Summary
Despite the fact that vocal folds are subjected to extensive mechanical forces, the role of mechanical strain in vocal fold wound healing has been overlooked. Recent studies on other tissues have demonstrated that low physiological levels of mechanical forces are beneficial to injured tissues, reduce inflammation, and induce synthesis of matrix-associated proteins essential for enhanced wound healing. In this study, we speculated that mechanical strain of low magnitudes also attenuates the production of inflammatory mediators and alters the extracellular matrix synthesis to augment wound healing in cultured vocal fold fibroblasts. To test this hypothesis, fibroblasts from rabbit vocal folds were isolated and exposed to various magnitudes of cyclic tensile strain (CTS) in the presence or absence of interleukin-1β (IL-1β). Results suggest that IL-1β activates proinflammatory gene transcription in vocal fold fibroblasts. Furthermore, CTS abrogates the IL-1β–induced proinflammatory gene induction in a magnitude-dependent manner. In addition, CTS blocks IL-1β–mediated inhibition of collagen type I synthesis, and thereby upregulates collagen synthesis in the presence of IL-1β. These findings are the first to reveal the potential utility of low levels of mechanical signals in vocal fold wound healing, and support the emerging on vivo data suggesting beneficial effects of vocal exercise on acute phonotrauma.
Keywords: Vocal fold fibroblasts, Inflammation, Interleukin-1β, Dynamic mechanical strain
INTRODUCTION
The pathophysiology of many voice disorders is related to the inherent impact stress, or phonotrauma, associated with vocal fold oscillation.1,2 These impact stresses lead to a variety of benign vocal fold lesions that pose significant impediments to communication, and may lead to a compromised quality of life.3,4 In severe cases, medical/surgical intervention of such lesion(s) is necessary. As the current standard of care, patients typically undergo behavioral voice therapy to reduce the acute inflammatory response associated with injury and to allow for optimal assessment and categorization of the underlying pathologic condition. Traditionally, voice therapy for phonotrauma emphasizes voice conservation,5,6 wherein patients are encouraged to minimize the quantity and/or loudness of phonation.7–9 Biomechanically, this approach is sensible, because use of quiet-breathy phonation should minimize pathogenesis by limiting impact stress between the vocal folds.10–12 However, there is little data to support this approach for the management of voice disorders.13
More recent approaches have challenged the traditional therapy of voice conservation.14 Emerging evidence emphasizes, that in other tissues, cyclic tensile strain (CTS) of low/physiological magnitudes attenuates the inflammatory response and augments repair by inducing synthesis of extracellular matrix in vitro15 and in vivo.16 For example, interleukin-1β (IL-1β), a proinflammatory cytokine in secretions localized to the surface of injured vocal folds,17,18 induces synthesis of a plethora of proinflammatory mediators including inducible-nitric oxide synthase (iNOS), nitric oxide (NO), cyclooxygenase-2 (COX-2), prostaglandin-E2 (PGE2), and matrix metalloproteinases (MMPs) in mesenchymal cells including, cartilage, tendons, and osteoblast-like cells of the periodontal ligament. However, cyclic tensile strain at low/physiological magnitudes inhibits IL-1β–induced synthesis of all of the above mediators, significantly.15,19–22 This marked inhibition of proinflammatory mediators is paralleled by an increase in the expression of matrix proteins such as type I and type II collagen, and proteoglycans, that are inhibited by IL-1β.23 These findings represent a paradigm shift, challenging the practice of immobilization for the therapeutic management of injured tissue. Although immobilization and voice rest are not homologous, these findings, along with anecdotal clinical observations, are the stimuli for a line of research currently under way with the goal of developing novel treatment modalities for patients with acute phonotrauma. Recent clinical observations suggest that the use of large-amplitude, low-impact vocal fold oscillations associated with “resonant voice” may assist in the recovery of vocal fold inflammation that is associated with acute phonotrauma.24 The underlying cellular mechanisms for such observations in the vocal folds have not been elucidated. In this study, it was hypothesized that low levels of mechanical strain may be anti-inflammatory on vocal fold fibroblasts and counteract the actions of IL-1β by inhibiting the expression of iNOS, NO, COX-2, PGE2, and MMP-1. Furthermore, it was speculated that low levels of mechanical forces may support tissue repair by augmenting the synthesis of matrix-associated proteins.
The long-term goals of this study are to understand the molecular basis of the mechanical signaling in the repair of inflamed vocal folds. During phonation, vocal fold fibroblasts experience various types of mechanical forces including tensile, shear, pulsatile, and compressive forces. One of the major mechanical forces experienced by vocal folds during phonation is tensile force. Therefore, to initiate the unraveling of the mechanisms of action of mechanical forces, a well-established in vitro model system to apply dynamic strain to vocal fold fibroblasts monolayers was used. However, this bioreactor does not have the capacity to simulate the forces associated with vocal fold oscillation. The current investigation seeks, instead, to provide proof of principle data to confirm that vocal fold fibroblasts respond similarly to other cells of mesenchymal origin. It is hoped that affirmative responses to the experimental questions will stimulate further investigation into the role of more physiologically relevant forces in vocal fold healing. Specifically, we examined (i) the actions of the dynamic strain on IL-1β–induced proinflammatory gene induction in vocal fold fibroblasts, (ii) the time, magnitude, and frequency-dependent effects of dynamic mechanical signals on vocal fold fibroblasts, and (iii) the actions of dynamic strain on the production of collagen type I (alpha1) required for tissue repair and critical for the biomechanical properties of the vocal folds in the presence or absence of IL-1β.25,26 Understanding the actions of mechanical signals on vocal fold fibroblasts would facilitate the development of therapeutic strategies to enhance resolution of vocal fold inflammation and wound healing following injury, based on sound scientific evidence.
MATERIALS AND METHODS
Isolation and culture of vocal fold fibroblast
Rabbit vocal fold fibroblasts (RVFF) were obtained from healthy 10–12 weeks old female New Zealand white rabbits (Harlan, Indianapolis, IN). All protocols were approved by the Institutional Animal Care and Use Committee at the Ohio State University. The posterior margin of the larynx was bisected between the arytenoids, the lamina propria gently peeled, minced into small pieces, and treated with 0.3% collagenase for 1 hour at 37°C. The cells released were centrifuged at 800 rpm for 10 minutes, washed, and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Grand Island, NY), 10% characterized fetal bovine serum (FBS) (Hyclone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine in a 37°C incubator with 5% CO2 and 95% humidity. Upon reaching 70–80% confluence, the cells were trypsinized and expanded. All cells used in the current investigation were first four passages.
Exposure of RVFF to cyclic tensile strain (CTS)
To expose RVFF to dynamic strain, cells were plated on collagen type I-coated Bioflex II plates (Flexcell International, Hillsborough, NC) at a density of 6 × 104 cells/well. The cells reached 80% confluence within 4–5 days. Subsequently, cells were washed with phosphate-buffered saline and incubated overnight in DMEM without FBS. The Bioflex II plates were placed on the Flexcell loading station equipped with a computer-assisted cyclic vacuum controller. This method of subjecting cells to uniform equibiaxial strain provided a nearly linear relationship between vacuum level and strain. The radial and circumferential strains exerted on the membrane were calculated as follows: circumferential strain=2π(change in radius)/2π(original radius)=change in radius/original radius=radial strain.
Preliminary experiments involved a cursory description of the inflammatory phenotype in vocal fold fibroblasts. Briefly, iNOS expression was assessed as a function of various concentrations of the IL-1β (FCRC, Frederick, MD) ranging from 0.1 to 10.0 ng/ml. Once complete, the optimal dosage established in the preliminary experiments (1.0 ng/ml) was used in all subsequent experiments. The experimental paradigm for the current investigation is as follows. RVFF were exposed to four treatment regimens: (i) untreated and unstressed control cells, (ii) cells treated with 1.0 ng/ml IL-1β, (iii) cells treated with CTS, and (iv) cells treated with both CTS and IL-1β. RVFF exhibited minimal cell deformation, cell detachment, or cell death following exposure to CTS at all the time points tested. Trypan blue exclusion assays confirmed greater than 99% cell viability in all treatment conditions.
Reverse transcriptase-polymerase chain reaction
Following treatment, RVFF were subjected to RNA extraction using Qiagen RNA extraction kit (Qiagen Inc., Santa Clara, CA) according to the manufacturer’s recommended protocols.22 Following extraction, the concentration of RNA was adjusted to 1 μg RNA/10 μl water and denatured for 15 minutes (65°C) in an Eppendorf Mastercycler. RNA was reverse transcribed in Reverse Transcriptase (RT) Mix containing dithiothreitol (10 mM) 2.5 μl; first strand buffer 5 μl; 25 mM MgCl2 3 μl; 2.5 mM deoxynucleoside-triphospate 7 μl; RNase inhibitor 1 μl; Moloney Murine Leukemia Virus 1 μl; and oligo (dT) 0.5 μl. Twenty microliters of RT Mix was added to 1 μg RNA, incubated at room temperature for 10 minutes, and placed in the thermalcycler to synthesize cDNA (25 minutes at 42°C, 5 minutes at 65°C). Subsequently, 21 μl of polymerase chain reaction (PCR) Supermix containing 2 μl of both sense and antisense primers was added to 2 μl of cDNA (Table 1). The samples were placed back in the thermalcycler, and the PCR was run for 35 cycles (2 minutes at 94°C, 45 seconds at 94°C, 1 minute at 72°C, and 10 minutes at 72°C), and held at 4°C upon completion. The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used to equalize all samples. The PCR products were separated on a 2% agarose gel and analyzed semiquantitatively by densitometry using ImageJ Software (National Institutes of Health, Bethesda, MD).
TABLE 1.
Abbreviations
| RVFF | Rabbit vocal fold fibroblasts |
| CTS | Cyclic tensile strain |
| IL-1β | Interleukin-1beta |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclo-oxygenase-2 |
| PGE-2 | Prostaglandin E2 |
| MMP | Matrix metalloproteinase |
| PCR | Polymerase chain reaction |
| NO | Nitric oxide |
Real-time PCR
Gene-specific primer sequences were selected using the Taqman Probe and Primer Design function of the Primer Express v1.5 software (Applied Biosystems, Foster City, CA). The sense and antisense sequences of primers used were as follows: iNOS sense 5′-TTCTGTGCTAATGCGGAAGGT-3′, antisense 5′-GCTTCCGACTTTCCTGTCTcA-3′, probe 6-FAMd (CCGCGTCAGAG-CCACAGTCCT) BHQ-1(D44591); COX-2 sense 5′-CTTTGGCAGGCTGGATTTTAA-3′, antisense 5′-AGAAGCCCACTGATACCTTT TGC-3′, probe 6-FAMd (TGCACAG-TATGACACAACAGCCCATCTCTC) BHQ-1; and GAPDH sense 5′-CTCAACTACATGGTCTACATGTTCCA-3′, antisense 5′-CTTCC CATTCTCAGCCTTGACT-3′, probe HEXd(ACCCACGGCAAGTTCAACGGCA) BHQ-1. Reverse transcription reactions were carried out using 2 μg RNA and Taq-Man Reverse Transcription reagents, followed by real-time PCR using Taqman PCR Master Mix and ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). Reactions were performed as follows. Cycle I (1×): 95°C for 3 minutes, Cycle II (50×): Step 1 at 95°C for 0.3 minutes, followed by Step 2 at 55°C for 0.3 minutes, and Step 3 at 72°C for 30 minutes, Cycle III at 40°C hold. Following amplification, a melting curve was obtained to ensure that primer-dimers or nonspecific products had been eliminated or minimized. The data, obtained by real-time PCR, were analyzed by the comparative threshold cycle (CT) method. In this method, the amount of the target, normalized to GAPDH, and relative to a calibrator (either untreated sample or IL-1β-stimulated cells), is given by 2ΔΔCT, where ΔΔCT=ΔCT (sample)–ΔCT (calibrator), and ΔCT is the CT of the target gene subtracted from the CT of GAPDH.
Analysis of cellular proteins
Western blot analysis was used for semiquantitative measurements of protein synthesis as described previously.27 Briefly, cells were lysed in ice cold Tris-buffered saline containing protease inhibitor cocktail (Roche, IN), and the extracted proteins were loaded on the sodium dodecyl sulfate-10% acrylamide gels. The proteins were electrophoretically transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) and identified by goat anticollagen type I-alpha1 IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Monoclonal mouse anti-β-actin IgG (1:20,000; Abcam, Cambridge, MA) was used to reprobe the same blots to equilibrate protein input in all lanes. Horseradish peroxidase (HRP)-labeled rabbit antigoat IgG (1:10,000 dilution; Chemicon, Temecula, CA) or HRP-labeled goat antimouse antibody (1:10,000 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used as a second antibody. The presence of HRP was detected by Luminol (Amersham, Arlington Heights, IL), and the semiquantitative analysis of luminescent bands was carried out with Kodak Image Station 1000 and Kodak 1D image analysis software.
Analysis of PGE2 and NO in culture supernatants of RVFF
Culture supernatants collected from all wells following a 24-hour treatment were analyzed by enzyme-linked immunosorbant assay (ELISA) for the presence of PGE2 (R&D, Minneapolis, MN) using the manufacturer’s recommended protocol. In addition, Griess reaction was used to determine the presence of NO secreted by vocal fold fibroblasts.28 The cells from each well were trypsinized and counted in triplicates by trypan blue exclusion test. All ELISA and NO data were standardized to total number of cells in culture.
Statistical analysis
All experiments were performed in triplicate. The results are presented either as digitized assessment of each band following densitometric analysis or as mean and standard error of the mean from triplicate values of real-time PCR analysis. Results from ELISA are presented as means and standard errors of means of triplicate values. The SPSS 13.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis. Each experiment was performed at least two times. For quantitative analysis, means and the standard errors of the means were calculated. To determine whether significant differences exist between groups, one-way analysis of variance (ANOVA) and the post hoc multiple comparison Tukey test were applied. To identify differences between IL-1β-treated cells in the absence or presence of CTS at various time points, one-way ANOVA and the post hoc multiple comparison Dunnett’s test were used. Differences were regarded as statistically significant at values of P<0.05.
RESULTS
IL-1β upregulates iNOS mRNA expression in RVFF
IL-1β, a proinflammatory cytokine, is shown to be increased in secretions localized to vocal fold injury.17,18,29 Therefore, the actions of IL-1β on RVFF were examined. iNOS is one of the major proinflammatory mediators induced by IL-1β in many types of cells, therefore its mRNA expression was used as a measure of inflammation in response to IL-1β actions. Examination of the effect of various concentrations of IL-1β on RVFF revealed that 1.0 ng/ml of IL-1β optimally stimulated iNOS expression and higher concentrations of IL-1β did not further increase iNOS expression (data not shown). Therefore, in all subsequent experiments, 1 ng/ml of IL-1β was used.
Dynamic strain suppresses IL-1β–induced iNOS expression in RVFF
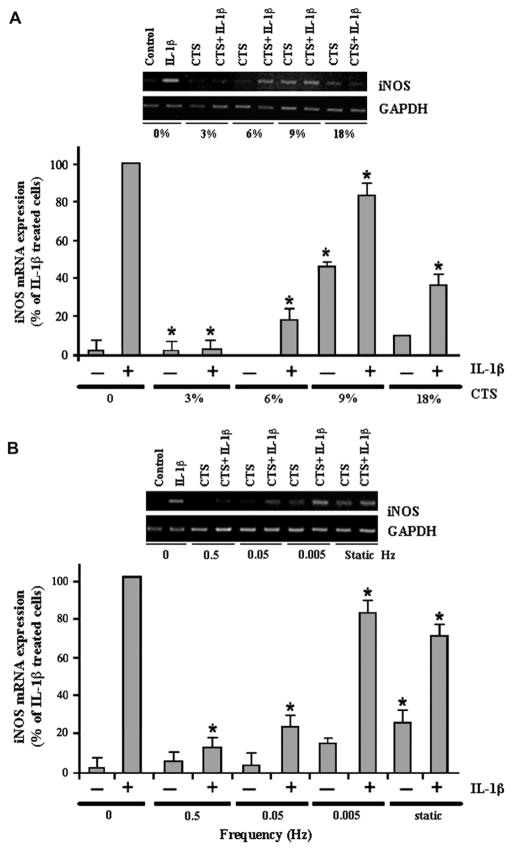
To examine the effects of various magnitudes of CTS on the proinflammatory responses of RVFF, RVFF were exposed to CTS of magnitudes between 0% and 18%. As shown in Figure 1, IL-1β–induced iNOS gene expression was significantly attenuated by CTS of 3–18% magnitudes within 4 hours. Nevertheless, CTS optimally blocked iNOS mRNA expression at magnitudes of 3% and 6%. Interestingly, although higher magnitudes of CTS (9% and 18%) inhibited iNOS mRNA expression, their effects were less pronounced. CTS alone was not proinflammatory at magnitudes of 3% or 6%. However, exposure of RVFF to increasing magnitudes of CTS (9% or 18%) resulted in iNOS mRNA expression.
FIGURE 1.
Effect of CTS of various magnitudes and frequencies on RVFF. (A) Effects of CTS of various magnitudes on iNOS mRNA expression in RVFF. RVFF grown on Bioflex II plates were subjected to 3%, 6%, 9%, or 18% CTS for 4 hours, in the presence or absence of IL-1β. Subsequently, RNA was extracted and analyzed for iNOS mRNA expression by real-time PCR and end point PCR (inset gel). (B) RVFF grown on Bioflex II plates were subjected to 6% CTS at frequencies of 0, 0.5, 0.05, 0.005 Hz, or static forces for 4 hours, in the presence or absence of IL-1β. Subsequently, RNA was extracted and analyzed for iNOS mRNA expression by real-time PCR and end point PCR (inset gel). Results represent mean and standard error of the mean (SEM) of triplicate values from one out of two separate experiments.
Because 6% CTS optimally inhibited IL-1β–induced iNOS mRNA expression, we examined the effects of CTS of various frequencies at a magnitude of 6%. The data revealed that the responses of RVFF to IL-1β are also regulated by the frequency of CTS. As shown in Figure 1B, a 4-hour exposure of RVFF to CTS of frequencies between 0.5 and 0.05 Hz inhibited IL-1β–dependent iNOS mRNA expression. However, exposure of RVFF to CTS of lower frequencies (0.005 Hz) for 4 hours, failed to inhibit IL-1β–induced iNOS mRNA expression. Similar to low frequencies, static CTS also was not effective in suppressing IL-1β–induced iNOS expression. It is also noteworthy that, exposure of RVFF to CTS at 0.5 and 0.05 Hz alone, did not induce iNOS expression. However, CTS at 0.005 Hz and static loading alone were proinflammatory and induced a significant upregulation of iNOS expression as compared to control cells (P<0.05).
Dynamic strain suppresses IL-1β–induced MMP-1, COX-2 gene induction
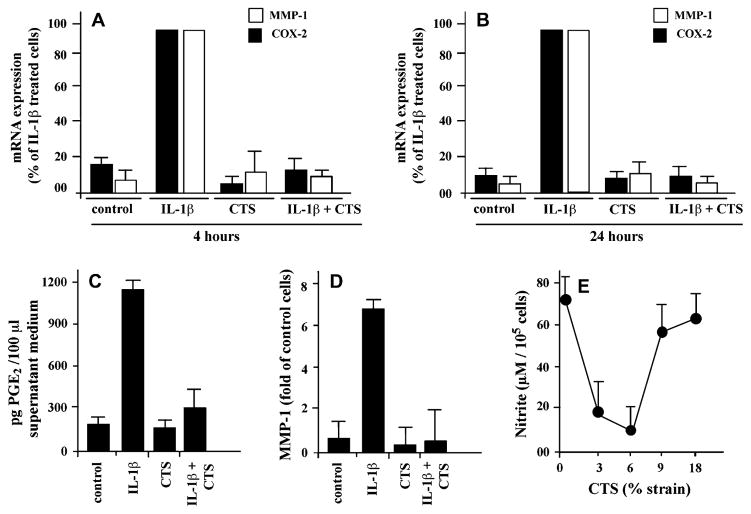
Next, the effects of CTS on other proinflammatory genes involved in vocal fold damage were examined. According to the results obtained above, CTS at a magnitude of 6% and a frequency of 0.5 Hz were used in the next experiments. As shown in Figure 2A, CTS significantly blocked IL-1β-induced MMP-1 and COX-2 mRNA expression over a period of 4 hours. Furthermore, these effects of CTS were sustained for the ensuing 20 hours, as evident from the suppression of both COX-2 and MMP-1 mRNA expression in Figure 2B. CTS alone in these experiments had no effect on either COX-2 or MMP-1 expression, suggesting that CTS at low magnitudes is not proinflammatory, but inhibits IL-1β–induced proinflammatory gene transcription.
FIGURE 2.
Effect of CTS on the MMP-1, COX-2, and NO induction in RVFF. RVFF grown on Bioflex II plates were exposed to CTS at a magnitude of 6% and 0.5 Hz for 4 hours or (A) 24 hours, in the presence or absence of IL-1β. (B) Subsequently, mRNA expression for MMP-1 and COX-2 was determined by real-time PCR. (C) PGE2 production by RVFF following exposure to 6% CTS at 0.5 Hz for 24 hours, in the presence or absence of IL-1β. The accumulation of PGE2 was assessed in the culture supernatants by ELISA. (D) MMP-1 synthesis by RVFF following exposure to 6% CTS at 0.5 Hz for 24 hours, in the presence or absence of IL-1β. The synthesis of MMP-1 was assessed by Western blot analysis, followed by densitometric analysis of each band. (E) NO synthesis in RVFF exposed to 6% CTS at 0.5 Hz for 36 hours, in the presence or absence of IL-1β. NO accumulation was measured in the culture supernatants and analyzed by Griess reaction. Results represent mean and SEM of triplicate values from one out of two separate experiments in A and B, and of duplicate values from one out of two separate experiments in C–E.
CTS blocks IL-1β–induced COX-2, iNOS, and MMP-1 synthesis
The CTS-mediated inhibition of COX-2 and MMP-1 synthesis was determined following continuous exposure of cells to CTS for 24 hours. As shown in Figure 2C, CTS blocked IL-1β–induced PGE2 accumulation in the culture supernatants as assessed by ELISA. Similarly, semiquantitative analysis of protein extracts from cells by Western blot analysis revealed that CTS also blocked MMP-1 synthesis that was upregulated by IL-1β (Figure 2D). Determination of the NO accumulation in the culture supernatants of cells exposed to various magnitudes of CTS for 36 hours revealed that CTS at magnitudes of 3% and 6% inhibited NO synthesis. However, CTS at magnitudes of 9% and 18% inhibited NO production to a lesser extent (Figure 2E). Furthermore as observed, treatment of cells with CTS alone did not affect the synthesis of PGE2, NO, or MMP-1.
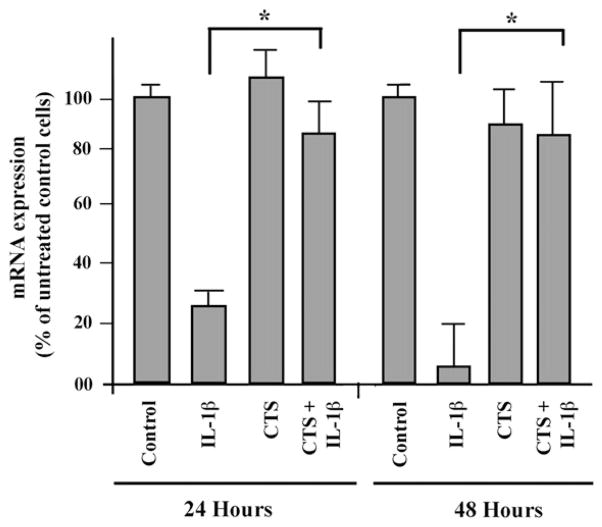
CTS abrogates IL-1β–induced inhibition of collagen I synthesis
In addition to proinflammatory gene induction, IL-1β has been shown to be associated with the inhibition of matrix protein synthesis, thereby augmenting tissue damage. The effects of CTS and IL-1β on matrix synthesis were examined. Western blot analysis showed that IL-1β inhibited the synthesis of collagen type I in RVFF (Figure 3) when examined after 24 or 48 hours. However, RVFF exposed to CTS in the presence of IL-1β, the levels of collagen type I were significantly upregulated as compared to cells treated with IL-1β alone. In these experiments, RVFF were subject to continuous exposure of CTS for 24 or 48 hours, and therefore, these results suggest that the effects of CTS were sustained.
FIGURE 3.
Effect of CTS on the procollagen type I induction. RVFF were exposed to 6% CTS at 0.5 Hz for 24 or 48 hours. The expression of procollagen type I mRNA was examined by end point PCR followed by densitometric analysis of the PCR products. The bars represent mean and SEM of triplicate values from one out of two separate experiments.
DISCUSSION
The primary question addressed in the current study was whether biomechanical signals alter the inflammatory responses in vocal fold fibroblasts as previously described in numerous other cell types. To answer this question, an in vitro system was used to delineate the mechanisms of actions of biomechanical signals on RVFF. The current data suggest that the inflammatory phenotype of vocal fold fibroblasts can be attenuated by mechanical signals. Furthermore, the responses of RVFF to biomechanical signals are magnitude dependent, ie, biomechanical signals of low magnitudes suppress IL-1β–induced proinflammatory gene induction, whereas, at higher magnitudes these signals have limited ability to inhibit IL-1β–induced proinflammatory gene induction. Interestingly, biomechanical signals applied in the absence of IL-1β are not proinflammatory at lower magnitudes, but induce iNOS mRNA expression at 9–18% tensile strain. These findings suggest that biomechanical signals at appropriate magnitudes may be able to attenuate inflammation induced by proinflammatory cytokines found in secretions localized to vocal fold injury.17,18,29 These findings are similar to findings from other cell types, where biomechanical signals are shown to inhibit proinflammatory responses of various cells of mesenchymal origin. Therefore, it is not surprising that RVFF also respond to biomechanical signals and exhibit proinflammatory or anti-inflammatory responses dependent upon the magnitude.
It is well known that vocal folds are exposed to various frequencies of biomechanical signals during phonation. Obviously, the bioreactor used in the current investigation does not produce physiologically relevant forces making any definitive statements about the role of mechanical forces in vocal fold healing premature. To address this issue, Titze et al30 recently described the development of a novel bioreactor approximating phonatory forces. However, this technology is not readily available at this time. With that limitation in mind, it appears that vocal fold fibroblasts respond similarly to other mesenchymal cells with regard to biomechanical signaling. These forces tend to be anti-inflammatory and inhibit IL-1β–induced iNOS mRNA synthesis. Although not physiologically relevant, exposure of RVFF to lower frequencies does not inhibit proinflammatory gene induction, rather these signals become proinflammatory even in the absence of IL-1β. These data suggest that these cells may respond in a dose-dependent manner planting the seed for further investigation on both the cellular level as well as in vivo.
To elucidate the effects of biomechanical signals on RVFF, three major mediators known to take part in inflammation were selected: iNOS, COX-2, and MMP-1. The current observations suggest that all of these mediators are produced by RVFF when exposed to IL-1β. These data are the first to characterize the inflammatory phenotype in vocal fold fibroblasts. More importantly, biomechanical signals of low magnitudes inhibit mRNA expression and synthesis of all of these mediators, by inhibiting their gene transcription. NO and PGE2 are known to amplify inflammatory responses and perpetuate inflammation. Furthermore, MMP-1 is associated with proteolysis of matrix proteins and thus tissue damage. Biomechanical signals, through the inhibition of these mediators, may not only suppress the immune response but also block MMP-1-mediated tissue damage. On the contrary, these signals induce matrix synthesis, by upregulating collagen type I. Collectively, by inhibiting inflammation and inducing matrix synthesis, biomechanical signals may play a potent role in the repair and regeneration of vocal folds.
Although the field of voice biology is considered to be in its infancy, the literature is rapidly growing due to an inherent interest in vocal fold wound healing and the potential for development of novel therapeutics to treat patients with dysphonia. Interestingly, most interventions described in the literature have attempted to augment the synthetic phenotype of vocal fold fibroblasts in an attempt to restore vocal fold mucosa following injury. This approach, although providing valuable insight into the synthetic mechanism of extracellular matrix repair following injury, ignores the inflammatory phase of healing. Our findings suggest that biomechanical signals of appropriate magnitudes have the capacity to curb inflammation, in addition to augmenting the synthetic phenotype of vocal fold fibroblasts. Because the immediate response to injury is thought to orchestrate later events in the wound-healing cascade and determine the ultimate outcome of wound healing, the theoretical justification for this type of early intervention following phonotrauma warrants further investigation. In fact, further validation of this finding comes from the investigation on fetal wound healing, where regenerative or scarless wounds exhibit an absence or decreased inflammatory response to injury.31
Although we have consistently observed that low levels of mechanical strain limit the inflammatory responses in vocal fold fibroblasts, it would be premature to make a definitive statement on the clinical implications of these findings. However, these data correspond well with emerging data from our laboratory suggesting that particular types of vocal exercise may limit inflammation in vivo.24 In short, patients present with improved inflammatory profiles when they perform resonant voice exercises as opposed to conversational speech or voice rest following vocal loading. Our data may provide the physiological basis of these observed effects of vocal exercise on acute inflammation of the vocal folds. Clearly, further investigation is warranted to translate the magnitudes used in the in vitro studies to those magnitudes that vocal fold fibroblasts are exposed to during voice exercises.
In summary, the results of the current investigation provide interesting insight into the mechanisms of actions of biomechanical signals on the proinflammatory and reparative responses of RVFF. Whether human vocal fold fibroblasts respond to biomechanical signals in a similar manner is yet to be elucidated. Nevertheless, the finding that inflammatory responses of RVFF are dramatically reduced in response to mechanical strain is novel and clinically relevant. These signals also upregulate matrix synthesis and thus may be critical in initiating wound repair following an injury to the vocal folds. Clearly, much work is needed to fully appreciate the potential of biomechanical signals in the repair of vocal folds.
Acknowledgments
The current project was funded in part by the National Institute of Health (F31 DC06764-01, DC5643, AT00646) and by the School of Health and Rehabilitation Sciences Research Development Fund.
The authors wish to acknowledge Dr. Vlad Sandulache, Asha John, and Dr. James Deschner for their advice and assistance.
References
- 1.Gunter HE. Modeling mechanical stresses as a factor in the etiology of benign vocal fold lesions. J Biomech. 2004;37:1119–1124. doi: 10.1016/j.jbiomech.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Gray SD. Benign pathologic responses of the larynx. NCVS Status Prog Rep. 1997;11:135–148. [Google Scholar]
- 3.Krischke S, Weigelt S, Hoppe U, Kollner V, Klotz M, Eysholdt U, Rosanowski F. Quality of life in dysphonic patients. J Voice. 2005;19:132–137. doi: 10.1016/j.jvoice.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Rasch T, Gunther S, Hoppe U, Eysholdt U, Rosanowski F. Voice-related quality of life in organic and functional voice disorders. Logoped Phoniatr Vocol. 2005;30:9–13. doi: 10.1080/14015430510006640. [DOI] [PubMed] [Google Scholar]
- 5.Colton R, Casper JK. Understanding Voice Problems: A Physiological Perspective for Diagnosis and Treatment. 2. Baltimore, MD: Williams & Wilkins; 1996. [Google Scholar]
- 6.Boone DR, McFarlane SC. The Voice and Voice Therapy. 5. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1994. [Google Scholar]
- 7.Froeschels E. Hygiene of the voice. Arch Otolaryngol. 1943;38:122–130. [Google Scholar]
- 8.Roy N, Weinrich B, Gray SD, et al. Voice amplification versus vocal hygiene instruction for teachers with voice disorders: a treatment outcomes study. J Speech Lang Hear Res. 2002;45:625–638. doi: 10.1044/1092-4388(2002/050). [DOI] [PubMed] [Google Scholar]
- 9.Roy N, Weinrich B, Gray SD, et al. An evaluation of the effects of two treatment approaches for teachers with voice disorders: a prospective randomized clinical trial. J Speech Lang Hear Res. 2001;44:286–296. doi: 10.1044/1092-4388(2001/023). [DOI] [PubMed] [Google Scholar]
- 10.Berry DA, Verdolini K, Montequin DW, Hess MM, Chan RW, Titze IR. A quantitative output-cost ratio in voice production. J Speech Lang Hear Res. 2001;44:29–37. doi: 10.1044/1092-4388(2001/003). [DOI] [PubMed] [Google Scholar]
- 11.Jiang JJ, Titze IR. Measurement of vocal fold intraglottal pressure and impact stress. J Voice. 1994;8:132–144. doi: 10.1016/s0892-1997(05)80305-4. [DOI] [PubMed] [Google Scholar]
- 12.Titze IR. Mechanical stress in phonation. J Voice. 1994;8:99–105. doi: 10.1016/s0892-1997(05)80302-9. [DOI] [PubMed] [Google Scholar]
- 13.Verdolini-Marston K, Burke MK, Lessac A, Glaze L, Caldwell E. Preliminary study of two methods of treatment for laryngeal nodules. J Voice. 1995;9:74–85. doi: 10.1016/s0892-1997(05)80225-5. [DOI] [PubMed] [Google Scholar]
- 14.Verdolini K. Resonant voice therapy. In: Stemple JC, editor. Voice Therapy: Clinical Studies. San Deigo, CA: Singular Publishing Group; 2000. pp. 46–62. [Google Scholar]
- 15.Deschner J, Hofman CR, Piesco NP, Agarwal S. Signal transduction by mechanical strain in chondrocytes. Curr Opin Clin Nutr Metab Care. 2003;6:289–293. doi: 10.1097/01.mco.0000068964.34812.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferretti M, Srinivasan A, Deschner J, et al. Anti-inflammatory effects of continuous passive motion on meniscal fibrocartilage. J Orthop Res. 2005;23:1165–1171. doi: 10.1016/j.orthres.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branski RC, Rosen CA, Verdolini K, Hebda PA. Cytokine analysis of acute wound healing in the larynx: a rabbit model. J Voice. 2005;19:283–289. doi: 10.1016/j.jvoice.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Verdolini K, Rosen CA, Branski RC, Hebda PA. Shifts in biochemical markers associated with wound healing in laryngeal secretions following phonotrauma: a preliminary study. Ann Otol Rhinol Laryngol. 2003;112:1021–1025. doi: 10.1177/000348940311201205. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal S. Low magnitude of tensile strain inhibits IL-1beta dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J Dent Res. 2001;80:1416–1420. doi: 10.1177/00220345010800050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal S, Long P, Gassner R, Piesco NP, Buckley NJ. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44:608–617. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gassner R, Buckley MJ, Georgescu H, et al. Cyclic tensile stress exerts anti-inflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163:2187–2192. [PMC free article] [PubMed] [Google Scholar]
- 22.Long P, Liu F, Piesco NP, Kapur R, Agarwal S. Signalling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone. 2002;30:547–552. doi: 10.1016/s8756-3282(02)00673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal S, Long P, Seyedain A, Piesco N, Shree A, Gassner R. A central role for nuclear factor-KB pathway in the antiinflammatory and proinflammatory actions of mechanical strain. FASEB J. 2003;17:899–901. doi: 10.1096/fj.02-0901fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdolini K, Rosen CA, Urban E, Branski RC, Steinhauer K, Hebda PA. The effect of targeted vocal exercise on recovery from acute inflammation. Presented at: The Voice Foundation’s 34th Annual Symposium: Care of the Professional Voice; 2005; Philadelphia, PA. [Google Scholar]
- 25.Gray SD, Titze IR, Chan R, Hammond TH. Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope. 1999;109:845–854. doi: 10.1097/00005537-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 27.Deschner J, Rath-Deschner B, Agarwal S. Regulation of matrix metalloproteinase expression by dynamic tensile strain in rat fibrochondrocytes. Osteoarthritis Cartilage. 2006;14:264–272. doi: 10.1016/j.joca.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt HHW. Determination of nitric oxide via measurement of nitrite and nitrate in culture media. Biochemica. 1995;(2):22. [Google Scholar]
- 29.Branski RC, Rosen CA, Verdolini K, Hebda PA. Markers of wound healing in vocal fold secretions from patients with laryngeal pathology. Ann Otol Rhinol Laryngol. 2004;113:23–29. doi: 10.1177/000348940411300105. [DOI] [PubMed] [Google Scholar]
- 30.Titze IR, Hitchcock RW, Broadhead K, Web K, Li W, Gray SD, Tresco PA. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37:1521–1529. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Dohar JE, Klein EC, Betsch JL, Hebda PA. Fetal airway repair: a new frontier. Arch Otolaryngol Head Neck Surg. 1998;124:25–29. doi: 10.1001/archotol.124.1.25. [DOI] [PubMed] [Google Scholar]