Abstract
Aims
To assess the effect of a multi-component primary care (PC)-delivered BI for reducing risky drug use (RDU) among patients identified by screening.
Design
Multicenter single-blind two-arm randomized controlled trial of patients enrolled from February 2011 to November 2012 with 3-month follow-up. Randomization and allocation to trial group were computer-generated.
Setting
Primary care waiting rooms of 5 federally qualified health centers (FQHCs) in Los Angeles County (LAC), USA.
Participants
334 adult primary care patients (171 intervention; 163 control) with RDU scores (4–26) on the WHO Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) self-administered on tablet PCs; 261 (78%) completed follow-up. Mean age was 41.7 years; 63% were male; 38% were Caucasian.
Intervention(s) and Measurement
Intervention patients received brief (typically 3–4 minutes) clinician advice to quit/reduce their drug use reinforced by a video doctor message, health education booklet, and up to two 20–30 minute follow-up telephone drug use coaching sessions. Controls received usual care and cancer screening information. Primary outcome was patient self-reported use of highest scoring drug (HSD) at follow-up.
Findings
Intervention and control patients reported equivalent baseline HSD use; at follow-up, after adjustment for covariates in a linear regression model, intervention patients reported using their HSD an average of 2.21 fewer days in the previous month than controls (p<0.005). No compensatory increases in use of other measured substances were found (p>0.10).
Conclusions
A clinician-delivered brief intervention with follow-up counseling calls may decrease drug use among risky users compared with usual care in low-income community health centers of Los Angeles County, USA.
Keywords: brief intervention, primary care, motivational interviewing, risky drug use, randomized controlled trial, community health centers
INTRODUCTION
The need for efficient interventions to reduce RDU (moderate use), particularly in vulnerable racial/ethnic populations, has been highlighted.(1–6) Early detection and management of RDU (7) might effectively reduce drug use and its consequences.(8, 9) About 68 million people in the United States (US) are estimated to be RDUs who might benefit from early BI.(10) Integrating substance use treatment into PC settings by conducting screening and BI has been encouraged by policymakers.(3, 11–15)
Although there is strong support for PC BI reducing risky alcohol use, harm, morbidity, and costs, and support for BI for reducing other drugs in various settings,(16–25) the efficacy of PC BIs for reducing nonmedical use of other drugs is less conclusive.(15, 26–33) One trial of urgent care outpatients found that heroin and cocaine abstinence were higher in those receiving a BI.(34) A multi-national trial of PC patients reporting risky drug use found marginal effects overall but no effect at the US site.(35) Similarly, two recent PC trials of BIs plus a booster session or phone contact showed no effect.(36, 37)
Because of these conflicting findings, we conducted the Quit Using Drugs Intervention Trial (QUIT), which provides a BI protocol for reducing RDU in PC populations. We tested whether receiving the QUIT BI reduced self-reported past-month highest scoring illicit drug (HSD) use compared to a control group among patients identified by screening.
METHODS
QUIT was a multicenter single-blind (patients were blinded) two-arm randomized controlled trial (RCT) of a BI designed to reduce RDU among adult PC patients in five FQHCs in LAC. Median follow-up time was 3.4 months (mean 3.9, SD 1.7).
SETTING AND PARTICIPANTS
Participating Clinics
Clinic selection was based on robust patient encounter volumes and areas most affected by drug use; clinics included the largest FQHC in LAC. All 5 clinics approached agreed to participate.
Participating Clinic Primary Care Providers (PCPs)
Inclusion criteria for clinicians were: (1) staff providers trained in PC; and (2) amenable to following the research protocol and to participating in a clinician group intervention training session averaging 15 minutes and a 1–2 minute one-on-one reminder session before conducting the first intervention. Of the 80 PCPs approached, 77 (96.3%) participated, including physicians (n=57), nurse practitioners (n=9), and physician assistants (n=11).
Participating Clinic Patients
Inclusion criteria included RDU in the prior 3 months; 18 or older; spoke English or Spanish; had a PC appointment; anticipated living in the LAC area for the next 3 months; and had an active phone number. Exclusion criteria included previously screened, under drug treatment starting more than 30 days ago or pregnant. Enrollment during the first 7 months was limited to patients with risky use of stimulants (cocaine or amphetamines) (enrolled 64 patients, 9/month); to facilitate recruitment, during the final 13-month period we included risky users of all illicit drug categories (enrolled an additional 270 patients, 21/month).
Procedure
Research assistants conducted enrollment. They greeted all adult patients in PC waiting rooms before their clinician appointment. To enhance portability and minimize staffing effort, patients self-administered all questionnaires on touch-screen “talking tablet PCs” tailored to the unique needs of low-literacy immigrant populations.(38)
The ASSIST identified RDU patients as “at moderate risk of health and other problems because of their drug use”.(7, 39) The ASSIST asks about tobacco, alcohol, marijuana, crack/cocaine, methamphetamine/amphetamine type stimulants, inhalants, sedatives, hallucinogens, and opioids. Its ability to classify patients based on degree of illicit drug use has been validated, including the self-administered version.(7, 35, 40–42) Patients' use of each drug category (excluding alcohol and tobacco) was coded as: no or low use (score 0–3); risky (moderate) use indicating clinician brief advice (score 4–26); or high use (score 27 and above). Patients were paid $30 for the initial assessment (average 42 minutes) and $50 for the follow-up assessment (average 50 minutes); those completing all study activities were eligible for a $500 lottery. Informed consent was obtained -- orally for screening and in writing if they qualified for enrollment.
Consenting patients were given tablets to answer screening and study questions and if study eligible, were assigned equally to the intervention (n=171) or control group (n=163) by an automated computer-generated adaptive urn randomization program that blocked onASSIST scores of 4–16 versus 17–26.(43) A central server was used to store the study data and to evaluate and perform the randomization.(38) The research assistants knew the ASSIST scores and randomization group, but they would not share this information with the participants. The consent and screener included 8 chronic conditions, exercise, tobacco and alcohol use to mask the purpose of the study, naming it the “Living Well Study” to promote healthy lifestyles. The research protocol was approved by UCLA’s Institutional Review Board.
Table 1 shows baseline characteristics, as delineated in the Behavioral Model for Vulnerable Populations. (44–46)
Table 1.
Baseline Characteristics of QUIT Study Participants by Treatment Group
| Characteristic | Total | Control | Intervention | Pa |
|---|---|---|---|---|
| All subjects | 334 | 163 | 171 | |
| PREDISPOSING | ||||
| Sociodemographics | ||||
| Age, mean (SD) | 41.7 (12.7) | 40.8 (13.1) | 42.4 (12.3) | .250 |
| Education ≥ 12yrs, n (%) | 253 (83.8) | 124 (84.4) | 129 (83.2) | .790 |
| Male, n (%) | 210 (62.9) | 97 (59.5) | 113 (66.1) | .214 |
| Race/Ethnicity, n (%) | .616 | |||
| White | 126 (37.7) | 63 (38.7) | 63 (36.8) | |
| African American | 76 (22.8) | 34 (20.9) | 42 (24.6) | |
| Hispanic | 113 (33.8) | 56 (34.4) | 57 (33.3) | |
| Other | 19 (5.7) | 10 (6.1) | 9 (5.3) | |
| U.S. Born, n (%) | 286 (87.2) | 140(87.5) | 146 (86.9) | .870 |
| Marital status, n (%) | .074 | |||
| Married | 39 (11.7) | 11 (6.8) | 28 (16.4) | |
| Widowed | 11 (3.3) | 5 (3.1) | 6 (3.5) | |
| Separated | 24 (7.2) | 10 (6.2) | 14 (8.2) | |
| Divorced | 67 (20.2) | 34 (21.0) | 33 (19.3) | |
| Never married | 191 (57.5) | 101(62.7) | 90 (52.6) | |
| Parenting status (children < 18 yo), n (%) | 71 (21.3) | 35 (21.6) | 36 (21.1) | .902 |
| Homeless history, n (%) | ||||
| Homeless, lifetime | 203 (61.0) | 94 (58.0) | 109 (63.7) | .285 |
| Homeless, current | 86 (26.2) | 36 (22.4) | 50 (29.9) | .119 |
| Highest Scoring Drug (HSD) History and Beliefs | ||||
| Duration of HSD use (mean years, SD) | 20.4 (13.4) | 19.1 (13.6) | 21.6 (13.1) | .081 |
| Counseled by clinician about HSD ever, n (%) | 82 (28.5) | 41 (29.3) | 41 (27.7) | .623 |
| Perception has problem with HSD n (%) | .360 | |||
| Do not have drug problem | 193 (67.0) | 98 (70.0) | 95 (64.2) | |
| Probably have drug problem | 66 (22.9) | 27 (19.3) | 39 (26.4) | |
| Definitely have drug problem | 29 (10.1) | 15 (10.7) | 14 (9.5) | |
| Interest in reducing/stopping HSD, n (%) | .645 | |||
| Very | 125 (37.4) | 60 (36.8) | 65 (38.0) | |
| Somewhat | 100 (29.9) | 46 (28.2) | 54 (31.6) | |
| Not at all | 109 (32.6) | 57 (35.0) | 52 (30.4) | |
| Confidence about reducing HSD Use, n (%) | .024 | |||
| Not at all confident | 23 (6.9) | 8 (4.9) | 15 (8.8) | |
| Somewhat confident | 108 (32.3) | 44 (27.0) | 64 (37.4) | |
| Totally confident | 203 (60.8) | 111(68.1) | 92 (53.8) | |
| Readiness to change, Mean (SD) | ||||
| Precontemplation | -0.65 (3.6) | -0.50 (3.7) | -0.79 (3.5) | .376 |
| Contemplation | 0.28 (4.2) | 0.06 (4.2) | 0.49 (4.3) | .403 |
| Action | 1.65 (4.2) | 1.91 (4.4) | 1.40 (4.0) | .252 |
| ENABLING | ||||
| Income ≤ $500/month, n (%) | 193 (58.0) | 93 (57.4) | 100 (58.5) | .843 |
| Insurance, past 3 months, n (%) | 109 (32.7) | 53 (32.7) | 56 (32.8) | .995 |
| NEED | ||||
| Fair or poor general health, n (%) | 137 (41.1) | 70 (43.2) | 67 (39.2) | .455 |
| Perceived physical health, on SF-12 (Likert scale 1–6), mean (SD) | 43.0 (12.0) | 43.1 (12.0) | 43.0 (12.1) | .922 |
| Perceived mental health status on SF-12 (Likert scale 1–6), mean (SD) | 42.8 (12.4) | 42.9 (12.3) | 42.7 (12.6) | .855 |
| # Chronic medical conditions, mean (SD)b | 1.1 (1.1) | 1.1 (1.1) | 1.1 (1.1) | .645 |
| Baseline Tobacco Use Days past month, mean (SD) | 12.08 (13.4) | 12.50 (13.4) | 11.68 (13.4) | .577 |
| Baseline Alcohol Use Days per week, past month, mean (SD) | 2.83 (1.5) c | 2.91 (1.5) c | 2.74 (1.5) c | .304 |
| Baseline Any binge drinking day, past month, n (%)d | 199 (59.6) | 102 (62.6) | 97 (56.7) | .276 |
| Baseline HSD ASSIST Score, mean (SD)e | 14.5 (6.6) | 14.3 (6.5) | 14.6 (6.7) | .609 |
| HSD, n (%) | .109 | |||
| Cannabis | 173 (51.8) | 82 (50.3) | 91 (53.2) | |
| Cocaine/Crack | 67 (20.1) | 26 (16.0) | 41 (24.0) | |
| Amphetamines | 41 (12.3) | 21 (12.9) | 20 (11.7) | |
| Sedatives | 29 (8.7) | 18 (11.0) | 11 (6.4) | |
| Opiates | 22 (6.6) | 14 (8.6) | 8 (4.7) | |
| Other (inhalants, hallucinogens) | 2 (0.6) | 2 (1.2) | 0 (0) |
Based on chi-square, two-sample t, or two-sample Wilcoxon test
Number of 8 chronic medical conditions in lifetime: asthma, hepatitis, epilepsy, cancer, tuberculosis, HTN, diabetes, or HIV/AIDS
Corresponding to about 1–2 drinking days per week in the past month
Binge drinking day is defined as 5+ drinks for men <65 yo, 4+ men >= 65yo and all women
Baseline ASSIST Score for Highest Scoring Drug on the ASSIST
INTERVENTIONS
Intervention Group
At baseline, these patients received a face-to-face brief intervention during their PCP visit. They subsequently received a Drug Health Education Booklet with a Report Card for their HSD, and viewed a video (2 minutes) reinforcing the clinician message.(47–49)
A research assistant gave the clinician a one-page summary; which advised that their patient scored in the risky range on the HSD; it also included a list of other substances used in the risky range and a suggested counseling script. Clinicians followed a scripted protocol based on the patients’ HSD; two-thirds of the interventions lasted at most 3–4 minutes; only 3 (1.5%) required more than 10 minutes. The message covered drug addiction as a chronic brain disease;(50) the need to quit or reduce using drugs to prevent this disease; the physical and mental consequences of drug use; and the potential accelerated progression towards addiction caused by poly-substance use. If a patient scored in the risky range on multiple drugs, clinicians focused on the HSD, but also recommended reduction of the other drugs. If a patient scored in the risky range for a stimulant (methamphetamine, amphetamines, cocaine), clinicians focused on that stimulant even if it was not the HSD, since prior investigation found that stimulants were the most common serious drugs used illicitly by the patient population. We intentionally targeted low-income populations in safety net clinics because there is a high rate of problem drug use(51) in these communities and a lack of attention paid to their drug use.(11, 52) It is an important setting to test the efficacy of brief intervention in patients identified by screening because of the large volume of patients seen in safety net clinics. Clinicians also told patients that they would receive telephone calls 2 and 6 weeks later from a drug-health educator. Clinicians were not incentivized for delivering their brief advice.
The 2- and 6-week telephone drug-use coaching sessions (20–30 minutes each) reinforced the clinicians’ message, and followed a scripted patient-centered interview protocol. Lay health educators (HEs) were initially trained over 4 sessions, totaling 12 hours. Training was supervised by the investigators including an addiction psychologist and included motivational interviewing, cognitive behavioral techniques,(53) and rehearsing mock health education calls until proficiency was obtained. Trainees shadowed experienced HEs (i.e., listened in on actual calls with patient permission), and their initial calls were observed for supervision and feedback. Weekly meetings with the PIs and project director fostered a HE “learning community,” where every case was discussed to maintain fidelity to the protocol. All 171 intervention patients received clinician brief advice and 134 (78%) had at least 1 telephone session (93 (54%) two sessions, 41 (24%) one session). Study instruments and intervention materials are available from the authors.
Control Group
Control patients completed the ASSIST but did not receive clinician BI or coaching sessions; they did receive a video and information booklet on cancer screening. At study exit, control patients were given all intervention materials and, if they scored 4 or higher for any drugs on the 3-month ASSIST, with their permission the results were given to their doctor. Usual care at the study clinics did not include SBI for drug use.
URINE DRUG SCREEN
Urine drug testing was conducted at baseline and follow-up to validate self-reported drug use. One clinic would not allow urine collection, citing concerns about patient sensitivity and privacy. The Confirm BioSciences, San Diego, Integrated QuickScreen™ CLIA Urine Cup was used since it reliably tests for up to 12 drugs (96–100% sensitivity). Of the patients in assenting clinics, 180/197 (91%) provided specimens at baseline and 143/150 (95%) did so at follow-up. Underreporting was calculated for cocaine/crack, cannabis, opiates and amphetamines (other categories were excluded due to inexact matches between urine testing and self-reports). At baseline, the proportion of patients testing positive who did not disclose use of their HSD varied from 0% (0/2) for opiates to 2.7% (2/74) cannabis, 12.5% (1/8) amphetamines, and 17.7% (3/17) for cocaine/crack. At follow-up, underreporting ranged from 0% for cocaine/crack (0/17) and opiates (0/1) to 10.4% (5/48) cannabis, and 14.3% (1/7) for amphetamines. No group differences were found at baseline or follow-up for underreporting one or more drugs, although at follow-up control patients had a numerically higher rate of underreporting: baseline 5.9% (I:5.0%, C:7.3%), 3 months 8.2% (I:5.3%, C:11.4%).
MEASUREMENTS
Outcome
The outcome measure was number of days in the 30 days preceding the follow-up interview (Addiction Severity Index)(54–56) that patients reported using their HSD. A similar question was asked at baseline. The ASI is a standardized data collection tool that has excellent psychometric properties.(57–59) For this study, we employed only self-reported use of substances for the past 30 days, which is identical to accepted timeline follow-back methodology.(60, 61)
Covariates
The Behavioral Model for Vulnerable Populations (Figure 1) guided selection of variables used as covariates in analyses.(45) All of the baseline variables in this figure were potential covariates. Single items elicited information about socio-demographics, past month use of substances in the ASSIST, history of substance use and quitting attitudes, family substance use, chronic conditions, victimization, socio-legal problems, criminal and HIV/STD risk behaviours and health service utilization. Past month income was dichotomized at ≤$500/month. The ASSIST was used to categorize patient drug use. Perceived general, physical and mental health status were assessed by the SF-12.(62, 63) Readiness to change drug use was assessed by the validated scales.(64, 65) Baseline and follow-up questionnaires were identical except fixed information (e.g., gender) was collected at baseline only.
Figure 1.
Behavioral Model of QUIT Intervention for Risky Drug Use
ANALYSIS
Primary Analysis
The intervention and control groups were contrasted with respect to HSD use at follow-up using a linear regression model that included an indicator variable for the intervention group and time between the baseline and follow-up surveys and adjusted for the fixed effects of baseline measures including initial HSD use. Although the residual distribution is somewhat asymmetric, using an untransformed outcome has the advantage of interpretive simplicity and still yields unbiased estimates while the large sample ensures sufficient power for statistical tests. Both complete sample and intent-to-treat (completed sample) analyses were conducted. In a preliminary two-way analysis of variance using treatment group and clinic, the clinic main effect and the clinic-by-treatment group interaction were not significant; consequently, clinic was excluded from the primary regression models. We also used fixed effects and a random intercept model as sensitivity analyses to ensure that intra-clinic correlation does inflate the type 1 error. The study goal was to detect a low-moderate between group difference of two-fifths of a standard deviation in HSD use at follow-up at an alpha level of 0.05. A sample of 400 patients divided about equally between groups yielded over 90% power to detect this difference allowing for 80% attrition.(66)
For both complete and completed sample analyses, baseline variables in Figure 1 associated with follow-up HSD use at the 0.15 level were candidate covariates. The initial regression models included 18 variables satisfying this criterion. Parsimonious final models were obtained by manually removing covariates one at a time in descending order of p values until only those associated with follow-up HSD use at the 0.10 level remained and multicollinearity wasn’t a problem. For the completed sample analysis, missing values of 3-month HSD use were replaced with baseline HSD use (last observation carried forward--LOCF); multiple imputation (SAS 9.3 PROCs MI and MIANALYZE) was used to estimate missing values for predictors. The multiple imputation model included all the initial covariates, plus variables in Figure 1 that were associated with loss-to-follow-up at the 0.15 level. Twenty sets of imputed values were produced.
Subgroup Analyses
To test whether intervention efficacy varied with baseline HSD use, an interaction between intervention group and baseline HSD use was added to the main effects models. It was significant at p<0.01, so baseline HSD use was dichotomized at the median of 5 days and stratified analyses were performed. The effects of the intervention in key demographic subgroups were also assessed by examining the fixed effects of these characteristics and their interactions with treatment group. The effect of different types of HSD was also investigated and completion of telephone sessions was assessed in a regression analysis controlling baseline HSD use.
Secondary Outcomes
Since reduction in HSD use could be accompanied by increased use of non-HSD substances, non-HSD changes when HSD use declined by at least 1 day were also examined.
RESULTS
PARTICIPANT FLOW
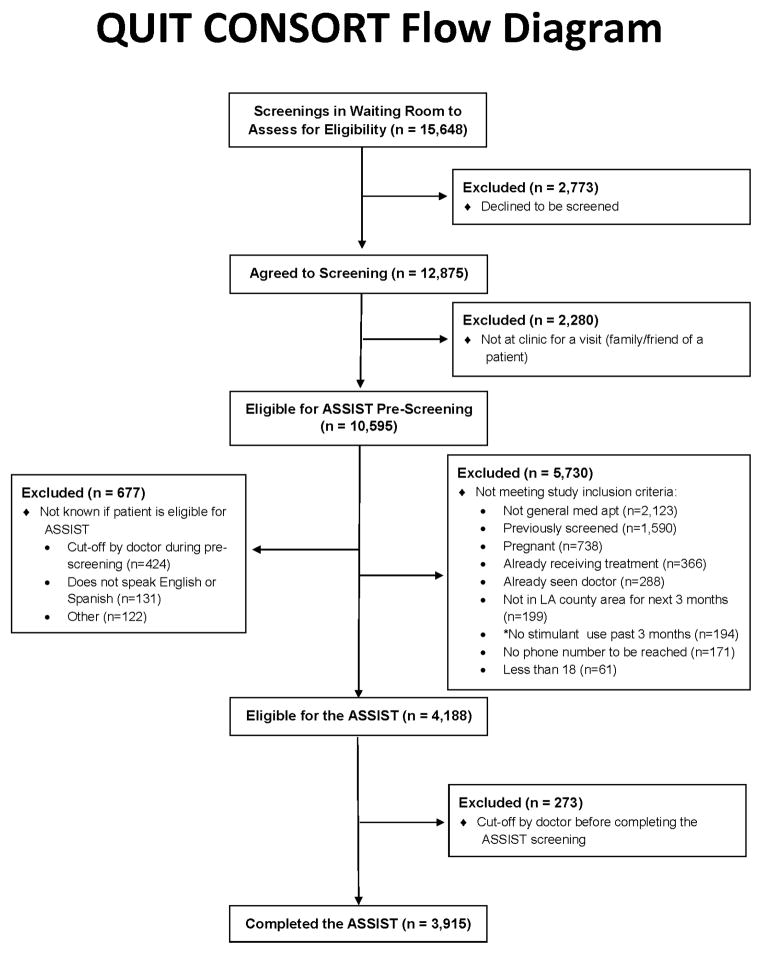
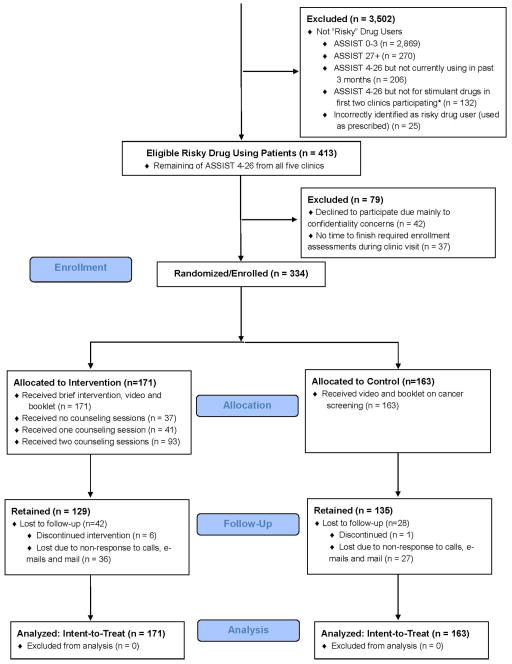
Enrollment occurred between February 25, 2011 and November 2, 2012. A total of 15,648 screenings were done in waiting rooms to identify adult patients with a PC appointment (Figure 2); 2,773 declined and 2,280 were not there for their own visit. Of 10,595 screenings, 5,730 were excluded, primarily for having a non-PC visit (37%) and having been previously screened (27%). The main cause of unknown eligibility was lack of time to complete the screener before their appointment (n=424). Of the 4,188 patients who remained eligible for the study, 3,915 (95%) completed the ASSIST. Of 3,915 ASSIST completers, 413 (11%) were RDUrs in the past 3 months; the remainder were excluded as follows: no/low use (74%), high use (7%), risky use but had not used in the past 3 months (5%), and non-stimulant risky use early in the study (3%).
Figure 2.
QUIT CONSORT Flow Diagram
Time to complete the ASSIST items averaged 4.9 minutes (SD: 5.6, median: 3.6); 75% of patients needed less than 7 minutes. The interview administered and self-administered pre-ASSIST screening questions took 1.6 minutes (SD: 1.4, median: 1.1) and 3.6 minutes (SD: 1.7, median: 3.2), respectively. Of the 413 qualifying patients, 334 (81%) enrolled (Figure 2), 42 (10%) declined to participate (due to confidentiality reasons), and 37 (9%) did not have time to finish the assessments. There was a non-significant trend (23.4% vs. 16%, p<.10) for higher dropout among intervention patients.
BASELINE CHARACTERISTICS (Table 1)
The sample was ethnically diverse. Despite using their HSD for about of 20.4 years, only 28% reported receiving clinician counseling. The mean HSD ASSIST score at baseline was 14.5 (range 4–26) and the most common HSD was cannabis (52%), followed by stimulants (32%).
AVAILABILITY OF OUTCOME INFORMATION
In total, 78% (n=261) (I: 129, C: 132) self-reported their HSD use at follow-up.
PRIMARY OUTCOME
Unadjusted Analyses
HSD use was balanced at baseline (Table 2, top panel). While control patients’ reported HSD use remained unchanged over time, intervention patients reported a significant mean reduction of 3.5 days (33% reduction). No group differences were found for less frequent users (middle panel). For more frequent users (bottom panel), intervention patients reported using less often than controls at follow-up and had a significant mean reduction of 7.8 days (41% reduction).
Table 2.
Profile of Past 30-Day Highest Scoring Druga (HSD) Use at Baseline andFollow-up among All Patients and by Baseline Level of HSD Use
| Total Sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # Days Used Highest Scoring Drug, Past 30 Daysb | ||||||||||
| Baseline | Follow-up | Reductionc Over Time | Adjusted HSD Group Difference at Follow-upf | |||||||
| Mean | Median | pd | Mean | Median | pd | Mean | pe | Mean | 95% CI | |
| Program: | .933 | .073 | 2.68* | 0.76, 4.60 | ||||||
| Control Group (N=132) | 10.7 | 5 | 9.9 | 4 | 0.83 | .211 | ||||
| Intervention Group (N=129) | 10.6 | 5 | 7.1 | 2 | 3.47 | .001 | ||||
| Patients Who Used their Highest Scoring Drug 0–4 Days at Baseline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # Days Used Highest Scoring Drug, Past 30 Daysb | ||||||||||
| Baseline | Follow-up | Reductionc Over Time | Adjusted HSD Group Difference at Follow-upf | |||||||
| Program | Mean | Median | pd | Mean | Median | pd | Mean | pe | Mean | 95% CI |
| Control Group (N=58) | 1.9 | 2 | .133 | 2.2 | 0.5 | .637 | -0.33 | .592 | -0.44 | -2.45, 1.58 |
| Intervention Group (N=62) | 1.5 | 1 | 2.7 | 0 | -1.15 | .170 | ||||
| Patients Who Used their Highest Scoring Drug ≥ 5 Days at Baseline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # Days Used Highest Scoring Drug, Past 30 Daysb | ||||||||||
| Baseline | Follow-up | Reductionc Over Time | Adjusted HSD Group Difference at Follow-upf | |||||||
| Program | Mean | Median | pd | Mean | Median | pd | Mean | pe | Mean | 95% CI |
| Control Group (N=74) | 17.6 | 15 | .419 | 15.9 | 15 | .015 | 1.74 | .109 | 5.52** | 2.43, 8.60 |
| Intervention Group (N=67) | 19.0 | 21 | 11.2 | 6 | 7.75 | .001 | ||||
p < .01
p < .001
Adjusted Analyses
Adjusting for 18 covariates in a linear regression model for the complete sample, intervention patients reported using their HSD at follow-up 3.9 fewer days than controls (p < 0.001) (adjusted means at follow up – I:7.1, 95% CI 5.8–8.5; C:9.8, 95% CI 8.5–11.2). In a reduced model adjusting for baseline HSD use, the baseline HSD ASSIST score, duration of HSD use, interest in quitting/reducing HSD use, gender, self-reported general health, history of tuberculosis, and the interval between surveys, the intervention group used their HSD an average of 3.5 fewer days at follow-up than the control group (p < 0.001).
Because the complete sample analysis assumed that data were missing completely at random, sensitivity analysis was conducted using LOCF for missing HSD use and multiple imputation for missing predictors. Using the completed sample produced a larger model with a treatment group effect of -2.4 (p=0.005) and a reduced model with an efficacy of -2.2 days (Table 3, top model). To ensure that clustering of patients within clinics was not causing the intervention effect to be overestimated, clinic was added to the larger regression model first as a fixed effect and then as a random effect. In both cases, the intervention effect remained strong (p <0.01).
Table 3.
| Total Sample (n=334) | ||||
|---|---|---|---|---|
| Measure | Estimate | s.e. | 95% CI | P |
| Intervention Program | -2.21 | 0.79 | -3.76, -0.65 | .005 |
| Baseline HSD Use | 0.69 | 0.04 | 0.61, 0.77 | .001 |
| HSD ASSIST Score | 0.14 | 0.07 | 0.01, 0.28 | .037 |
| Interval between baseline and follow-up surveysc | 0.68 | 0.25 | 0.20, 1.16 | .006 |
| General Health Statusd | -0.73 | 0.40 | -1.50, 0.04 | .064 |
| Interest in quitting/reducing HSD usee | 1.44 | 0.69 | 0.09, 2.79 | .037 |
| Patients with Baseline Highest Scoring Drug Use 0–4 Days, Past 30 Days (n=154) | ||||
|---|---|---|---|---|
| Measure | Estimate | s.e. | 95% CI | P |
| Intervention Program | 0.08 | 0.75 | -1.39, 1.55 | .915 |
| Intervalc | 1.29 | 0.24 | 0.83, 1.17 | .001 |
| Healthd | -1.06 | 0.37 | -1.79, -0.33 | .004 |
| Patients with Baseline Highest Scoring Drug Use 5 or More Days, Past 30 Days (n=180) | ||||
|---|---|---|---|---|
| Measure | Estimate | s.e. | 95% CI | P |
| Intervention Program | -4.01 | 1.33 | -6.63, -1.40 | .003 |
| Baseline HSD Use | 0.69 | 0.07 | 0.56, 0.83 | .001 |
| HSD ASSIST Score | 0.21 | 0.12 | - 0.02, 0.45 | .075 |
| Male | 2.94 | 1.41 | 0.19, 5.70 | .036 |
| Actionf | -0.32 | 0.18 | -0.67, 0.04 | .078 |
| TB History | -7.82 | 4.59 | -16.82, 1.17 | .088 |
After multiple imputation of missing predictor values
Interval in days between baseline and follow-up surveys
General health (1 = Excellent, 5 = Poor)
Interest in quitting/reducing HSD use (1 = very interested, 3 = not at all interested)
Score on the Action Readiness to change scale
Subgroup Analyses
Adding a baseline HSD use x group interaction to the reduced models in both the complete and completed samples showed that intervention patients reduced use more than controls as baseline frequency of HSD use increased (p < 0.05 for both samples). When baseline HSD use was divided at the median of 5 days, intervention patients with higher frequency HSD use in both the complete (p < 0.001) and completed sample (Table 3, bottom model) had lower HSD use at follow-up compared to control patients. No group differences were found for lower frequency HSD users in the complete or completed (Table 3, middle model) samples. Interactions of group with age, gender, race/ethnicity, homelessness, and mental health status were tested, but were not significant. However, in the completed sample, there was a trend (p=0.074) for the interaction involving gender. When the reduced model was rerun by gender, female intervention patients used 4 fewer days at follow-up than female controls (p = 0.005), while intervention males only used 1.2 days less than control males (p = 0.221) (not shown).
Effect of Telephone Education
Intervention patients were divided into those who had completed both telephone education sessions (n=93) and those who had not (n=78) and compared to controls in a regression model controlling baseline HSD use. No group differences were found in either the complete or completed sample for patients with at most one telephone session, but strong effects were found for intervention patients with two telephone sessions versus controls (both samples, p<0.001, not shown).
Effect of Drug Category
Table 4 shows that intervention patients, compared to control patients, had the lowest numerical follow-up HSD use in all drug categories except methamphetamine/amphetamines. When the complete sample reduced regression model was fit to data for patients whose HSD was cannabis, and for those whose HSD was cocaine, a sedative or an opiate, intervention patients again used less than controls (both p<0.01, not shown).
Table 4.
Past 30-Day Use of Highest Scoring Drug (HSDa) at Follow-up as a Function of Drug Type
| Number of Drug Use Days, Past 30 Days, at Follow- up | ||||
|---|---|---|---|---|
| HSD Drug Type | Control Group meanb (n) | Intervention Group meanb (n) | Group Difference | 95% CI |
| Cannabis | 11.72 (68) | 9.22 (69) | 2.51 | -0.34, 5.35 |
| Cocaine/Crack | 6.16 (20) | 3.39 (30) | 2.77 | -0.08, 5.63 |
| Methamphetamine/Amphetamine-Type Stimulants | 6.86 (14) | 6.85 (14) | 0.01 | -7.57, 7.58 |
| Sedatives | 9.40 (16) | 5.20 (8) | 4.19 | -2.92, 11.31 |
| Opiates | 11.63 (12) | 3.81 (8) | 7.82 | -0.44, 16.08 |
Highest scoring drug in problem range (4–26) on WHO ASSIST
Adjusted for baseline HSD use (NOTE: due to small sample sizes none of these results are significant, so no p values are shown)HKVB
SECONDARY OUTCOMES
Results of Wilcoxon signed rank tests in the complete sample indicated that patients who reduced (baseline – follow-up) their HSD use by a day or more also had reductions of 1.2 days for alcohol, 3.7 days for tobacco, use and 4.1 days for cannabis (all p<0.001, not shown). No significant changes were observed for other non-HSD drugs.
DISCUSSION
This study had four key findings. First, BI patients reported a 33% decline in mean HSD use, and had an adjusted 2-day reduction in reported past month HSD use at follow-up compared to controls; there was no compensatory increase in use of other substances. Second, for more frequent users, BI patients reported a 41% decline in mean HSD use and had an adjusted 4-day reduction in reported past month HSD use at follow-up compared to controls. Possibly due to a floor effect for infrequent users, QUIT efficacy was muted by including both low and high frequency risky users. Third, QUIT appeared to work best with risky cannabis, sedative and opiate users and with two telephone sessions. The stronger effect with two sessions is consistent with results of other studies that have found greater alcohol and drug reduction efficacy with multiple contacts.(67, 68)
Our positive findings for PC BI for risky drug use contrast with those of previous primary care-based BI trials. A trial conducted in inner-city outpatient clinics found higher abstinence in hair testing confirmed abstinence and reductions in cocaine and opiate use after six months.(34) In contrast, two recent PC trials showed negative effects.(36, 37) In one trial, there were no differences in hair-testing confirmed drug use at 6-month follow-up.(37) In the other trial, which featured a brief motivational intervention and a telephone-booster at 2 weeks, there were no differences in hair-testing confirmed drug use abstinence or severity during the one-year follow-up.(36)
Several study differences may explain the different results. Perhaps most important, PCP involvement in the BI was a key component of QUIT, but PCPs were not included in the BI of the negative trials. This is consistent with the alcohol BI trials where the biggest effect size and most robust outcomes were conducted in PC with the patients’ primary care clinicians conducting the intervention.(15, 29, 32) Perhaps the most important variable that helps people change their health behaviors is empathy and the relationship and trust with their family doctor. Further, the latter two trials(36, 37) used hair testing, which maybe more valid, rather than urine to validate self-report drug use and both had longer follow-ups (6 and 12 months) than QUIT.(35)
QUIT appeared to work regardless of age and race/ethnicity and for all drug classes except for stimulants (particularly the amphetamines). Had we not initially only enrolled patients with risky stimulant use, we might have found stronger results given the greater reduction for cannabis, opiates, and sedatives. Stronger BIs may be required for risky use of amphetamines. Women appeared to respond better than men, possibly due to different drug use profiles.
Study limitations included 1) The intervention effect for drug, alcohol, and tobacco use reduction may be due to reporting bias. However, compared to urine drug testing, there were low rates of HSD underreporting. Further, since there is substantially more incentive to underreport use of illegal drugs than to underreport use of alcohol or tobacco, it is also possible that the reductions in alcohol and tobacco were real and part of an effort to improve health. Some underreporting may reflect use of medications that can cross-react with the amphetamine test, such as over-the-counter cough syrups and cold/allergy preparations. Moreover, underreporting of one or more drugs at follow-up was numerically greater among control patients, potentially leading to conservative relative reduction findings. 2) Representativeness/Generalizability - limited by the 10% who declined enrollment and one geographic setting. Further, 18% of people in the waiting room declined screening. However, many were probably not patients or would have been excluded if screened; indeed, most of the 6,018 excluded patients were non-PC patients, repeaters, or currently receiving substance use treatment. 3) Attrition; however, our 75% follow-up was comparable to other studies of low-income patients and drug use.(36, 37) 4) Relatively short 3-month follow-up. 5) Small sample size made it difficult to detect interactions. 6) In the future, screening tools using the ASSIST strategy should add a question about recency of use of substance (e.g., past 3 months) at the end to identify the most appropriate candidates for BI or referral to treatment.
CONCLUSIONS
QUIT findings show one way to integrate screening and a multi-component BI protocol for reducing RDU into PC, and if confirmed in additional studies, might be used to inform guidelines on integrating screening for illicit drug use into primary care, to optimize use of information technology (computer tablets) for behavioral health screening in safety net clinics,(69–71) to advise clinician BI intervention techniques, and to improve the health of the individual and potentially the health of the public. Additional larger RCTs are needed to address questions of efficacy in similar and other settings and populations, costs, sustainability, and longer term effects. QUIT, together with these future studies, has the potential to fill an important gap in care - regarding whether to integrate BIs for RDU into FQHCs and other PC settings.(1, 3)
Acknowledgments
We gratefully acknowledge the following, without whose support we could not have conducted this study. The National Institute on Drug Abuse of the US National Institutes of Health, our funder and greatest supporter: Nora Volkow, MD, Wilson Compton, MD, MPE, Eve Reider, PhD, and Jacqueline Lloyd, PhD. The hard working partners: the clinicians and staff of our study clinics, and the patients – who were willing to consider drug use behavior change. Our colleagues and collaborators: Robert Ali, MD, University of Adelaide; Corey Arnold, PhD, UCLA Medical Imaging Informatics; C. Hendricks Brown, PhD, Northwestern University; Alex Bui, PhD, UCLA Medical Imaging Informatics; Sonya Gabrielian, MD, MPH, VA Greater Los Angeles Healthcare System; Mars Lan, PhD, UCLA Department of Computer Science; Michael McCoy, MD, Regenstrief Institute; Adeline Nyamathi, ANP PhD FAAN, UCLA School of Nursing; Majid Sarrafzadeh, PhD, UCLA Department of Computer Science. The medical directors of our study clinics: Maria Chandler, MD, MBA; Michael Eaton, PA; Paul Gregerson, MD, MBA; Michael Hochman, MD, MPH; Karen Lamp, MD; Elisa Nicholas, MD, MSPH; Jehni Robinson, MD; and Martin Serota, MD. Senior Statistician: Yihang Liu. Health Educators: Nell Baldwin, Yohanna Barth-Rogers, Janet Beyan, Jacqueline Euan, Peggy Leung, Yu-Ming Ni, Alex Rice, and Kelly Townsend. Research Assistants: Rahul Abraham, Maureen Albia, Claire Alvarenga, Esther Baek, Ryan Bakhit, Nataly Barragan, Daniel Benhuri, Ben Benhuri, Vasthi Becerra, Ashley Brumell, Cindy Chen-Wu, Ryane Daniels, Evelyn Diego, Pauline Do, Colleen Duro, Phil Garrity, Rahel Gebregziabher, Nora Ghodousi , Kidan Habtay, Lea Heller, Marissa Hernandez, Niree Hindoyan, Aida Martinez, Sareen Melikian, Hannah Mendoza, Edward Mezian, Blake Johnson, Aram Kim, Camille King, Jinsol (Gene) Lee, Jordan McCrary, Angel Mendoza, Jose Muniz Castro, Maki Nakazato, Christian Neckelmann, Gem Nelson, Osose Oboh, Beverly Okereke , Daniel Pak, Hahnnah Park, Rakshya Pokharel, Francesca Rozo, Desiree Sanchez, Henry Teaford, Ashley Torkan, Daniel Toro-Lira, Clara Tsou, Gina Tucker, Anmy Vu, Christine Vu, Hugo Yepez, and Charlette Yoon.
This research was primarily funded by a grant from NIDA (“Preventing Drug Use in Low Income Clinic Populations.” R01 DA022445-01). Special thank you to Hendricks Brown and Juan Villamar from the Center for Prevention Implementation Methodology (Ce-PIM) NIDA P30 DA027828.
ABBREVIATIONS
- ASSIST
W.H.O. Alcohol, Smoking and Substance Involvement Screening Test
- BI
Brief intervention
- FQHC
Federally qualified health centers
- HSD
Highest scoring drug
- NIDA
National Institute on Drug Abuse
- QUIT
Quit Using Drugs Intervention Trial
- RCT
Randomized controlled trial
- RDU
Risky drug use
- LAC
Los Angeles County
- PC
Primary Care
- PCPs
Primary Care Providers
Footnotes
Based on complete cases with follow-up data
Baseline minus follow-up HSD use
Two-sample t-test for group difference
Paired t test for change
Program difference at follow-up adjusted for baseline HSD use
Conflict of Interest Declaration: None
Trial Registration:
Brief Title: Preventing Drug Use in Low Income Clinic Populations (QUIT)
Protocol ID: DESPR DA022445
ClinicalTrials.gov ID: NCT01942876
REFERENCE LIST
- 1.Pating DR, Miller MM, Goplerud E, Martin J, Ziedonis DM. New systems of care for substance use disorders: treatment, finance, and technology under health care reform. Psychiatr Clin North Am. 2012;35(2):327–56. doi: 10.1016/j.psc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Nida. Strategic plan on reducing health disparities 2003. [updated 2007/01/24]. Available from: http://www.nida.nih.gov/StrategicPlan/HealthStratPlan.html. (Archived by WebCite® at http://www.webcitation.org/6Xn8kjhWD)
- 3.Buck JA. The looming expansion and transformation of public substance abuse treatment under the Affordable Care Act. Health Aff (Millwood) 2011;30(8):1402–10. doi: 10.1377/hlthaff.2011.0480. [DOI] [PubMed] [Google Scholar]
- 4.Saitz R, Alford DP, Bernstein J, Cheng DM, Samet J, Palfai T. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4(3):123–30. doi: 10.1097/ADM.0b013e3181db6b67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nida. Five Year Strategic Plan: Bringing the power of science to bear on drug abuse and addiction. [updated 2007/01/24]. Available from: http://www.drugabuse.gov/StrategicPlan/Index.html (Archived by WebCite® at http://www.webcitation.org/6Xn92b8uR)
- 6.Hingson R, Compton WM. Screening and brief intervention and referral to treatment for drug use in primary care: back to the drawing board. JAMA. 2014;312(5):488–9. doi: 10.1001/jama.2014.7863. [DOI] [PubMed] [Google Scholar]
- 7.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008;103(6):1039–47. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Justice, National Drug Intelligence Center. The Economic Impact of Illicit Drug Use on American Society. Product No. 2011-Q0317–0022011. [Google Scholar]
- 9.Kamerow DB. Research on mental disorders in primary care settings: rationale, topics, and support. Fam Pract Res J. 1986;6(1):5–11. [PubMed] [Google Scholar]
- 10.McLellan AT. Demand Reduction in the 2010 Drug Control Strategy: Prevention, Intervention, Treatment & Recovery. New York Soceity of Addiction Medicine (NYSAM) Annual Medical Scientific Conference; February 5–6; New York, NY: White House Office of National Drug Control Policy (ONDCP); 2010. [Google Scholar]
- 11.Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus. 2007;28(3):7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- 12.Humphreys K, McLellan AT. Brief Intervention, Treatment, and Recovery Support Services for Americans Who Have Substance Use Disorders: An Overview of Policy in the Obama Administration. Psychological Services. 2010;7:275–84. [Google Scholar]
- 13.Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99(1–3):280–95. doi: 10.1016/j.drugalcdep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Squires LE, Alford DP, Bernstein J, Palfai T, Saitz R. Clinical case discussion: screening and brief intervention for drug use in primary care. J Addict Med. 2010;4(3):131–6. doi: 10.1097/ADM.0b013e3181f59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace P, Culter S, Haines A. Randomized controlled trial of general practitioner intervention in patients with excessive alcohol consumption. Br Med J. 1988;297:663–8. doi: 10.1136/bmj.297.6649.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68(5):898. [PubMed] [Google Scholar]
- 17.Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72(3):455–66. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- 18.Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treat. 2001;21(2):55–64. doi: 10.1016/s0740-5472(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 19.Martin G, Copeland J. The adolescent cannabis check-up: randomized trial of a brief intervention for young cannabis users. J Subst Abuse Treat. 2008;34(4):407–14. doi: 10.1016/j.jsat.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 20.McCambridge J, Strang J. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: Results from a multi-site cluster randomized trial. Addiction. 2004;99(1):39–52. doi: 10.1111/j.1360-0443.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 21.Saunders B, Wilkinson C, Phillips M. The impact of a brief motivational intervention with opiate users attending a methadone programme. Addiction. 1995;90(3):415–24. doi: 10.1046/j.1360-0443.1995.90341510.x. [DOI] [PubMed] [Google Scholar]
- 22.Ritter A, Cameron J. A review of the efficacy and effectiveness of harm reduction strategies for alcohol, tobacco and illicit drugs. Drug and alcohol review. 2006;25(6):611–24. doi: 10.1080/09595230600944529. [DOI] [PubMed] [Google Scholar]
- 23.Gates S, McCambridge J, Smith LA, Foxcroft DR. Interventions for prevention of drug use by young people delivered in non-school settings. The Cochrane database of systematic reviews. 2006;(1):Cd005030. doi: 10.1002/14651858.CD005030.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Tait RJ, Hulse GK. A systematic review of the effectiveness of brief interventions with substance using adolescents by type of drug. Drug and alcohol review. 2003;22(3):337–46. doi: 10.1080/0959523031000154481. [DOI] [PubMed] [Google Scholar]
- 25.Dunn C, Deroo L, Rivara FP. The use of brief inteventions adapted from motivational interviewing across behavioral domains: A systematic review. Addiction. 2001;96(12):1725–42. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- 26.Young MM, Stevens A, Galipeau J, Pirie T, Garritty C, Singh K, et al. Effectiveness of brief interventions as part of the Screening, Brief Intervention and Referral to Treatment (SBIRT) model for reducing the nonmedical use of psychoactive substances: a systematic review. Systematic reviews. 2014;3:50. doi: 10.1186/2046-4053-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saitz R. Screening and brief intervention for unhealthy drug use: little or no efficacy. Frontiers in psychiatry. 2014;5:121. doi: 10.3389/fpsyt.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitlock EP, Polen MR, Green CA, Orleans T, Klein J Force USPST. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(7):557–68. doi: 10.7326/0003-4819-140-7-200404060-00017. [DOI] [PubMed] [Google Scholar]
- 29.Ockene JK, Adams A, Hurley TG, Reed GW. Brief physician- and nurse practitioner-delivered counseling for high risk drinkers: Does it work? Arch Intern Med. 1999;159(18):2198–205. doi: 10.1001/archinte.159.18.2198. [DOI] [PubMed] [Google Scholar]
- 30.Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: A meta-analytic review of controlled interventions in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97(3):279–92. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- 31.Fleming MT, Mundt MP, French MT, Manwell LB, Stauffacher EA, Barry KL. Brief physician advice for problem drinkers: Long-term efficacy and benefit-cost analysis. Alcoholism Clinical and Experimental Research. 2002;26(1):36–43. [PubMed] [Google Scholar]
- 32.Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers: A randomized controlled trial in community-based primary care practices. JAMA. 1997;277(13):1039–45. [PubMed] [Google Scholar]
- 33.Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99(7):839–45. doi: 10.1111/j.1360-0443.2004.00778.x. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein J, Bernstein E, Tassiopoulos K, Heeren T, Levenson S, Hingson R. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77(1):49–59. doi: 10.1016/j.drugalcdep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Humeniuk R, Ali R, Babor T, Souza-Formigoni ML, de Lacerda RB, Ling W, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107(5):957–66. doi: 10.1111/j.1360-0443.2011.03740.x. [DOI] [PubMed] [Google Scholar]
- 36.Roy-Byrne P, Bumgardner K, Krupski A, Dunn C, Ries R, Donovan D, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312(5):492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, et al. Screening and brief intervention for drug use in primary care: the ASPIRE randomized clinical trial. JAMA. 2014;312(5):502–13. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singleton KW, Lan M, Arnold C, Vahidi M, Arangua L, Gelberg L, et al. Wireless data collection of self-administered surveys using tablet computers. AMIA Annu Symp Proc. 2011;2011:1261–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Humeniuk R, Dennington V, Ali R. The effectiveness of a brief intervention for illicit drugs linked to the alcohol, smoking and substance involvement screening test (ASSIST) in primary health care settings: a technical report of phase III findings of the WHO ASSIST randomized controlled trial [Google Scholar]
- 40.WHO ASSIST Working Group. The alcohol, smoking and substance involvement screening test (ASSIST): Development, reliability and feasibility. Addiction. 2002;97(9):1183–94. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 41.Humeniuk R, Ali R World Health Organization ASSIST Phase II Study Group. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) and pilot brief intervention [electronic resource]: a technical report of phase II findings of the WHO ASSIST Project/prepared by Rachel Humeniuk & Robert Ali, on behalf of the WHO ASSIST Phase II Study Group. [Google Scholar]
- 42.McNeely J, Strauss SM, Wright S, Rotrosen J, Khan R, Lee JD, et al. Test-retest reliability of a self-administered Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in primary care patients. J Subst Abuse Treat. 2014 doi: 10.1016/j.jsat.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol/Supplement. 1994;12:70–5. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 44.Andersen R, Davidson P, Baumeister S. Improving access to care in America. In: Kominski J, editor. Changing the US health care system. 4. San Francisco, CA: Jossey-Bass; 2014. [Google Scholar]
- 45.Gelberg L, Andersen RM, Leake BD. The Behavioral Model for Vulnerable Populations: application to medical care use and outcomes for homeless people. Health Serv Res. 2000;34(6):1273–302. [PMC free article] [PubMed] [Google Scholar]
- 46.Stein JA, Andersen R, Gelberg L. Applying the Gelberg-Andersen behavioral model for vulnerable populations to health services utilization in homeless women. Journal of health psychology. 2007;12(5):791–804. doi: 10.1177/1359105307080612. [DOI] [PubMed] [Google Scholar]
- 47.Gerbert B, Berg-Smith S, Mancuso M, Caspers N, McPhee S, Null D, et al. Using innovative video doctor technology in primary care to deliver brief smoking and alcohol intervention. Health Promot Pract. 2003;4(3):249–61. doi: 10.1177/1524839903004003009. [DOI] [PubMed] [Google Scholar]
- 48.Gerbert B, Danley DW, Herzig K, Clanon K, Ciccarone D, Gilbert P, et al. Reframing “prevention with positives”: incorporating counseling techniques that improve the health of HIV-positive patients. AIDS Patient Care STDS. 2006;20(1):19–29. doi: 10.1089/apc.2006.20.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilbert P, Ciccarone D, Gansky SA, Bangsberg DR, Clanon K, McPhee SJ, et al. Interactive “Video Doctor” counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3(4):e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–95. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 51.Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States. Arch Gen Psychiatry. 2007;64:566–78. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 52.Saitz R. Introduction: screening and brief intervention. Subst Abus. 2007;28(3):1–2. doi: 10.1300/J465v28n03_01. [DOI] [PubMed] [Google Scholar]
- 53.Miller W, Rollnick S. Motivational interviewing: Preparing people for change. New York, NY: Guilford Press; 2002. [Google Scholar]
- 54.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 55.McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The Addiction Severity Index at 25: origins, contributions and transitions. Am J Addict. 2006;15(2):113–24. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- 56.McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Leonhard C, Mulvey K, Gastfriend DR, Shwartz M. The Addiction Severity Index: a field study of internal consistency and validity. J Subst Abuse Treat. 2000;18(2):129–35. doi: 10.1016/s0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- 58.Moos RH, Finney JW, Federman EB, Suchinsky R. Specialty mental health care improves patients' outcomes: findings from a nationwide program to monitor the quality of care for patients with substance use disorders. J Stud Alcohol. 2000;61(5):704–13. doi: 10.15288/jsa.2000.61.704. [DOI] [PubMed] [Google Scholar]
- 59.Rosen CS, Henson BR, Finney JW, Moos RH. Consistency of self-administered and interview-based Addiction Severity Index composite scores. Addiction. 2000;95(3):419–25. doi: 10.1046/j.1360-0443.2000.95341912.x. [DOI] [PubMed] [Google Scholar]
- 60.Sobell LC, Sobell MB. In: Timeline follow-back: A technique for assessing self-reported ethanol consumption. Allen J, Litten RZ, editors. Totowa, NJ: Human Press; 1992. pp. 41–72. [Google Scholar]
- 61.Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behav Res Ther. 1979;17(2):157–60. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- 62.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Ware JE, Kosinski M, Keller SD. SF-12: How to score the SF-12 physical and mental health summary scales. Health Institute, New England Medical Center; 1995. [Google Scholar]
- 64.Rollnick S, Heather N, Gold R, Hall W. Development of a short 'readiness to change' questionnaire for use in brief, opportunistic interventions among excessive drinkers. Br J Addict. 1992;87(5):743–54. doi: 10.1111/j.1360-0443.1992.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 65.Hile MG, Adkins RE. The impact of substance abusers' readiness to change on psychological and behavioral functioning. Addict Behav. 1998;23(3):365–70. doi: 10.1016/s0306-4603(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 66.Cohen J. Statistical Power: Analysis for the Behavioral Sciences. New York, NY: Academic Press; 1969. [Google Scholar]
- 67.Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers. A randomized controlled trial in community-based primary care practices. JAMA : the journal of the American Medical Association. 1997;277(13):1039–45. [PubMed] [Google Scholar]
- 68.McKay JR, Lynch KG, Shepard DS, Pettinati HM. The effectiveness of telephone-based continuing care for alcohol and cocaine dependence: 24-month outcomes. Arch Gen Psychiatry. 2005;62(2):199–207. doi: 10.1001/archpsyc.62.2.199. [DOI] [PubMed] [Google Scholar]
- 69.Hahn EA, Cella D, Dobrez D, Shiomoto G, Marcus E, Taylor SG, et al. The talking touchscreen: A new approach to outcomes assessment in low literacy. Psychooncology. 2004;13(2):86–95. doi: 10.1002/pon.719. [DOI] [PubMed] [Google Scholar]
- 70.Karlsson A, Bendtsen P. Acceptability of a computerized alcohol screening and advice routine in an emergency department setting--a patient perspective. Addict Behav. 2005;30(4):767–76. doi: 10.1016/j.addbeh.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 71.Hahn EA, Choi SW, Griffith JW, Yost KJ, Baker DW. Health literacy assessment using talking touchscreen technology (Health LiTT): a new item response theory-based measure of health literacy. Journal of health communication. 2011;16 (Suppl 3):150–62. doi: 10.1080/10810730.2011.605434. [DOI] [PMC free article] [PubMed] [Google Scholar]