Abstract
Bone mineral density (BMD) and geometric bone measures are individually associated with prevalent osteoporotic fractures. Whether an aggregate of these measures would better associate with fractures has not been examined. We examined relationships between self-reported fractures and selected bone measures acquired by quantitative computerized tomography (QCT), a composite bone score, and QCT-acquired dual-energy X-ray absorptiometry–like total femur BMD in 2110 men and 2682 women in the Age, Gene/Environment Susceptibility-Reykjavik Study. The combined bone score was generated by summing gender-specific Z-scores for 4 QCT measures: vertebral trabecular BMD, femur neck cortical thickness, femur neck trabecular BMD, and femur neck minimal cross-sectional area. Except for the latter measure, lower scores for QCT measures, singly and combined, showed positive (p < 0.05) associations with fractures. Results remained the same in stratified models for participants not taking bone-promoting medication. In women on bone-promoting medication, greater femur neck cortical thickness and trabecular BMD were significantly associated with fracture status. However, the association between fracture and combined bone score was not stronger than the associations between fracture and individual measures or total femur BMD. Thus, the selected measures did not all similarly associate with fracture status and did not appear to have an additive effect on fracture status.
Keywords: Bone mineral density, bone strength, elderly, fracture, quantitative computerized tomography
Introduction
Fracture is a common adverse consequence of age-related osteoporosis (1,2). Clinically, skeletal risk of fracture is assessed from bone mineral density (BMD), which is measured by dual-energy X-ray absorptiometry (DXA) (3,4). A greater fracture risk is expected in those with decreased BMD who have osteoporotic T-scores (3,4). However, there is a relatively high frequency of fractures even in people with nonosteoporotic BMD, which raises concerns about the accuracy of BMD alone in calculating fracture risk (5).
Because age-associated structural changes affect bone strength, the addition of a measure of bone strength to BMD has been recommended for fracture risk assessment (5). Geometric bone measures (e.g., cross-sectional area and cortical thickness) have been described as individual surrogates of bone strength (3,6,7) and have also been associated with prevalent osteoporotic fractures (3,6,8–11). However, DXA provides a 2-dimensional measurement of average bone mineral content (in grams per centimeter square area of the bone) and is not designed to measure geometric parameters of bone (3,4,12).
Quantitative computerized tomography (QCT) is a nonprojectional technique that has several advantages over other densitometric methods of measuring BMD (6,8,9,13). Cortical and trabecular bone can be separated, trabecular volumes of interest can be estimated independent of degenerative changes in the spine, and 3-dimensional geometric measures for computing bone strength can be determined. Although studies of associations between individual QCT measures and fracture have been carried out (3,6,8–11), none has yet resulted in improved fracture risk assessment. Whether a combined score of selected QCT bone measures would better associate with prevalent fractures than an individual measure has not been examined. The more than 4000 QCT bone measures from older participants of the Age, Gene/Environment Susceptibility-Reykjavik (AGES-Reykjavik) Study (14) provide a unique opportunity to conduct such an investigation.
We analyzed data from the AGES-Reykjavik Study to determine whether an aggregate of QCT-derived bone measures would better associate with prevalent osteoporotic fractures than individual QCT bone measures would. Specifically, we investigated (1) associations between self-reported fracture status and selected individual QCT bone measures and (2) associations between self-reported fracture status and a composite bone score generated by combining the selected bone measures. In addition, we compared associations between fracture status and QCT bone measures (both individual and combined) with associations between fracture status and DXA-like QCT-derived total femoral BMD.
Materials and Methods
Study Subjects
Participants were part of the AGES-Reykjavik Study, a longitudinal population-based project nested in the Reykjavik Study. Details about the setting, follow-up, and data collection have been reported previously (14). Briefly, the Reykjavik Study was a prospective study of cardiovascular disease in Iceland that comprised a random sample of 30,795 men and women who were born in 1907–1935 and were living in Reykjavik in 1967. The sample was divided into 6 groups by birth date, and each group was invited to participate in specific stages of the study. AGES-Reykjavik examinations began in 2002, when 11,549 previously examined Reykjavik Study cohort members were still alive. The single AGES-Reykjavik examination was completed over the course of 3 clinic visits, which were finished within 4–6 wk for each participant.
The AGES-Reykjavik Study was approved by the National Bioethics Committee in Iceland (#VSN00-063) and by the National Institute on Aging—Intramural Institutional Review Board. The present study proposal was also approved by the University of Texas Houston Health Science Center Committee for Protection of Human Subjects and the Executive Committee for the AGES-Reykjavik Study in Iceland. All participants signed an informed written consent form before participating in the AGES-Reykjavik Study.
QCT Measures and Composite Bone Score
As part of the AGES-Reykjavik Study, the lumbar spine (L1, L2) and the proximal femur (from above the head in the acetabulum to below the lesser trochanter) were scanned with 1-mm slice thickness for acquiring QCT bone measures. Bone measures were calculated using a standard QCT imaging protocol and analysis software described elsewhere (6,11,15). The QCT measures used in the composite bone score were vertebral trabecular BMD, femoral neck trabecular BMD, femoral neck cortical thickness (calculated as femoral neck cortical volume/femoral neck integral volume), and femoral neck minimal cross-sectional area. The traditional total femoral BMD was represented by the QCT-derived DXA-like total femoral BMD.
The 4 selected measures were each standardized by generating a gender-specific Z-score variable (X [data point] − population mean/[standard deviation {SD} for population mean]) and were then summed into a composite bone score. We divided the individual and combination bone scores into quartiles and defined the highest quartile as “high bone score” and the lower 3 quartiles as “low bone score.” Low bone score was used as the reference.
Covariates
We used questionnaire data on age, weight, height, education, smoking (defined as ever smoking at least 100 cigarettes in life), current alcohol use, social and medical history, bone-promoting medication use, and lifestyle practices, including physical activity (<4 h/wk vs ≥4 h/wk). Other covariates were ability to perform certain activities of daily living (any difficulty with eating, bathing, dressing, or transferring from bed to chair vs no difficulty), leg and hand muscle strength (maximal isometric muscle strength of the knee and hand, collected using computerized isometric dynamometry), and walking speed (recorded in meters per second based on time to walk 6 m at normal speed). Use of bone-promoting medications was ascertained based on medications brought to the clinic at the time of the examination: bisphosphonates; selective estrogen-receptor modulators; hormonal preparations including norethisterone, estradiol, dienogest, and levonorgestrel with estrogen; angiotensin receptor blockers; and diuretics, including hydrochlorothiazide. For women, we collected ages at menarche and menopause, history of use of oral contraceptives, and history of hormone replacement therapy (not considered in bone-promoting medications). The questionnaire used closed-ended designated response categories.
Fracture Status
Fracture status, the outcome measure of our study, was defined based on self-report of fractures. Fracture status was defined as having a first-ever vertebral or wrist fracture after 65 yr or a hip fracture at any age (yes and no categories).
Data Analysis
Because of known differences in bone mineral distribution, data were analyzed separately for men and women using Stata, version 9.2 (StataCorp LP, College Station, TX). Correlations between the selected bone measures were tested. All bone measures had a relatively normal distribution except the femoral neck minimal cross-sectional area, which required log transformation. The composite bone score was generated by combining the Z-scores for the 4 individual bone measures as continuous variables. The composite bone scores were divided into quartiles. Men and women in the highest quartile of bone scores represented the participants with the best quality of bone in the cohort.
An age-adjusted univariate logistic regression model was developed to determine the gender-specific relationship between fracture status and bone measures (both individual and combined), comparing the high bone score (highest quartile) to the low bone score (lower 3 quartiles). A backward stepwise multivariate regression model was then developed with 4 individual bone measures to determine which of them would most closely associate with fracture status. The multivariate models were adjusted for the following covariates as described previously: age, weight, height, education, smoking, current alcohol use, physical activity, bone-promoting medication use, activities of daily living, muscle strength, and walking speed. Regression models for the women were also adjusted for ages of menarche and menopause, history of oral contraceptive use, and history of hormone replacement therapy. Additional models, using the same covariates, were developed after stratifying the data by bone-promoting medication use (yes/no). The results were reported in odds ratios (ORs) and 95% confidence intervals (CIs).
Results
A total of 5764 subjects (2438 men and 3326 women) participated in the AGES-Reykjavik Study (14). For this analysis, we excluded 972 participants (642 women and 328 men) due to missing QCT data. About 21% of the missing QCT data were for people who were homebound and were not able to come for QCT measures. Other persons missing QCT data included those with metal implants that prevented adequate scan analysis, those with poor-quality scans, and those who did not have QCT scans because of equipment failure. A total of 4792 participants (2110 men and 2682 women) were included in the final analysis.
Excluded participants due to missing data were older (mean ± SD: 80 ± 6 yr for men and 81 ± 7 yr for women), were weaker in terms of isometric leg strength (mean ± SD: 357 ± 100 N for men and 228 ± 73 N for women), and had slower walking speeds (mean ± SD: 8 ± 2 s for both men and women) than those who were included in the analysis.
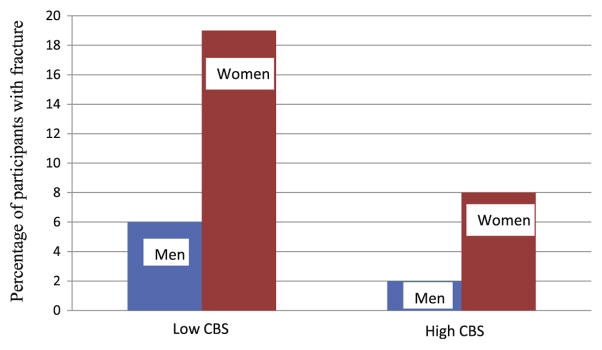
For both men and women in our study, the mean age (±SD) was 76 ± 5 yr. The prevalence of fractures was greater in women (16%) than in men (5%) (Table 1). Relevant population characteristics of the study participants by composite bone score categories are described in Table 1. Men and women with a low composite bone score were older, had less education (Table 1), and had a higher prevalence of fracture than those with a high composite bone score (Fig. 1). We found expected associations between higher bone score (p < 0.001) and younger age at menarche and older age at menopause. Bone-promoting medication use had a positive association with the composite bone score and oral steroid use had a negative association (p < 0.001), but there were too few participants taking oral steroids (≤3%) to analyze this separately.
Table 1.
Distribution of Population Characteristics by Composite Bone Score Category
| Variables | Category | Men (n = 2110)
|
Women (n = 2682)
|
||||
|---|---|---|---|---|---|---|---|
| Total N in each category | Low CBS (%) | High CBS (%) | Total N in each category | Low CBS (%) | High CBS (%) | ||
| Age (yr) | 65–74 | 830 | 36 | 49 | 1143 | 36 | 63 |
| 75–84 | 1106 | 55 | 46 | 1346 | 56 | 33 | |
| ≥85 | 174 | 9 | 5 | 193 | 8 | 4 | |
| Weight (kg) | Mean ± SD | 83 ± 13 | 81 ± 13 | 88 ± 13 | 70 ± 13 | 68 ± 12 | 77 ± 15 |
| Height (m) | Mean ± SD | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 |
| Education | Primary | 328 | 17 | 13 | 796 | 31 | 26 |
| Secondary | 1112 | 53 | 52 | 1271 | 47 | 50 | |
| College | 648 | 30 | 35 | 600 | 22 | 24 | |
| Smoked ≥100 cigarettes in life | Yes | 1496NS | 72 | 71 | 1414NS | 53 | 53 |
| Alcohol use, current | Yes | 1497NS | 72 | 73 | 1572 | 58 | 64 |
| Current physical activity | ≥4 h/wk | 385NS | 18 | 21 | 341 | 12 | 16 |
| Bone-promoting medication use | Yes | 475NS | 22 | 23 | 1293 | 47 | 53 |
| ADL (transfer + bathe + eat + dress) | No difficulty | 1686NS | 80 | 82 | 1965NS | 74 | 73 |
| Hand muscle grip strength (N) | Mean ± SD | 387 ± 99 | 380 ± 100 | 409 ± 92 | 234 ± 67 | 229 ± 56 | 250 ± 90 |
| Knee extension strength (N) | Mean ± SD | 400 ± 113 | 394 ± 113 | 437 ± 109 | 229 ± 66 | 253 ± 76 | 277 ± 75 |
| Time to walk 6 m (s) | Mean ± SD | 7 ± 2 | 7 ± 2 | 6 ± 2 | 7 ± 2 | 7 ± 2 | 7 ± 2 |
| Fracture status | Yes | 108 | 6 | 2 | 436 | 19 | 8 |
Note:
p > 0.05; other results were statistically significant at p < 0.05.
Abbr: ADL, activities of daily living (transfer, bathing, eating, and dressing); CBS, composite bone score; high CBS, highest quartiles; low CBS, lowest 3 quartiles; SD, standard deviation.
Fig. 1.
Distribution of fracture status by composite bone score category. CBS, composite bone score.
All bone measures including total femoral BMD were positively correlated with each other (r2 ≥ 0.51) except femoral neck minimal cross-sectional area (r2 of −0.05 to 0.1). Except for femoral neck minimal cross-sectional area, each individual QCT measure and the composite bone score were inversely related (p < 0.001) to age, that is, older age was associated with lower bone score.
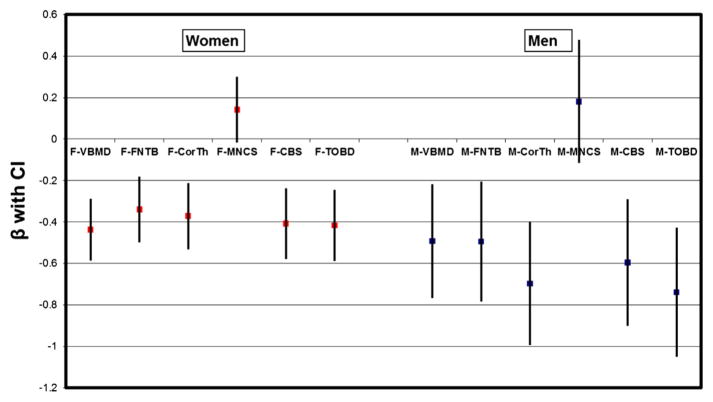
Each bone measure except femoral neck minimal cross-sectional area showed an inverse relationship (p < 0.001 for both genders) with fracture status as well (lower bone score was associated with greater risk of having a fracture). The femoral neck minimal cross-sectional area showed a positive association with fracture status (not statistically significant). For both men and women, the relationships between fracture status and bone measures, both individually (except femoral neck minimal cross-sectional area) and as the composite bone score, were similar in strength based on regression coefficient (β) and 95% CI (Fig. 2). Total femoral BMD and fracture status were likewise inversely related at approximately the same strength as the individual QCT measures (Fig. 2). Femoral neck minimal cross-sectional area had a minimal impact (β close to 0 for both genders) on the composite bone score.
Fig. 2.
Associations between fracture status and QCT bone measures (individual and combined) in age-adjusted regression analysis. BMD, bone mineral density; CBS, composite bone score; CI denotes 95% confidence interval; CorTh, femoral neck cortical thickness; FNTB, femoral neck trabecular BMD; M and F represent measures in male and female participants, respectively; MNCS, femoral neck minimal cross-sectional area; QCT, quantitative computerized tomography; TOBD, QCT-derived DXA-like total femur BMD; VBMD, vertebral trabecular BMD. Each bone variable had 2 categories (highest quartile and lowest 3 quartiles); for each bone score variable tested, the reference group was the lowest 3 quartiles. β is the regression coefficient.
Of the 4 individual bone measures, vertebral trabecular BMD was most closely associated with fracture status in both men and women (Table 2). Femoral neck trabecular BMD was significantly associated with fracture status in women. Women with high vertebral trabecular BMD and high femoral neck trabecular BMD were 32% (OR: 0.68; 95% CI: 0.47–0.98) and 36% (OR: 0.64; 95% CI: 0.44–0.92) less likely to have reported fractures compared with those with low vertebral trabecular BMD and low femoral neck trabecular BMD, respectively (Table 2).
Table 2.
Associations Between Fracture Status and QCT Bone Measures in Backward Stepwise Multivariate Logistic Regression Models
| Gender/type of model | Bone measure | Self-reported fracture status, OR (95% CI)
|
|
|---|---|---|---|
| 1st step—all bone measures | Last step—the most significant measure remaining in the model | ||
| Men/general (n = 1857) | VBMD | 0.57 (0.29–1.10) | 0.52 (0.29–0.92) |
| FNTB | 0.75 (0.39–1.42) | — | |
| MNCS | 1.33 (0.78–2.28) | — | |
| CorTh | 0.80 (0.43–1.50) | — | |
| Women/general (n = 2105) | VBMD | 0.71 (0.49–1.03) | 0.68 (0.47–0.98) |
| FNTB | 0.67 (0.46–0.97) | 0.64 (0.44–0.92) | |
| MNCS | 0.10 (0.73–1.36) | — | |
| CorTh | 0.82 (0.57–1.18) | — | |
| Men/without bone-promoting medication (n = 1553) | VBMD | 0.49 (0.21–1.16) | 0.45 (0.22–0.93) |
| FNTB | 0.62 (0.27–1.42) | — | |
| MNCS | 1.34 (0.70–2.59) | — | |
| CorTh | 0.96 (0.46–2.02) | — | |
| Women/without bone-promoting medication (n = 1366) | VBMD | 0.78 (0.47–1.3) | 0.64 (0.41–0.99) |
| FNTB | 0.76 (0.45–1.29) | — | |
| MNCS | 1.15 (0.76–1.74) | — | |
| CorTh | 1.15 (0.71–1.88) | — | |
| Men/with bone-promoting medication (n = 463) | VBMD | 0.70 (0.24–2.07) | — |
| FNTB | 1.08 (0.38–3.07) | — | |
| MNCS | 1.55 (0.58–4.12) | — | |
| CorTh | 0.59 (0.18–1.91) | 0.46 (0.15–1.37) | |
| Women/with bone-promoting medication (n = 1258) | VBMD | 0.67 (0.38–1.16) | — |
| FNTB | 0.57 (0.33–0.96) | 0.46 (0.28–0.77) | |
| MNCS | 0.86 (0.53–1.39) | — | |
| CorTh | 0.59 (0.34–1.03) | 0.52 (0.32–0.86) | |
Note: Each bone variable had 2 categories (highest quartile and lowest 3 quartiles); for each bone measure tested, the reference group was the lowest 3 quartiles.
Abbr: BMD, bone mineral density; CI, confidence interval; CorTh, femoral neck cortical thickness; FNTB, femoral neck trabecular BMD; MNCS, femoral neck minimal cross-sectional area; OR, odds ratio; QCT, quantitative computerized tomography; VBMD, vertebral trabecular BMD.
Of the 4 individual bone measures, only higher vertebral trabecular BMD was protective against fracture in men and women not using bone-promoting medication (Table 2). Women using bone-promoting medication and having a high femoral neck cortical thickness (OR: 0.52; 95% CI: 0.32–0.86) or high femoral neck trabecular BMD (OR: 0.46; 95% CI: 0.28–0.77) were less likely to report fracture compared with those with low femoral neck cortical thickness or low femoral neck trabecular BMD (Table 2). No bone measure was statistically significantly associated with fracture status in men treated with bone-promoting medication.
Discussion
The goal of this study was to determine whether an aggregate of QCT-derived bone measures would better associate with prevalent osteoporotic fractures than individual QCT bone measures would. We found that self-reported fracture was associated with a low score on 3 of 4 individual QCT measures, a low composite bone score generated from the sum of the QCT measures, and a lower total femoral BMD. However, the associations did not differ significantly in strength based on β regression coefficient (Fig. 2).
Our findings on the associations of individual bone measures with fracture status are consistent with previous research, including reports from the Osteoporotic Fractures in Men Study (MrOS) (3). In that study, baseline QCT scans of the hips were obtained in 3347 men aged 65 yr and older, who were then followed for an average of 5.5 yr. Although some QCT measures were independently associated with increased hip fracture risk, multivariate analysis indicated that the overall ability to predict fracture risk in MrOS was not improved by considering QCT measures in addition to DXA results.
Few other reports are available in the literature on relationships between QCT bone measures and fracture (16). Our study adds valuable information about the value of QCT-acquired individual bone measures for fracture risk assessment in a large population of older (≥65 yr) men and women.
Although it was not significant, the positive relationship in our study between fracture status and increased cross-sectional area of the femoral neck (highest quartile) (Fig. 2) is consistent with previous reports (3,6,17). In theory, larger femoral neck cross-sectional area is thought to be a compensatory change to offset age-related bone loss (10) and should be protective against fracture (9,18). However, larger cross-sectional area of the femoral neck also has been associated with lower BMD (6). Larger cross-sectional area may be associated with increased fracture risk due to its association with poorer-quality bone and the bone loss that triggers periosteal apposition in aging bone. Remodeling-based resorption without modeling-based formation, reported in the periosteal area of bone apposition in animal studies (19), perhaps indicates a weaker structure in this bone region compared with the area developed with a natural “modeling process.” No information is yet available on the quality of bone accumulated by periosteal apposition, which increases femoral neck cross-sectional area with advancing age in humans. Riggs et al (9) suggested that decreased cortical volumetric BMD is due to increased cortical porosity in perimenopausal women. Investigation of the structural quality of bone accumulated through periosteal apposition in humans is recommended.
Our findings from the univariate analysis on relationships between fracture status and QCT bone measures are similar to those from the multivariate analysis. Both vertebral BMD and femoral neck trabecular BMD were significantly associated with fracture status in women. However, only vertebral trabecular BMD showed a significant positive association with fracture status in men. Women are known to have an earlier and greater loss of trabecular bone (lumbar > femoral) than men do, as well as greater loss of trabecular bone than cortical bone (9,20,21). Thus, accelerated bone loss during perimenopause leads to greater disruption of bone architecture and lower bone strength in older women than in older men (9,22,23). Similarly, weaker trabecular BMD in women than in men may have contributed to the findings in our study that fracture in women was associated with lower vertebral trabecular BMD and lower femoral neck BMD (Table 2).
Lower vertebral trabecular BMD remained significantly associated with fracture status in men and women not taking any bone-promoting medication. No QCT measure was associated with fracture status in men taking bone-promoting medications, whereas lower cortical thickness and lower trabecular bone density of the femoral neck were significantly associated with fracture status in women taking the same medications. In both genders, trabecular bone is known to show a faster response to antiresorptive therapy than cortical bone does (24). This phenomenon may underlie the lack of association between higher vertebral trabecular BMD and fracture status in men and women taking bone-promoting medications. Femoral neck measures (cortical thickness and trabecular BMD) may be relatively more important for addressing fracture risk in this group, particularly for women.
A potential limitation of our analysis is that fracture status was coded according to patient self-report. However, data from the Icelandic Fracture Registry show that self-report is highly reliable (25). Caution is warranted for making assumptions about how the composite bone score would generalize to fracture status in other races and ethnicities and in younger population. Still, Iceland, like the United States, has a large population of white people of European ancestry who have a high risk of bone loss and fracture, and the results from this article should be generalizable to that population. Due to the cross-sectional nature of our analysis, we cannot estimate how the composite bone score would fare in its relationship to fracture status with age-related bone changes over time.
Bone-promoting medication use is strongly related to fracture and can modify the associations between bone measures and fracture. Therefore, we stratified the data by bone-promoting medication use, adjusting for the same covariates used in earlier models. Different levels of BMD changes were reported in postmenopausal women receiving different kinds of bone-promoting medication for varied lengths of time (26). A collection of bone-promoting medication was simply coded as “bone-promoting medication” in our study. It is outside the scope of this article to determine whether the outcome differences in our stratified models are attributable to the type and length of medication taken by the participants. Long-term follow-up on bone strength with and without bone-promoting medication, and by type of medication, is indicated to check for associations between fracture status and each QCT measure in the elderly.
In conclusion, the selected measures did not all similarly associate with fracture status and did not appear to have an additive effect on fracture status. Measurable mechanical properties generated by combining QCT bone measures in a biomechanical model using finite element analysis (16) or composite beam theory (7) have been associated with fracture in previous studies. Calculated strength measures using the selected bone properties in a more complex biomechanical model may be better able than a simple additive model to predict fracture risk.
Acknowledgments
The Age, Gene/Environment Susceptibility-Reykjavik Study is funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). Genotyping was conducted at the NIA IRP Laboratory of Neurogenetics. The authors thank Faith Reidenbach, Caley-Reidenbach Consulting, LLP, for editorial assistance.
References
- 1.US Department of Health and Human Services. [Accessed: May 17, 2011];Bone health and osteoporosis: a surgeon general’s report. 2004 Available at: http://www.surgeongeneral.gov/library/reports/index.html.
- 2.National Osteoporosis Foundation. [Accessed: May 17, 2011];Bone health basics: get the facts. Available at: http://www.nof.org/aboutosteoporosis/bonebasics/whybonehealth.
- 3.Black DM, Bouxsein ML, Marshall LM, et al. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res. 2008;23:1326–1333. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dargent-Molina P, Benhamou CL, Cortet B, et al. Devising global strategies for fracture-risk evaluation. Joint Bone Spine. 2007;74:240–244. doi: 10.1016/j.jbspin.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health. Osteoporosis prevention, diagnosis, and therapy. [Accessed: June 26, 2011];NIH Consensus Statement. 2000 17(1):1–36. Available at: http://consensus.nih.gov/2000/2000Osteoporosis111html.htm. [PubMed] [Google Scholar]
- 6.Cheng X, Li J, Lu Y, et al. Proximal femoral density and geometry measurements by quantitative computed tomography: association with hip fracture. Bone. 2007;40:169–174. doi: 10.1016/j.bone.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Whealan KM, Kwak SD, Tedrow JR, et al. Noninvasive imaging predicts failure load of the spine with simulated osteolytic defects. J Bone Joint Surg Am. 2000;82:1240–1251. doi: 10.2106/00004623-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lang TF, Li J, Harris ST, Genant HK. Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr. 1999;23:130–137. doi: 10.1097/00004728-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Riggs BL, Melton Iii LJ, 3rd, Robb RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 10.Meta M, Lu Y, Keyak JH, et al. Young-elderly differences in bone density, geometry and strength indices depend on proximal femur sub-region: a cross sectional study in Caucasian-American women. Bone. 2006;39:152–158. doi: 10.1016/j.bone.2005.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigurdsson G, Aspelund T, Chang M, et al. Increasing sex difference in bone strength in old age: the Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK) Bone. 2006;39:644–651. doi: 10.1016/j.bone.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Beck TJ. Extending DXA beyond bone mineral density: understanding hip structure analysis. Curr Osteoporos Rep. 2007;5:49–55. doi: 10.1007/s11914-007-0002-4. [DOI] [PubMed] [Google Scholar]
- 13.Engelke K, Adams JE, Armbrecht G, et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123–162. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang TF, Leblanc AD, Evans HJ, et al. Adaptation of the proximal femur to skeletal reloading after long-duration space-flight. J Bone Miner Res. 2006;21:1224–1230. doi: 10.1359/jbmr.060509. [DOI] [PubMed] [Google Scholar]
- 16.Orwoll ES, Marshall LM, Nielson CM, et al. Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res. 2009;24:475–483. doi: 10.1359/JBMR.081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahlborg HG, Nguyen ND, Nguyen TV, et al. Contribution of hip strength indices to hip fracture risk in elderly men and women. J Bone Miner Res. 2005;20:1820–1827. doi: 10.1359/JBMR.050519. [DOI] [PubMed] [Google Scholar]
- 18.Center JR, Nguyen TV, Pocock NA, Eisman JA. Volumetric bone density at the femoral neck as a common measure of hip fracture risk for men and women. J Clin Endocrinol Metab. 2004;89:2776–2782. doi: 10.1210/jc.2003-030551. [DOI] [PubMed] [Google Scholar]
- 19.Orwoll ES. Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res. 2003;18:949–954. doi: 10.1359/jbmr.2003.18.6.949. [DOI] [PubMed] [Google Scholar]
- 20.Zhai G, Hart DJ, Valdes AM, et al. Natural history and risk factors for bone loss in postmenopausal Caucasian women: a 15-year follow-up population-based study. Osteoporos Int. 2008;19:1211–1217. doi: 10.1007/s00198-008-0562-x. [DOI] [PubMed] [Google Scholar]
- 21.Sirola J, Kroger H, Honkanen R, et al. Factors affecting bone loss around menopause in women without HRT: a prospective study. Maturitas. 2003;45:159–167. doi: 10.1016/s0378-5122(03)00150-6. [DOI] [PubMed] [Google Scholar]
- 22.Geusens P, Dinant G. Integrating a gender dimension into osteoporosis and fracture risk research. Gend Med. 2007;4(Suppl B):S147–S161. doi: 10.1016/s1550-8579(07)80055-6. [DOI] [PubMed] [Google Scholar]
- 23.Seeman E. Clinical review 137: sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab. 2001;86:4576–4584. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 24.Engelke K, Kemmler W, Lauber D, et al. Exercise maintains bone density at spine and hip EFOPS: a 3-year longitudinal study in early postmenopausal women. Osteoporos Int. 2006;17:133–142. doi: 10.1007/s00198-005-1938-9. [DOI] [PubMed] [Google Scholar]
- 25.Siggeirsdottir K, Aspelund T, Sigurdsson G, et al. Inaccuracy in self-report of fractures may underestimate association with health outcomes when compared with medical record based fracture registry. Eur J Epidemiol. 2007;22:631–639. doi: 10.1007/s10654-007-9163-9. [DOI] [PubMed] [Google Scholar]
- 26.Ettinger MP. Aging bone and osteoporosis: strategies for preventing fractures in the elderly. Arch Intern Med. 2003;163:2237–2246. doi: 10.1001/archinte.163.18.2237. [DOI] [PubMed] [Google Scholar]