Abstract
Background
Diabetes, heart failure (HF), and chronic kidney disease (CKD) are common co-morbidities, but overall use and safety of anti-hyperglycemic medications (AHM) among patients with these co-morbidities are poorly understood.
Methods and Results
Using Get With the Guidelines-Heart Failure (GWTG-HF) and linked Medicare Part D data, we assessed AHM use within 90 days of hospital discharge among HF patients with diabetes discharged from GWTG-HF hospitals between 1-1-2006 and 10-1-2011. We further summarized use by renal function and assessed renal contraindicated AHM use for patients with eGFR <30mL/min/1.73m2. Among 8,791 patients meeting inclusion criteria, the median age was 77 (interquartile range [IQR] 71-83), 62.3% were female, median BMI was 29.7 (IQR 25.5-35.3), median HbA1c was 6.8 (IQR 6.2-7.8), and 34% had ejection fraction <40%. 74.9% of patients filled a prescription for an AHM, with insulin (39.5%), sulfonylureas (32.4%), and metformin (17%) being the most commonly used AHMs. Insulin use was higher and sulfonylurea/metformin use was lower among patients with lower renal function classes. Among 1,512 patients with eGFR <30mL/min/1.73m2, 35.4% filled prescriptions for renal contraindicated AHMs per prescribing information, though there was a trend toward lower renal contraindicated AHM use over time (Cochran-Mantel-Haenszel row-mean score test p=0.048). While use of other AHMs was low overall, thiazolidinediones were used in 6.6% of HF patients and DPP4-inhibitors were used in 5.1%, with trends for decreasing thiazolidinedione use and increased DPP4-inhibitor use over time (p<0.001).
Conclusions
Treatment of diabetes in patients with HF and CKD disease is complex, and these patients are commonly treated with renal contraindicated AHMs, including over 6% receiving a thiazolidinedione despite known concerns regarding heart failure. More research regarding safety and efficacy of various AHMs among HF patients is needed.
Keywords: heart failure, diabetes mellitus, chronic kidney disease
Diabetes is common among heart failure patients, occurring in 28-44% of heart failure patients, and is associated with adverse outcomes regardless of ejection fraction, including increased risk of both cardiovascular death and heart failure hospitalization.1-3 Despite the high prevalence of comorbid heart failure and diabetes, there are few randomized clinical trial data to inform the management of diabetes among patients with heart failure.4
While improved glucose control is associated with fewer microvascular complications from diabetes, clinicians must balance these potential benefits with the risk of adverse effects from anti-hyperglycemic medications (AHMs). For example, hypoglycemic episodes are more frequent among heart failure patients with diabetes than in patients without heart failure, and certain AHMs such as thiazolidinediones and dipeptidyl peptidase-4 (DPP4) inhibitors may increase the risk of heart failure, which led the Food and Drug Administration to consider manufacturer labelling outlining this risk.5-8 For the DPP-4 inhibitor saxagliptin in particular, the risk of heart failure may increase with chronic kidney disease (CKD), which is present in up to half of ambulatory patients with heart failure, though the drug has been studied and does not appear to increase ischemic events in patients with renal insufficiency.9, 10
The treatment of diabetes in patients with acute decompensated heart failure (ADHF) presents a particular challenge since nearly 2/3 of these patients have impaired renal function, which affects the safety of many AHMs used in the treatment of diabetes.11, 12 In particular, metformin, sulfonylureas, meglitinides, incretin mimetics (glucagon-like peptide-1 receptor agonists), DPP4-inhibitors, and alpha-glucosidase inhibitors carry specific cautions in their prescribing information recommending against use or cautious dose adjustment in patients with renal impairment. While impaired renal function is prevalent in patients hospitalized with acute decompensated heart failure, it is unclear how this affects the choice of AHMs for patients with diabetes. Moreover, the overall use and outcomes associated with use of AHMs with renal contraindications among heart failure patients are poorly understood. We performed an analysis of Get With The Guidelines-Heart Failure (GWTG-HF) linked with Medicare claims data to characterize utilization of AHMs after hospitalization for heart failure based on renal function.
Methods
GWTG-HF
GWTG-HF is a national quality improvement initiative aimed at improving heart failure care, and details of the program have been previously published.13, 14 Participating hospitals must receive approval through their local institutional review boards or a waiver of individual consent under the common rule. Trained personnel regularly review hospital records and identify patients admitted with heart failure. Medical history and demographic data are abstracted, and de-identified data are entered into a central database using a web-based patient management tool. Quintiles (Cambridge, MA) is the data collection coordination center for the American Heart Association/American Stroke Association GWTG programs. The Duke Clinical Research Institute (Durham, NC) serves as the data analysis center and has an agreement to analyze the aggregate, de-identified data for research purposes.
Study Population
Using GWTG-HF, we identified patients with heart failure who had either a prior medical history of diabetes or new diagnosis of diabetes established during the index heart failure hospitalization and were discharged from hospital between 01/01/2006 and 10/01/2011. Patients had to be continuously enrolled in Medicare fee-for-service and Medicare Part D at index discharge and for the 90 days post discharge. Among a total of 61,093 Medicare beneficiaries identified in GWTG-HF during this study period, 22,873 (37.4%) had diabetes and were discharged prior to 10/1/2011. We excluded patients who died in hospital (n=671), transferred out of index hospital (n=587), who were discharged to hospice (n=585), had end stage renal disease (n=1387), or had transplant or LVAD implantation during hospitalization (n=22). Of the remaining patients, 8,791/19,621 (44.8%) were enrolled in Medicare fee-for-service and Medicare Part D continuously within the 90 days post index discharge and were included in the final study population.
Statistical Analysis
Patients were classified into three renal function classes (eGFR<30mL/min/1.73m2, 30≤ eGFR<60mL/min/1.73m2, and eGFR≥60mL/min/1.73m2) using the Modification of Diet in Renal Disease (MDRD) formula and serum creatinine on admission to hospital. Percentages and medians with 25th and 75th percentiles (IQRs) were reported to describe the distribution of categorical and continuous variables, respectively. The patient characteristics, including demographics, clinical data, medical history, and hospital characteristics as captured in GWTG-HF were compared between the three renal function groups. Pearson Chi-square and Kruskal-Wallis tests were used to test the difference for categorical and continuous variables.
To assess use of AHMs, we linked GWTG-HF data to Medicare Part D claims to identify diabetes medications filled within 90 days of index discharge for heart failure, including metformin/biguanide, sulfonylurea, meglitinide, glucagon like peptide-1 (GLP-1) agonist, DPP4-inhibitor, alpha-glucosidase inhibitor, thiazolidinedione, insulin, and amylin analog. For patients with greater than one hospitalization, only the first claim for AHM and its corresponding hospitalization and was used for analysis. If multiple claims for one AHM class were found within 90 days, that AHM was counted only once. If a patient had claims for two or more medications in different AHM classes, for example both insulin and metformin, it was counted for each of the AHM classes. AHMs with labeling to avoid or reduce dosage in chronic kidney disease were categorized as renal contraindicated AHMs, including metformin, sulfonylureas, meglitinides, incretin mimetics (GLP-1 agonists), DPP4-inhinitors, and alpha-glucosidase inhibitors. Renal contraindicated use was defined as the use of a renal contraindicated AHM among patients with eGFR<30mL/min/1.73m2 on admission to hospital, as captured in GWTG-HF. The trend in renal contraindicated AHM use from 2006 to 2011 was tested using Cochran-Mantel-Haenzsel row-mean score statistic.
A multivariable logistic regression analysis was conducted to examine the factors associated with renal contraindicated AHM use among the patients with eGFR<30mL/min/1.73m2. Generalized estimating equation (GEE) methods were used to account for the within-hospital clustering of patients. The regression model included demographics, age, gender, non-Caucasian race, Hispanic ethnicity, prior medical histories (atrial fibrillation/flutter, chronic obstructive pulmonary disease [COPD], hyperlipidemia, hypertension, peripheral vascular disease [PVD], ischemic heart disease including prior myocardial infarction [MI], coronary artery disease [CAD], percutaneous coronary intervention [PCI], or coronary artery bypass grafting [CABG], cerebrovascular accident/transient ischemic attack [CVA/TIA], implantable-cardioverter defibrillator [ICD], heart failure, anemia, pacemaker, renal insufficiency, depression and smoking history in past 6 months), ejection fraction, BMI, respiratory rate at admission and heart rate, systolic blood pressure at discharge, sodium, eGFR, blood urea nitrogen (BUN), hemoglobin at admission, and potassium at discharge, length of stay, and hospital characteristics, including region, number of beds, rural (vs. urban) location, and hospitals teaching status. Most variables had missing rate less than 5% except Hispanic ethnicity had 12% missing and BMI had 13% missing and potassium had 16% missing. Therefore, multiple imputation was used to address missing variables.
A p value <0.05 was considered statistically significant for all tests. All analyses were performed with SAS software version 9.2 (SAS Institute, Cary, NC).
Results
Among 8,791 Medicare patients with heart failure and diabetes included in our study, the median age was 77 (IQR 71-83), 62.3% female, with median EF 47% (IQR 30-60). The median BMI was 29.7 (IQR 25.5-35.3) and median HbA1c was 6.8 (IQR 6.2-7.8). In this cohort, 17.2% had eGFR<30mL/min/1.73m2, 49.1% had 30≤ eGFR<60mL/min/1.73m2, and 32.5% had eGFR≥60mL/min/1.73m2 (Table 1). Patients in lower renal function classes included higher proportion of females (71.1 vs. 64.9 vs. 53.6, p<0.001), more patients with multiple comorbidities (4 vs. 3 vs. 3, p<0.001), lower proportion of patients with reduced ejection fraction (30.4 vs. 33.7 vs. 36.2, p=0.001), and slightly longer lengths of stay (5 (IQR 3-7.5) vs. 4 (IQR 3-7) vs. 4 (IQR 3-6), p<0.001).
Table 1. Characteristics of Heart Failure Patients with Diabetes Categorized by Renal Function.
| Variable | eGFR<30 mL/min/1.73m2 N=1,512 |
30≤ eGFR <60 mL/min/1.73m2 N=4,313 |
eGFR≥60 mL/min/1.73m2 N=2,861 |
p-value |
|---|---|---|---|---|
| Age (years) | 77 (71-83) | 78 (71-84) | 76 (70-82) | <0.001 |
| Female Sex | 71.1 | 64.9 | 53.6 | <0.001 |
| Race | <0.001 | |||
| White | 76.1 | 77 | 72.4 | |
| Black | 14.2 | 14.4 | 19.2 | |
| Other/Unknown | 9.7 | 8.6 | 8.4 | |
| Medical History | ||||
| CKD (Cr >2 mg/dL) | 57.1 | 19.7 | 3.4 | <0.001 |
| Diabetes – insulin treated | 53.6 | 44.3 | 36.8 | <0.001 |
| Ischemic heart disease | 65.2 | 67.3 | 63.1 | <0.001 |
| CVA/TIA | 16.5 | 18.1 | 18.0 | 0.354 |
| Atrial fibrillation/flutter | 26.5 | 34.1 | 33.5 | <0.001 |
| Hyperlipidemia | 54.5 | 55.7 | 52.9 | 0.061 |
| Hypertension | 83.9 | 82.8 | 83.6 | 0.474 |
| Peripheral vascular disease | 19.8 | 15.3 | 13.5 | <0.001 |
| Pulmonary disease | 28.8 | 30.9 | 33.2 | 0.010 |
| Smoking | 7.2 | 8.7 | 12.4 | <0.001 |
| Median number of comorbidities | 4 | 3 | 3 | <0.001 |
| 0 comorbidities | 1.3 | 1.3 | 1.9 | |
| 1-2 comorbidities | 17.4 | 26.1 | 30.9 | |
| 3-4 comorbidities | 45.6 | 46.5 | 48.3 | |
| >4 comorbidities | 35.6 | 26.1 | 18.9 | |
| Other history | ||||
| Ejection Fraction <40% or moderate or severe dysfunction | 30.4 | 33.7 | 36.2 | 0.001 |
| Length of Stay (days) | 5 (3-7) | 4 (3-7) | 4 (3-6) | <0.001 |
| Admission Exam | ||||
| Systolic Blood Pressure (mmHg) | 143 (124-165) | 143 (124-162) | 144 (128-164) | 0.028 |
| Heart Rate (beats/min) | 75 (65-88) | 79 (68-92) | 82 (70-95) | <0.001 |
| Body Mass Index | 30.1 (25.9-35.9) | 29.8 (25.7-35.4) | 29.3 (25.0-35.0) | <0.001 |
| Labs | ||||
| Creatinine (mg/dL) | 2.4 (2.1-3) | 1.4 (1.2-1.6) | 0.9 (0.8-1.0) | <0.001 |
| eGFR (mL/min/1.73m2) | 23.2 (18.4-26.8) | 44.0 (37.3-51.2) | 75.2 (66.2-87.7) | <0.001 |
| HbA1c (%) | 6.7 (6.1-7.5) | 6.9 (6.2-7.8) | 6.8 (6.2-7.8) | 0.017 |
| Meds at Discharge | ||||
| ACE/ARB | 42.2 | 65.0 | 77.6 | <0.001 |
| Beta-blocker | 80.6 | 80.6 | 78.7 | 0.090 |
| Aldosterone Antagonist | 9.1 | 15.3 | 17.1 | <0.001 |
| Hydralazine/Nitrate | 26.9 | 17.2 | 9.0 | <0.001 |
| Number of meds prescribed | <0.001 | |||
| 0 | 0.8 | 0.6 | 0.7 | |
| 1-2 | 8.6 | 6.5 | 5.6 | |
| 3-4 | 27.1 | 22.3 | 22.7 | |
| >4 | 62.8 | 70.2 | 70.6 | |
| Hospital Characteristics | ||||
| Academic | 59.3 | 59.9 | 61.7 | 0.256 |
| Urban | 89.6 | 89.7 | 90.1 | 0.204 |
Categorical variables are presented as percentages, and continuous variables are presented as medians (interquartile ranges). eGFR= estimated glomerular filtration rate, CKD=chronic kidney disease, Cr=creatinine, CVA/TIA=cerebrovascular accident/transient ischemic attack, Hba1c=hemoglobin a1c, ACE/ARB=angiotensin converting enzyme inhibitor/angiotensin receptor blocker, min = minute
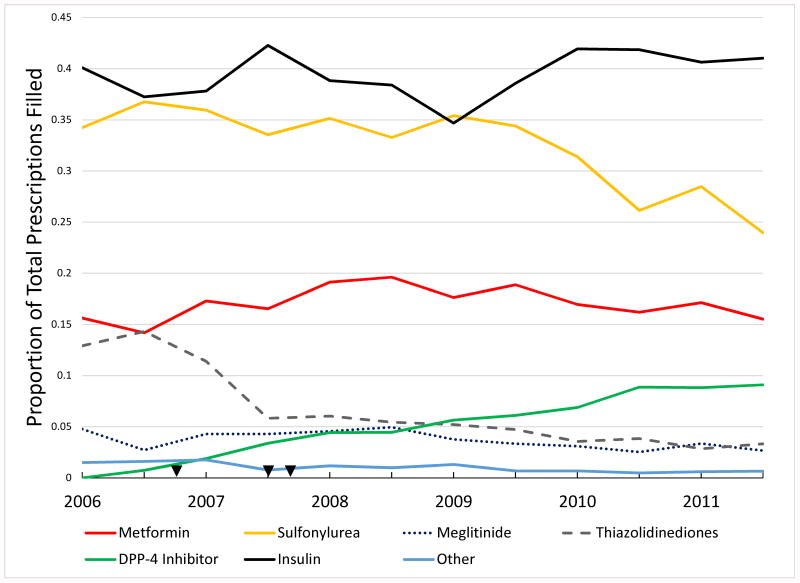
AHM use varied considerably by renal function (Table 2). The proportion of patients filling prescriptions for insulin, which is generally used as monotherapy, was higher among patients with more severe renal dysfunction (47.4% vs. 40.9% vs. 33%, p<0.001 among patients with eGFR<30mL/min/1.73m2 vs. 30≤ eGFR<60mL/min/1.73m2 vs. eGFR≥60mL/min/1.73m2, respectively). In contrast, metformin use (3.5% vs. 13.5% vs. 29.5%) and sulfonylurea use (26.9% vs. 34.3% vs. 32.9%) was lower among patients with more severe renal dysfunction. Prescription fills for other AHMs were low overall, though thiazolidinediones were surprisingly used in 6.6% of patients with heart failure despite the known risk for worsening heart failure. DPP-4 inhibitors were likewise used in 5.1% of patients overall, though there was a trend towards increased use of DPP-4 inhibitors and decreased use of thiazolidinediones among all patients over time (p<0.001) (Figure 1).
Table 2. Overall AHM Use at within 90 Days of Discharge for Heart Failure.
| Variable | Overall | eGFR<30 mL/min/1.73m2 N=1,512 |
30≤ eGFR <60 mL/min/1.73m2 N=4,313 |
eGFR≥60 mL/min/1.73m2 N=2,861 |
p-value |
|---|---|---|---|---|---|
| Therapy | |||||
| Metformin | 17.0 | 3.5 | 13.5 | 29.5 | <0.001 |
| Sulfonylurea | 32.4 | 26.9 | 34.3 | 32.9 | <0.001 |
| Meglitinide | 3.7 | 4.9 | 3.6 | 3.0 | 0.005 |
| DPP4-inhibitor | 5.1 | 4.9 | 5.3 | 4.7 | 0.477 |
| Thiazolidinedione | 6.6 | 5.7 | 7.2 | 6.4 | 0.117 |
| Insulin | 39.5 | 47.4 | 40.9 | 33.0 | <0.001 |
| GLP-1 agonist | 0.4 | 0.2 | 0.4 | 0.5 | 0.284 |
| Alpha-glucosidase inhibitor | 0.5 | 0.6 | 0.6 | 0.4 | 0.426 |
| Amylin Analog | 0.1 | 0.1 | 0.1 | 0.1 | 0.999 |
| Number of AHMs Filled | <0.001 | ||||
| 0 | 25.1 | 25.0 | 24.3 | 26.4 | |
| 1 | 50.4 | 58.8 | 51.6 | 44.3 | |
| 2 | 19.3 | 13.5 | 18.9 | 22.9 | |
| >2 | 5.2 | 2.7 | 5.2 | 6.5 |
Values represent percentages. AHM=antihyperglycemic medication, DPP4=dipeptidyl-peptidase 4 inhibitor, GLP-1 agonist=glucagon like peptide 1 agonist
Figure 1. Overall Semi-Annual AHM Use.
This graph describes overall use of AHMs on a semi-annual basis among all patients included in this study. Arrowheads represent, in chronological order from left to right, the publication of DREAM trial (DREAM=Diabetes REduction Assessment with ramipril and rosiglitazone Medication Trial) showing higher numerical risk of heart failure by rosiglitazone, publication of meta-analysis suggesting HF risk by thiazolidinediones, and date of FDA black box warning for thiazolidinediones for heart failure15, 16, 17. Information regarding potential HF risk for DPP4-inhibitors was not available until late 2013.7 Cochran-Mantel-Haenszel Row Mean Score Test for Trend: metformin p=0.433, sulfonylurea p<0.001, meglitinide p=0.024, GLP-1 agonist p=0.035, DPP-4 inhibitor p<0.001, alpha-glucosidase inhibitor p=0.017, thiazolidinedione p<0.001, insulin p=0.113, amylin analog p=0.280. Abbreviations: AHM=antihyperglycemic medication, DPP4=dipeptidyl-peptidase 4 inhibitor, GLP-1 agonist=glucagon like peptide 1 agonist
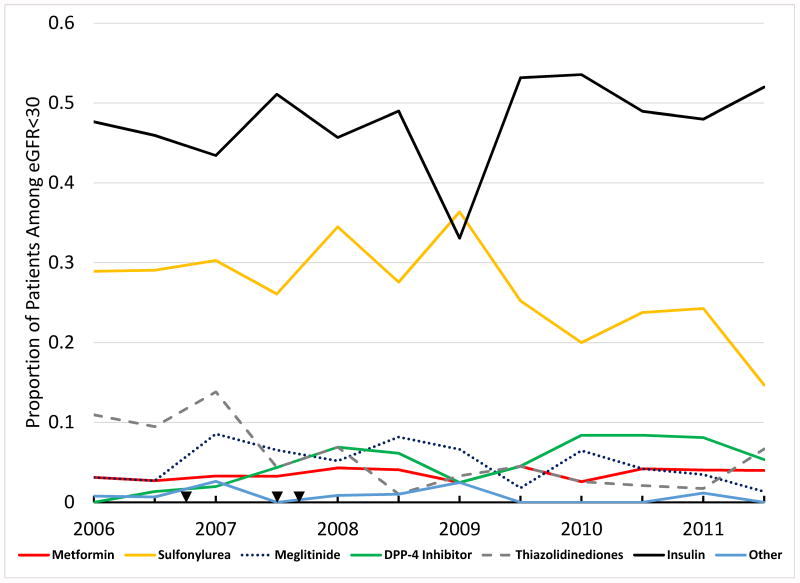
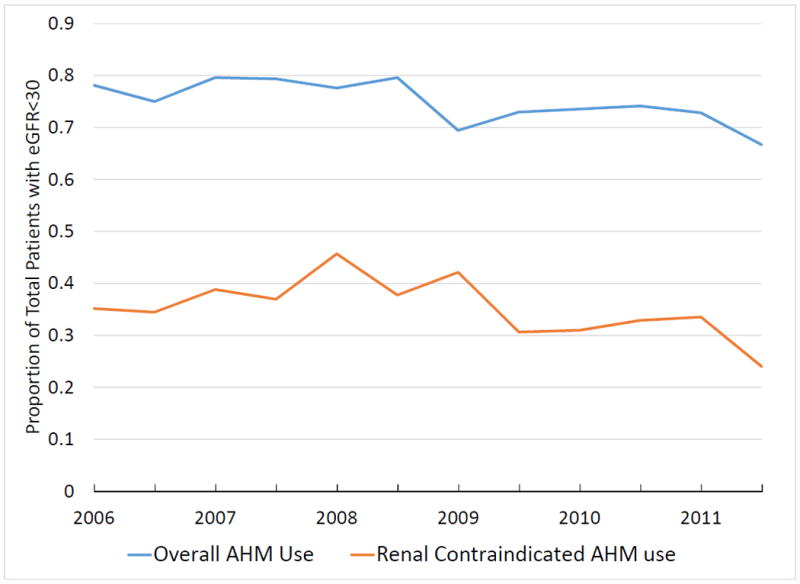
Among 1,512 patients with eGFR<30mL/min/1.73m2, 35.4% received renal contraindicated AHMs, though there was a trend toward less renal contraindicated AHM use over time during the study period (p=0.048) (Figure 2A). In particular, there was a temporal trend towards less sulfonylurea use (p=0.005), more DPP-4 inhibitor use (p<0.001) and less thiazolidinedione use (p<0.001) (Figure 2B). The proportion of patients with a prior history of insulin treated diabetes was lower (32.3% vs. 65.3%, p<0.001) and age slightly higher (77 (IQR 72-84) vs. 76 (IQR 70-83), p<0.005) among patients receiving renal contraindicated AHMs compared to those not (Table 3). There was also a slightly higher HbA1c (6.9 (IQR 6.2-7.7) vs. 6.6 (IQR 6-7.3), p=0.030) and slightly greater proportion of patients with low ejection fraction among patients receiving renal contraindicated AHMs (33.6% vs. 28.7%, p=0.049). Surprisingly, patients receiving renal contraindicated AHMs had less number of comorbidities (p=0.03). Among hospital characteristics, only hospital region showed an association with renal contraindicated AHM use. We performed a multivariable logistic regression analysis to identify independent predictors of renal contraindicated AHM use, but no clinically meaningful variables were found to have clear association with renal contraindicated AHM use (Supplement Table).
Figure 2.
Panel A: Use of AHMs Among Patients with eGFR<30mL/min/1.73m2. Use of renal contraindicated AHM was 35.4% overall, with a trend towards lower renal contraindicated use over time (Cochran-Mantel-Haenszel row-mean score test p=0.048). Panel B: Overall Use of AHMs Among Patients with eGFR<30mL/min/1.73m2. Cochran-Mantel-Haenszel row-mean score tests: metformin p=0.542, DPP-4 inhibitors p<0.001, thiazolidinediones p<0.001, sulfonylurea p=0.005, meglitinide p=0.381. Arrowheads represent, in chronological order from left to right, the publication of DREAM trial showing higher numerical risk of heart failure by rosiglitazone, publication of meta-analysis suggesting HF risk by thiazolidinediones, and date of FDA black box warning for thiazolidinediones for heart failure15, 16, 17. Information regarding potential HF risk for DPP4-inhibitors was not available until late 2013.7 Abbreviations: AHM=antihyperglycemic medication, eGFR=estimated glomerular filtration rate, DPP4=dipeptidyl-peptidase 4 inhibitor
Table 3. Factors Associated with Renal Contraindicated AHM Use Among Patients with eGFR<30mL/min/1.73m2.
| Variable | Renal contraindicated AHM use (N=535) |
No renal contraindicated AHM use (N=977) |
p-value |
|---|---|---|---|
| Age (years) | 77 (72-84) | 76 (70-83) | 0.005 |
| Female Sex | 71.2 | 71.0 | 0.941 |
| Race | 0.560 | ||
| White | 77.0 | 75.6 | |
| Black | 13.8 | 14.4 | |
| Other/Unknown | 9.2 | 9.9 | |
| Medical History | |||
| CKD (Cr >2mg/dL) | 53.1 | 59.3 | 0.020 |
| Diabetes – Insulin treated | 32.3 | 65.3 | <0.001 |
| Ischemic heart disease | 64.3 | 65.7 | 0.582 |
| CVA/TIA | 12.5 | 18.6 | 0.002 |
| Atrial fibrillation/flutter | 29.2 | 25.1 | 0.086 |
| Hyperlipidemia | 53.8 | 54.9 | 0.701 |
| Hypertension | 84.3 | 83.6 | 0.733 |
| Peripheral Vascular Disease | 16.5 | 21.7 | 0.014 |
| Pulmonary disease | 26.5 | 30.1 | 0.145 |
| Adult history of smoking | 8.8 | 6.4 | 0.084 |
| Number of comorbidities | 0.030 | ||
| 0 comorbidities | 1.5 | 1.2 | |
| 1-2 comorbidities | 21.1 | 15.4 | |
| 3-4 comorbidities | 44.7 | 46.2 | |
| >4 comorbidities | 32.7 | 37.3 | |
| Ejection Fraction <40% or moderate or severe dysfunction | 33.6 | 28.7 | 0.049 |
| Length of Stay (days) | 5 (3-7) | 5 (3-8) | 0.108 |
| Admission Exam | |||
| Systolic Blood Pressure (mmHg) | 142 (123-162) | 144 (124-167) | 0.068 |
| Heart Rate (beats/minute) | 74 (64-88) | 76 (65-88) | 0.502 |
| Body Mass Index | 29.6 (25.9-34.6) | 30.4 (25.9-36.8) | 0.038 |
| Labs at admission | |||
| eGFR (mg/dL/1.73m2) | 23.7 (18.8-27.2) | 23 (18.0-26.6) | 0.043 |
| Hba1c (%) | 6.9 (6.2-7.7) | 6.6 (6-7.3) | 0.030 |
| Meds at Discharge | |||
| ACE/ARB | 44.9 | 40.7 | 0.130 |
| Beta-blocker | 80 | 80.9 | 0.561 |
| Aldosterone Antagonist | 9.4 | 9.0 | 0.886 |
| Hydralazine/Nitrate | 23.7 | 28.6 | 0.032 |
| Number of meds prescribed (as captured in GWTG-HF) | 0.377 | ||
| 0 | 1.3 | 0.5 | |
| 1-2 | 8.6 | 8.7 | |
| 3-4 | 26.3 | 27.9 | |
| >4 | 63.8 | 63.0 | |
| Hospital Characteristics | |||
| Academic | 58.3 | 59.9 | 0.430 |
| Urban | 90.1 | 89.4 | 0.934 |
| Region | 0.023 | ||
| West | 10.3 | 8.9 | |
| South | 33.5 | 38.8 | |
| Midwest | 24.5 | 26.8 | |
| Northeast | 31.2 | 24.7 |
Categorical variables are presented as percentages, and continuous variables are presented as medians (interquartile ranges). AHM=antihyperglycemic medication, eGFR=estimated glomerular filtration rate, CKD=chronic kidney disease, Cr=creatinine, CVA/TIA=cerebrovascular accident/transient ischemic attack, Hba1c=hemoglobin a1c, ACE/ARB=angiotensin converting enzyme inhibitor/angiotensin receptor blocker, GWTG-HF=Get With The Guidelines Heart Failure
Discussion
In this study, we have reported novel data regarding usage patterns of AHMs among Medicare patients with heart failure and diabetes. AHMs are used in ∼75% of patients with heart failure and diabetes, with insulin being the most commonly prescribed medication regardless of renal function class, followed by sulfonylureas and metformin. Moreover, we show that AHMs that may worsen heart failure, including thiazolidinediones and potentially some DPP4-inhibitors based on data that emerged after the study period, are used in one-tenth of patients with heart failure and that over one-third of patients with eGFR <30mL/min/1.73m2 are treated with renal contraindicated AHMs. There were strikingly few differences among patients receiving renal contraindicated AHMs vs. not receiving renal contraindicated AHMs among patients with eGFR<30mL/min/1.73m2, suggesting that provider choices, rather than patient factors, may be driving these prescribing patterns.
Current guidelines provide little guidance for managing complex comorbidities such as diabetes and chronic kidney disease in heart failure patients, reflecting the relatively paucity of data regarding management of these complex comorbidities. Where data are lacking, safety is generally the primary driver for prescribing, yet we showed that 35.4% of prescriptions for AHMs among patients with diabetes and eGFR<30mL/min/1.73m2 are renal contraindicated, thereby potentially exposing patients to a higher risk of adverse events, particularly hypoglycemia. We suspect this reflects a lack of awareness regarding the safe use of various AHM classes among patients with diabetes and CKD. Moreover, we observed an approximate 10% prescription rate for AHMs that may worsen heart failure. While data regarding the potential increased risk of heart failure with the DPP-4 inhibitor saxagliptin did not emerge until 2013, data regarding the heart failure risk with thiazolidinediones was available throughout most of the study period.7, 15
The impact of renal contraindicated AHM prescription on healthcare utilization and outcomes are poorly understood and need further investigation. This is particularly important because heart failure patients are prone to fluctuations in renal function because neurohormonal and hemodynamic derangements, volume status, and even the medicines used to treat heart failure or concomitant comorbidities can have significant effects on renal function and clearance of AHMs. While it is possible that renal contraindicated AHMs were being used as a strategy to obtain better glycemic control (Hba1c 6.9 vs. 6.6 among patients with renal contraindicated AHM vs. not), glycemic control was good overall and data is mixed regarding blood glucose control and heart failure outcomes. Though some data suggest improvement in diastolic dysfunction with enhanced blood glucose control, large randomized trials have shown higher risks of hypoglycemia with intensive blood glucose control strategies without improvements in macrovascular outcomes or incident heart failure.18-20 As such, we need more data to guide diabetes management strategies for patients with heart failure, including specific glycemic control targets and recommendations for medication choices, especially among patients with CKD who are prone to adverse medication effects from renal contraindicated AHMs.
Unfortunately, the optimal diabetes treatment strategy among patients with heart failure is unclear, even among patients without CKD. Thiazolidinediones have been shown to increase risk of heart failure in case reports from as early as 2002, which has been supported by clinical trial and observational data and led to an FDA issued black box warning in 2007 against their use in heart failure patients.5, 21-24 Observational studies suggest that insulin and sulfonylureas may be associated with higher risks for fluid retention and heart failure respectively, and the initial results of the FIGHT trial suggest that liraglutide provides no cardiovascular outcome benefit to heart failure patients.25-27 Recently, heart failure risk with DPP4 inhibitors has been rigorously studied, with one study showing no increased risk of heart failure (TECOS – Trial Evaluating Cardiovascular Outcomes with Sitagliptin) and two showing neutral to higher risk (EXAMINE – Examination of cArdiovascular outcoMes with alogliptIN vs standard of carE in patients with type 2 diabetes and acute coronary syndrome and SAVOR-TIMI 53 – Saxagliptin Assesment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus).8, 28, 29 These studies did not specifically enroll heart failure patients, and the TECOS trial excluded patients with eGFR <30mL/min/1.73m2. Nevertheless, for saxagliptin patients with CKD had a greater risk for heart failure.7, 8 Metformin potentially has the best observational data supporting its use in heart failure patients, but it continues to carry warnings against its use in CKD patients despite evidence suggesting it may be cautiously used in setting of mild to moderate CKD.30, 31 Unfortunately there is little data on how CKD affects heart failure risk among other AHMs. While choice of an optimal AHM in heart failure patients is not straightforward, it should be noted that thiazolidinediones were prescribed in ≥3% of patients during the last two years of our study despite strong evidence against their use. Moreover, 32.3% of patients who received renal contraindicated AHMs were also prescribed insulin.
Despite a growing body of knowledge addressing the complex interplay between metabolic derangements of heart failure and diabetes, a safe and effective AHM remains elusive.32, 33 The SGLT-1 inhibitor empaglaflozin may be promising based on the recent results of EMPA-REG trial, which showed a lower risk of heart failure hospitalization (hazard ratio 0.65) and death from cardiovascular causes (hazard ratio 0.62).34 Unfortunately, SGLT-1 inhibitor use was not assessed in this study because it was not FDA approved during the study period. Additional studies are underway to assess cardiovascular outcomes with nearly all classes of AHMs.35 Nevertheless, these trials are not specifically designed to recruit heart failure patients and many limit enrollment of patients with CKD, so we believe there need to be more dedicated trials of AHMs specifically for heart failure patients with complex comorbidities such as CKD.
This study has a number of limitations that must be mentioned. GWTG-HF is a voluntary quality improvement program and may not represent prescribing patterns at non-participating hospitals. Also, care for patients with Medicare and Part D may differ from care of younger patients in different care settings. It is possible that renal function at baseline in our patients with acute decompensated heart failure did not reflect baseline outpatient renal function; nevertheless we believe that our results are still applicable to a broad population of patients because labile renal function should itself be considered when prescribing AHMs with renal contraindications to mitigate risks of adverse drug effects. In addition, AHM use was assessed in the first 90 days after hospital discharge. We were therefore unable to capture if AHM therapy was altered by outpatient providers after this time frame or if patients continued to fill prescriptions for AHMs. It should be noted that nearly one quarter of patients failed to fill a prescription for AHM regardless of renal function class, which could signify either lack of adherence, diet-control, or sufficient pre-existing supply of AHMs. Due to limitations of data, we are unable to identify whether factors that are not captured in GWTG-HF may have affected choice and safety of AHMs used to treat patients in this study. Lastly, these data are observational and hypothesis generating. While nearly one-third of AHMs prescribed among patients with eGFR <30mL/min/1.73m2 have renal contraindications, further research is needed to assess how this affects outcomes.
In conclusion, AHMs are used in a majority of patients with heart failure and diabetes, though a considerable proportion of prescriptions are renal contraindicated or thought to exacerbate heart failure. There is a strong need to increase awareness among both providers and patients to prevent and investigate the incidence and impact of adverse events based on these prescribing patterns. Moreover, there are few data to guide management of diabetes in the setting of comorbid heart failure and CKD. Future clinical trials are needed to assess outcomes among patients receiving specific classes of AHMs, including renal contraindicated AHMs.
Supplementary Material
Clinical Perspective.
Diabetes and chronic kidney disease are common comorbidities among heart failure patients and are associated with adverse outcomes including death and heart failure hospitalization. This is particularly challenging because many anti-hyperglycemic medications (AHMs) including metformin, sulfonylureas, meglitinides, incretin mimetics (glucagon-like peptide-1 receptor agonists), DPP4-inhibitors, and alpha-glucosidase inhibitors carry specific cautions in their prescribing information recommending against use or cautious dose adjustment in patients with renal impairment. We used Get With The Guidelines Heart Failure and Medicare Part D to study and report novel data regarding AHM prescribing patterns among heart failure patients with diabetes and CKD. We found that 75% of patients with heart failure and diabetes were treated with AHMs, and that over one-third of patients with estimated glomerular filtration rate (eGFR) <30mL/min/1.73m2 were treated with renal contraindicated AHMs. This may reflect a lack of awareness regarding the safe use of various AHM classes among patients with diabetes and CKD and could potentially expose patients to a higher risk of adverse events, particularly hypoglycemia. There is a strong need to increase awareness among both providers and patients to prevent and investigate the impact of renal contraindicated AHM prescriptions on healthcare utilization and outcomes. Moreover, there is little randomized data to guide management of diabetes in heart failure patients, and there need to be more dedicated trials of AHMs specifically for heart failure patients, including patients with complex comorbidities such as CKD.
Acknowledgments
Sources of Funding: This project was supported in part by grant number U19HS021092 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. PA Patel was additionally supported by National Institutes of Health grant T32-HL1007101.
References
- 1.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) American Heart Journal. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Cavender MA, Steg PG, Smith SC, Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PW, Bhatt DL. Impact of diabetes on hospitalization for heart failure, cardiovascular events, and death: Outcomes at 4 years from the REACH registry. Circulation. 2015;132:923–31. doi: 10.1161/CIRCULATIONAHA.114.014796. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the candesartan in heart failure: Assessment of reduction in mortality and morbidity (CHARM) programme. European Heart Journal. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC. Diabetes medications and heart failure: Recognizing the risk. Circulation. 2014;130:1565–7. doi: 10.1161/CIRCULATIONAHA.114.012883. [DOI] [PubMed] [Google Scholar]
- 5.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J, investigators PR Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (prospective pioglitazone clinical trial in macrovascular events): A randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 6.Gitt AK, Bramlage P, Binz C, Krekler M, Deeg E, Tschope D. Comorbidity, hypoglycaemia and appropriate selection of antidiabetic pharmacotherapy in diabetic patients with heart failure in clinical practice in germany. Results of the DiaRegis registry. Herz. 2012;37:294–300. doi: 10.1007/s00059-012-3611-3. [DOI] [PubMed] [Google Scholar]
- 7.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I, Committee S-TS, Investigators Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. The New England Journal of Medicine. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 8.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL, for the S-TSC, Investigators Heart failure, saxagliptin and diabetes mellitus: Observations from the SAVOR - TIMI 53 randomized trial. Circulation. 2014;130:1579–88. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 9.Heywood JT, Fonarow GC, Yancy CW, Albert NM, Curtis AB, Stough WG, Gheorghiade M, McBride ML, Mehra MR, O'Connor CM, Reynolds D, Walsh MN. Influence of renal function on the use of guideline-recommended therapies for patients with heart failure. The American Journal of Cardiology. 2010;105:1140–1146. doi: 10.1016/j.amjcard.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Udell JA, Bhatt DL, Braunwald E, Cavender MA, Mosenzon O, Steg PG, Davidson JA, Nicolau JC, Corbalan R, Hirshberg B, Frederich R, Im K, Umez-Eronini AA, He P, McGuire DK, Leiter LA, Raz I, Scirica BM, Committee S-TS, Investigators Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: Observations from the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:696–705. doi: 10.2337/dc14-1850. [DOI] [PubMed] [Google Scholar]
- 11.Patel UD, Hernandez AF, Liang L, Peterson ED, LaBresh KA, Yancy CW, Albert NM, Ellrodt G, Fonarow GC. Quality of care and outcomes among patients with heart failure and chronic kidney disease: A Get With The Guidelines -- Heart Failure program study. American Heart Journal. 2008;156:674–681. doi: 10.1016/j.ahj.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, Committee ASA, Investigators High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: A report from the ADHERE database. Journal of Cardiac Failure. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 13.LaBresh KA, Gliklich R, Liljestrand J, Peto R, Ellrodt AG. Using “Get With The Guidelines” to improve cardiovascular secondary prevention. Joint Commission Journal on Quality and Safety. 2003;29:539–550. doi: 10.1016/s1549-3741(03)29064-x. [DOI] [PubMed] [Google Scholar]
- 14.Smaha LA, American Heart A. The American Heart Association Get With The Guidelines Program. American Heart Journal. 2004;148:S46–48. doi: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: A meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 16.Investigators DT. Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 17.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England Journal of Medicine. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 18.Action to Control Cardiovascular Risk in Diabetes Study G. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. The New England Journal of Medicine. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Investigators OT. Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. The New England Journal of Medicine. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 20.von Bibra H, Hansen A, Dounis V, Bystedt T, Malmberg K, Ryden L. Augmented metabolic control improves myocardial diastolic function and perfusion in patients with non-insulin dependent diabetes. Heart. 2004;90:1483–1484. doi: 10.1136/hrt.2003.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher AM, Smeeth L, Seabroke S, Leufkens HG, van Staa TP. Risk of death and cardiovascular outcomes with thiazolidinediones: A study with the general practice research database and secondary care data. PloS one. 2011;6:e28157. doi: 10.1371/journal.pone.0028157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: An observational study. Circulation. 2005;111:583–590. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- 23.Scheen AJ. DREAM study: Prevention of type 2 diabetes with ramipril and/or rosiglitazone in persons with dysglycaemia but no cardiovascular desease. Revue medicale de Liege. 2006;61:728–732. [PubMed] [Google Scholar]
- 24.Wooltorton E. Rosiglitazone (avandia) and pioglitazone (actos) and heart failure. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2002;166:219. [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert RE, Krum H. Heart failure in diabetes: Effects of anti-hyperglycaemic drug therapy. Lancet. 2015;385:2107–2117. doi: 10.1016/S0140-6736(14)61402-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhong J, Goud A, Rajagopalan S. Glycemia lowering and risk for heart failure: Recent evidence from studies of dipeptidyl peptidase inhibition. Circulation Heart failure. 2015;8:819–825. doi: 10.1161/CIRCHEARTFAILURE.114.001967. [DOI] [PubMed] [Google Scholar]
- 27.Late-breaking clinical trial abstracts. Circulation. 2015;132:2267–2285. doi: 10.1161/CIR.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 28.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR, Group TS Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. The New England Journal of Medicine. 2015;373:232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 29.Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Lam H, White WB, Investigators E Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in examine: A multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–2076. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 30.Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, Vanderloo SE, McAlister FA. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: Systematic review of observational studies involving 34,000 patients. Circulation Heart failure. 2013;6:395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162. [DOI] [PubMed] [Google Scholar]
- 31.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: A systematic review. JAMA : the Journal of the American Medical Association. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dei Cas A, Fonarow GC, Gheorghiade M, Butler J. Concomitant diabetes mellitus and heart failure. Current Problems in Cardiology. 2015;40:7–43. doi: 10.1016/j.cpcardiol.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, Tschoepe D, Doehner W, Greene SJ, Senni M, Gheorghiade M, Fonarow GC. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Failure. 2015;3:136–145. doi: 10.1016/j.jchf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. The New England Journal of Medicine. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 35.Lathief S, Inzucchi SE. Approach to diabetes management in patients with CVD. Trends in Cardiovascular Medicine. 2016;26:165–79. doi: 10.1016/j.tcm.2015.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.