Abstract
Semaphorins and their receptors are abnormally expressed in various cancers, but little is known about the expression and function of semaphorin 3E (SEMA3E) and its receptor, plexin D1 (PLXND1), in gastric cancer development or metastasis. We evaluated SEMA3E and PLXND1 expression by quantitative RT-PCR in gastric tissues from 62 patients who underwent gastrectomy and analyzed the correlation between their expression and clinicopathological variables. To assess the function of SEMA3E, we generated human gastric cancer cell lines with suppressed or increased SEMA3E expression. The expression level of SEMA3E, but not PLXND1, was correlated with lymph node involvement and metastatic progression in gastric cancer. A significant association was observed between a high level of SEMA3E expression and poor differentiation or poor survival in the intestinal type of gastric cancer. SEMA3E knockdown in gastric cancer cells attenuated cell proliferation and metastatic ability in vitro and in vivo. Moreover, SEMA3E caused cell proliferation and anchorage-independent cell growth in the intestinal type of gastric cancer. These results suggested that SEMA3E is likely to be involved in the development of gastric cancer and might also be a therapeutic target for its treatment.
Keywords: semaphorin 3E, plexin D1, gastric cancer, proliferation, metastasis
Introduction
Although the prevalence of gastric cancer has decreased in most areas of the world (1), it is still the third leading cause of cancer death in both genders worldwide (2). Despite recent advances in diagnostic techniques, such as magnifying endoscopy with narrow band imaging (NBI) (3), and in treatment including target therapy (4,5), there are still many gastric cancer patients with a poor prognosis. The pathogenic mechanism contributing to the aggressive biological feature in this cancer needs to be clarified.
Semaphorins are secreted and membrane-bound proteins and were originally implicated in the control of axonal wiring (6). They comprise a wide protein family and are involved in a range of functions, from tissue morphogenesis to the immune response. Recently they have also been shown to function in the regulation of various phases of cancer development and have become potential therapeutic targets in various types of cancer (7).
The high-affinity functional receptor of semaphorin 3E (SEMA3E) is plexin D1 (PLXND1), and SEMA3E-PLXND1 signaling is required in developmental angiogenesis (8). Moreover, PLXND1 expression is enhanced in endothelial cells of tumor vessels and in cancer cells (9,10). The activation of PLXND1 by SEMA3E on tumor cells facilitates the invasive migration of cancer cells through the complex formation and transactivation of the epidermal growth factor receptor 2 (ErbB2) (11), or through regulation of the subcellular location and activity of Snail, which is a transcriptional repressor that acts during epithelial-to-mesenchymal transition (EMT) (12). Luchino et al reported that SEMA3E/PLXND1 signaling has a critical role in tumor cell resistance to apoptosis via NR4A1 binding to PLXND1 receptor (13).
Actually, the expression levels of SEMA3E appear to be positively correlated with increased metastases in ovarian, melanoma, and colon cancers and with poor patient survival in colorectal and pancreatic cancers (11,12,14). However, little is known about the expression and function of SEMA3E and PLXND1 in the development or metastasis of gastric cancer. In this study, we investigated the involvement of SEMA3E/PLXND1 signaling in the development of gastric cancer.
Materials and methods
Tissues
A total of 124 gastric tissues, 62 matched normal and carcinoma pairs, were obtained from patients who underwent surgery at Miyagi Cancer Center (Natori, Japan), between 2007 and 2013. All samples were immediately frozen after resection in liquid nitrogen and stored at −80°C or fixed in 10% buffered formalin and embedded in paraffin wax.
The gastric cancers were histopathologically classified as the intestinal type and diffuse type according to the classification of the World Health Organization, and were additionally classified according to the pathologic tumor-node-metastasis (TNM) Classification (15). No patients received chemotherapy or radiotherapy before surgery. For statistical analysis, overall survival was defined by death from any cause, and Kaplan-Meier survival curves were used.
Cell lines
The gastric cancer cell lines MKN74 (intestinal type), GCIY (diffuse type) and HGC-27 (diffuse type) were obtained from RIKEN BioResource Center (Tsukuba, Japan). MKN74 was maintained in RPMI-1640 (Wako Pure Chemical Industries, Osaka, Japan) and GCIY and HGC-27 were maintained in DMEM (Wako Pure Chemical Industries), containing 10% inactivated FBS (EuroClone, Milan, Italy) with 100 U/ml penicillin and 100 μg/ml streptomycin (Nacalai Tesque, Kyoto, Japan) and were cultured in a humidified 5% CO2 incubator at 37°C.
RNA preparation, reverse transcription, and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from frozen samples and cell lines using RNeasy Mini kit (Qiagen, Tokyo, Japan) according to the manufacturer’s protocol. First-strand cDNAs from all samples were synthesized from 1.0 μg of total RNA by PrimeScript® 1st strand cDNA Synthesis kit (Takara Bio, Shiga, Japan) following the manufacturer’s protocol. The expression of PLXND1 and SEMA3E was quantified by LightCycler Brilliant SYBR Green qRT-PCR kit (Roche Applied Science, IN, USA) according to the manufacturer’s protocol with the specific primer sets (Table I). The levels of PLXND1 and SEMA3E expression in each sample were normalized to the respective GAPDH expression levels. The specificity of each PCR reaction was confirmed by melting curve analyses.
Table I.
Primers used in this study.
| Gene | Sequence |
|---|---|
| PLXND1 | Forward: cagcgctactacaagcagatca Reverse: gctcaaacttgtgctgcagttgtgt |
| SEMA3E | Forward: aagtcagattccatcactgtgacat Reverse: agcaaagtactgttgttctctatgc |
| GAPDH | Forward: tgaaggtcggagtcaacgg Reverse: agagttaaaagcagccctggtg |
Phosphorylation of extracellular signal-regulated kinase (Erk)
To assess the phosphorylation of Erk in MKN74 by SEMA3E, MKN74 cells were plated in the culture medium without FBS overnight. The culture medium was aspirated from the dish and cells were washed using PBS. Culture medium with or without recombinant SEMA3E was injected. Five minutes post-injection, the cells were harvested and western blot analysis was performed using antibodies of α-tubulin, Erk and phosphorylated Erk.
RNA interference
To knockdown SEMA3E in GCIY and HGC-27, we used Knockout™ RNAi systems (Clontech Laboratories, Mountain View, CA, USA) according to the manufacturer’s protocol. We designed seven shRNA sequences targeting SEMA3E according to a previous study (11). After annealing of the complementary shRNA oligonucleotides, we ligated those oligonucleotides into pSIREN vector (sh1 and 2). Then, we transfected Platinum-A packaging cell lines (Provided by Professor Kitamura) with shSEMA3E or pSIREN Vector (control) to produce recombinant retroviruses. Stably infected GCIY and HGC-27 cells with the recombinant retrovi-ruses were selected with puromycin. The cells transduced with the sh1 and -2 vector were selected for further study since these cells showed effective reduction of SEMA3E by qRT-PCR. The sequences of shRNA targeted SEMA3E are shown in Table II. All the experiments in vitro were performed in triplicate, repeated three times and representative data are shown.
Table II.
Sequences of shRNA inserts for the shSEMA3E expressing vector.
| shRNA | Sequence |
|---|---|
| shSEMA3E-1 | |
| Sense | gatccGTGCTGAAAGTAATCACAATaCTC GAGaATTGTGATTACTTTCAGCACttttttG |
| Antisense | AATTCaaaaaaGTGCTGAAAGTAATCACA ATtCTCGAGtATTGTGATTACTTTCAGCACg |
| shSEMA3E-2 | |
| Sense | gatccGCCACGATCTTTACAAGCGAAAaC TCGAGaTTTCGCTTGTAAAGATCGTGGCttttttG |
| Antisense | AATTCaaaaaaGCCACGATCTTTACAAGC GAAAtCTCGAGtTTTCGCTTGTAAAGATCGTGGCg |
SEMA3E expression retroviral vector
Human SEMA3E cDNA (Kazusa DNA Research Institute, Chiba, Japan) was amplified by PCR and inserted into the pcDNA4.0/3xHA-HisA vector (pcDNA4.0/3xHA-HisA-SEMA3E). Then, human SEMA3E cDNA with HA tag was amplified by PCR and inserted into the pBabe puro vector (pBabe-SEMA3E-HA). Recombinant retroviruses were produced with Platinum-A packaged cell lines. Briefly, Plat-A cells were transfected with pBabe-SEMA3E-HA or pBabe-puro Vector (EV). FuGENE-6 (Roche Applied Science) and Opti-MEM I (Gibco/Life Technologies Co.) were added following the manufacturer’s protocol. Forty-eight hours after transfection, the retrovirus-containing supernatant was collected and passed through a 0.45-μm filter. MKN74 cells were infected with the recombinant retroviruses and then selected with puromycin.
Antibodies and recombinant protein
Antibodies used in this study were obtained from the following sources: α-tubulin (Santa Cruz Biotechnology, CA, USA): Hemagglutinin (HA) (Roche); Erk, and phosphorylated Erk (Cell Signaling Technology, MA, USA). Recombinant SEMA3E was purchased from R&D Systems (R&D Systems, MN, USA).
Western blot analysis
Cells were harvested in RIPA buffer supplemented with protease and phosphatase inhibitors (Roche). The lysates were pre-cleared by centrifugation (10,000 × g) for 15 min at 4°C, and supernatants were subjected to 5–20% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinyliden difluoride membranes. After blocking with 5% non-fat milk in Tris-buffered saline (TBS) containing 0.1% Tween-20, the membranes were incubated overnight at 4°C with the primary antibody. After washing with TBS buffer, they were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies. Reactive bands were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific Inc., MA, USA).
Cell growth assay
The cell growth rate was evaluated using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). Cells (3×103) of GCIY, HGC-27 and MKN74 per well were seeded in 96-well plates in normal cell growth medium. After incubation for 24, 48 or 72 h, Cell Counting Kit-8 solution (10 μl) was added and the cells were further incubated for 2 h at 37°C. Then, measurements of absorbance at 450 nm by VersaMax (Molecular Devices, CA, USA) were measured after 24-, 48- and 72-h incubation. The index evaluated at 48 and 72 h was normalized to that at 24 h.
Soft agar colony formation assay
Soft agar assays were conducted in 6-well plates. The base layer of each well consisted of 1 ml with final concentrations of 1X medium (DMEM or RPMI plus 10% FBS) and 0.6% low melting point agarose. Plates were cooled at room temperature until solid, at which point a 1 ml growth agar layer was poured, consisting of 5×104 cells of GCIY and 1×104 cells of MKN74 suspended in 1X media and 0.3% low melting point agarose. Plates were again cooled at room temperature until the growth layer congealed. A further 1 ml of 1X medium without agarose was added on top of the growth layer on day 0 and 0.5 ml was applied once a week. Cells were allowed to grow at 37°C for 4 weeks and total colonies were counted.
Animal experiments
Six-week-old female NOD/Shi-scid-IL2Rγnull (NOG) mice were purchased from Central Institute for Experimental Animals (CIEA, Kawasaki, Japan) and used in this experiment. They were allowed to acclimate for a week before the experiments. They were housed in a clean room of the animal care facility at Miyagi Cancer Research Center and kept under standard temperature, humidity and timed lighting conditions and provided mouse chow/water ad libitum. The approval of the Animal Welfare Committee at the Institute was obtained for all studies.
To investigate the effect of SEMA3E expression in tumor formation and growth in vivo, 1×105 cells of GCIY-control (n=6) or GCIY-sh1 (n=6) were injected subcutaneously with 100 μl PBS into NOG mice. Then we measured the volume of the subcutaneous tumors formed once a week for 8 weeks. The tumor volume was calculated by the formula: [(long diameter] × (short diameter)2] × 1/2.
To assess the effect of SEMA3E expression on peritoneal metastasis, we injected 1.0×106 cells of GCIY-control (n=6) or GCIY-sh1 (n=6) with 200 μl PBS into the peritoneum of mice. After 5.5 weeks, the mice were sacrificed, and the presence of nodules and ascites in the peritoneal cavity was investigated macroscopically.
Statistical analysis
The correlation of PLXND1 and SEMA3E expression with the patient’s clinicopathological variables was analyzed by Fisher’s exact test. Statistical significance of differences between 2 groups was determined using Student’s t-test or Wilcoxon rank sum test and p-value (<0.05) was regarded as statistically significant. Statistical difference between 3 or 4 groups was evaluated using Tukey’s test and a p-value <0.05 was regarded as statistically significant. All statistical analyses were performed using JMP® 10 (SAS Institute Inc., Cary, NC, USA).
Results
SEMA3E and PLXND1 expression in human gastric cancer samples
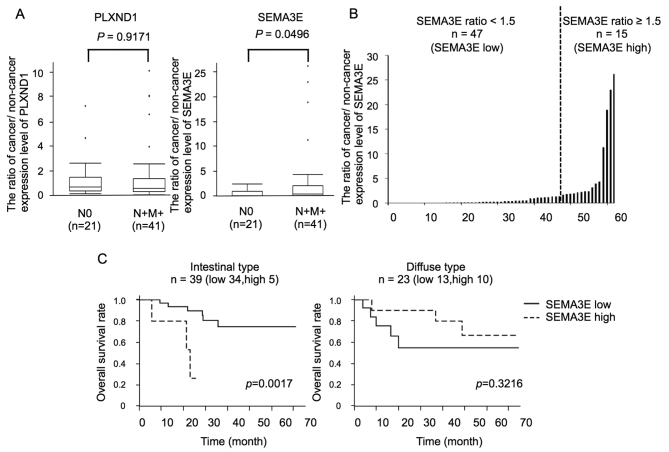
The SEMA3E and PLXND1 expressions of cancerous and adjacent non-cancerous tissues from the gastric cancer patients were examined by qRT-PCR and normalized by the GAPDH expression levels, and we evaluated the ratios of cancer/non-cancer expression levels of SEMA3E and PLXND1. The human gastric cancer samples were obtained from patients with tumors localized to the stomach (N0) or with any lymph node involvement or distant metastases at diagnosis (N+M+). The mean expression ratio of PLXND1 was 1.27±1.73 and 1.50±2.40 (cancer/non-cancer of PLXND1 ± standard deviation) in N0 (n=21) and N+M+ (n=41) patients, respectively. The mean expression ratio of SEMA3E was 0.52±0.72 and 2.73±6.05 (cancer/non-cancer of SEMA3E ± standard deviation) in N0 (n=21) and N+M+ (n=41) patients, respectively. While there were no significant differences in the PLXND1 expression levels between N0 and N+M+ patients (p=0.9171), the SEMA3E expression levels in N+M+ patients were significantly higher than those in N0 patients (p=0.0496) (Fig. 1A).
Figure 1.
The expression of PLXND1 and SEMA3E in human gastric carcinoma tissues. (A) PLXND1 and SEMA3E expression levels of cancerous and adjacent non-cancerous tissues from the gastric cancer patients were examined by qRT-PCR and normalized by the GAPDH expression level, and the ratios of cancer/non-cancer expression levels of PLXND1 and SEMA3E were evaluated. There were no significant differences in the PLXND1 expression ratios between the N0 and N+M+ patients (p=0.9171). The SEMA3E expression ratios in N+M+ patients was significantly higher than those in the N0 patients (p=0.0496). (B) The human gastric cancer tissues were classified according to the SEMA3E expression ratio into a high-SEMA3E group (cancer/non-cancer ≥1.5, n=15) and low-SEMA3E group (cancer/non-cancer <1.5, n=47). (C) In the intestinal type gastric cancer patients, the high-SEMA3E group (n=5) showed shorter survival than the low-SEMA3E (n=32) group (p=0.0017), while no significant relation was seen between the SEMA3E expression ratio and the overall survival in the diffuse type gastric cancer patients.
Moreover, the human gastric cancer tissues were classified according to the SEMA3E expression ratio into a high-SEMA3E group (cancer/non-cancer ≥1.5, n=15) and low-SEMA3E group (cancer/non-cancer <1.5, n=47) (Fig. 1B). The association between the SEMA3E expression ratio and the clinicopathological features is summarized in Table III. A significant association was found between the SEMA3E expression ratio and the diffuse type gastric cancer. The PLXND1 expression ratio was higher in the high-SEMA3E group than in the low-SEMA3E group, but there was no significant difference.
Table III.
The association between the SEMA3E expression ratio and the clinicopathological features.
| SEMA3E low (n=47) | SEMA3E high (n=15) | P-value | |
|---|---|---|---|
| Gender | |||
| Male | 32 | 10 | |
| Female | 15 | 5 | 1 |
| Histopathology | |||
| Intestinal | 34 | 5 | |
| Diffuse | 13 | 10 | 0.012 |
| pT | |||
| T1 | 7 | 1 | |
| T2–4 | 40 | 14 | 0.6665 |
| pN | |||
| N0 | 18 | 4 | |
| N1–3 | 29 | 11 | 0.5409 |
| pM | |||
| M0 | 40 | 13 | |
| M1 | 7 | 2 | 1 |
| ly | |||
| ly0 | 20 | 5 | |
| ly1–3 | 27 | 10 | 0.5638 |
| v | |||
| v0 | 21 | 10 | |
| v1–3 | 26 | 5 | 0.2351 |
| PLXND1 ratio | |||
| Mean ± SD | 0.99±1.33 | 2.78±3.51 | 0.1127 |
In addition, in the intestinal type gastric cancer patients, the high-SEMA3E group (n=5) showed shorter survival than the low-SEMA3E (n=32) group (p=0.0017, Fig. 1C).
Activation of Erk by SEMA3E in gastric cancer cells
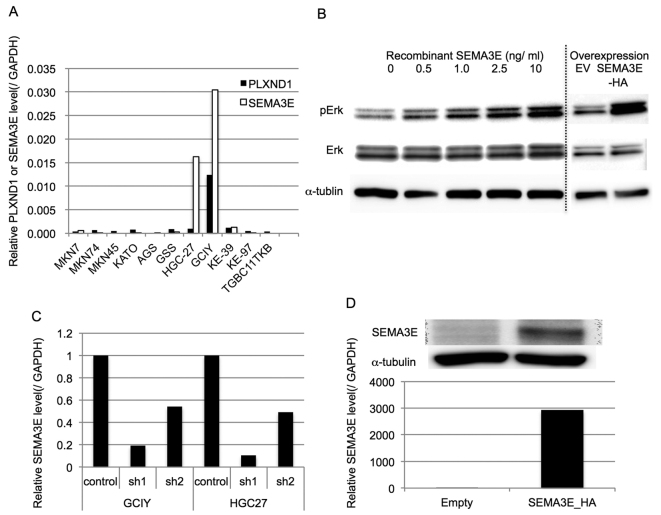
At first, the PLXND1 and SEMA3E expression levels of several gastric cancer cell lines were examined by qRT-PCR and normalized by the GAPDH expression level (Fig. 2A). Since PLXND1 expression was not associated with the biological aggressiveness of gastric cancer, we examined whether SEMA3E could activate Erk, which is the one of the representative growth promoting oncogenic signals, in gastric cancer cells with low level expression of PLXND1. As shown in Fig. 2B, SEMA3E could phosphorylate Erk in MKN74 cells, which endogenously express very low level of PLXND1, depending on the concentration of recombinant SEMA3E.
Figure 2.
The expression of PLXND1 and SEMA3E in human gastric cancer cell lines, and activation of Erk by SEMA3E in human gastric cancer cell lines. The generation of SEMA3E up- and downregulated gastric cancer cells. (A) PLXND1 and SEMA3E expression levels of several gastric cancer cell lines were examined by qRT-PCR and normalized by the GAPDH expression level. GCIY expressed high levels of PLXND1 and SEMA3E. (B) SEMA3E phosphorylated Erk in MKN74 cells, which endogenously express very low levels of PLXND1. Moreover, MKN74-SEMA3E-HA cells phosphorylated Erk more than MKN74-EV. (C) Two types of stably shSEMA3E-expressing GCIY and HGC-27 cells were generated and the qRT-PCR revealed that the SEMA3E expression levels were effectively reduced in these knockdown cells. (D) Stably SEMA3E-overexpressing MKN74 cells (MKN74-SEMA3E-HA) were generated and the qRT-PCR and western blot analysis revealed that the SEMA3E expression levels were effectively increased in MKN-SEMA3E-HA.
Generation of SEMA3E up- and downregulated cells
To assess the functional role of SEMA3E in gastric cancer, we generated stably shSEMA3E-expressing GCIY and HGC-27 cells, which express very high levels of endogenous PLXND1 as well as SEMA3E. The qRT-PCR revealed that the SEMA3E expression levels were effectively reduced in the knockdown cells (Fig. 2C).
MKN74, a gastric cancer cell line derived from intestinal adenocarcinoma, expressed low levels of PLXND1 and SEMA3E. Therefore, we generated stably SEMA3E-overexpressing MKN74 cells (MKN74-SEMA3E-HA). The qRT-PCR and western blot analysis revealed that the SEMA3E expression levels were effectively increased in MKN-SEMA3E-HA (Fig. 2D). Consistent with activation of Erk by SEMA3E in MKN74, MKN74-SEMA3E-HA cells phosphorylated Erk more than MKN74-EV (Fig. 2B).
Cell growth
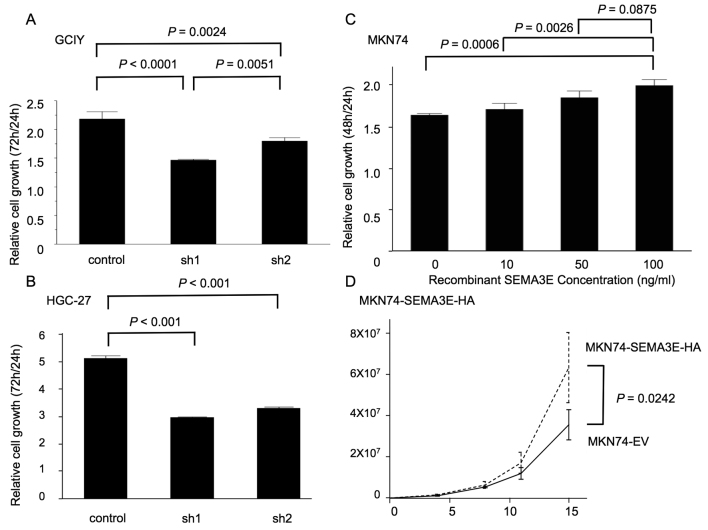
To assess the functional role of SEMA3E in gastric cancer development, we utilized Cell Counting Kit-8 assays. The results of these assays showed that cell growth was significantly reduced in SEMA3E knockdown GCIY and HGC-27 cells (sh1 and sh2) (Fig. 3A and B). In addition, the cellular proliferation of MKN74 cells was significantly increased in a SEMA3E-dependent manner (Fig. 3C) and MKN74-SEMA3E-HA significantly proliferated more than MKN74-EV (Fig. 3D).
Figure 3.
Cell growth of GCIY and MKN74 using Cell Counting Kit-8 assays. (A and B) Cell growth was significantly reduced in SEMA3E knockdown GCIY and HGC-27 cells (sh1 and sh2). (C and D) MKN74 cells showed significantly increased cellular proliferation in an SEMA3E-dependent manner and MKN74-SEMA3E-HA significantly proliferated more than MKN74-EV.
Soft agar colony formation assay
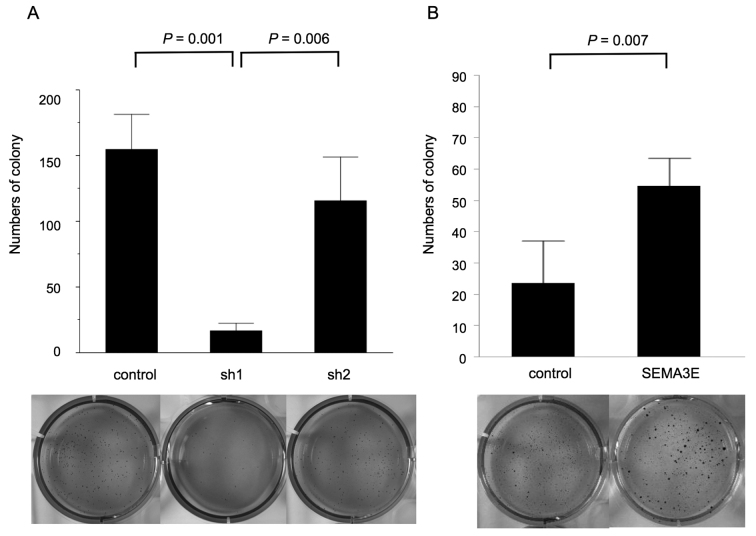
To evaluate the function of SEMA3E in the anchorage-independent cell growth, we utilized a soft agar assay. GCIY-sh1 cells formed a smaller numbers of colonies than respective control cells (p=0.001, Fig. 4A). Consistent with this finding, MKN74-SEMA3E-HA cells formed larger numbers of colonies than MKN74-EV in anchorage-independent cell growth (p=0.007, Fig. 4B).
Figure 4.
Soft agar colony formation assay of GCIY and MKN74. (A) GCIY-sh1 cells formed fewer colonies than the respective control cells (p=0.001). (B) SEMA3E-overexpressing MKN74 cells formed larger numbers of colonies than MKN74-EV in anchorage-independent cell growth (p=0.007).
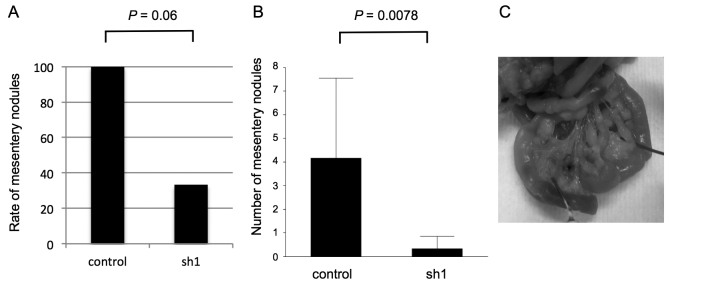
The effects of SEMA3E expression on gastric cancer growth and peritoneal dissemination in vivo. We examined the functional role of SEMA3E in gastric cancer development by subcutaneous injection assay of NOG mice. The tumor growth in NOG mice was significantly impaired in SEMA3E knockdown GCIY cells (sh1) (p<0.001) (Fig. 5). Next, we examined the metastatic capacity of SEMA3E knockdown gastric cancer cells by intraperitoneal administration assay. GCIY-sh1 cells macroscopically metastasized more slowly (Fig. 5A) and in smaller numbers (Fig. 5B) of mesentery nodules in the peritoneal cavity compared to control cells (p=0.06, 0.008) (Fig. 6).
Figure 5.
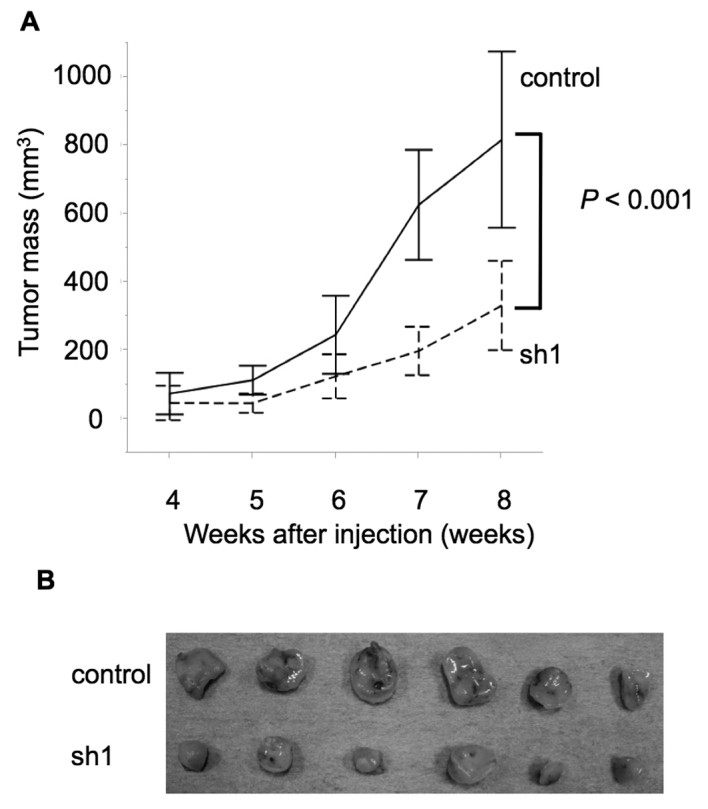
Tumor mass after injection into subcutaneous of NOG mice. (A) The volume of the subcutaneous tumors was measured once a week for 8 weeks. The tumor growth in NOG mice was significantly impaired in SEMA3E knockdown GCIY cells (sh1) 8 weeks after subcutaneous injection (p<0.001). (B) Dissected tumor tissues from NOG mice. The tumors derived from SEMA3E knockdown cells are smaller than than those from the control cells.
Figure 6.
Nodules in the peritoneal cavity after injection into peritoneum of NOG mice. We measured the number of nodules in the peritoneal cavity macroscopically 5.5 weeks after peritoneum injection. GCIY-sh1 cells macroscopically metastasized more slowly (A) and in smaller numbers (B) of mesentery nodules compared to control cells (p=0.06, 0.008). (C) The mesentery nodules in the peritoneal cavity could be clearly recognized macroscopically.
Discussion
We investigated the involvement of SEMA3E-PLXND1 signaling in gastric cancer development and clearly revealed that: i) the expression level of SEMA3E but not PLXND1 was significantly associated with lymph node involvement and metastatic progression; ii) the high level expression of SEMA3E was associated with poor differentiation and poor survival in the intestinal type of gastric cancer; iii) SEMA3E could activate Erk signalling even when the PLXND1 expression level was very low in gastric cancer; iv) SEMA3E knockdown in gastric cancer cells attenuated cell proliferation and metastatic ability in vitro and in vivo; v) SEMA3E enhanced cell proliferation and the anchorage-independent cell growth in the intestinal type of gastric cancer. These results suggest that SEMA3E plays a pivotal role in gastric cancer progression and metastasis.
In several types of human cancers such as colon cancer, melanoma and breast cancer, elevated expression levels of SEMA3E correlate with increased malignancy (11,13). Moreover, SEMA3E is associated with poor patient survival in pancreatic cancer (14). Therefore, our present findings are consistent with previous studies indicating that the expression of SEMA3E is involved in the enhanced aggressiveness of various types of carcinoma. In this study, the high-SEMA3E group of intestinal type gastric cancer patients showed significantly shorter survival than the low-SEMA3E group. In addition, forced expression of SEMA3E in intestinal gastric cancer cells enhanced both the anchorage-dependent and -independent growth of these cells, suggesting that the over-expression of SEMA3E accelerated the aggressiveness of this type of gastric cancer cell via the induction of cellular growth. On the other hand, no relation between the SEMA3E expression level and the overall survival was found in the diffuse type of gastric cancer. However, knockdown of this molecule in this type of gastric cancer cells caused a reduction of the cellular proliferation in vitro and in vivo, and suppressed peritoneal disseminations. These findings indicate that SEMA3E is involved in the development of both types of gastric cancer.
In this study, no association was found between SEMA3E expression and gastric cancer cell migration. In ovarian, colon, lung and breast cancer and melanoma, SEMA3E expression promoted trans-endothelial migration (11,12,16). In particular the SEMA3E-induced activation of MAPK promoted epithelial-mesenchymal transition (EMT) and cancer cell migration in a human ovarian endometrioid carcinoma cell line (12). Although SEMA3E could not affect gastric cancer cell migration, SEMA3E expression enhanced the anchorage-dependent as well as -independent cell growth, subcutaneous tumor growth and peritoneal dissemination in NOG mice, suggesting that SEMA3E is involved in gastric cancer development through enhancing cell growth rather than cell migration.
Recently, it was reported that the expression level of SEMA3E was inversely associated with tumor progression in gastric cancer and that SEMA3E overexpression inhibited the migration and invasion of gastric cancer in vitro and in vivo (17). These results conflict with our current findings. We cannot explain the reason for this contradiction, but our results are not an artifact since enhanced expression of SEMA3E in both clinical samples and cultured cells was clearly associated with aggressive biological behavior. In addition to the cancer promoting effect, SEMA3E showed inhibition of the PDGF-mediated proliferation and migration of human smooth muscle cells (18), suggesting that this molecule has multifunctional effects that differ according to the cellular type. Semaphorins were shown to have both promoting and suppressive effects in gastric cancer development. For example, SEMA3A was shown to be significantly decreased as gastric cancer progressed and metastasized (19). In contrast, SEMA3C and SEMA5A were demonstrated to be involved in the progression of gastric cancer (20,21). Therefore, it is not surprising that SEMA3E has both promoting and suppressive effects on gastric cancer cells because cancer cells consist of heterogenetic cells. Accordingly, SEMA3E might give rise to distinct results since the clinical samples and cultured cells used in our studies and those by Chen et al (17) were quite different. Further study will be required to reveal the function of SEMA3E in gastric cancer.
In conclusion, enhanced SEMA3E expression in the intestinal type of gastric cancer was associated with a high rate of the diffuse type of gastric cancer and poor prognosis. SEMA3E knockdown in gastric cancer cells attenuated the cell proliferation and metastatic ability in vitro and in vivo, and SEMA3E promoted gastric cancer cell growth in the intestinal type of gastric cancer. These results indicate that SEMA3E is likely to be involved in the development of gastric cancer and might also be a therapeutic target in the treatment of gastric cancer as well as breast cancer.
Acknowledgements
We thank Professor Kitamura (The University of Tokyo, Tokyo, Japan) for providing Platinum-A cells. This study was supported by Grants-in-Aid for Scientific Research (KAKENHI)(15K19080) and (24591022) to M.Y. and K.S., respectively.
Abbreviations
- EMT
epithelial-to-mesenchymal transition
- ErbB2
epidermal growth factor receptor 2
- Erk
extracellular signal-regulated kinase
- NBI
narrow band imaging
- NOG
NOD/Shi-scid-IL2Rγnull
- qRT-PCR
quantitative real-time PCR
- PLXND1
plexin D1
- SEMA3E
semaphorin 3E
References
- 1.Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156–169. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Yagi K, Nozawa Y, Endou S, Nakamura A. Diagnosis of early gastric cancer by magnifying endoscopy with NBI from viewpoint of histological imaging: Mucosal patterning in terms of white zone visibility and its relationship to histology. Diagn Ther Endosc. 2012;2012:954809. doi: 10.1155/2012/954809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boku N. Past and present achievements, and future direction of the Gastrointestinal Oncology Study Group (GIOSG), a Division of Japan Clinical Oncology Group (JCOG) Jpn J Clin Oncol. 2011;41:1315–1321. doi: 10.1093/jjco/hyr129. [DOI] [PubMed] [Google Scholar]
- 5.Boku N. HER2-positive gastric cancer. Gastric Cancer. 2014;17:1–12. doi: 10.1007/s10120-013-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: Progress made and promises ahead. Trends Biochem Sci. 2008;33:161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Rehman M, Tamagnone L. Semaphorins in cancer: Biological mechanisms and therapeutic approaches. Semin Cell Dev Biol. 2013;24:179–189. doi: 10.1016/j.semcdb.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 9.Moriya J, Minamino T, Tateno K, Okada S, Uemura A, Shimizu I, Yokoyama M, Nojima A, Okada M, Koga H, et al. Inhibition of semaphorin as a novel strategy for therapeutic angiogenesis. Circ Res. 2010;106:391–398. doi: 10.1161/CIRCRESAHA.109.210815. [DOI] [PubMed] [Google Scholar]
- 10.Roodink I, Raats J, van der Zwaag B, Verrijp K, Kusters B, van Bokhoven H, Linkels M, de Waal RM, Leenders WP. Plexin D1 expression is induced on tumor vasculature and tumor cells: A novel target for diagnosis and therapy? Cancer Res. 2005;65:8317–8323. doi: 10.1158/0008-5472.CAN-04-4366. [DOI] [PubMed] [Google Scholar]
- 11.Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, et al. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest. 2010;120:2684–2698. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng CH, Murray KD, Jou MF, Hsu SM, Cheng HJ, Huang PH. Sema3E/plexin-D1 mediated epithelial-to-mesenchymal transition in ovarian endometrioid cancer. PLoS One. 2011;6:e19396. doi: 10.1371/journal.pone.0019396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luchino J, Hocine M, Amoureux MC, Gibert B, Bernet A, Royet A, Treilleux I, Lécine P, Borg JP, Mehlen P, et al. Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell. 2013;24:673–685. doi: 10.1016/j.ccr.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, et al. Australian Pancreatic Cancer Genome Initiative. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 16.Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, Guldberg P, Lukanidin E. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer Res. 2005;65:6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Xie GH, Wang WW, Yuan XL, Xing WM, Liu HJ, Chen J, Dou M, Shen LS. Epigenetically downregulated Semaphorin 3E contributes to gastric cancer. Oncotarget. 2015;6:20449–20465. doi: 10.18632/oncotarget.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Movassagh H, Shan L, Halayko AJ, Roth M, Tamm M, Chakir J, Gounni AS. Neuronal chemorepellent Semaphorin 3E inhibits human airway smooth muscle cell proliferation and migration. J Allergy Clin Immunol. 2014;133:560–567. doi: 10.1016/j.jaci.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Tang C, Gao X, Liu H, Jiang T, Zhai X. Decreased expression of SEMA3A is associated with poor prognosis in gastric carcinoma. Int J Clin Exp Pathol. 2014;7:4782–4794. [PMC free article] [PubMed] [Google Scholar]
- 20.Pan G, Zhu Z, Huang J, Yang C, Yang Y, Wang Y, Tuo X, Su G, Zhang X, Yang Z, et al. Semaphorin 5A promotes gastric cancer invasion/metastasis via urokinase-type plasminogen activator/phosphoinositide 3-kinase/protein kinase B. Dig Dis Sci. 2013;58:2197–2204. doi: 10.1007/s10620-013-2666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyato H, Tsuno NH, Kitayama J. Semaphorin 3C is involved in the progression of gastric cancer. Cancer Sci. 2012;103:1961–1966. doi: 10.1111/cas.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]