey Hayes M, Dina C, Froguel P, et al. Association of the calpain-10 gene with type 2 diabetes in Europeans: results of pooled and meta-analyses. Mol Genet Metab. 2006;89(1–2):174–84. 10.1016/j.ymgme.2006.05.013 . [DOI] [PubMed] [Google Scholar]
- 17.Black BL, Croom J, Eisen EJ, Petro AE, Edwards CL, Surwit RS. Differential effects of fat and sucrose on body composition in A/J and C57BL/6 mice. Metabolism. 1998;47(11):1354–9. . [DOI] [PubMed] [Google Scholar]
- 18.Nakama K. Studies on diabetic syndrome and influences of long-term tolbutamide administration in Mongolian gerbils (Meriones unguiculatus). Endocrinol Jpn. 1977;24(5):421–33. . [DOI] [PubMed] [Google Scholar]
- 19.Nishigaki R, Guo F, Onda M, Yamada N, Yokoyama M, Naito Z, et al. Ultrastructural changes and immunohistochemical localization of nitric oxide synthase, advanced glycation end products and NF-kappa B in aorta of streptozotocin treated Mongolian gerbils. Nihon Ika Daigaku Zasshi. 1999;66(3):166–75. . [DOI] [PubMed] [Google Scholar]
- 20.Shafrir E. Contribution of animal models to the research of the causes of diabetes. World J Diabetes. 2010;1(5):137–40. 10.4239/wjd.v1.i5.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafrir E, Ziv E. A useful list of spontaneously arising animal models of obesity and diabetes. Am J Physiol Endocrinol Metab. 2009;296(6):E1450–2. 10.1152/ajpendo.00113.2009 . [DOI] [PubMed] [Google Scholar]
- 22.Ikeda Y, Olsen GS, Ziv E, Hansen LL, Busch AK, Hansen BF, et al. Cellular mechanism of nutritionally induced insulin resistance in Psammomys obesus: overexpression of protein kinase Cepsilon in skeletal muscle precedes the onset of hyperinsulinemia and hyperglycemia. Diabetes. 2001;50(3):584–92. . [DOI] [PubMed] [Google Scholar]
- 23.Miura T, Suzuki W, Ishihara E, Arai I, Ishida H, Seino Y, et al. Impairment of insulin-stimulated GLUT4 translocation in skeletal muscle and adipose tissue in the Tsumura Suzuki obese diabetic mouse: a new genetic animal model of type 2 diabetes. Eur J Endocrinol. 2001;145(6):785–90. . [DOI] [PubMed] [Google Scholar]
- 24.Myers MG Jr., Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21(11):643–51. 10.1016/j.tem.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–7. 10.1056/NEJMoa010492 . [DOI] [PubMed] [Google Scholar]
- 26.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–71. 10.1093/eurheartj/ehn387 . [DOI] [PubMed] [Google Scholar]
- 27.Washington RE, Andrews RM, Mutter R. Emergency Department Visits for Adults with Diabetes, 2010: Statistical Brief #167 Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD)2006. [PubMed] [Google Scholar]
- 28.Liu Y, Sweeney G. Adiponectin action in skeletal muscle. Best Pract Res Clin Endocrinol Metab. 2014;28(1):33–41. 10.1016/j.beem.2013.08.003 . [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. 10.1038/nm788 . [DOI] [PubMed] [Google Scholar]
- 30.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–43. 10.1038/415339a . [DOI] [PubMed] [Google Scholar]
- 31.Baier LJ P P, Yang XL, Pratley RE, Hanson RL, Shen GQ et al. A calpain-10 gene polymorphism is associated with reduced muscle mRNA levels and insulin resistance. J CLIN INVEST. 2000;106(7):69–73. 10.1172/JCI10665 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Pratley RE, Baier LJ, Horikawa Y, Bell GI, Bogardus C, et al. Reduced skeletal muscle calpain-10 transcript level is due to a cumulative decrease in major isoforms. Mol Genet Metab. 2001;73(1):111–3. 10.1006/mgme.2001.3171 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All EST files are available from the GenBank database (accession number(s) KT377188-KT377192.