Abstract
In temporomandibular joint disorders, the release of proinflammatory cytokines such as interleukin-1 (IL-1) initiates an inflammatory process disrupting cartilage homeostasis, ultimately leading to cartilage destruction. Additionally, mechanical stimuli affect articular chondrocyte metabolism. While articular chondrocytes generate nitric oxide (NO) in the presence of IL-1 proteoglycan synthesis is consecutively suppressed. The purpose of this study was to assess the effects of proinflammatory cytokines and mechanical strain in the form of cyclic tensile stretch on proteoglycan synthesis in chondrocytes, as compared to the NO competitive inhibitor L-N-monomethyl arginine (LMA), and to assess whether this effect is secondarily related to the activity of growth factors such as transforming growth factor beta (TGF-β). Lapine articular chondrocytes were exposed to one of four different treatment regimens: no cyclic tensile stretch, IL-1, cyclic tensile stretch, or IL-1 plus cyclic tensile stretch. NO production was determined as medium nitrite accumulation. TGF-β-bioactivity in chondrocyte conditioned medium was measured with the mink-lung epithelial cell bioassay. Proteoglycan synthesis was measured as the incorporation of 35-[S]-sodium sulfate into macromolecules separated from unincorporated label by gel filtration on PD-10 columns. In resting chondrocyte cultures, only baseline levels of NO were measured and the application of stretch for 24 h did not affect NO production. Addition of IL-1 provoked a large increase in NO synthesis which was abrogated in the presence of LMA. Application of stretch decreased the IL-1 induced NO synthesis, but did not modify the effect of LMA (being a competitive inhibitor of the inducible NO synthase) inhibiting IL-1 induced NO production. Glucosaminoglycan production was noted as proteoglycan synthesis showing almost no effect of cyclic stretch alone in comparison to the control condition, which correlates with the missing NO production in control and stretch conditions. Addition of IL-1 strongly inhibited proteoglycan synthesis, which was partly restored in the presence of LMA. However, cyclic stretch acted as a stronger restorer of proteoglycan synthesis in IL-1 treated conditions in the absence, and even more in the presence, of LMA. It was concluded that motion in the form of cyclic tensile stretch is a remarkable anti-inflammatory stimulus reversing the IL-1 induced suppression of proteoglycan synthesis in chondrocytes. These findings have therapeutic implications for the treatment of temporomandibular joint disorders, supporting early onset of postoperative and post-traumatic continuous passive motion therapy.
Keywords: articular cartilage, mechanical strain, cytokines, interleukin-1, nitric oxide, proteoglycans, temporomandibular joint
Articular cartilage maintains normal temporomandibular joint (TMJ) function through a specialized, hydrated extracellular matrix17. The major constituents of fibro-cartilage include type II collagen fibrils providing tensile strength, and aggregating proteoglycans (aggrecans), nonaggregating proteoglycans, and water providing compressive resilience16. Less abundant components, types IX and XI collagen7 and small proteoglycans19, also influence matrix organization.
Glucosaminoglycans are the polysaccharide sidechains attached to the core proteins of proteoglycans. The distribution of proteoglycans close to the inferior and superior surfaces16,18 in a given articular tissue is not uniform17,18. Chondroitin sulfate is evenly distributed in cartilage; dermatan sulfate is largely concentrated in the periphery, and keratan sulfate in the middle of TMJ discs17,18. This may reflect different loading patterns in various regions of the TMJ disc.
Chondrocytes embedded in the articular cartilage express distinct functional properties in development and aging, while maintaining homeostasis in the mature organism and during cartilage remodeling after traumatic or inflammatory injury14. Physiologically, these responses lead to the formation or restitution of cartilage extracellular matrix (ECM). Dysregulation of these responses can lead to qualitatively or quantitatively abnormal formation or degradation of ECM, resulting in impaired joint function16.
Additionally, mechanical stimuli affect articular chondrocyte metabolism. During normal daily activity, a variety of mechanical forces including stresses, strains, and pressures are distributed within the joint; and the long-term stability of the cartilage matrix depends mainly on the response of chondrocytes to mechanical stimuli5,10 and their limited capability to balance between anabolic and catabolic processes12,22. However, continuous passive motion in vivo and cyclic tensile stretch in vitro revealed anabolic, anti-inflammatory effects on chondrocyte metabolism and reparative effects in cartilage healing10,13,20,25.
Chondrocyte activation and the induction of anabolic or catabolic responses are thought to be primarily the function of cytokines and growth factors14. The catabolic cascade is induced by proinftammatory stimuli like interleukin-1 (IL-1) and characterized by the secretion of proteases, suppression of matrix synthesis, and inhibition of chondrocyte proliferation14. The anabolic program is associated with the secretion of antagonistic cytokines, synthesis of protease inhibitors, production of extracellular matrix, and cell replication14.
During inflammation in degenerative TMJ diseases, proinflammatory cytokines such as IL-1 decrease synthesis and increase degradation of proteoglycans and collagens5,12,22, and growth factors like TGF-β are unable to stimulate chondrocyte synthesis of collagens and proteoglycans, ultimately to reduce the activity of IL-1 stimulated proteinases4,15,24.
As first shown by Stadler et al.21, articular chondrocytes synthesize copious amounts of free radical nitric oxide (NO) following activation by IL-1 (Fig. 1). NO is generated by the NO synthase (NOS) group of enzymes which synthesize NO by combining molecular oxygen with the terminal guanidino nitrogen of the amino acid L-arginine, yielding L-citrulline as a coproduct. This reaction requires several cofactors and can be inhibited by substituted arginine derivatives such as L-N-monomethyl arginine (LMA), L-arginine-methyl ester (L-NAME), and aminoguanidine. NO can also be generated pharmacologically by compounds such as sodium nitroprusside which are used clinically as vasodilators9.
Fig. 1.
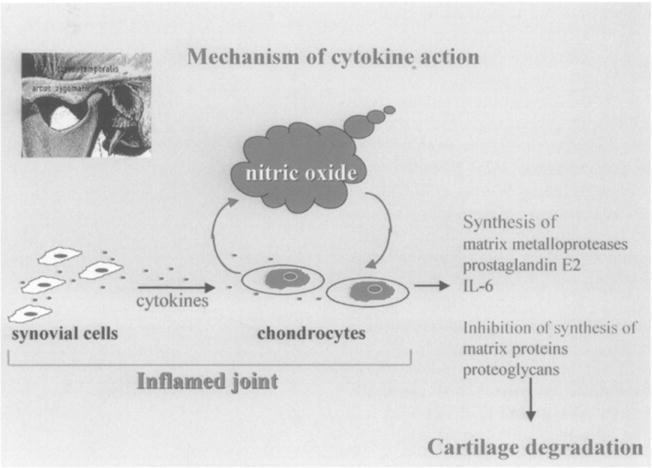
Mechanism of cytokine action: proinflammatory cytokines (such as IL-1) start the catabolic pathways in chondrocytes via nitric oxide leading to synthesis of matrix metalloproteases, prostaglandin E2, interleukin-6 and inhibition of synthesis of matrix proteins and proteoglycans.
Under aerobic conditions, NO spontaneously oxidizes to its stable metabolites, nitrate (NO3) and nitrite (NO2). Since the half-life of NO is in the order of seconds, NO production is assessed indirectly by measuring the conversion of 4C-labeled L-arginine to L-citrulline in tissue homogenates or by measuring the accumulation of the stable NO metabolites NO3 and NO2 in biological fluids such as plasma, urine and synovial fluid10,12,21,22.
To date, three distinct isoforms of NOS have been identified: a neuronal form (nNOS) initially isolated from brain, an endothelial form (ecNOS) initially isolated from the endothelium, and an inducible form (iNOS) initially isolated from macrophages12,22.
The iNOS pathway is principally regulated at the transcriptional level. Proinflammatory cytokines and endotoxin are potent inducers of iNOS in a wide variety of cell types, whereas glucocorticoids and the anti-inflammatory cytokines interleukin-4 (IL-4), interleukin-10 (IL-10), and the growth factor TGF-β suppress NO production. Although the profile of stimuli which activate iNOS are broadly similar in different cells, there are important interspecies and tissue-specific differences in iNOS regulation. NO inhibits proteoglycan synthesis directly12,21,22 whereas TGF-β suppresses that NO production, reversing the NO-mediated inhibition of proteoglycan synthesis22.
We report on the effect of mechanical strain on the proteoglycan synthesis of articular chondrocytes in the face of inflammatory mediators. This effect may be modulated via NO synthase regulation.
Material and methods
The following materials were obtained from the indicated suppliers:
New Zealand white rabbits, 5–6 lbs (Myrtle’s Rabbitry, Thompson Station, TN, USA); tissue culture media, sera, antibiotics, trypsin, Gey’s solution, etc. (from GIBCO, Grand Island, NY or Sigma Chemical Co, St Louis, MO, USA); 35-[S] sodium sulfate, 1 Ci/mmol (550 mCi mmol 1; DuPont, Wilmington, DE, USA) {(New England Nuclear, Boston, MA)}; Human platelet Transforming Growth Factor β-1, antibodies to TGF-β1, and TGF-β1 ELISA Kit (R&D Systems, Minneapolis, MN, USA); recombinant human interleukin-1β (rhIL-1β) (Genentech, Boston, MA, USA); NG-monomethyl-L-arginine (LMA) was synthesized by Drs. Paul Dowd and Wei Zhang (Department of Chemistry, University of Pittsburgh, PA, USA) and purchased from Sigma Chemical Co.), tissue culture plastic from Fisher Scientific (Pittsburgh, PA); six-well culture dishes (Flex I+II) and Flexercell® Strain unit from Flexcell Corp. (McKeesport, PA, USA).
Rabbit articular cartilage culture
Primary monolayer cultures of articular chondrocytes were established from the pooled articular cartilages of the knee and shoulder joints of young adult New Zealand white rabbits. As described in detail previously11, cartilage was cut into small pieces before digestion sequentially with trypsin (0.2% w/v) and collagenase (0.2% w/v). Cells were seeded into six-well plates at a density of 105 cells/well in Ham’s F12 medium supplemented with 10% (v/v) fetal bovine serum (FBS), 100 units of penicillin/ml, and 100 μg streptomycin/ml. Cultures of chondrocytes were used when almost confluent, without subculture. In these primary cultures, chondrocytes retain their differentiated phenotype and produce chondroitin sulfate proteoglycans and type II collagen10,12,14. Prior to the experiments, growth medium was removed from the cultures of chondrocytes, the cells were washed with Gey’s balanced salt solution, and 1.5 ml of the serumless Neumann Tytell medium was added to each well. At this time 1 ng/ml/well rhIL-1β was added to the cultures in the presence or absence of LMA (0.5 mM), which is a competitive inhibitor of NO production.
Application of mechanical strain
The Flexercell Strain unit was used for application of mechanical cyclic tensile stretch on chondrocytes3. Cells to be subjected to stretch were grown on specially designed six-well tissue culture dishes with flexible silicon bottoms (Flex I, Flexcell®). Through an air pump, a negative pressure of 12 kPa was applied to the flexible-bottomed wells at 3 cycles per minute; each cycle consisted of a 10 s stretch period followed by a 10 s relaxation period. As the flexible bottoms were pulled downward by the negative pressure, the cells attached to their upper surface were stretched by the deformation of the rubber3. Control cultures were grown under the same conditions on dishes of the same size containing a silicone disc identical to the one used for stretch, sitting on a rigid plastic bottom (Flex II, Flexcell®).
Based on the strain profile reported for the Flex I dishes at 22 kPa3,10, the degree of strain applied to the cells using 12 kPa negative pressure can be calculated to range between 160 000 microstrain (μE) at the edge to less than 20 000 μE at the center of the well, and the average strain along the radius of the dish is approximately 50 000 μE. Because microstrain is defined as a millionth of the change in length divided by the initial length of the cell, cells in the concentric area between 0.5 and 2 cm from the center are subjected to 5% average elongation, with 12% maximal elongation at the edge, down to a mininmm 1–2% elongation at the center of the dish3,8,10. The detachment rate was less than 1%. Real-time video microscopy has proven that cells remain adherent to the plate and are stretched, demonstrating that deformation to the flexible membrane was translated to the cells3,8
Measurement of nitrite
Nitrite was determined in conditioned media, as a stable end-product of NO generated by the cells. Nitrite accounts for approximately 50% of the NO generated in cultures of chondrocytes21. These determinations used a spectrophotometric assay based upon the Griess reaction21,22 measuring the absorbance at 550 nm.
Measurement of proteoglycan synthesis
Proteoglycan synthesis was determined in chondrocytes cultured for 24 hours in low serum medium with agonists and antagonists and in the presence and absence of mechanical cyclic tensile stress as noted12,22. The cells were labeled with 35-[S]-sodium sulfate, 40 μCi/ml, for the final 8 hours of incubation. Aliquots of conditioned media (CM) and cell extracts (0.5 M NaOH, 4°C, 48 h) were separated from unincorporated precursors by size-exclusion chromatography with Sephadex G-25M in PD-10 columns (Pharmacia, Piscataway, NJ, USA) with 4 M guanidinium hydrochloride, 50 mM sodium sulfate, and 50 mM trizma buffer pH 7. The large proteoglycan molecules were excluded from this column and were collected in the void volume, whereas unincorporated 35SO42− was eluted later. The radioactivity in newly synthesized proteoglycans was determined by scintillation counting. Synthesis was expressed as the percentage of values of control cells incubated with vehicle alone.
Bioassay of TGF-β activity
TGF-β bioactivity in conditioned media (CM) was determined from the inhibition of [3-H] thymidine incorporation into trichloracetic acid (TCA) precipitable DNA of mink-lung epithelial cells as previously reported in detail 22. Latent TGF-β was activated by heating aliquots of CM to 80°C for 10 rain. Values shown are normalized/106 cells and expressed as picomoles/L in the CM. The contribution of TGF-β1 to the bioactivity measured was determined as described earlier22. Aliquots of heat-activated CM were prereacted (45 rain, room temperature) with antibodies specific for TGF-β1 at concentrations which blocked the bioactivity of this isoform (1 μg/ml) without interfering with the assay of other isoforms. The bioassay standard curves for TGF-β1 were from 0.05 to 5 pM, the range used for these assays, allowing a quantitative estimation. When CM samples were incubated with antibodies to TGF-β simultaneously, negligible bioactivity was detectable, suggesting little if any contribution from other isoforms of TGF-β to the activity assayed.
Statistics
Each experiment had duplicate samples, and each was repeated five times. Pooled measurements were used to generate the data presented here (i.e. n=5). Student’s t-test for unpaired data was used for statistical analysis to compare strain versus non-strain groups, and P<0.05 was considered significant.
Results
NO production
In resting chondrocyte cultures, only baseline levels of NO (1.24±0.38 μm) were measured and application of stretch for 24 h did not affect NO production (1.05±0.31 μm) (Fig. 2). Addition of IL-1 provoked a large increase in NO synthesis (35.3±7.75 μm) which was abrogated in the presence of the competitive NO inhibitor LMA (4.26±1.18 μm). Application of stretch decreased the IL-1 induced NO synthesis (21.8±3.78 μm), but did not modify the effect of LMA inhibiting IL-1 induced NO production (3.5±1.01 μm) (Fig. 2).
Fig. 2.
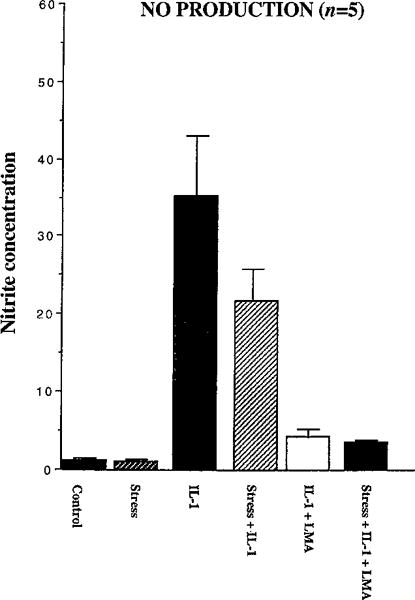
NO production (as nitrite concentration in μM) of chondrocytes in 6 different treatment regimens (control, stress, IL-1, IL-1 plus stress, IL-1 plus LMA, stress plus IL-1 plus LMA) after 24 hours: values shown are mean±SE (duplicate samples, each repeated five times; n=5). In resting chondrocyte cultures, only baseline levels of NO were measured and application of stretch did not affect NO production. Addition of IL-1 provoked a large increase in NO synthesis which was abrogated in the presence of the competitive NO inhibitor LMA. Application of stretch decreased the IL-1 induced NO synthesis but did not modify the effect of LMA inhibiting IL-1 induced NO production.
Proteoglycan synthesis
Glucosaminoglycan production was noted as proteoglycan synthesis showing almost no effect of the applied frequency of cyclic stretch alone (102.3±13.5%) in comparison to the control condition (100%), which correlates with the missing NO production in control and stretch conditions. Addition of IL-1 strongly inhibited proteoglycan synthesis (62.4±11%) which was partly restored in the presence of LMA (70.3±11.35%). However, cyclic stretch acted as a stronger restorer of proteoglycan synthesis in IL-1 treated conditions in the absence (75.43±13%), and even more in the presence, (85.7±12.3%) of LMA (Fig. 3).
Fig. 3.
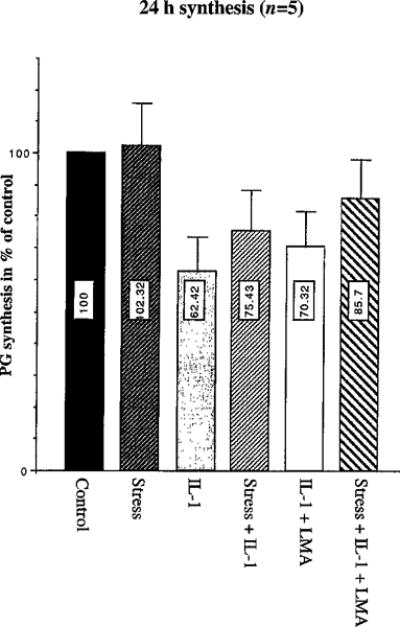
Proteoglycan (PG) synthesis (as % of control) in chondrocytes treated as in Fig. 2: values shown are mean±SE (duplicate samples, each repeated five times; n=5). Differences between the percentages of proteoglycan synthesis across stretch regimens versus no-stretch regimens were statistically significant for the IL-1 treatment groups (P=0.001) and IL-1 in conjunction with LMA treatment groups (P=0.047). Groups without IL-1 treatment (control versus stretch alone) yielded no significant differences (P=0.804).
Differences between the percentages of proteoglycan synthesis across stretch regimens versus no-stretch regimens were statistically significant for the IL-1 treatment groups (P=0.001) and IL-1 treatment groups in conjunction with LMA (P=0.047). Groups without IL-1 treatment yielded no significant differences (P=0.804).
Transforming growth factor-p values
We measured TGF-β in the conditioned medium in the form of a descriptive analysis (Fig. 4). Presence of IL-1 led to an additional increase of 66% of total TGF-β (16 pM) as compared to the control group (9 pM). Cyclic tensile stretch provoked a 400% increase (36 pM). IL-1 plus cyclic tensile stretch revealed the highest level of total TGF-β (40 pM).
Fig. 4.
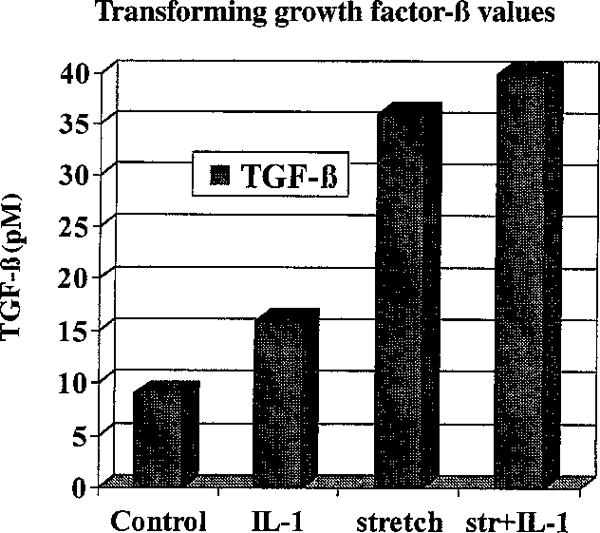
Transforming growth factor-β values (in pM) measured in the conditioned medium of 4 different treatment regimens in the form of a descriptive analysis.
Discussion
Elevated levels of nitric oxide have been detected in synovial fluid from patients with temporomandibular joint disorders23 and the possible role of nitric oxide in the physiopathology of pain associated with temporomandibular joint disorders has already been illustrated2. Chondrocytes are known as multifunctional cells, capable of synthesis and degradation of all ECM components and, to a certain extent, having control over the location of these components in the ECM6,17. Physical therapy, in particular continuous passive motion, has been shown to have positive effects on temporomandibular joint disease and to improve function probably through inhibition of NO production10,13,20,25. This manuscript deals with the effects of mechanical strain on the regulation of proteoglycan synthesis via NO in chondrocytes. Therefore we created the experimental setting of this study at the cellular level to analyze and understand the possible effects of NO in chondrocytes.
Resting chondrocyte cultures produced only baseline levels of NO, a fact which coincides with numerous other reports4,10,12,21,22. Our data show that chondrocytes exposed to cyclic stretch still did not synthesize more NO than the control cells10. However, in the presence of IL-1, articular chondrocytes generated large amounts of NO by an inducible form of nitric oxide synthase, which is consistent with earlier reports10,12,21,22. NO production in IL-1 treated chondrocytes was abrogated in the presence of the competitive NO inhibitor LMA21,22. Application of stretch decreased the IL-1 induced NO synthesis in chondrocytes about twenty-five percent, thus confirming our previous findings10, but did not modify the effect of LMA on IL-1 induced NO production.
Glucosaminoglycan production was noted as proteoglycan synthesis. Fukuda et al.8 have shown that low frequency and percentage of cyclic stretch enhance proteoglycan synthesis and higher stress regimens reduce proteoglycan synthesis. Our stretch regimen, midway between those used by Fukuda et al.8, showed almost no effect of cyclic stretch alone on proteoglycan synthesis in comparison to the control condition.
Proteoglycan synthesis was partly restored in the additional presence of LMA, as reported earlier12,22. However, our results revealed that cyclic stretch acted as a stronger restorer of proteoglycan synthesis in IL-1 treated cells (Fig. 3). Mechanical stress in the form of cyclic stretch may explain the beneficial action of continuous passive motion on joints13,20,25 as a downregulator of NO, while other forms of stress, such as shear stress, induce the release of nitric oxide6.
Chondrocyte activation and the induction of catabolic responses are thought to be primarily the function of cytokines. The catabolic program is induced by proinflammatory stimuli like IL-1 and is characterized by the secretion of proteases, the suppression of matrix synthesis, and the inhibition of chondrocyte proliferation. Mechanical stress in the form of cyclic stretch has an anabolic effect and might be associated with the secretion of antagonistic cytokines, the synthesis of protease inhibitors, the production of extracellular matrix, and cell replication3,5.
Growth factors like TGF-β are known to stimulate chondrocyte synthesis of collagens and proteoglycans, and to reduce the activity of IL-1 stimulated proteinases4,15,24. Recently, we published findings on TGF-β production in NO-treated chondrocytes22. TGF-β production was inhibited, downregulating matrix synthesis22. Therefore we additionally measured TGF-β in the conditioned medium, performing descriptive analysis. The presence of IL-1 led to an additional increase of 66% of total TGF-β as compared to the control group. The anabolic effect of mechanical stress in the form of cyclic stretch provoked a fourfold increase in total TGF-β. Treatment with IL-1 plus cyclic tensile stretch revealed the highest level of total TGF-β. Nevertheless, effects of IL-1 and TGF-β are dependent on the differentiation status of the cells and related to inducible nitric oxide synthase expression 4. An increase in apoptosis associated with aging may contribute to the greater risk for cartilage degeneration in adults1.
Häuselmann et al.12 reported on different nitric oxide production by superficial and deep articular chondrocytes and its implications for proteoglycan turnover in inflammatory joint diseases. The effects we report here are on pooled cells and may be different in cells from superficial and deep layers in cartilage.
Cartilage covering the articulating surfaces of the rigid bony skeleton is of critical interest in arthritic diseases and TMJ disorders, where therapies such as continuous passive motion stimulate repair of articular cartilage after matrix proteoglycan loss10,13,20,25. Because of the complex role of NO in arthritis5,10,12,21,22,23, we sought to determine whether mechanical strain in the form of cyclic tensile stretch affects IL-1 induced suppression of proteoglycan synthesis. It was revealed that cyclic tensile stretch might have more beneficial effects on cartilage healing than pharmacological applications of NO inhibitors.
Mechanical cyclic tensile stretch is a remarkable anti-inflammatory stimulus reversing the IL-1 induced suppression of proteoglycan synthesis in chondrocytes. Although mechanical stretch inhibits NO production only in part, it overruns the capabilities of competitive NO inhibitors like LMA in restoring proteoglycan synthesis. These findings suggest that physical therapies such as continuous passive motion may be as effective in TMJ disorders as pharmacological applications of anti-inflammatory drugs such as NO inhibitors.
Acknowledgments
Thanks to John M. Close, MA, staff biostatistician for the University of Pittsburgh School of Dental Medicine, for his advice and comparative statistical analysis of strain groups versus non-strain groups.
The research reported here has been funded, in part, by a grant from the United States National Institutes of Health Academic Research Enhancement Award 1 R15 DE1297–01, and an American Association of Oral and Maxillofacial Surgery Foundation 1999 Nobel Biocare USA Inc. Research Support Grant.
References
- 1.Adams SC, Horton WE., Jr Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec. 1998;250:418–25. doi: 10.1002/(SICI)1097-0185(199804)250:4<418::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Anbar M, Gratt BM. The possible role of nitric oxide in the physiopathology of pain associated with temporomandibular joint disorders. J Oral Maxillofac Surg. 1998;56:872–82. doi: 10.1016/s0278-2391(98)90018-0. [DOI] [PubMed] [Google Scholar]
- 3.Banes AJ, Link GW, Tran Son Tay R, Gilbert JW, Monbureau O. Culturing cells in a mechanically active environment. Am Biotechnol Lab. 1990;8:12–22. [PubMed] [Google Scholar]
- 4.Blanco FJ, Geng Y, Lotz M. Differentiation-dependent effects of IL-1 and TGF-β on human articular chondrocyte proliferation are related to inducible nitric oxide synthase expression. J Immunol. 1995;154:4018–26. [PubMed] [Google Scholar]
- 5.Das P, Schurman DJ, Smith RL. Nitric oxide and G proteins mediate response of bovine articular chondrocytes to fluid-induced shear. J Bone Joint Surg. 1997;15:87–93. doi: 10.1002/jor.1100150113. [DOI] [PubMed] [Google Scholar]
- 6.Dijkgraaf LC, deBont LGM, Boehring G, Liem RSB. Normal cartilage structure, biochemistry, and metabolism: a review of the literature. J Oral Maxillofac Surg. 1995;53:924–9. doi: 10.1016/0278-2391(95)90283-x. [DOI] [PubMed] [Google Scholar]
- 7.Eyre DR. The collagens of articular cartilage. Semin Arthritis Rheum. 1991;21:2–11. doi: 10.1016/0049-0172(91)90035-x. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda K, Asada S, Kumano F, Saitoh M, Otani K, Tanaka S. Cyclic tensile stretch on bovine articular chondrocytes inhibits protein kinase C activity. J Lab Clin Med. 1997;130:209–15. doi: 10.1016/s0022-2143(97)90098-6. [DOI] [PubMed] [Google Scholar]
- 9.Furchgott RE. The 1996 Albert Lasker Medical Research Awards. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. JAMA. 1996;276:1186–8. [PubMed] [Google Scholar]
- 10.Gassner R, Buckley MJ, Evans CH, et al. Cyclic tensile stretch exerts anti-inflammatory properties by inhibiting iNOS induction in chondrocytes. J Immunol. 1999;163:2187–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Green WT. Behavior of articular chondrocytes in cell culture. Clin Orthop. 1971;75:248–60. doi: 10.1097/00003086-197103000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Häuselmann HJ, Stefanovic-Racic M, Michel BA, Evans CH. Differences in nitric oxide production by superficial and deep human articular chondrocytes: implications for proteoglycan turnover in inflammatory joint diseases. J Immunol. 1998;160:1444–8. [PubMed] [Google Scholar]
- 13.Kim HKW, Kerr RG, Cruz TF, Salter RB. Effects of continuous passive motions and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J Rheumatol. 1995;22:1714–21. [PubMed] [Google Scholar]
- 14.Lotz M, Blanco FJ, von Kempis J, et al. Cytokine regulation of chondrocyte functions. J Rheumatol. 1995;22:104–8. [PubMed] [Google Scholar]
- 15.Lum ZP, Hakala BE, Mort JS, Recklms AD. Modulation of the catabolic effects of interleukin-1β on human articular chondrocytes by transforming growth factor β. J Cell Physiol. 1996;166:351–9. doi: 10.1002/(SICI)1097-4652(199602)166:2<351::AID-JCP13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Mgo JJ, Rahemtulla F, Scott PG. Proteoglycan expression in the rat temporomandibular joint in response to unilateral bite raise. J Dent Res. 1998;77:1520–8. doi: 10.1177/00220345980770070701. [DOI] [PubMed] [Google Scholar]
- 17.Mow VC, Zhu W, Ratcliffe A. Structure and function of articular cartilage and meniscus. In: Mow VC, Hayes WC, editors. Basic orthopaedic biomechanics. New York: Raven Press; 1991. p. 143. [Google Scholar]
- 18.Nakano T, Scott PG. A quantitative chemical study of glucosaminoglycans in the articular disc of bovine temporomandibular joint. Arch Oral Biol. 1989;34:749–57. doi: 10.1016/0003-9969(89)90082-4. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg L. Structure and function of dermatan sulfate proteoglycans in articular cartilage. In: Kuettner K, Schleyerbach R, Peygon JG, Hascall VC, editors. Articular cartilage and osteoarthritis. New York: Raven Press; 1992. pp. 45–62. [Google Scholar]
- 20.Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10:211–9. [PubMed] [Google Scholar]
- 21.Stadler J, Stefanovic-Racic M, Billiar TR, et al. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991;147:3915–20. [PubMed] [Google Scholar]
- 22.Studer RK, Georgescu HI, Miller LA, Evans CH. Inhibition of transforming growth factor beta production by nitric oxide-treated chondrocytes: implications for matrix synthesis. Arthritis Rheum. 1999;42:248–57. doi: 10.1002/1529-0131(199902)42:2<248::AID-ANR6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Kondoh T, Kamei K, et al. Elevated levels of nitric oxide in synovial fluid from patients with temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:505–9. doi: 10.1016/s1079-2104(96)80194-6. [DOI] [PubMed] [Google Scholar]
- 24.Van Beuningen JM, van der Kraan PM, Arntz OJ, van den Berg WB. Protection from interleukin-1 induced destruction of articular cartilage by transforming growth factor β: studies in anatomically intact cartilage in vitro and in vivo. Ann Rheum Dis. 1993;52:185–91. doi: 10.1136/ard.52.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JM, Moran M, Thonar EJA, Salter R. Continuous passive motion stimulates repair of rabbit knee articular cartilage after matrix proteoglycan loss. Clin Orthop Rel Res. 1994;304:252–62. [PubMed] [Google Scholar]


