Purpose of review
Physical therapies and exercise are beneficial not only for physiological recovery in inflamed or injured joints, but also for promoting a homeostatic equilibrium in healthy joints. Human joints provide the pivot points and physiological hinges essential for ambulation and movement to the body, and it is this mobility that in return promotes the health of the joints. But how mobilization regulates the joint microenvironment at the molecular level has remained enigmatic for many years. Recent advances in joint biomechanics and molecular approaches have facilitated an enriched understanding of how joints operate. Consequently, the mechanisms active during joint inflammation that lead to arthritic conditions, both in vivo in animal models, and in vitro at cell and tissue levels, have become increasingly detailed and defined. These efforts have produced mounting evidences supporting the premise that biomechanical signals play a fundamental role in both the etiopathogenesis of arthritic diseases and in the physiological restoration of joints. This report aims to summarize current peer-reviewed literature and available experimental data to explain how the signals generated by mechanical forces/joint mobilization generate beneficial effects on inflamed articular cartilage, and to propose the basis for using appropriate physical therapies for the optimal benefit to the patient suffering from joint associated injuries.
Keywords: Cartilage, chondrocytes, mechanical strain, NF-κB, signal transduction, inflammation
1. Introduction
Articular cartilage constantly experiences biomechanical forces during joint movement and is built to bear sustained heavy loads. The major forces experienced by cartilage are absorbed by the matrix composed of fluid-rich proteoglycans, and the anisotropic and heterogeneous fibrous network of collagen type II that provides the tensile and shear strength [59,80]. Chondrocytes, located in the gelatinous pericellular matrix of lacunae, constantly experience compressive, tensile and shear forces during joint movement [34,78]. These cells are mechanosensitive and maintain the cartilage matrix in a state of constant turnover by a balance of anabolic and catabolic activities [2,35,36]. Therefore, understanding the mechanisms by which chondrocytes sense mechanical signals and respond to those signals is essential in order to incorporate optimal levels of mechanical stimuli for maintaining and improving cartilage health.
2. Mechanical loading at low (physiological) levels inhibits proinflammatory gene induction and upregulates matrix synthesis
Mechanical loading within normal physiological limits is an important regulatory stimulus for cartilage biosynthesis [3,73] and tissue maintenance in vivo. During joint movement, chondrocytes experience dynamic compressive, tensile and shear forces (see Fig. 1). In vivo and in vitro studies have shown that the magnitude, frequency and duration of mechanical forces are all important determinants of the chondrocytic responses and ultimate fate of the articular cartilage [45,67,68]. Dynamic mechanical forces of low/physiologic magnitudes induce anti-inflammatory and anabolic responses in cartilage [8, 5,11,12,18,29,39,40,48,50,64,73,77,79]. Compressive forces suppress expression of matrix metallopeptidase (MMP)-1, MMP-3, MMP-9 and MMP-13 gene expression, as well as prevent the down-regulation of aggrecan in chondrocytes stimulated by exogenous IL-1β [16,20,21,52,58]. Similarly, compressive forces inhibit interleukin (IL)-1β-induced nitric oxide synthase 2A (iNOS/NOS2A) and cyclooxygenase 2 (COX2/PTGS2) expression [11,12] and up-regulate proteoglycan synthesis and cell division in the presence or absence of IL-1. Similarly, dynamic tensile forces of low magnitudes induce anti-inflammatory responses by suppressing IL-1β, tumor necrosis factor-α (TNF-α) and lipopolysaccharide (LPS)-dependent iNOS, COX2, MMP-13 and MMP-1 expression, as well as prostaglandin E2 (PGE2) and nitric oxide (NO) production in articular chondrocytes [18,29,42,53,54,79] (Table 1).
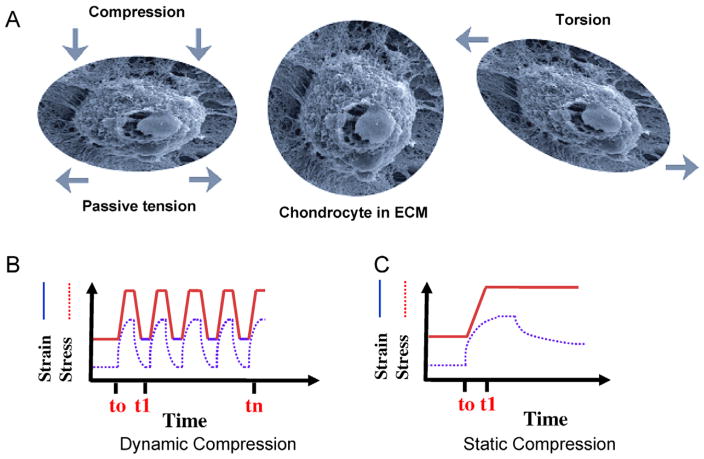
Fig. 1.
Types of biomechanical forces exerted on chondrocytes. (A) A scanning electron micrograph of a normal chondrocyte in a 3D construct surrounded by extra cellular matrix (ECM) is shown in the middle. Chondrocyte and ECM deformation after active compression that consequently results in passive tensile forces and radial fluid flow, is shown in the left panel. Chondrocytes and ECM deformation in response to shear forces, is shown in the right panel. (B) A representation of dynamic compressive forces that can be of equal or varying time intervals (frequencies), and lead to cyclic matrix and chondrocyte deformation. (C) A representation of static compressive forces, which involves a ramp and hold type effect that increases hydrostatic pressure and induces matrix deformation [33,65].
Table 1.
Molecules that are regulated by dynamic tension or compression of low/physiological magnitudes
| Molecule | Experimental system | Up/down regulation | Presence of stimulus | CF/TF | References |
|---|---|---|---|---|---|
| ADAMTS 4/5 | Down | CF | [20] | ||
| Aggrecan | C, F, CE | Up | IL-1β/TNF-α | CF/TF | [18,20,50,73,79] |
| Cell proliferation | C | Up | CF | [48] | |
| Collagen type II | C, CE | Up | IL-1β/TNF-α | CF/TF | [16,18,20,50,52,58,73,76,77,79] |
| COX-1/PGE2 | C, CE, in vivo | Down | IL-1β/TNF-α | CF/TF | [11,12,18,29,50,79] |
| IL-1β | C, CE, in vivo | Down | IL-1β | CF/TF | [18,79] |
| iNOS/NO | C, CE | Down | IL-1β/TNF-α/LPS | CF/TF | [11,12,18,29,44,45,50,53,54,79] |
| MMP-1 | C, F, in vivo | Down | IL-1β/TNF-α | CF/TF | [16–18,50,52,58,79] |
| MMP-3 | C | Down | IL-1β/TNF-α | CF/TF | [16–18,52,58,79] |
| MMP-7 | C | Down | IL-1β | TF | [17] |
| MMP-8 | C | Down | IL-1β | TF | [17] |
| MMP9 | C | Down | IL-1β | CF/TF | [17] |
| MMP-13 | C | Down | IL1β/TNFα/LPS | TF | [16,17,52–54] |
| MMP-16 | C | Down | IL-1β | TF | [17] |
| MMP-17 | C | Down | IL-1β | CF/TF | [17] |
| Proteoglycans | C, F, CE | Up | IL-1β/None/IGF | CF/TF | [16,18,20,29,39,40,52,58,64,76,77,79] |
| TIMP II | C | Up | IL-1β/TNF-α | TF | [18,50,79] |
| TNF-α | C, F, CE | Down | IL-1β | CF/TF | [18,54] |
Abbreviations: C, chondrocytes; CE, cartilage explants; CF, compressive forces; F, fibrochondrocytes; TF, tensile forces.
Dynamic compression has been shown to up-regulate the expression of anabolic genes such as Aggrecan (ACAN), collagen type II (COL2A1) and TIMP metallopeptidase inhibitor 3 (TIMP3) [26], while down-regulating specific genes of the matrix metallopeptidase (MMP) family [27,47,57]. Furthermore, cyclic tensile strain could augment cartilage repair by inducing ACAN, TIMP2 and COL2A1, as well as proteoglycan mRNA expression and synthesis by attenuation of IL-1β-induced suppression of these genes [1,17,79] (Table 1).
3. Biomechanical signals of high (traumatic) magnitude are pro-inflammatory
Exposure of cartilage to mechanical strain of high magnitudes leads to inflammation and synthesis of mediators of tissue destruction, such as IL-1 and TNF-α [24,68]. IL-1β and TNF-α actions lead to expression of multiple pro-inflammatory genes, including iNOS/NOS2A, COX2/PTGS2, and MMP-1, MMP-3, MMP-9 and MMP-13 and down-regulate proteoglycans production. These mediators cause matrix degradation and inhibition of synthesis of matrix-associated proteins [22,23,25,31,51,65,66].
Immobilization of healthy joints also results in cartilage matrix loss [38]. Static compressive strain exerts proinflammatory effects, inhibits the anabolic responses of cartilage to growth factors and increases catabolism, i.e., up-regulates the levels of MMPs, augments matrix loss, promotes proteoglycan and collagen type II degradation, as well as contributes to chondrocyte apoptosis [6,10,24,33,46,55,60,66,68] (Table 2).
Table 2.
Molecules that are regulated by dynamic/static tension or compression of high/hyperphysiologic magnitudes
| Molecule | Experimental system | Up/down regulation | CF/TF | References |
|---|---|---|---|---|
| ADAMTS 4/5 | C, EC | Up | CF | [27,46,47,52] |
| Aggrecan | C, F, EC, in vivo | Down | CF/TF/in vivo | [8,27,33,67,68] |
| Collagen II | C, EC, in vivo | Down | CF | [10,25,27,52,67,68] |
| COX-2 | C, F, EC, in vivo | Up | CF/TF | [22,23] |
| IL-1β | C, F, EC, in vivo | Up | CF/TF/in vivo | [46,60,65] |
| iNOS/NO | C, F, EC, | Up | CF/TF | [23,24] |
| MMP-1 | C, F, EC, in vivo | Up | CF/TF | |
| MMP-3 | C, F, CE | Up | TF/in vivo | [16,27,31,46,52,65] |
| MMP-9 | C | Up | TF/in vivo | [52] |
| MMP-13 | C | Up | CF/TF | [16,65] |
| Proteoglycan | C, F, EC, in vivo | Down | CF/TF/in vivo | [25,27,33,52,55,60,65,66,69] |
| TIMP I/II | EC | CF | [27,46] |
Abbreviations: C, chondrocytes; CE, cartilage explants; CF, compressive forces; F, fibrochondrocytes; TF, tensile forces.
4. Intracellular mechanisms of actions of mechanical signals in chondrocytes
The preceding observations clearly demonstrate that tissue trauma, physiologically damaging forces, and restricted joint mobility are significant contributing factors in the etiopathogenesis of osteoarthritis (OA). On the other hand, patients with arthritic diseases benefit from rehabilitative physical therapies designed to reduce inflammation and improve joint function [4,15,32,56]. Thus, one of the striking properties of biomechanical signals is to activate or inhibit pro-inflammatory signaling responses. Since Nuclear Factor-kappaB (NF-κB) is an indispensable transcription factor for the regulation of pro-inflammatory gene induction, attention has turned to this signaling pathway as a possible mechanism for modulating biomechanical signals. Clinically, NF-κB is known to be constitutively activated in some rheumatic conditions such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), as well as following traumatic injury of the joint. Furthermore, a number of anti-RA compounds have been shown to exhibit anti-NF-κB activities. These findings further emphasize the importance of detailed investigations into the pivotal role of the NF-κB activation pathway when examining the effects of biomechanical signals.
5. Transcriptional regulation of pro-inflammatory genes by NF-κB
NF-κB transcription factors regulate a wide range of pro-inflammatory and anti-apoptotic genes, and are involved in both acute and chronic inflammatory responses. NF-κB is a rapid response, inducible, transcription factor that is controlled by sequential signal activation cascades. In physiologically resting cells heterodimers of the NF-κB/REL protein family are sequestered in the cytoplasm in an inactive form via interactions with members of the I-κB (NFKBI, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor) proteins [30,37,49,71]. In the classical NF-κB signaling pathway, binding of pro-inflammatory mediators, such as IL-1β, TNF-α, and/or LPS to their cognate receptors leads to activation of a series of receptor-associated signaling molecules that converge at the common kinase, mitogen-activated protein kinase kinase kinase 7 (MAP3K7/TAK1). Phosphorylation of MAP3K7/TAK1 at Threonine 187, leads to activation of kinase activity of the central signalosome complex containing IκB Kinase (IKK, inhibitor of kappa light polypeptide gene enhancer in B-cells kinase). This kinase complex is comprised of three related molecules, inhibitor of kappa light polypeptide gene enhancer in B-cells kinase alpha (IKBKA/IKKA/IKK-α/CHUK), beta (IKBKB/IKKB/IKK-β), and gamma (IKBKG/IKKG/IKK-γ), which then phosphorylate I-κBα and/or I-κBβ proteins. Following phosphorylation I-κB proteins are targeted for ubiquitin-mediated degradation, freeing the bound and inactivated NF-κB and faciliating its phosphorylation. Subsequently, the activated NF-κB translocates to the nucleus, where it binds to the consensus sequences of several genes including pro-inflammatory cytokines and mediators, as well as some of the molecules required for the activation of NF-κB signaling cascade itself, intitiating a series of positive and negative feedback signaling loops [30,37].
6. Biomechanical signals of high (traumatic) magnitudes induce NF-κB transcriptional activation and pro-inflammatory gene induction
Consistent with the observation that cyclic tensile strain (CTS) and cyclic compressive forces (CCF) of high magnitude induce pro-inflammatory gene induction in chondrocytes, are the findings that these signals initiate the nuclear translocation of p65/p50 heterodimers of NF-κB (Fig. 2). Furthermore, caffeic acid phenethyl ester (CAPE), a cell-permeable specific inhibitor of NF-κB, completely abrogates mechanical strain-induced NF-κB nuclear translocation and iNOS mRNA expression, confirming that the actions of these mechanical signals are mediated by NF-κB family transcription factors [1,61]. Which specific proteins within the NF-κB signaling cascade are distinctively activated by biomechanical CTS or CCF of high magnitudes remains to be further elucidated.
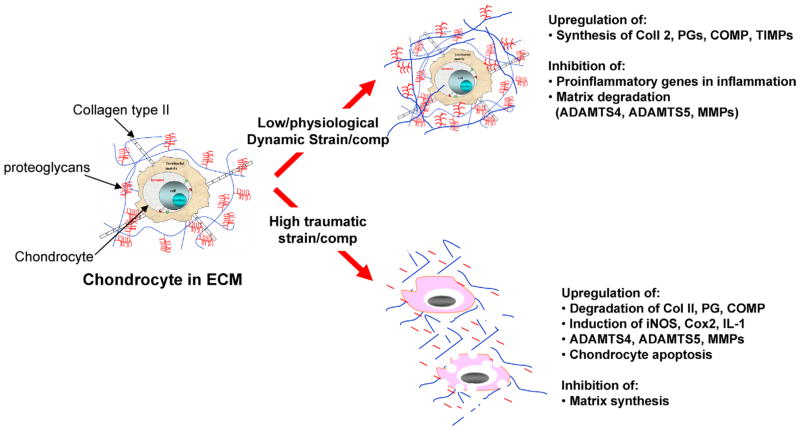
Fig. 2.
Transcriptional regulation of various genes in response to mechanical forces. Cyclic/dynamic compressive and tensile forces of low magnitudes upregulate synthesis of matrix associated proteins such as proteoglycans, collagen type II, aggrecan, TIMPs and COMP. In addition, biomechanical signals of low magnitudes inhibit IL-1β-induced expression of proinflammatory genes as well as abrogate cytokine mediated inhibition of synthesis of matrix associated proteins. Contrarily, dynamic/static compressive and tensile forces of high magnitudes and static forces induce expression of proinflammatory genes that are associated with matrix destruction such as iNOS, COX-2, MMPs, ADAMTS-4 and ADAMTS-5. In parallel, these forces inhibit expression of matrix associated molecules such as aggrecan, collagen type II, TIMPs and COMP.
7. Biomechanical signals of low (physiological) magnitude inhibit NF-κB nuclear translocation and suppress IL-1β-mediated proinflammatory gene induction
Mechanical signals of low/physiological magnitudes block the IL-1β-induced transcriptional activity of NF-κB by intercepting multiple steps in the NF-κB signaling cascade (Fig. 3). In both chon-drocytes and fibrochondrocytes, CTS of low magnitudes does not appear to inhibit IL-1β, TNF-α, or LPS receptor-mediated pro-inflammatory gene induction [1,19,53]. These findings suggest that mechanical signals use specific target sites to trigger NF-κB signaling. However, all of these signals inhibit MAP3K7/TAK1 activation, a common converging point of signal transduction generated by all three receptors, CTS at low magnitudes inhibits IL-1β-induced phosphorylation of MAP3K7/TAK1 at Thre-onine 187, blocking its kinase activity. The suppression of MAP3K7/TAK1 activation leads to inhibition of IL-1β-induced phosphorylation of IKK-β, a key regulatory molecule in the signalosome complex that modulates several functions within the NF-κB signaling cascade. CTS-mediated inhibition of IKK-β activity leads to a marked reduction in the phosphorylation and failure of subsequent degradation of I-κBα and I-κBβ. Consequently, NF-κB remains inactive and sequestered in the cytoplasm by I-κBα and I-κBβ preventing its nuclear translocation. Finally, inhibition of the nuclear translocation of NF-κB results in suppression of the transcriptional activation of several additional pro-inflammatory genes.
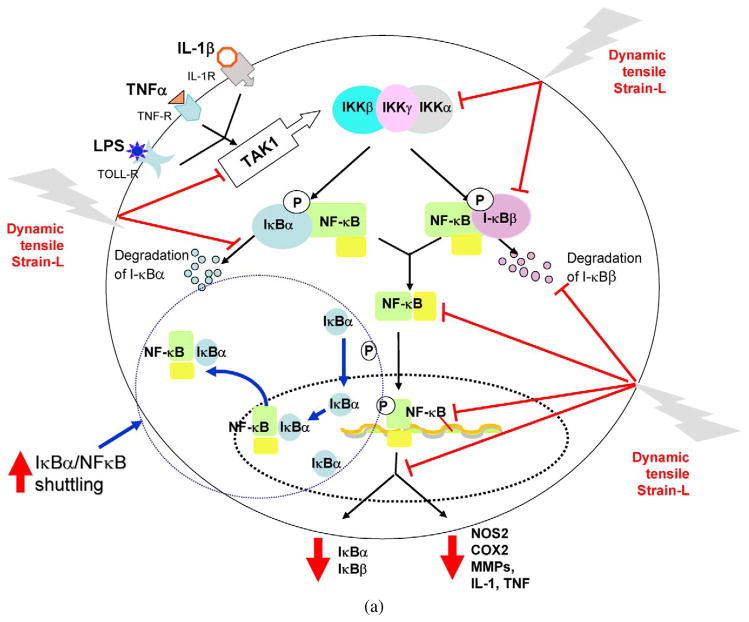
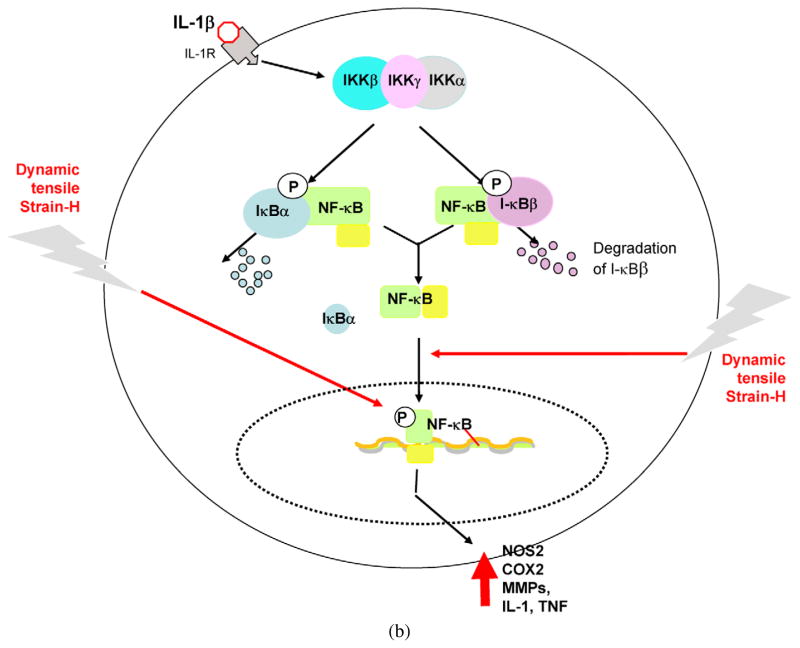
Fig. 3.
Schematic respresentation of the mechanisms of intracellular actions of dynamic tensile strain. (a) Cyclic/dynamic tensile strain of low magnitudes (CTS-L) suppresses IL-1β induced proinflammatory gene induction by intercepting salient steps in the NF-κB signaling cascade to inhibit its transcriptional activity. (i) CTS inhibits TAK1 activation, the point of convergence of signals generated by IL-1β, TNF-α or LPS, to subsequently inhibit IKK. Inhibition of IKK activation leads to suppression of phosphorylation and degradation of I-κBα and I-κBβ. This is followed by the failure of NF-κB dissociation from I-κBα and I-κBβ and thus inhibition of its nuclear translocation. (ii) At the initial stages of IL-1β-mediated activation of cells, CTS-L upregulates I-κBα nuclear translocation to prevent NF-κB binding to the DNA and facilitate export of nuclear NF-κB, that may enter the nucleus. (iii) CTS also suppresses IL-1β-induced I-κBα and I-κBβ mRNA expression. These actions collectively inhibit proinflammatory gene induction as well as expression of molecules involved in NF-κB signaling cascade to suppress inflammation. Thin arrows indicate NF-κB signaling pathway activated by IL-1β. The stop arrows indicate the points where CTS-L intercepts the NF-κB signaling cascade. The arrows in I-κBα/NF-κB shuttling circle show the mechanisms by which I-κBα shuttles the NF-κB out of the nucleus. (b) Cyclic/dynamic tensile strain of high magnitudes (DTS-H) upregulates proinflammatory gene transcription by inducing I-κBα and I-κBβ degradation and subsequent nuclear translocation of NF-κB. This results in the transcriptional activation of proinflammatory mediators including NOS2, COX-2, MMPs, IL-1β and TNF-α, and inhibition of the expression of matrix associate proteins, aggrecan, collagen type II and TIMPs. Thin arrows indicate pathway regulated by IL-1β. Heavy arrows indicate points where CTS-H is as yet known to activate NF-κB signaling cascade.
Interestingly, CTS regulates the NF-κB signaling cascade at multiple steps to prevent NF-κB-mediated pro-inflammatory gene transcription. One of the important roles of I-κBα is to shuttle intranuclear NF-κB across the nuclear membrane and back into the cytoplasm. CTS rapidly promotes I-κBα nuclear import to complex any available translocated NF-κB, and export it out of the nucleus to terminate its transcriptional activity (Fig. 3(a)).
In addition to the pro-inflammatory genes previously described, IL-1β induces the expression and eventual synthesis of multiple proteins involved in the maintenance of NF-κB signaling, perpetuating the inflammatory response. Inhibiting the expression of these molecules within the NF-κB signaling pathway is yet another mechanism by which CTS inhibits the pro-inflammatory gene response. For example, CTS readily inhibits I-κBα mRNA expression as a direct consequence of I-κBα falling under the transcriptional control of NF-κB (Fig. 3(b)).
8. Conclusion
In summary, it is apparent that the mechanical loading of chondrocytes is a key element for the both the regulation of healthy cartilage homestasis and regeneration, as well possible repair in response to traumatic damage. Here we have summarized how signals generated by biomechanical forces regulate the NF-κB signaling pathways to exert their pro- and anti-inflammatory effects. Chondrocytes respond to biomechanical forces in a magnitude- and frequency-dependent manner. Cyclic forces of physiologic levels suppress pro-inflammatory gene inductions, while static forces invariably induce pro-inflammatory gene expressions. Biomechanical signals initiated by cyclic tensile forces of high (traumatic) magnitudes induce pro-inflammatory genes by activating the NF-κB signaling cascade. On the other hand, at lower (physiological) magnitudes these signals attenuate the expression of cytokine-induced pro-inflammatory genes by inhibiting NF-κB at multiple steps within the signaling cascade.
NF-κB is constitutively activated following traumatic joint injury as well as in some rheumatic conditions, strongly implicating its role in joint inflammation [7,9,43,62,70,72]. Additionally, application of inhibitors of IKK or NF-κB is shown to be efficacious in suppressing inflammation of arthritic joints [13, 14,28,41,63,74,75]. Present investigations reveal a fundamental role for the signals generated by tensile forces in inhibiting NF-κB signaling and its subsequent pro-inflammatory gene induction. Thus, biomechanical signals appear to be the one of the most potent modulators of cartilage/joint inflammation and regeneration yet characterized. In addition to contributing to fundamental advances in the basic science of cartilage biomechanical signaling, further understanding of the biomechanical and mechanotransduction roles of chondrocytes in vivo could lead to the development of appropriate physical therapies. These clinical intervention strategies could be rationally and systematically designed to provide patient-specific, magnitude- and dosage-dependent, applications of biomechanical stimuli so as to generate those signals optimal for the therapeutic management of the arthritic joint microenvironment.
References
- 1.Agarwal S, et al. Role of NF-kappaB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals. Arthrit Rheum. 2004;50:3541–3548. doi: 10.1002/art.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aigner T, Kurz B, Fukui N, Sandell L. Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr Opin Rheumatol. 2002;14:578–584. doi: 10.1097/00002281-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Behrens F, Kraft EL, Oegema TR., Jr Biochemical changes in articular cartilage after joint immobilization by casting or external fixation. J Orthop Res. 1989;7:335–343. doi: 10.1002/jor.1100070305. [DOI] [PubMed] [Google Scholar]
- 4.Bennell K, Hinman R. Exercise as a treatment for osteoarthritis. Curr Opin Rheumatol. 2005;17:634–640. doi: 10.1097/01.bor.0000171214.49876.38. [DOI] [PubMed] [Google Scholar]
- 5.Bonassar LJ, et al. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19:11–17. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 6.Bonassar LJ, Grodzinsky AJ, Srinivasan A, Davila SG, Trippel SB. Mechanical and physicochemical regulation of the action of insulin-like growth factor-I on articular cartilage. Arch Biochem Biophys. 2000;379:57–63. doi: 10.1006/abbi.2000.1820. [DOI] [PubMed] [Google Scholar]
- 7.Bondeson J, et al. Adenoviral gene transfer of the endogenous inhibitor IkappaBalpha into human osteoarthritis synovial fibroblasts demonstrates that several matrix metalloproteinases and aggrecanases are nuclear factor-kappaB-dependent. J Rheumatol. 2007;34:523–533. [PubMed] [Google Scholar]
- 8.Buschmann MD, et al. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366:1–7. doi: 10.1006/abbi.1999.1197. [DOI] [PubMed] [Google Scholar]
- 9.Calzado MA, Bacher S, Schmitz ML. NF-kappaB inhibitors for the treatment of inflammatory diseases and cancer. Curr Med Chem. 2007;14:367–376. doi: 10.2174/092986707779941113. [DOI] [PubMed] [Google Scholar]
- 10.Chen CT, Bhargava M, Lin PM, Torzilli PA. Time, stress, and location dependent chondrocyte death and collagen damage in cyclically loaded articular cartilage. J Orthop Res. 2003;21:888–898. doi: 10.1016/S0736-0266(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury TT, Bader DL, Lee DA. Dynamic compression counteracts IL-1beta induced iNOS and COX-2 activity by human chondrocytes cultured in agarose constructs. Biorheology. 2006;43:413–429. [PubMed] [Google Scholar]
- 12.Chowdhury TT, Bader DL, Lee DA. Dynamic compression counteracts IL-1 beta-induced release of nitric oxide and PGE2 by superficial zone chondrocytes cultured in agarose constructs. Osteoarthr Cartilage. 2003;11:688–696. doi: 10.1016/s1063-4584(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 13.Clohisy JC, et al. Direct inhibition of NF-kappa B blocks bone erosion associated with inflammatory arthritis. J Immunol. 2003;171:5547–5553. doi: 10.4049/jimmunol.171.10.5547. [DOI] [PubMed] [Google Scholar]
- 14.Dale J, Alcorn N, Capell H, Madhok R. Combination therapy for rheumatoid arthritis: methotrexate and sul-fasalazine together or with other DMARDs. Nat Clin Pract Rheumatol. 2007;3:450–458. doi: 10.1038/ncprheum0562. quiz, following 478. [DOI] [PubMed] [Google Scholar]
- 15.Das UN. Anti-inflammatory nature of exercise. Nutrition. 2004;20:323–326. doi: 10.1016/j.nut.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 16.De Croos JN, Dhaliwal SS, Grynpas MD, Pilliar RM, Kandel RA. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biol. 2006;25:323–331. doi: 10.1016/j.matbio.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Deschner J, Rath-Deschner B, Agarwal S. Regulation of matrix metalloproteinase expression by dynamic tensile strain in rat fibrochondrocytes. Osteoarthr Cartilage. 2006;14:264–272. doi: 10.1016/j.joca.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschner J, Hofman CR, Piesco NP, Agarwal S. Signal transduction by mechanical strain in chondrocytes. Curr Opin Clin Nutr Metab Care. 2003;6:289–293. doi: 10.1097/01.mco.0000068964.34812.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dossumbekova A, Anghelina M, Madhavan S, He L, Quan N, Knobloch TJ, Agarwal S. Biomechanical signals inhibit IKK activity to attenuate NF-κB transcriptional activity in inflamed chondrocytes. Arthrit Rheum. 2007;56:3284–3296. doi: 10.1002/art.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehrenbacher A, Steck E, Roth W, Pahmeier A, Richter W. Long-term mechanical loading of chondrocyte–chitosan biocomposites in vitro enhanced their proteoglycan and collagen content. Biorheology. 2006;43:709–720. [PubMed] [Google Scholar]
- 21.Fehrenbacher A, Steck E, Rickert M, Roth W, Richter W. Rapid regulation of collagen but not metalloproteinase 1, 3, 13, 14 and tissue inhibitor of metalloproteinase 1, 2, 3 expression in response to mechanical loading of cartilage explants in vitro. Arch Biochem Biophys. 2003;410:39–47. doi: 10.1016/s0003-9861(02)00658-6. [DOI] [PubMed] [Google Scholar]
- 22.Fermor B, et al. The effects of cyclic mechanical strain and tumor necrosis factor alpha on the response of cells of the meniscus. Osteoarthr Cartilage. 2004;12:956–962. doi: 10.1016/j.joca.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Fermor B, et al. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthr Cartilage. 2002;10:792–798. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- 24.Fermor B, et al. The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. J Orthop Res. 2001;19:729–737. doi: 10.1016/S0736-0266(00)00049-8. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 26.Fitzgerald JB, Jin M, Grodzinsky AJ. Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J Biol Chem. 2006;281:24095–24103. doi: 10.1074/jbc.M510858200. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald JB, et al. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem. 2004;279:19502–19511. doi: 10.1074/jbc.M400437200. [DOI] [PubMed] [Google Scholar]
- 28.Fusconi M, Vannini A, Dall’aglio AC, Pappas G, Bianchi FB, Zauli D. Etanercept and infliximab induce the same serological autoimmune modifications in patients with rheumatoid arthritis. Rheumatol Int. 2007;28:47–49. doi: 10.1007/s00296-007-0379-5. [DOI] [PubMed] [Google Scholar]
- 29.Gassner R, et al. Cyclic tensile stress exerts antiinflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163:2187–2192. [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 31.Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40:1–11. doi: 10.3109/03008209909005273. [DOI] [PubMed] [Google Scholar]
- 32.Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 34.Guilak F. The deformation behavior and viscoelastic properties of chondrocytes in articular cartilage. Biorheology. 2000;37:27–44. [PubMed] [Google Scholar]
- 35.Hardingham TE, Rayan V, Lewthwaite JC. Regulation of cartilage matrix synthesis by chondrocytes. Rev Rhum Ed Fr. 1994;61:93S–98S. [PubMed] [Google Scholar]
- 36.Heinegard D, Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989;3:2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB–NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 38.Hudelmaier M, Glaser C, Hausschild A, Burgkart R, Eckstein F. Effects of joint unloading and reloading on human cartilage morphology and function, muscle cross-sectional areas, and bone density – a quantitative case report. J Musculoskel Neur Interact. 2006;6:284–290. [PubMed] [Google Scholar]
- 39.Jin M, Emkey GR, Siparsky P, Trippel SB, Grodzinsky AJ. Combined effects of dynamic tissue shear deformation and insulin-like growth factor I on chondrocyte biosynthesis in cartilage explants. Arch Biochem Biophys. 2003;414:223–231. doi: 10.1016/s0003-9861(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 40.Jin M, Frank EH, Quinn TM, Hunziker EB, Grodzinsky AJ. Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch Biochem Biophys. 2001;395:41–48. doi: 10.1006/abbi.2001.2543. [DOI] [PubMed] [Google Scholar]
- 41.Jue DM, Jeon KI, Jeong JY. Nuclear factor kappaB (NF-kappaB) pathway as a therapeutic target in rheumatoid arthritis. J Korean Med Sci. 1999;14:231–238. doi: 10.3346/jkms.1999.14.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knobloch TJ, Madhavan S, Nam J, Agarwal SK, Agarwal S. Regulation of chondrocytic gene expression by biomechanical signals. Crit Rev Eukaryot Gene Expr. 2008;18:139–150. doi: 10.1615/critreveukargeneexpr.v18.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lauder SN, et al. Interleukin-1beta induced activation of nuclear factor-kappab can be inhibited by novel pharmacological agents in osteoarthritis. Rheumatology (Oxford) 2007;46:752–758. doi: 10.1093/rheumatology/kel419. [DOI] [PubMed] [Google Scholar]
- 44.Lee DA, Noguchi T, Frean SP, Lees P, Bader DL. The influence of mechanical loading on isolated chondrocytes seeded in agarose constructs. Biorheology. 2000;37:149–161. [PubMed] [Google Scholar]
- 45.Lee DA, Frean SP, Lees P, Bader DL. Dynamic mechanical compression influences nitric oxide production by articular chondrocytes seeded in agarose. Biochem Biophys Res Commun. 1998;251:580–585. doi: 10.1006/bbrc.1998.9520. [DOI] [PubMed] [Google Scholar]
- 46.Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthrit Rheum. 2005;52:2386–2395. doi: 10.1002/art.21215. [DOI] [PubMed] [Google Scholar]
- 47.Lee TC, O’Brien FJ, Gunnlaugsson T, Parkesh R, Taylor D. Microdamage and bone mechanobiology. Technol Health Care. 2006;14:359–365. [PubMed] [Google Scholar]
- 48.Li KW, et al. Mechanical compression modulates proliferation of transplanted chondrocytes. J Orthop Res. 2000;18:374–382. doi: 10.1002/jor.1100180308. [DOI] [PubMed] [Google Scholar]
- 49.Liacini A, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp Cell Res. 2003;288:208–217. doi: 10.1016/s0014-4827(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 50.Long P, Gassner R, Agarwal S. Tumor necrosis factor alpha-dependent proinflammatory gene induction is inhibited by cyclic tensile strain in articular chondrocytes in vitro. Arthrit Rheum. 2001;44:2311–2319. doi: 10.1002/1529-0131(200110)44:10<2311::aid-art393>3.0.co;2-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001;391(Suppl):S108–S115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 52.Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–1200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Madhavan S, Anghelina M, Sjostrom D, Dossumbekova A, Guttridge DC, Agarwal S. Biomechanical signals suppress TAK1 activation to Inhibit NF-kB transcriptional activation in fibrochondrocytes. J Immunol. 2007;179:6246–6254. doi: 10.4049/jimmunol.179.9.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madhavan S, Anghelina M, Rath-Deschner B, Wypasek E, John A, Deschner J, Piesco N, Agarwal S. Biomechanical signals exert sustained attenuation of proinflammatory gene induction in articular chondrocytes. Osteoarthr Cartilage. 2006;14:1023–1032. doi: 10.1016/j.joca.2006.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milentijevic D, Rubel IF, Liew AS, Helfet DL, Torzilli PA. An in vivo rabbit model for cartilage trauma: a preliminary study of the influence of impact stress magnitude on chondrocyte death and matrix damage. J Orthop Trauma. 2005;19:466–473. doi: 10.1097/01.bot.0000162768.83772.18. [DOI] [PubMed] [Google Scholar]
- 56.Milne S, Brosseau L, Robinson V, Noel MJ, Davis J, Drouin H, Wells G, Tugwell P. Continuous passive motion following total knee arthroplasty. Cochrane Database Syst Rev. 2003;2:CD004260. doi: 10.1002/14651858.CD004260. [DOI] [PubMed] [Google Scholar]
- 57.Mio K, Saito S, Tomatsu T, Toyama Y. Intermittent compressive strain may reduce aggrecanase expression in cartilage: a study of chondrocytes in agarose gel. Clin Orthop Relat Res. 2005;433:225–232. doi: 10.1097/01.blo.0000150466.30696.c6. [DOI] [PubMed] [Google Scholar]
- 58.Monfort J, et al. Decreased metalloproteinase production as a response to mechanical pressure in human cartilage: a mechanism for homeostatic regulation. Arthrit Res Ther. 2006;8:R149. doi: 10.1186/ar2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 60.Murata M, Bonassar LJ, Wright M, Mankin HJ, Towle CA. A role for the interleukin-1 receptor in the pathway linking static mechanical compression to decreased proteoglycan synthesis in surface articular cartilage. Arch Biochem Biophys. 2003;413:229–235. doi: 10.1016/s0003-9861(03)00129-2. [DOI] [PubMed] [Google Scholar]
- 61.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okamoto T. NF-kappaB and rheumatic diseases. Endocr Metab Immun Disord Drug Targets. 2006;6:359–372. doi: 10.2174/187153006779025685. [DOI] [PubMed] [Google Scholar]
- 63.Ostensen M, Motta M. Therapy insight: the use of antirheumatic drugs during nursing. Nat Clin Pract Rheumatol. 2007;3:400–406. doi: 10.1038/ncprheum0532. [DOI] [PubMed] [Google Scholar]
- 64.Parkkinen JJ, Lammi MJ, Helminen HJ, Tammi M. Local stimulation of proteoglycan synthesis in articular cartilage explants by dynamic compression in vitro. J Orthop Res. 1992;10:610–620. doi: 10.1002/jor.1100100503. [DOI] [PubMed] [Google Scholar]
- 65.Patwari P, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthrit Rheum. 2003;48:1292–1301. doi: 10.1002/art.10892. [DOI] [PubMed] [Google Scholar]
- 66.Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19:242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 67.Ragan PM, et al. Chondrocyte extracellular matrix synthesis and turnover are influenced by static compression in a new alginate disk culture system. Arch Biochem Biophys. 2000;383:256–264. doi: 10.1006/abbi.2000.2060. [DOI] [PubMed] [Google Scholar]
- 68.Ragan PM, et al. Down-regulation of chondrocyte aggrecan and type-II collagen gene expression correlates with increases in static compression magnitude and duration. J Orthop Res. 1999;17:836–842. doi: 10.1002/jor.1100170608. [DOI] [PubMed] [Google Scholar]
- 69.Sah RL, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Effects of compression on the loss of newly synthesized proteoglycans and proteins from cartilage explants. Arch Biochem Biophys. 1991;286:20–29. doi: 10.1016/0003-9861(91)90004-3. [DOI] [PubMed] [Google Scholar]
- 70.Saklatvala J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr Drug Targets. 2007;8:305–313. doi: 10.2174/138945007779940115. [DOI] [PubMed] [Google Scholar]
- 71.Seguin CA, Bernier SM. TNFalpha suppresses link protein and type II collagen expression in chondrocytes: Role of MEK1/2 and NF-kappaB signaling pathways. J Cell Physiol. 2003;197:356–369. doi: 10.1002/jcp.10371. [DOI] [PubMed] [Google Scholar]
- 72.Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappaB activation by cur-cumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73:1434–1445. doi: 10.1016/j.bcp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Shieh AC, Athanasiou KA. Dynamic compression of single cells. Osteoarthr Cartilage. 2007;15:328–334. doi: 10.1016/j.joca.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 74.Tarner IH, Muller-Ladner U, Gay S. Emerging targets of biologic therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2007;3:336–345. doi: 10.1038/ncprheum0506. [DOI] [PubMed] [Google Scholar]
- 75.Toussirot E, Wendling D. The use of TNF-alpha blocking agents in rheumatoid arthritis: an update. Expert Opin Pharmacother. 2007;8:2089–2107. doi: 10.1517/14656566.8.13.2089. [DOI] [PubMed] [Google Scholar]
- 76.Waldman SD, Couto DC, Grynpas MD, Pilliar RM, Kandel RA. Multi-axial mechanical stimulation of tissue engineered cartilage: review. Eur Cell Mater. 2007;13:66–73. doi: 10.22203/ecm.v013a07. discussion 73–74. [DOI] [PubMed] [Google Scholar]
- 77.Waldman SD, Couto DC, Grynpas MD, Pilliar RM, Kandel RA. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthr Cartilage. 2006;14:323–330. doi: 10.1016/j.joca.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 78.Wan LQ, Miller C, Guo XE, Mow VC. Fixed electrical charges and mobile ions affect the measurable mechano-electrochemical properties of charged-hydrated biological tissues: the articular cartilage paradigm. Mech Chem Biosyst. 2004;1:81–99. [PMC free article] [PubMed] [Google Scholar]
- 79.Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes. J Immunol. 2000;165:453–460. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu W, Iatridis JC, Hlibczuk V, Ratcliffe A, Mow VC. Determination of collagen–proteoglycan interactions in vitro. J Biomech. 1996;29:773–783. doi: 10.1016/0021-9290(95)00136-0. [DOI] [PubMed] [Google Scholar]