Summary
Objective
We sought to determine the molecular basis for the anticatabolic effects of mechanical signals on fibrocartilage cells by studying the expression of a variety of matrix metalloproteinases (MMPs). Furthermore, we examined whether the effects of biomechanical strain on MMP gene expression are sustained.
Methods
Fibrochondrocytes from temporomandibular joint (TMJ) discs were exposed to dynamic tensile strain for various time intervals in the presence of interleukin (IL)-1β. The regulation of the messenger RNA (mRNA) expression and synthesis of MMPs and tissue inhibitors of MMPs (TIMPs) were examined by end-point and real-time reverse transcriptase-polymerase chain reaction (RT-PCR) as well as Western blot analysis.
Results
Fibrochondrocytes expressed mRNA for MMP-2, -3, -7, -8, -9, -11, -13, -14, -16, -17, and -19 as well as TIMP-1, -2, and -3, IL-1β induced a significant (P <0.05) upregulation of mRNA for MMP-3, -7, -8, -9, -13, -16, -17, and -19. The IL-1β-stimulated upregulation of these MMPs was significantly (P <0.05) abrogated by dynamic tensile strain. However, MMP-2, -11, -14, and TIMPs were not affected by either IL-1β or tensile strain. Biomechanical strain also inhibited the IL-1β-stimulated protein synthesis of MMP-3, -7, -8, -9, -13, -16, and -17. Application of mechanical strain for various time intervals during a 24-h incubation with IL-1β showed that the suppressive effects of mechanical signals are sustained.
Conclusions
The data provide evidence that biomechanical signals can downregulate the catabolic activity of fibrocartilage cells in an inflammatory environment by inhibiting the expression of a variety of MMPs. Furthermore, the matrix-protective effects of biomechanical signals are sustained even in an inflammatory environment.
Keywords: Mechanical strain, Fibrocartilage, TMJ, Matrix metalloproteinases
Introduction
Arthritis is an inflammatory disorder that is associated with the loss of structure and function of the joint. The articular inflammatory process causes synovial cells to proliferate and synthesize proinflammatory and chemotactic cytokines, thereby promoting the recruitment of additional immune cells and perpetuating inflammation1,2. The altered balance of inflammatory mediators and proteolytic fragments of the extracellular matrix under inflammatory conditions changes the levels of matrix-degrading enzymes, e.g., matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs), both required for normal tissue homeostasis and remodeling3. MMPs not only mediate cartilage degradation but also amplify immune response by proteolytic cleavage and activation of cytokines. Additionally, their cleavage products act as chemoattractants, thereby also serving as proinflammatory signals4.
Current treatment concepts for arthritic patients are based on our understanding of the pathological processes associated with arthritis. For the treatment of arthritis, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs (DMARDs) are currently used. NSAIDs provide partial relief of pain and stiffness but do not slow the progression of the disease. Corticosteroids are suppressors of inflammation but, like DMARDs and NSAIDs, have side effects which limit their usefulness5–7.
One of the most promising approaches to successfully inhibit pain and catabolic processes in arthritis is exercise or mechanical loading of inflamed joints. It has been demonstrated that articular cartilage is better preserved in knees treated with continuous passive motion than with immobilization in arthritis8. Clinical applications of continuous passive motion in various joints have also produced very satisfactory results9,10.
Little is known about the effects of mechanical loading on knee menisci, or intervertebral and temporomandibular joint (TMJ) discs, all of which consist of fibrocartilage11. Fibrocartilage is degraded by MMPs that are elevated in the synovial fluid of arthritic patients12. Fibrochondrocytes participate in the inflammation-driven matrix degradation by production and activation of MMPs13. In intervertebral discs, static compression has been shown to induce MMP-2 activation14. Similarly, immobilization or dynamic compression affects catabolic genes with an overall downregulation of collagen and upregulation of aggrecanase, MMP-13, and MMP-315. In addition, abnormal hydrostatic pressure accelerates disc degeneration by reduction of proteoglycan synthesis and increase in MMP-3 production16.
Interestingly, cyclic tensile strain (CTS) abolishes the interleukin (IL)-1β-induced MMP-1 synthesis in rabbit fibrocartilage17. However, little is known about the regulation of other MMPs by mechanical loading during inflammation in fibrocartilage. It is also not clear whether biomechanical forces can exert a sustained effect on the MMP expression in fibrocartilaginous tissue. Therefore, in this report we sought to determine if biomechanical strain suppresses the catabolic phenotype of fibrochondrocytes under inflamed conditions by studying the expression of a variety of MMPs. Furthermore, we investigated whether the effects of biomechanical strain are sustained under these conditions.
Materials and methods
CELL CULTURES
Fibrocartilage was harvested from TMJ discs of 10–12 week old Sprague–Dawley rats (Harlan, Indianapolis, IN). Discs were cleaned, minced and transferred onto macroporous filters (Spectra/Mesh®, Spectrum Laboratories, Rancho Dominguez, CA) and placed in a digestion chamber. After incubating with 0.2% trypsin for 10 min and 0.2% collagenase I (Worthington®, Lakewood, NJ) for 3 h, the cells were centrifuged and the pellet was resuspended in DMEM/F12 (Cellgro® by Mediatech, Herndon, VA), supplemented with 10% FBS (Hyclone®, Logan, UT), 1% Penicillin/Streptomycin (Cellgro® by Mediatech, Herndon, VA), and 1% L-Glutamine (Gibco by Invitrogen, Grand Island, NY). Cells were grown to 80–90% confluence and used between third and fifth passage. Cells expressed messenger RNA (mRNA) for aggrecan, biglycan, versican, and collagen type I.
APPLICATION OF CTS
Cells (5 ×105/well) were seeded on collagen I-coated BioFlex® 6-well culture plates (Flexcell® International Corp., Hillsborough, NC) and grown to 80% confluence (7–8 days) in 5% CO2 and 37°C. One day before experiments, the FBS concentration was decreased to 1%. To study the anticatabolic effects of mechanical load on fibrochondrocytes, BioFlex plates were placed onto the Loading Stations™ (Flexcell International Corp., Hillsborough, NC) and subjected to CTS at 20% and 0.05 Hz in the presence or absence of recombinant human interleukin-1β (1 ng/ml; Calbiochem, CA).
To identify MMPs and TIMPs that are expressed and regulated by mechanical loading in inflamed fibrocartilage, cells were subjected to CTS in the presence and absence of IL-1β for 4 and 24 h. In order to determine whether the regulatory effect of mechanical loading on the MMP expression under inflamed conditions is sustained, cells were stimulated with IL-1β for 24 h while being subjected to mechanical strain only for the initial 1, 2, 4, 8, 12, 16, and 20 h. In addition, cells were incubated with IL-1β for 24, 36, and 48 h, while CTS was applied only for the first 8 h of the entire incubation time. Unstretched cells in the presence or absence of IL-1β were used as controls.
END-POINT REVERSE TRANSCRIPTASE-POLYMERASE CHAIN REACTION (RT-PCR)
The screening for MMPs and TIMPs that are expressed in fibrochondrocytes was performed by end-point RT-PCR. Primers specific for members of the MMP and TIMP families (Table I) were designed with Primer Express® (Applied Biosystems, Foster City, CA). RNA was extracted from untreated controls and cells that were subjected to CTS and/ or IL-1β treatment for 24 h by an RNA extraction kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s recommended protocols. A total of 1.0 μg of RNA was reverse transcribed with 200 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA) at 42°C for 25 min followed by 65°C for 5 min. The cDNA was amplified with 0.1 μg of specific primers in a reaction mixture (PCR supermix, Invitrogen, Carlsbad, CA) containing Taq DNA polymerase, Tris–HCl, KCl, MgCl2, and dNTPs. Amplification was carried out for 30 cycles of 45 s at 94°C, 45 s at 59°C, and 60 s at 72°C by Mastercycler Gradient (Eppendorf, Hamburg, Germany). The bands of ethidium bromide-stained PCR products on agarose gels were visualized by a Kodak Image Station 1000 (Eastman Kodak Company, Rochester, NY).
Table I.
Primer sequences for end-point RT-PCR
| Sense (5′–3′) | Antisense (5′–3′) | Length (bp) | Accession no. | |
|---|---|---|---|---|
| MMP-2 | CCC ATA CTT TAC TCG GAC CA | TGA CCT TGA CCA GAA CAC CA | 420 | U65656 |
| MMP-3 | CTG GAA TGG TCT TGG CTC AT | CTG ACT GCA TCG AAG GAC AA | 370 | NM_133523 |
| MMP-7 | GCA GAA GTT CTT CGG TTT GC | TCT GCA GTC CCC CAA CTA AC | 273 | NM_012864 |
| MMP-8 | TGG TCT TCA GGC TGC TTA TG | CTT GGA CAC TCC TTG GGA AT | 146 | AJ007288 |
| MMP-9 | CCA CCG AGC TAT CCA CTC AT | GTC CGG TTT CAG CAT GTT TT | 159 | NM_031055 |
| MMP-10 | TGC TTT GTC CTT TGA TGC AG | GTC TCG GGA AGC CTT TAT CC | 398 | NM_133514 |
| MMP-11 | GCC AGA TTT GGT TCT TCC AA | AGT AGG CAT AGC CCT CAG CA | 291 | NM_012980 |
| MMP-12 | TGC AGC TGT CTT TGA TCC AC | TCC AAT TGG TAG GCT CCT TG | 204 | NM_053963 |
| MMP-13 | CCC TCG AAC ACT CAA ATG GT | GAG CTG CTT GTC CAG GTT TC | 312 | XM_343345 |
| MMP-14 | AGG GAC CCT CAT AGC TTG GT | TAG GGC TCA TAT GCC CAA AG | 190 | NM_031056 |
| MMP-16 | CAG CTC TGG AAG AAG GTT GG | GAG CTG CCT CTT GTT TGG TC | 315 | D85509 |
| MMP-17 | AGT TTG GCT ACC TCC CAC CT | CCC TCC AAG AAA GGT TCC TC | 252 | XM_239639 |
| MMP-19 | GGC TTT CCC ATG AAA CTC AA | TAG CTG CTG AGG GTT GGT CT | 196 | XM_222317 |
| TIMP-1 | GGT TCC CTG GCA TAA TCT GA | GTC ATC GAG ACC CCA AGG TA | 246 | NM_053819 |
| TIMP-2 | GCA TCA CCC AGA AGA AGA GC | TGA TGC AGG CAA AGA ACT TG | 174 | NM_021989 |
| TIMP-3 | CAC GGA AGC CTC TGA AAG TC | GTA CCC GAA ATT GGA GAG CA | 274 | U27201 |
REAL-TIME RT-PCR
In order to quantitatively analyze the regulatory effects of IL-1β and/or CTS on the expression of MMPs that we had identified in fibrochondrocytes, real-time RT-PCR was performed by a Biorad iCycler iQ (Biorad, Hercules, CA) and specific primers (Table II). Two microliters of cDNA as a template was amplified with SYBR Green Supermix (Biorad Hercules, CA) in a 25 μl reaction containing 1× SYBR Green Supermix, 0.3 μM of each primer, and de-ionized water. The mixture was heated initially at 95°C for 3 min and then followed by 40 cycles with denaturation at 95°C for 30 s, annealing at 59°C for 30 s, and extension at 72°C for 30 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. Following amplification, melt curve protocols were performed to ensure that primer-dimers or non-specific products had been eliminated or minimized. To analyze the data obtained by real-time PCR, the comparative threshold cycle (CT) method was applied18,19. Briefly, the amount of a target, normalized to GAPDH, and relative to a calibrator (either untreated sample or IL-1β-stimulated cells), is given by 2−ΔΔCT, where ΔΔCT = ΔCT (sample) − ΔCT (calibrator), and ΔCT is the CT of the target gene subtracted from the CT of GAPDH18.
Table II.
Primer sequences for real-time RT-PCR
| Sense (5′–3′) | Antisense (5′–3′) | Length (bp) | Accession no. | |
|---|---|---|---|---|
| MMP-2 | TGG GGG AGA TTC TCA CTT TG | CCA TCA GCG TTC CCA TAC TT | 87 | U65656 |
| MMP-3 | TGG GAA GCC AGT GGA AAT G | CCA TGC AAT GGG TAG GAT GAG | 81 | NM_133523 |
| MMP-7 | TCG GCG GAG ATG CTC ACT | TGG CAA CAA ACA GGA AGT TCA C | 81 | NM_012864 |
| MMP-8 | ACC TAC GAA AAT TCT ACC ACT TAC CAA | CCT TAA GCT TCT CGG CAA TCA | 81 | AJ007288 |
| MMP-9 | TGC TCC TGG CTC TAG GCT AC | TTG GAG GTT TTC AGG TCT CG | 88 | NM_031055 |
| MMP-11 | ACT TTC CGC TGA CAA CAC CT | GAA GCC CAG GTC CAC AAA TA | 85 | NM_012980 |
| MMP-13 | CTG ACC TGG GAT TTC CAA AA | ACA CGT GGT TCC CTG AGA AG | 90 | XM_343345 |
| MMP-14 | TGG GGT CAT CTG CTT CTC TT | TAG GGC TCA TAT GCC CAA AG | 90 | NM_031056 |
| MMP-16 | GAG CTC GTC CAT CCG TTG AG | GAA CGA TGC ACG AAA TCC AA | 85 | D85509 |
| MMP-17 | TCA TGT ACT ACG CCC TCA AAG TCT | ATC TGG ATG TCC GCC ATG TT | 85 | XM_239639 |
| MMP-19 | GGA AAC AAG GTG TGG CGG TAT | CAT CTA GGT TGG GTT CCA CTC TGT | 85 | XM_222317 |
| TIMP-1 | CTG AGA AGG GCT ACC AGA GC | GTC ATC GAG ACC CCA AGG TA | 88 | NM_053819 |
| TIMP-2 | AGG ACC TGA CAA GGA CAT CG | TTC TTT CCT CCA ACG TCC AG | 84 | NM_021989 |
| TIMP-3 | TAC ACA GGG CTG TGC AAC TT | CCA GGT GGT AGC GGT AAT TG | 85 | U27201 |
| GAPDH | CTC AAC TAC ATG GTC TAC ATG TTC CA | CTT CCC ATT CTC AGC CTT GAC T | 81 | X02231 |
WESTERN BLOT ANALYSIS
In order to study whether the regulatory effects of IL-1β and/or CTS on the expression of several MMPs are also reflected at the protein level, Western blot analysis was performed. MMPs were analyzed from whole lysate of cells (2 ×106) subjected to the regimens described above. Total protein concentration was determined with a BCA Protein Assay Reagent Kit (Pierce Biotechnology, Rockford, IL) and a VICTOR3™ plate reader (Perkin–Elmer, Boston, MA) that measured the absorbance at 562 nm. Equal amount of proteins in each lane was resolved on SDS-10%-PAGE under reducing conditions. After electrophoresis, the proteins were electrotransferred to ImmunBlot™ polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked with 5% nonfat milk and probed with goat polyclonal anti-rat MMP-3, -7, -8, -9, -13, or -16 antibodies or with a rabbit polyclonal anti-rat MMP-17 antibody (Santa Cruz Biotechnology, CA). These antibodies recognize pro- and active forms of MMPs (Santa Cruz Biotechnology, CA). Horseradish peroxidase (HRP) conjugated donkey anti-goat immunoglobulin G (IgG) or HRP-conjugated donkey anti-rabbit IgG were applied for detection (Chemicon International, Temecula, CA). To confirm equal input in each lane, blots were also probed with a mouse monoclonal anti-rat β-actin antibody (Abcam, Cambridge, MA) and an HRP-conjugated donkey anti-mouse IgG. Lightening Chemiluminescence reagent (Perkin–Elmer Life Sciences, Boston, MA) was used as substrate for HRP.
STATISTICAL ANALYSIS
The SPSS 13.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis. For quantitative analysis of the mRNA expression, mean values and standard errors of the mean (S.E.M.) were calculated (n = 6/group) and a statistical analysis was performed for each experiment. Each experiment was performed at least three times. To determine whether significant differences exist between untreated cells, IL-1β-stimulated cells, cells subjected to CTS, and IL-1β-stimulated cells simultaneously subjected to CTS, One-Way analysis of variance (ANOVA) and the post hoc multiple comparison Tukey’s test were applied. To compare different groups of stretched IL-1β-treated cells with unstretched IL-1β-treated cells, One-Way ANOVA and the post hoc multiple comparison Dunnett’s test were used. Differences were regarded as statistically significant at values of P <0.05.
Results
FIBROCHONDROCYTES EXPRESS A VARIETY OF MMPS AND TIMPS
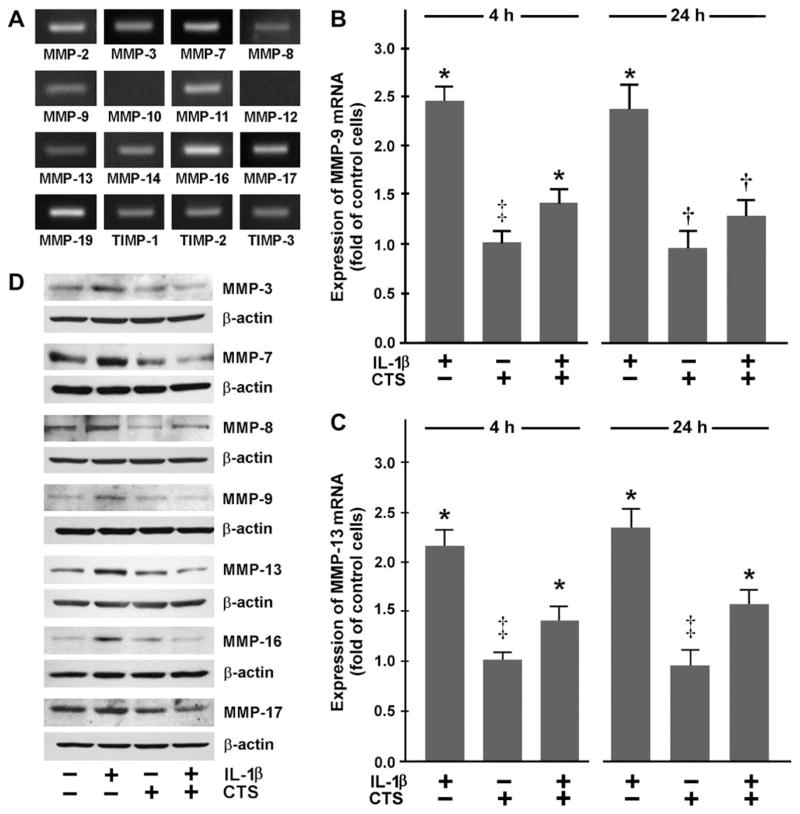
First, we sought to determine what members of the MMP and TIMP families are expressed in fibrocartilage. Fibrochondrocytes constitutively expressed mRNA for MMP-2, -3, -7, -8, -9, -11, -13, -14, -16, -17, and -19 as well as TIMP-1, -2, and -3. However, untreated cells did not express MMP-10 and -12 mRNA [Fig. 1(A)]. Furthermore, expression of both MMPs could also not be observed when cells were exposed to IL-1β and/or CTS for 24 h (data not shown).
Fig. 1.
(A) Constitutive expression of mRNA for MMPs and TIMPs in fibrochondrocytes, as analyzed by end-point RT-PCR. Representative data from one of three experiments are shown. (B) MMP-9 and (C) MMP-13 mRNA expression in fibrochondrocytes at 4 and 24 h, as determined by real-time PCR. Cells were treated with 1 ng/ml of IL-1β and/or subjected to CTS at 20% and 0.05 Hz. Results are shown as means ±S.E.M. (n = 6/group). For statistical analysis, the One-Way ANOVA and the post hoc multiple comparison Tukey’s test were employed. *Significantly (P <0.05) different from all other groups including control, †significantly (P <0.05) different from IL-1β-treated cells, ‡significantly (P <0.05) different from IL-1β-treated cells in the presence and absence of CTS. Representative data from one of four experiments are shown. (D) Protein synthesis of MMP-3, -7, -8, -9, -13, -16, and -17 at 24 h, as determined by Western blot analysis. Cells were treated with 1 ng/ml of IL-1β and/or subjected to CTS at 20% and 0.05 Hz for 24 h. Representative gels from one of three experiments are shown.
MECHANICAL SIGNALS REGULATE MMP-9 AND -13 EXPRESSION
As MMP-9 and -13 play a key role in cartilage degradation associated with arthritis, we first sought to determine whether dynamic tensile strain modulates the expression of these MMPs in fibrochondrocytes under inflammatory conditions. IL-1β that was used as an inflammatory agent, significantly (P <0.05) upregulated the expression of MMP-9 and -13 at 4 and 24 h, as demonstrated by real-time PCR. However, when IL-1β-treated cells were simultaneously subjected to CTS, the IL-1β-induced upregulation of MMP-9 and -13 was significantly (P <0.05) inhibited at both time points [Fig. 1(B, C)]. Western blot analysis revealed that the IL-1β-stimulated upregulation of mRNA for both MMPs was paralleled by their protein synthesis at 24 h. Figure 1(D) shows the presence of bands at approximately 82 kDa and 48 kDa, representing active forms of MMP-9 and -13. More importantly, the IL-1β-stimulated synthesis of both MMPs was inhibited by CTS [Fig. 1(D)].
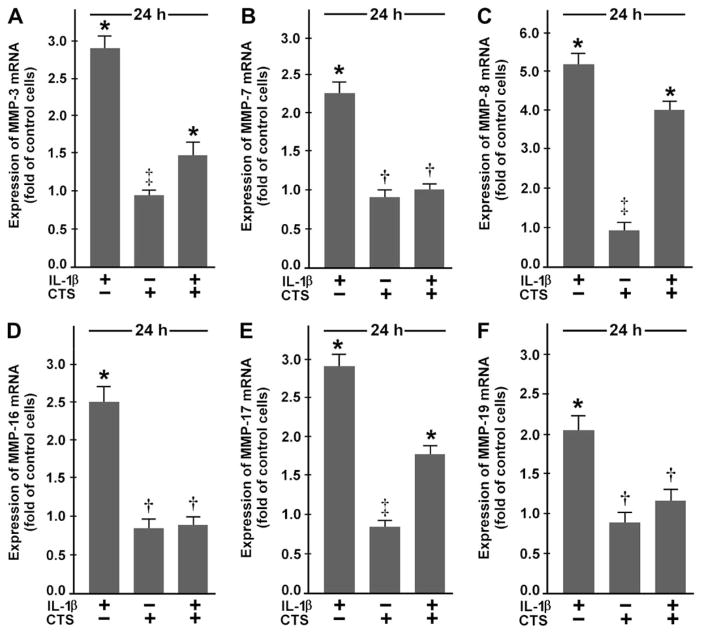
CTS ALSO AFFECTS EXPRESSION OF MMP-3, -7, -8, -16, -17, AND -19
Since fibrocartilage cells also express other members of the MMP and TIMP families [Fig. 1(A)], we studied the effect of CTS on their expression in IL-1β-treated fibrochondrocytes. IL-1β significantly (P <0.05) upregulated the constitutive mRNA expression for MMP-3, -7, -8, -16, -17, and -19. Nevertheless, the upregulation of the mRNA expression for these MMPs was only observed when cells were exposed to IL-1β for 24 h [Fig. 2(A–F)] but not after 4 h (data not shown). Interestingly, CTS also significantly (P <0.05) suppressed the IL-1β-induced upregulation of these MMPs when examined at 24 h [Fig. 2(A–F)]. As demonstrated by Western blot analysis, the IL-1β-induced upregulation of mRNA for these MMPs was paralleled by an increase in the synthesis of their proteins. Furthermore, the IL-1β-stimulated synthesis of these MMPs was inhibited by CTS. Protein bands at approximately 45 kDa for MMP-3, 20 kDa for MMP-7, 50 kDa for MMP-8, 55 kDa for MMP-16, and 67 kDa for MMP-17 were observed, representing active forms of these MMPs [Fig. 1(D)].
Fig. 2.
Expression of mRNA for (A) MMP-3, (B) MMP-7, (C) MMP-8, (D) MMP-16, (E) MMP-17, and (F) MMP-19 in fibrochondrocytes at 24 h, as determined by real-time PCR. Cells were treated with 1 ng/ml of IL-1β and/or subjected to CTS at 20% and 0.05 Hz. Results are shown as means ±S.E.M. (n = 6/group). For statistical analysis, the One-Way ANOVA and the post hoc multiple comparison Tukey’s test were employed. *Significantly (P <0.05) different from all other groups including control, †significantly (P <0.05) different from IL-1β-treated cells, ‡significantly (P <0.05) different from IL-1β-treated cells in the presence and absence of CTS. Representative data from one of four experiments are shown.
Interestingly, MMP-2, -11, and -14 as well as TIMPs were not regulated by IL-1β and/or CTS at either 4 or 24 h (data not shown).
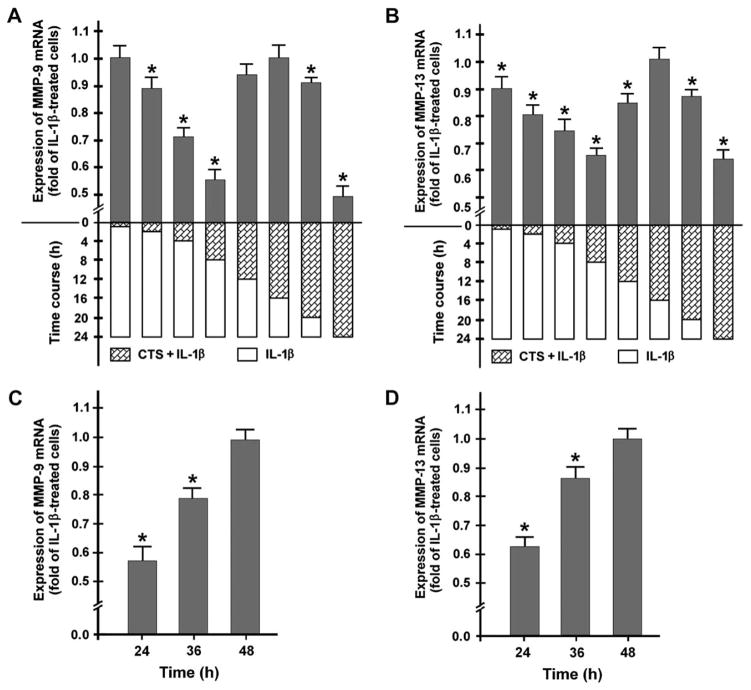
SUPPRESSION OF IL-1-INDUCED MMP-9 AND -13 EXPRESSION IS SUSTAINED AFTER REMOVAL OF CTS
Next, we sought to determine whether the inhibitory effect of CTS on the MMP expression in the presence of IL-1β is sustained. Since MMP-9 and -13 are major key players in arthritic diseases, we focused on the expression of these MMPs. Cells were stimulated with IL-1β for 24 h while being subjected to mechanical strain either only for the initial 1, 2, 4, 8, 12, 16, and 20 h or for the entire 24-h incubation time. Application of CTS for 1 h was not sufficient to inhibit the IL-1β-induced MMP-9 expression when examined 23 h later. Nevertheless, exposure of cells to CTS for 2, 4, and 8 h significantly (P <0.05) inhibited the IL-1β-stimulated MMP-9 expression. Interestingly, CTS applied for 12 h or 16 h did not induce a significant decrease in the IL-1β-induced MMP-9 expression, whereas a significantly (P <0.05) inhibitory effect of CTS was observed again when CTS was applied for 20 h or 24 h [Fig. 3(A)]. Application of CTS for 1, 2, 4, and 8 h also significantly (P <0.05) reduced the IL-1β-stimulated MMP-13 expression. Similar to MMP-9, IL-1β-induced MMP-13 expression was not significantly decreased when CTS was applied for 16 h. However, for 20 and 24 h of CTS application a significant (P <0.05) inhibition of the IL-1β-induced MMP-13 expression was again observed [Fig. 3(B)].
Fig. 3.
Expression of mRNA for (A) MMP-9 and (B) MMP-13 at 24 h, as analyzed by real-time RT-PCR. Fibrochondrocytes were stimulated with IL-1β for 24 h while being subjected to CTS (20%, 0.05 Hz) either only for the initial 1, 2, 4, 8, 12, 16, and 20 h or for the entire 24 h-interval. Results are shown as means ±S.E.M. (n = 6/group). For statistical analysis, the One-Way ANOVA and the post hoc multiple comparison Dunnett’s test were employed. *Significantly (P <0.05) different from unstretched IL-1β-treated cells. Representative data from one of three experiments are shown. (C) MMP-9 and (D) MMP-13 mRNA expression at 24, 36, and 48 h. Cells were incubated with 1 ng/ml of IL-1β for 24, 36, and 48 h, respectively, while CTS (20%, 0.05 Hz) was applied only for the initial 8 h of the entire incubation time. Results are shown as means ±S.E.M., (n = 6/group). For statistical analysis, the One-Way ANOVA and the post hoc multiple comparison Dunnett’s test were employed. *Significantly (P <0.05) different from unstretched IL-1β-treated cells. Representative data from one of four experiments are shown.
Since CTS applied only for the initial 8 h during the entire 24-h incubation with IL-1β had a profound and sustained suppressive effect on MMP-9 and -13, we wondered how long this effect would be sustained. Cells were incubated with IL-1β for 24, 36, and 48 h while CTS was applied only for the initial 8 h. The inhibition of the IL-1β-stimulated mRNA expression for MMP-9 and -13 was statistically significant (P <0.05) at 24 and 36 h [Fig. 3(C, D)]. However, no significant suppression by CTS was observed at 48 h [Fig. 3(C, D)].
Discussion
Our data provide evidence that biomechanical strain downregulates the expression of MMPs in fibrocartilage cells in an inflammatory environment. This suggests that mechanical loading is not only critical to fibrocartilage homeostasis and remodeling but also to prevent extracellular matrix degradation in arthritic diseases. MMPs are zinc-dependent endopeptidases which can cleave constituents of the extracellular matrix of fibrocartilage as well as non-matrix proteins. Increased intraarticular levels of MMPs, in response to proinflammatory cytokines such as IL-1β, are hallmarks of arthritis4,20. We have shown that IL-1β not only induces upregulation of MMPs like MMP-3, -7, -8, -9 and -13 that are known to be present in fibrocartilage but also MMP-19 and membrane-type MMPs (MMP-16 and -17) that have not been as yet identified in this tissue. This study confirms the role of IL-1β as a critical key procatabolic mediator in arthritic diseases. More importantly, these actions of IL-1β are effectively abolished by CTS, which provide strong evidence for a possible role of CTS as an anticatabolic signal in arthritic diseases.
MMP-9 is a matrix-degrading MMP that increases with the progression of arthritis12. MMP-9 knockouts have milder arthritis proving a detrimental role of MMP-921. Similarly, MMP-13 has a crucial role in cartilage degradation and sub-chondral bone resorption by its ability to cleave collagen, gelatin, and aggrecan, as well as to activate proteolytic enzymes22–24. The observations that intraarticular injections of MMP-13 induce collagen cleavage in the joints25, transgenic mice overexpressing MMP-13 show cartilage degradation26, and MMP-13 is found in synovial fluids of arthritic patients12 further suggest its importance in cartilage destruction. Our in vitro observations suggest that mechanical signals generated by CTS may be extremely significant in matrix protection by abrogating the synthesis of both these key enzymes involved in cartilage destruction.
MMP-3 associated tissue destruction is due to its ability to cleave collagen, gelatin, proteoglycans, and glycoproteins27. Furthermore, MMP-3 has an established role as an activator of other MMPs, such as MMP-1, -7, -8, -9, and -1324. The upregulation of MMP-3 by IL-1β is well documented28. Our data revealed that CTS can suppress the IL-1β-induced synthesis of this MMP. While little is known about MMP-7, its role in cartilage destruction involves its ability to cleave a variety of extracellular matrix constituents, to activate gelatinases, and to function as a pro-tumor necrosis factor-α (pro-TNF-α) convertase29. This enzyme was also upregulated by IL-1β and downregulated by the actions of CTS on fibrochondrocytes in the present study. MMP-8 has the ability to cleave collagen, gelatin, and other matrix molecules. However, the involvement of MMP-8 in enhanced cartilage matrix breakdown in osteoarthritic cartilage degeneration is debated30. Interestingly, our data show that MMP-8 is also produced by fibrochondrocytes and regulated by IL-1β and biomechanical signals. Membrane-anchored MMP-16 and -17 are also increased in the synovial membrane of arthritic patients31,32. MMP-16 not only cleaves collagen, gelatin, casein, fibronectin, and activates gelatinase but also cleaves focal adhesion kinase33. MMP-17 has a role in angiogenesis, gelatin degradation, and cleavage of pro-TNF-α, thus also functioning as a proinflammatory mediator34,35. MMP-19 is widely expressed in quiescent conditions36 and is involved in matrix degradation, angiogenesis, neovasculation, and lymphocyte extravasation. It is found in blood vessel walls of inflamed synovium of rheumatoid arthritis patients37. The present study provides evidence that CTS suppresses IL-1β-induced expression of MMP-16, -17, and -19.
This study also showed that the suppression of the IL-1β-upregulated mRNA expression by CTS is also reflected at the protein level for MMP-3, -7, -8, -9, -13, -16, and -17. Since an antibody recognizing rat MMP-19 is commercially unavailable, the examination of this MMP could not be performed. Interestingly, CTS alone decreased the constitutive protein synthesis of several MMPs but did not change their mRNA expression, indicating that CTS might also regulate MMPs at the post-transcriptional level. In view of the fact that mechanical signals were able to regulate the expression and protein synthesis of these MMPs in an inflammatory environment, the significance of these signals should not be underestimated.
Interestingly, MMP-2, -11, and -14 were constitutively expressed in fibrochondrocytes but were not affected by IL-1β and/or CTS. Little is known about the actions of MMP-11 and -14 or their involvement in arthritis. MMP-2 is widely expressed by most cell types but appears to be only marginally induced or repressed by growth factors and cytokines38. The same may also apply to its regulation by CTS.
The reason why the mRNA expression for some MMPs was significantly upregulated by IL-1β at 4 h but not for others until 24 h has yet to be elucidated. Although not studied here, it might be that the effect of IL-1β on those MMPs that were not significantly changed until 24 h was rather indirect.
This study also demonstrated that all three members of the TIMP family are synthesized by fibrochondrocytes. However, neither IL-1β nor CTS affected their expression. This is in contrast to our earlier findings in rabbits17 where mechanical forces upregulated TIMP-2. Species-related differences might be responsible for these findings.
Little is known about the regulatory role of mechanical strain in fibrocartilage. Fibrocartilage of the human TMJ is markedly thinner in edentate individuals where occlusal forces are substantially reduced, suggesting that maintenance of the fibrocartilage depends on intermittent mechanical loading39. Furthermore, high and low hydrostatic pressures are suggested to accelerate intervertebral disc degeneration, whereas physiologic levels act as an anabolic signal for stimulation of proteoglycan synthesis and TIMP-1 production16. In accordance, tensile strain of physiological levels inhibits MMP-1 synthesis and promotes TIMP-2 production in rabbit TMJ disc cells17. These findings indicate that there are distinct thresholds of mechanical signals for modeling and remodeling on the one side, and tissue destruction and cell death on the other side40. In the present study fibrochondrocytes of the TMJ dics were subjected to CTS at a relatively high strain. We found that CTS at this strain was most efficient in counteracting the effects of inflammatory mediators on rat fibrochondrocytes, even if CTS at lower strain also had an inhibitory effect (unpublished data). Several investigators have sought to determine what tensile stress fibrochondrocytes of the human TMJ discs experience during mandibular movements. By finite element analysis it has been demonstrated that normal motion results in relatively high stresses deep in the TMJ joint41. Interestingly, almost four times higher tensile stresses are found in discs when compared to condyles42. Furthermore, large disc deformations occur for relatively small joint loads43. Although these studies suggest that relatively high strain can be observed, the physiological strain that disc cells of the rat TMJ experience during joint loading has yet to be determined. The present in vitro study on the effects of mechanical signals on the MMP expression is the first step in understanding the actions of mechanical loading. Whether similar forces are effective in regulating inflammation in vivo is yet to be determined.
While our data demonstrate that mechanical signals suppress MMP expression in the presence of an inflammatory stimulus, it was not clear whether these responses are sustained after removal of CTS. Intriguingly, the suppressive effect of biomechanical strain on MMPs was sustained even if CTS was applied only for the initial 2 h of the 24-h incubation with IL-1β. In general, the longer the cells were subjected to CTS the higher the suppressive effect. Surprisingly, the MMP suppression was less after exposure of cells to CTS for 12 or 16 h when compared to other time points. Interestingly, we have observed the same cell response in similar experiments on articular chondrocytes and meniscal cells (unpublished observations). However, the mechanism behind this observation is unclear. More importantly, the suppressive effects of biomechanical strain applied for the initial 8 h were recognizable until 36 h. Taken together, these findings emphasize that the anticatabolic effects of biomechanical strain are sustained and this information may be relevant for a clinical application. Especially, in view of the fact that mechanical load or motion as a therapeutic measure is difficult to apply in patients over long periods of time.
This in vitro study provides evidence that biomechanical strain can downregulate the catabolic phenotype of fibrocartilage cells in an inflammatory environment by inhibiting the expression and synthesis of a variety of MMPs. Furthermore, this study showed that the matrix-protective effect of biomechanical loading under an inflammatory environment is sustained. Thus it can be concluded that the effects of mechanical loading/motion are critical to prevent extracellular matrix degradation in inflammatory diseases and might have a potential application in the treatment of patients afflicted with arthritic diseases.
Acknowledgments
The authors are thankful to Dr F.M. Beck for his valuable advise on the statistical analysis. This study was supported by grants from the National Institutes of Health: DE15399, DE13799, and AT000646.
References
- 1.Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004;36:372–8. doi: 10.1016/s1357-2725(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–46. [PubMed] [Google Scholar]
- 3.Iannone F, Lapadula G. The pathophysiology of osteoarthritis. Aging Clin Exp Res. 2003;15:364–72. doi: 10.1007/BF03327357. [DOI] [PubMed] [Google Scholar]
- 4.Mohammed FF, Smookler DS, Khokha R. Metalloproteinases, inflammation, and rheumatoid arthritis. Ann Rheum Dis. 2003;62(Suppl 2):ii43–7. doi: 10.1136/ard.62.suppl_2.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591–602. doi: 10.1056/NEJMra040226. [DOI] [PubMed] [Google Scholar]
- 6.Trippel SB. The unmet anti-inflammatory needs in orthopedics. Am J Orthop. 1999;28:3–7. [PubMed] [Google Scholar]
- 7.Schurman DJ, Smith RL. Osteoarthritis: current treatment and future prospects for surgical, medical, and biologic intervention. Clin Orthop Relat Res. 2004;427(Suppl):S183–9. [PubMed] [Google Scholar]
- 8.Kim HK, Kerr RG, Cruz TF, Salter RB. Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J Rheumatol. 1995;22:1714–21. [PubMed] [Google Scholar]
- 9.Salter RB. History of rest and motion and the scientific basis for early continuous passive motion. Hand Clin. 1996;12:1–11. [PubMed] [Google Scholar]
- 10.Johnson DP, Eastwood DM. Beneficial effects of continuous passive motion after total condylar knee arthroplasty. Ann R Coll Surg Engl. 1992;74:412–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamin M, Ralphs JR. Biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- 12.Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–61. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–13. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh AH, Lotz JC. Prolonged spinal loading induces matrix metalloproteinase-2 activation in intervertebral discs. Spine. 2003;28:1781–8. doi: 10.1097/01.BRS.0000083282.82244.F3. [DOI] [PubMed] [Google Scholar]
- 15.MacLean JJ, Lee CR, Grad S, Ito K, Alini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003;28:973–81. doi: 10.1097/01.BRS.0000061985.15849.A9. [DOI] [PubMed] [Google Scholar]
- 16.Handa T, Ishihara H, Ohshima H, Osada R, Tsuji H, Obata K. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine. 1997;22:1085–91. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal S, Long P, Gassner R, Piesco NP, Buckley MJ. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44:608–17. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol. 2001;15:805–29. doi: 10.1053/berh.2001.0195. [DOI] [PubMed] [Google Scholar]
- 21.Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. 2002;169:2643–7. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–8. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 24.Dreier R, Grassel S, Fuchs S, Schaumburger J, Bruckner P. Pro-MMP-9 is a specific macrophage product and is activated by osteoarthritic chondrocytes via MMP-3 or a MT1-MMP/MMP-13 cascade. Exp Cell Res. 2004;297:303–12. doi: 10.1016/j.yexcr.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 25.Otterness IG, Bliven ML, Eskra JD, te Koppele JM, Stukenbrok HA, Milici AJ. Cartilage damage after intra-articular exposure to collagenase 3. Osteoarthritis Cartilage. 2000;8:366–73. doi: 10.1053/joca.1999.0311. [DOI] [PubMed] [Google Scholar]
- 26.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada Y, Shinmei M, Tanaka O, Naka K, Kimura A, Nakanishi I, et al. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest. 1992;66:680–90. [PubMed] [Google Scholar]
- 28.Schrier DJ, Flory CM, Finkel M, Kuchera SL, Lesch ME, Jacobson PB. The effects of the phospholipase A2 inhibitor, manoalide, on cartilage degradation, stromelysin expression, and synovial fluid cell count induced by intraarticular injection of human recombinant interleukin-1 alpha in the rabbit. Arthritis Rheum. 1996;39:1292–9. doi: 10.1002/art.1780390805. [DOI] [PubMed] [Google Scholar]
- 29.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements JM, Crimmin M, et al. Matrix metalloproteinases and processing of pro-TNF-alpha. J Leukoc Biol. 1995;57:774–7. doi: 10.1002/jlb.57.5.774. [DOI] [PubMed] [Google Scholar]
- 30.Stremme S, Duerr S, Bau B, Schmid E, Aigner T. MMP-8 is only a minor gene product of human adult articular chondrocytes of the knee. Clin Exp Rheumatol. 2003;21:205–9. [PubMed] [Google Scholar]
- 31.Pap T, Shigeyama Y, Kuchen S, Fernihough JK, Simmen B, Gay RE, et al. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 2000;43:1226–32. doi: 10.1002/1529-0131(200006)43:6<1226::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Yamanaka H, Makino K, Takizawa M, Nakamura H, Fujimoto N, Moriya H, et al. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in rheumatoid synovium. Lab Invest. 2000;80:677–87. doi: 10.1038/labinvest.3780071. [DOI] [PubMed] [Google Scholar]
- 33.Shofuda T, Shofuda K, Ferri N, Kenagy RD, Raines EW, Clowes AW. Cleavage of focal adhesion kinase in vascular smooth muscle cells overexpressing membrane-type matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2004;24:839–44. doi: 10.1161/01.ATV.0000126680.78500.4c. [DOI] [PubMed] [Google Scholar]
- 34.Plaisier M, Kapiteijn K, Koolwijk P, Fijten C, Hanemaaijer R, Grimbergen JM, et al. Involvement of membrane-type matrix metalloproteinases (MT-MMPs) in capillary tube formation by human endometrial microvascular endothelial cells: role of MT3-MMP. J Clin Endocrinol Metab. 2004;89:5828–36. doi: 10.1210/jc.2004-0860. [DOI] [PubMed] [Google Scholar]
- 35.English WR, Puente XS, Freije JM, Knauper V, Amour A, Merryweather A, et al. Membrane type 4 matrix metalloproteinase (MMP17) has tumor necrosis factor-alpha convertase activity but does not activate pro-MMP2. J Biol Chem. 2000;275:14046–55. doi: 10.1074/jbc.275.19.14046. [DOI] [PubMed] [Google Scholar]
- 36.Sedlacek R, Mauch S, Kolb B, Schatzlein C, Eibel H, Peter HH, et al. Matrix metalloproteinase MMP-19 (RASI-1) is expressed on the surface of activated peripheral blood mononuclear cells and is detected as an autoantigen in rheumatoid arthritis. Immunobiology. 1998;198:408–23. doi: 10.1016/S0171-2985(98)80049-1. [DOI] [PubMed] [Google Scholar]
- 37.Kolb C, Mauch S, Peter HH, Krawinkel U, Sedlacek R. The matrix metalloproteinase RASI-1 is expressed in synovial blood vessels of a rheumatoid arthritis patient. Immunol Lett. 1997;57:83–8. doi: 10.1016/s0165-2478(97)00057-6. [DOI] [PubMed] [Google Scholar]
- 38.Ijima Y, Kobayashi M, Kubota E. Role of interleukin-1 in induction of matrix metalloproteinases synthesized by rat temporomandibular joint chondrocytes and disc cells. Eur J Oral Sci. 2001;109:50–9. doi: 10.1034/j.1600-0722.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 39.Taddei C, Frank RM, Cahen PM. Effects of complete denture wearing on temporomandibular joints: a histomorphometric study. J Prosthet Dent. 1991;65:692–8. doi: 10.1016/0022-3913(91)90208-e. [DOI] [PubMed] [Google Scholar]
- 40.Milentijevic D, Torzilli PA. Influence of stress rate on water loss, matrix deformation and chondrocytes viability in impacted articular cartilage. J Biomech. 2005;38:493–502. doi: 10.1016/j.jbiomech.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 41.DeVocht JW, Goel VK, Zeitler DL, Lew D. A study of the control of disc movement within the temporomandibular joint using the finite element technique. J Oral Maxillofac Surg. 1996;54:1431–7. doi: 10.1016/s0278-2391(96)90259-1. discussion 1437–1438. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Akyuz U, Xu L, Pidaparti RM. Stress analysis of the human temporomandibular joint. Med Eng Phys. 1998;20:565–72. doi: 10.1016/s1350-4533(98)00070-8. [DOI] [PubMed] [Google Scholar]
- 43.Beek M, Koolstra JH, van Ruijven LJ, van Eijden TM. Three-dimensional finite element analysis of the human temporomandibular joint disc. J Biomech. 2000;33:307–16. doi: 10.1016/s0021-9290(99)00168-2. [DOI] [PubMed] [Google Scholar]