Abstract
Objective
To understand the intracellular mechanisms of the action of mechanical strain on articular chondrocytes during inflammation.
Methods
One of the major mediators responsible for cartilage destruction in inflamed articular joints is tumor necrosis factor α (TNFα). Therefore, in this study we examined the intracellular mechanisms of actions of cyclic tensile strain (CTS) on the recombinant human TNFα (rHuTNFα)–induced proinflammatory pathways in primary cultures of chondrocytes. The expression of messenger RNA (mRNA) for TNFα-dependent proinflammatory proteins was examined by semiquantitative reverse transcriptase–polymerase chain reaction. The synthesis of proinflammatory proteins was examined by Western blot analysis in cell extracts, followed by semiquantitative measurement of bands using densitometric analysis. Nitric oxide production was measured by Griess reaction, and prostaglandin E2 production was assessed by radioimmunoassays. The proteoglycan synthesis in chondrocytes was assessed by incorporation of Na235SO4 in chondroitin sulfate proteoglycans.
Results
By exposing chondrocytes to CTS in the presence of TNFα in vitro, we showed that CTS is an effective antagonist of TNFα actions and acts as both an antiinflammatory signal and a reparative signal. CTS of low magnitude suppresses TNFα-induced mRNA expression of multiple proinflammatory proteins involved in catabolic responses, such as inducible nitric oxide synthase, cyclooxygenase 2, and collagenase. CTS also counteracts cartilage degradation by augmenting induction of tissue inhibitor of metalloproteinase 2. Additionally, CTS augments the reparative process via abrogation of TNFα-induced suppression of proteoglycan synthesis. Nonetheless, CTS acts on chondrocytes in a TNFα-dependent manner, since exposure of chondrocytes to CTS alone had no effect on these parameters.
Conclusion
CTS of low magnitude acts as an effective antagonist of TNFα not only by inhibiting the TNFα-dependent induction of proinflammatory proteins upstream of mRNA transcription, but also by augmenting the proteoglycan synthesis that is inhibited by TNFα.
Osteoarthritis (OA) and rheumatoid arthritis (RA) are characterized by consistent inflammation of multiple joints and marked destruction of cartilage and bone (1–3). Accumulated evidence suggests that tumor necrosis factor α (TNFα) and interleukin-1 (IL-1) are the pivotal mediators of the disease processes (1,4–6). Both of these cytokines are found in the synovial fluid of arthritic joints. Furthermore, the arthritogenic potential of TNFα has been demonstrated by the presence of severe joint inflammation and cartilage destruction in mice overexpressing TNFα (7,8). TNF neutralization by anti-TNF antibodies has been shown to reduce inflammation and provide pain relief in patients with RA (9). TNFα is involved primarily in the onset of arthritis, and induces catabolic responses in chondrocytes by stimulating expression of inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), and proteases, including stromelysin and collagenase (10–12). TNFα also synergizes with IL-1 to enhance cartilage destruction in vivo (7). Furthermore, in addition to stimulating cartilage degradation, TNFα inhibits synthesis of aggrecan and type II collagen (CII) (13,14). Collectively, induction of catabolic enzymes and inhibition of matrix synthesis by TNFα and IL-1β drive cartilage destruction in chronic inflammatory diseases such as RA or OA (1–14).
A number of antiarthritic therapies aimed at neutralizing the effects of cytokines are currently being investigated. Physical therapies such as continuous passive motion have been shown to mediate reparative/anabolic effects on diseased or inflamed synovial joints, although only limited information is available regarding the mechanisms of their intracellular actions (15–20). Their effects have been attributed mainly to increased circulation and dissemination of inflammatory mediators from the inflamed joint (17,21). We have recently shown that, in vitro, cyclic tensile strain (CTS) suppresses actions of IL-1β on chondrocytes by inhibiting expression of multiple proinflammatory genes such as iNOS, COX-2, and matrix metalloproteinase 1 (MMP-1) (22,23). Additionally, CTS actions include proteoglycan synthesis and induction of reparative proteins such as tissue inhibitors of metalloproteinase 2 (TIMP-2). Because of the pivotal role of TNFα in the pathogenesis of inflammatory joint diseases, in this study we examined whether the antiinflammatory effects of CTS are also mediated via suppression of TNFα actions. By exposing articular chondrocytes to CTS in vitro, we demonstrate that CTS is a potent antagonist of TNFα actions and exerts its effects via transcriptional regulation of TNFα response elements.
MATERIALS AND METHODS
Isolation and characterization of rabbit articular chondrocytes
Slices (~70–100 μm thick) of hyaline articular cartilage were aseptically shaved from the surface of shoulder and knee joints of young adult NZW rabbits (10–12 pounds). Chondrocytes were released using 0.2% trypsin, followed by digestion using 0.2% clostridial collagenase (Sigma, St. Louis, MO) (22). Cells were washed in tissue culture medium (F-12 medium; Gibco, Grand Island, NY) supplemented with 10% fetal calf serum, 100 units/ml penicillin, and 10 μg/ml streptomycin, then adjusted to 105 cells/2 ml, transferred to 6-well, pronectin-coated (22) BioFlex culture plates (Flexcell International, McKeesport, PA), and cultured at 37°C in 5% CO2 for 8 days (23). The cultures reached 90% confluence in 6–8 days. In primary cultures, these chondrocytes retain their differentiated phenotype and synthesize chondroitin sulfate (CS) proteoglycans and CII (24), as well as expression of messenger RNA (mRNA) for aggrecan, biglycan, transforming growth factor β1 and CII. Four- to 8-week cultures of such chondrocytes exhibit synthesis of a cartilaginous matrix with tensile stiffness similar to that found in vivo (24–26). Since cartilage lacks blood, nerves, and lymphatics, it is highly unlikely that these cultures would be contaminated by other cell types. Further, such chondrocytes respond to IL-1β in a manner similar to that of cartilage explants (24,25).
Exposure of chondrocytes to equibiaxial CTS and TNFα
Confluent primary chondrocyte monolayers grown on BioFlex 6-well culture plates were subjected to equibiaxial strain in a Flexercell strain unit (Flexcell International) (22,27). Chondrocytes were grown on a rubber membrane coated with ProNectin F (Protein Polymer Technologies, San Diego, CA). To provide uniform radial and circumferential strain on the membrane, the plates were placed on a loading station (located in an incubator with 5% CO2 and 95% humidity), such that when vacuum was applied to the loading station, the membrane deformed across the post face, creating uniform biaxial strain. The radial and circumferential strains exerted on this membrane at various vacuum levels were experimentally determined by the displacement of 3 dots made on the BioFlex membrane relative to vacuum pressure. A metric scale was used to calibrate the distances in the imaging software (Optimas; Media Cybernetics, Silver Spring, MD).
In this system, adherent chondrocytes were subjected to a strain identical to that exerted on the membrane, as determined by 2-dimensional microscopic examination using a 20× objective, followed by morphometric analysis of the images by Optimas software (22,23,28). The strain was calculated as circumferential strain = 2π (change in radius)/2π (original radius) = (change in radius)/(original radius) = radial strain. The results showed a nearly linear relationship between the vacuum level and percentage of strain exerted on the membrane.
Earlier, we observed that CTS with a magnitude of 10% or higher is proinflammatory in nature, whereas 3–6% CTS is antiinflammatory and inhibits IL-1–induced proinflammatory gene induction (22,23). Therefore, in these studies we exposed the cells to 6% CTS in a Flexercell unit at a rate of 3 cycles per minute (0.05 Hz), i.e., 10 seconds of a maximum of 6% equibiaxial stress followed by 10 seconds of relaxation per cycle (180 cycles/hour), providing reproducible suppression of TNFα-induced iNOS mRNA expression and NO production. In all experiments, cells located on the membrane across the post face were scraped and used in all experiments, whereas cells located in the periphery of the membrane were discarded.
In a majority of experiments, chondrocytes were divided into 4 groups: untreated and unstressed control cells, cells treated with CTS alone, cells treated with TNFα (1 ng/ml) alone, and cells treated with CTS and TNFα (1 ng/ml). The cells were subjected to CTS when TNFα was added. Studies with various concentrations of recombinant human TNFα (rHuTNFα) (0.1, 0.5, 1.0, 5.0, 10.0 ng/ml) as a stimulus indicated that 1 ng/ml TNFα optimally induced iNOS mRNA expression within 4 hours of incubation. Trypan blue exclusion confirmed the viability of >99% of cells in culture following all treatments.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA was extracted with an RNA extraction kit (Qiagen, Valencia, CA). A total of 0.5 μg of RNA was mixed with 1 μg oligo(dT) (12–18 oligomers; Perkin-Elmer Cetus, Norwalk, CT) in reverse transcription buffer and incubated for 10 minutes at room temperature (23). Thereafter, the reaction mixture was cooled on ice and incubated with 200 units of Moloney murine leukemia virus RT for 60 minutes at 37°C. The complementary DNA thus obtained was amplified with 0.1 μg of specific primers in a reaction mixture containing 200 μM deoxynucleoside triphosphates and 0.1 units of Taq polymerase in PCR buffer (Perkin-Elmer Cetus). PCR was performed in a DNA thermal cycler (Perkin-Elmer Cetus) for 30 cycles of 40 seconds at 94°C, 40 seconds at 62°C, and 60 seconds at 72°C.
The sequence of sense and antisense rabbit primers used was as follows: GAPDH (548 bp) sense 5′-GGTGAAGGTCGGAGTCAACGG-3′, antisense 5′-GGTCATGAGTCCTTCCACGAT-3′; iNOS (243 bp) sense 5′-CGCCCTTCCGCAGTTTCT-3′, antisense 5′-TCCAGGAGGACATGCAGCAC-3′; MMP-1 (322 bp) sense 5′-TCAGTTCGTCCTCACTCCAG, antisense 5′-TTGGTCCACCTGTCATCTTC; TIMP-1 (326 bp) sense 5′-GCAACTCCGACCTTGTCATC-3′, antisense 5′-AGCGTAGGTCTTGGTGAAGC-3′; TIMP-2 (414 bp) sense 5′-GTATGATCAGGGCCAAG-3′, antisense 5′-TTCTCTGTGACCCAGTCCAT-3′; and COX-2 (282 bp) sense 5′-TCAGCCACGCAGCAAATCCT-3′, antisense 5′-GTCATCTGGATGTCAGCACG-3′ (23). PCR products on agarose gels were subjected to semiquantitative image analysis using a Fluor-S MultiImager system (Bio-Rad, Hercules, CA). In each case, photographic images are presented from 1 representative experiment out of a total of 3 experiments. The significance of differences between mean values of experimental and control groups was determined by analysis of variance.
Prostaglandin E2 (PGE2) measurements
PGE2 was measured in the culture supernatants of chondrocytes at various intervals by radioimmunoassay (Amersham Pharmacia Biotech, Piscataway, NJ).
Western blot analysis
After various treatments, chondrocytes (3 × 106) were washed with ice-cold phosphate buffered saline scraped from Flex II plates (Flexcell), immediately lysed in 200 μl of ice-cold cell lysis buffer (20 mM HEPES [pH 7.5], 150 mM NaCl, 1% Nonidet P40, and 1 mM Na3VO4) containing EDTA-free complete protease inhibitor cocktail (1 mM benzamidine, 0.4 mM phenylmethylsulfonyl fluoride, 1 mM sodium metabisulfite, 10 μg ml leupeptin, and 10 μg aprotinin [all from Sigma]), and centrifuged at 16,000g for 10 minutes. The supernatant was cleared twice with 50 μl of GammaBind G–Sepharose slurry (Amersham Pharmacia Biotech). The lysate was aliquoted in 50-μl samples, frozen quickly, and placed in a −70°C freezer until use. Total protein in lysates was determined by Bio-Rad protein assay using bovine serum albumin (0–100 μg) as a standard. The synthesis of iNOS and collagenase was assessed in 50 μg of protein extracts by Western blot analysis (23), using goat anti-iNOS (Transduction Laboratories, Lexington, KY) or anti–MMP-1 (Santa Cruz Biotechnology, Santa Cruz, CA) as the primary antibodies, donkey anti-goat horseradish peroxidase (HRP) as secondary antibodies, and luminol as a chemiluminescent HRP substrate. The semiquantitative assessment of the luminescence in each band was performed by exposing the blots to Reflection autoradiographic film, followed by semiquantitative analysis of the luminescent bands using a Fluor-S MultiImager (Bio-Rad).
Proteoglycan synthesis
Total proteoglycan synthesis was measured by incorporation of Na235SO4 into CS proteoglycans during the last 8 hours of the experiment. Subsequently, culture supernatants were extracted with 0.5M NaOH, and the incorporated precursor was separated by size-exclusion chromatography using a PD-10 column (Amersham Pharmacia Biotech). The 35S incorporation in proteoglycans was measured by scintillation counting (22).
RESULTS
CTS suppresses rHuTNFα-dependent induction of iNOS
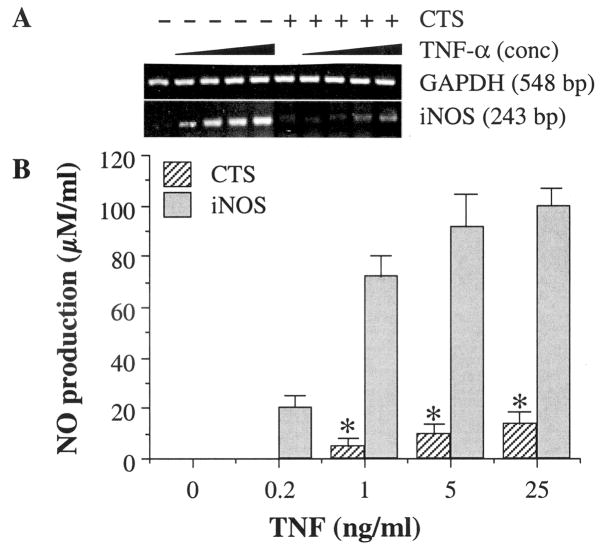
TNFα activates multiple proinflammatory genes (9–12), such as COX-2, iNOS, and metalloproteinases, which initiate cartilage destruction, whereas NO and PGE2 generated by COX-2 and iNOS further amplify and regulate cellular metabolism. We have previously observed that 6% CTS optimally inhibits IL-1β–induced proinflammatory responses (22,23). Therefore, to evaluate the effects of CTS on TNFα actions, chondrocytes were first exposed to various concentrations of TNFα in the presence of 6% CTS for 24 hours. Afterwards, the level of iNOS mRNA expression was analyzed by RT–quantitative competitive–PCR. As shown in Figure 1A, TNFα induced significant levels of iNOS mRNA in a dose-dependent manner, which reached an optimal level with 1 ng/ml TNFα. In parallel experiments, simultaneous exposure of cells to 6% CTS in the presence of various concentrations of TNFα consistently resulted in the suppression of iNOS mRNA expression, which was paralleled by inhibition of NO production (Figure 1B). Since CTS-mediated abrogation of TNFα-induced iNOS mRNA expression results in decreased synthesis of iNOS, this inhibition in NO synthesis can be attributed to suppression of iNOS synthesis. Under these conditions, chondrocytes subjected to CTS exhibited negligible cell detachment (<0.1%) versus unstressed control cells, as assessed by counting nonadherent cells in the wells, or cell death, as assessed by DNA fragmentation (results not shown).
Figure 1.
Effect of 6% cyclic tensile strain (CTS) on various concentrations of recombinant human tumor necrosis factor α (rHuTNFα)–induced nitric oxide synthase (iNOS) messenger RNA (mRNA) expression and NO production. A, Inhibition of iNOS mRNA expression in the presence of 0, 0.2, 1.0, 5.0, or 25.0 ng/ml rHuTNFα by 6% CTS (0.05 Hz), as assessed by reverse transcriptase–quantitative competitive–polymerase chain reaction. B, Accumulation of NO in the culture supernatants of the chondrocytes during a 24-hour period, showing inhibition of rHuTNFα-dependent NO production. The control unstressed chondrocytes(s) treated with CTS alone did not exhibit iNOS mRNA expression or NO production. The data in A represent 1 out of 3 separate experiments. Data in B represent means and SEM of triplicate values. * = P ≤ 0.05 versus cells treated with rHuTNFα alone.
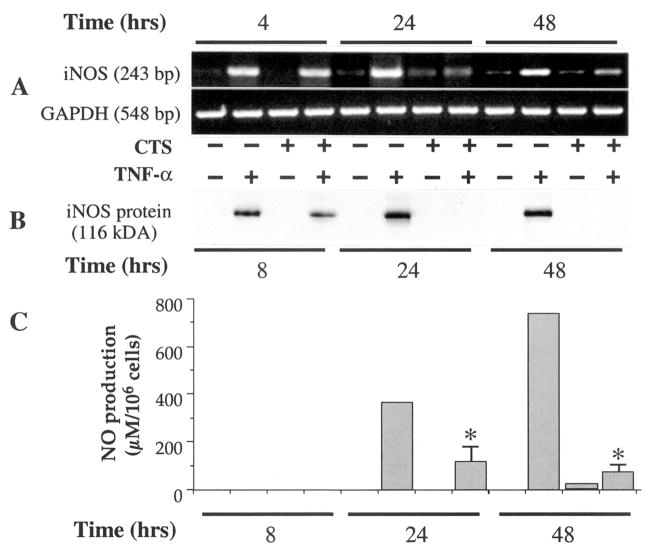
CTS down-regulates iNOS synthesis in rHuTNFα-treated chondrocytes
Since the effects of TNFα are sustained over a long period, we next determined whether the effects of CTS in inhibiting TNFα actions are also sustained. Therefore, chondrocytes were subjected to CTS regimens for 4, 8, 24, or 48 hours in the presence of TNFα. Thereafter, the cells were harvested, and total iNOS mRNA expression, iNOS synthesis, and NO production were assessed by RT-PCR, Western blot analysis, and Griess reaction, respectively. As expected, untreated control cells and cells subjected to stress alone exhibited no iNOS mRNA expression, protein synthesis, or NO production (Figures 2A–C). However, chondrocytes exposed to TNFα exhibited increased iNOS mRNA expression within 4 hours, which was sustained over the next 48 hours (Figure 2A). We did not observe significant iNOS synthesis during the first 4 hours of exposure of chondrocytes to TNFα. However, iNOS synthesis was apparent after 8 hours and continued during the ensuing 40 hours (Figure 2B). Accumulation of NO in the culture supernatants was not observed during the first 8 hours, but was significantly elevated at 24 hours and 48 hours (Figure 2C). More important, when chondrocytes were simultaneously exposed to TNFα and CTS, the iNOS mRNA expression was suppressed by 34%, 72%, and 79% at 4, 24, and 48 hours, respectively. The suppression of iNOS mRNA by CTS was paralleled by a significant decrease in iNOS synthesis and NO production (Figures 2A–C).
Figure 2.
Inhibition of rHuTNFα-dependent iNOS mRNA expression, iNOS synthesis, and NO production by CTS in articular chondrocytes. A, Expression of iNOS mRNA in chondrocytes either untreated or subjected to rHuTNFα (1 ng/ml) alone, CTS alone, or rHuTNFα and CTS for 4, 24, or 48 hours. B, Inhibition of iNOS synthesis by CTS in chondrocytes subjected to treatment regimens as described in A for 8, 24, or 48 hours. C, NO synthesis in chondrocytes subjected to treatment regimens described in A for 8, 24, or 48 hours, as measured in the culture supernatants by Griess reaction. Data in A and B represent 1 of 3 separate experiments. Data in C represent means and SEM of triplicate values. * = P ≤ 0.05 versus cells treated with rHuTNFα alone. See Figure 1 for definitions.
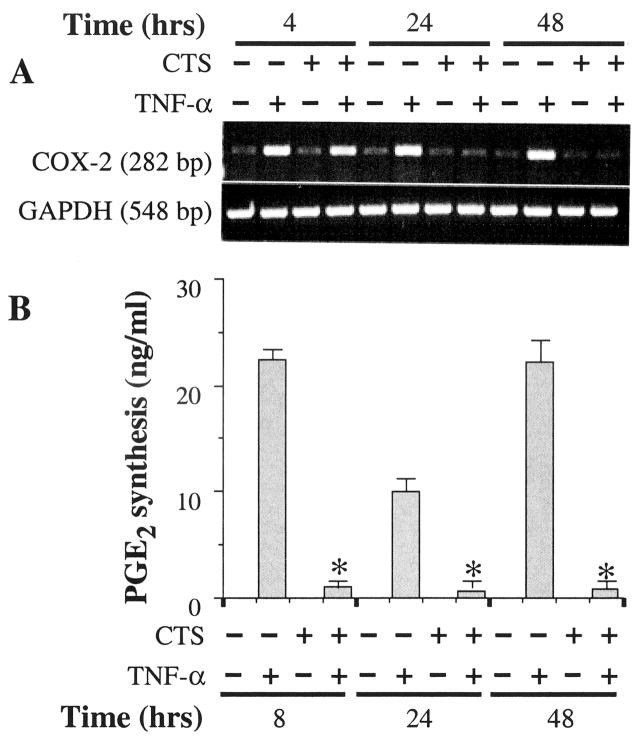
CTS suppresses TNFα-dependent induction of COX-2
Chondrocytes exhibit a marked increase of PGE2 synthesis following exposure to TNFα (12). To determine whether the physiologic consequences of CTS actions also involve inhibition of TNFα-dependent COX-2 induction and parallel reduction in PGE2 production, chondrocytes were subjected to TNFα and CTS simultaneously or individually for 4, 24, or 48 hours. As is apparent from Figure 3A, CTS significantly suppressed TNFα-induced COX-2 mRNA expression (P < 0.01) The measurement of the PCR products in each band by semiquantitative densitometric analysis revealed a 42%, 96%, and 92% inhibition of COX-2 mRNA expression within 4, 24, and 48 hours, respectively. This significant (P < 0.05) inhibition of COX-2 mRNA induction by CTS was paralleled by >99% inhibition of TNFα-dependent PGE2 production at all time points tested (Figure 3B), suggesting that CTS substantially contributes to the inhibition of proinflammatory actions of TNFα under these conditions. Unactivated chondrocytes, or those exposed to CTS alone, did not express COX-2 mRNA (Figures 3A and B). This was not surprising, since mechanical strain at moderate intensities has not been reported to exhibit proinflammatory effects.
Figure 3.
Inhibition of rHuTNFα-dependent cyclooxygenase 2 (COX-2) mRNA expression and prostaglandin E2 (PGE2) synthesis by CTS in articular chondrocytes. A, COX-2 mRNA expression in chondrocytes either untreated or subjected to rHuTNFα (1 ng/ml), CTS alone, or rHuTNFα and CTS for 4, 24, or 48 hours. B, PGE2 synthesis in chondrocytes subjected to treatment regimens described in A for 8, 24, or 48 hours, as measured in the culture supernatants by radioimmunoassay. Data in A represent 1 of 3 separate experiments. Data in B represent means and SEM of triplicate values. * = P ≤ 0.05 versus cells treated with rHuTNFα alone. See Figure 1 for other definitions.
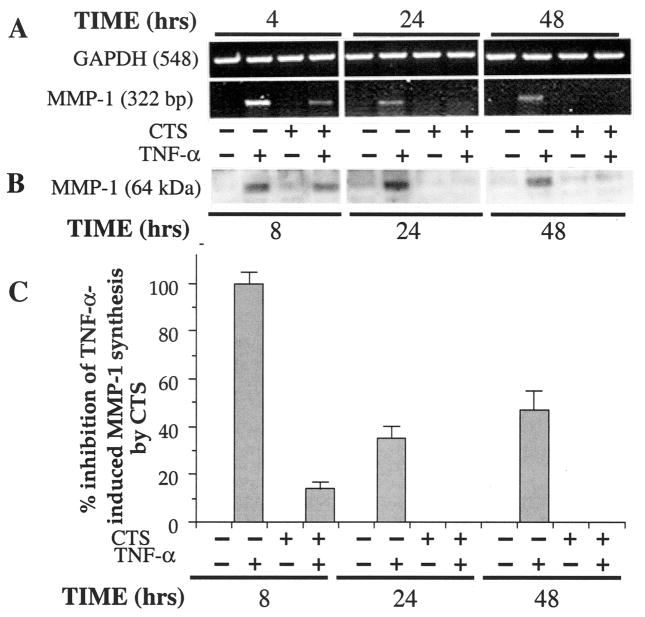
CTS suppresses TNFα-dependent induction of collagenase (MMP-1)
Synthesis of MMP-1 in response to IL-1β and TNFα plays a pivotal role in cartilage degradation in rheumatic diseases (10,11,28). Therefore, for effective control of TNFα-induced catabolism, CTS actions must include suppression of collagenase production. The examination of the effects of CTS on TNFα-dependent MMP-1 induction showed that, while chondrocytes exposed to TNFα expressed significant amounts of MMP-1, cells subjected to TNFα and CTS exhibited a significant reduction of MMP-1 mRNA expression, i.e., 63% during the first 4 hours, 98% after 24 hours, and >98% after 48 hours (Figure 4A). More important, the suppression of MMP-1 mRNA expression was reflected in the reduction in MMP-1 synthesis as assessed by Western blot analysis (showing an 86% inhibition during the first 8 hours and >98% after 24 and 48 hours [Figures 4B and C]). Untreated control cells did not express MMP-1 mRNA constitutively. CTS alone did not induce mRNA expression or synthesis of MMP-1, demonstrating that the actions of CTS on chondrocytes were totally dependent on the presence of an inflammatory signal, such as TNFα.
Figure 4.
Inhibition of rHuTNFα-dependent collagenase mRNA expression and synthesis by CTS in articular chondrocytes. A, Expression of collagenase mRNA in chondrocytes either untreated or treated with rHuTNFα, CTS alone, or rHuTNFα and CTS for 4, 24, or 48 hours. B, Collagenase synthesis by chondrocytes subjected to treatment regimens described in A. C, Percentage inhibition of collagenase synthesis, as measured by the relative intensity of each band in Western blot analysis. Data in A and B are representative of 1 of 3 separate experiments. Data in C represent means and SEM of triplicate values. * = P ≤ 0.05 versus cells treated with rHuTNFα alone. See Figure 1 for definitions.
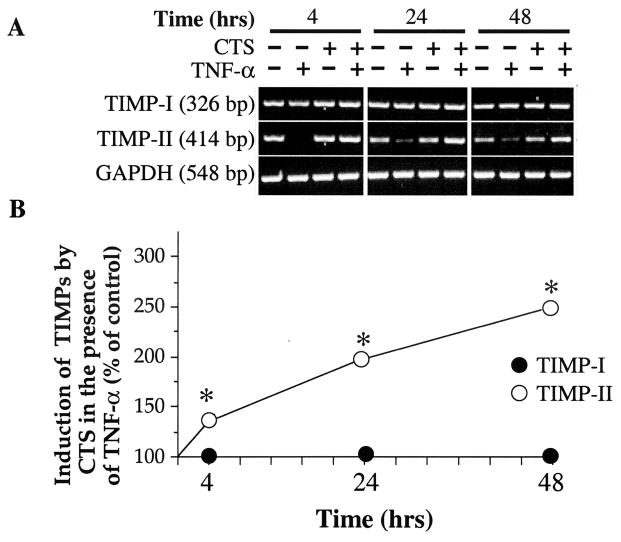
CTS induces expression of TIMPs in the presence of TNFα
Proinflammatory cytokines not only induce the synthesis of matrix-degrading enzymes but also inhibit TIMP induction (29,30). Inhibition of TIMPs prolongs the enzymatic activity of metalloproteinases. To examine whether CTS also abrogates TNFα-induced suppression of gene transcription for TIMPs, chondrocytes were exposed to TNFα in the presence as well as absence of CTS. The densitometric analysis of the RT-PCR products for TIMP-1 revealed that exposure to TNFα did not inhibit TIMP-1 mRNA expression significantly after 4, 24, or 48 hours of exposure (Figure 5A). Furthermore, CTS alone or in the presence of IL-1β did not affect TIMP-1 mRNA expression (Figures 5A and B).
Figure 5.
Effect of CTS on rHuTNFα-dependent inhibition of tissue inhibitors of metalloproteinases (TIMPs) mRNA expression. A, Expression of mRNA for TIMP-I and TIMP-II in chondrocytes either untreated or exposed to rHuTNFα, CTS alone, or rHuTNFα and CTS. The mRNA expression was assessed by reverse transcriptase–polymerase chain reaction using 1 μg RNA from cells in each group. Amplification of GAPDH mRNA was used to standardize equal input in all lanes. Data in A represent 1 of 3 separate experiments. Data in B represent means and SEM of triplicate values. * = P ≤ 0.05 versus cells treated with rHuTNFα alone. See Figure 1 for other definitions.
On the other hand, TNFα treatment of chondrocytes resulted in a consistent and significant (P < 0.05) inhibition of the constitutive expression of TIMP-2 mRNA (Figure 5A). Coexposure of chondrocytes to TNFα and CTS resulted in hyperinduction of TIMP-2 mRNA, as evidenced by observation of a mean ± SEM 2.2 ± 0.33–fold increase in TIMP-2 mRNA during the first 24 hours and a 2.7 ± 0.24–fold increase after 24 hours versus untreated control cells (P < 0.05). CTS alone did not affect TIMP-2 mRNA expression, demonstrating that CTS acts on chondrocytes in a TNFα-dependent manner.
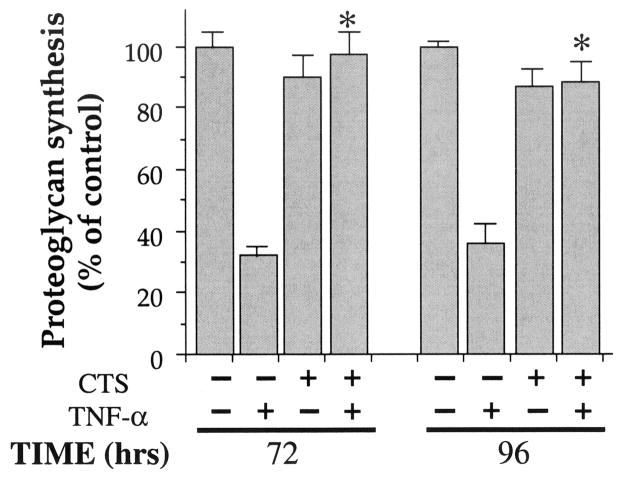
CTS abrogates TNFα-dependent inhibition of proteoglycan synthesis
The hallmark of rheumatic diseases is inhibition of proteoglycan synthesis in inflamed joints, which is attributed mainly to IL-1β and TNFα (1–7,31). Therefore, we next determined whether CTS also abrogates the effects of TNFα on proteoglycan synthesis. Chondrocytes exposed to TNFα showed a marked decrease in proteoglycan synthesis, as observed by incorporation of Na235SO4 in CS proteoglycans during the last 8 hours of the experiment (Figure 6). This decrease in proteoglycan synthesis was evident after incubation of chondrocytes with TNFα for 72 hours (67% reduction), and further reduction after 96 hours (63% reduction). Nevertheless, coexposure of chondrocytes with CTS completely abrogated TNFα-induced inhibition of proteoglycan synthesis during the first 72 hours, and reduced it by 84% after 96 hours. Interestingly, cells treated with CTS alone also exhibited a 10 ± 2% to 14 ± 3% (mean ± SEM) down-regulation of proteoglycan synthesis after 72 and 96 hours, versus untreated controls.
Figure 6.
Effect of CTS on rHuTNFα-dependent inhibition of proteoglycan synthesis. Total chondroitin sulfate proteoglycans in cell culture supernatants of chondrocytes either untreated or treated with rHuTNFα (1 ng/ml), CTS alone, or rHuTNFα and CTS for 72 or 96 hours. Proteoglycans were assessed by incorporation of Na235SO4 during the last 8 hours of incubation. Data represent means and SEM of triplicate values in 1 of 3 separate experiments. * = P ≤ 0.05 versus cells treated with rHuTNFα alone.
DISCUSSION
Our observations provide evidence that signals generated by CTS act as powerful antagonists of the actions of TNFα, one of the proinflammatory cytokines implicated in the etiology of rheumatic diseases (1–13). Interestingly, the observations that continuous passive motion reduces inflammation and facilitates physiologically sound recovery in orthopedic patients also point to the possible antiinflammatory/reparative actions of mechanical strain in vivo (15–20). We have used equibiaxial CTS on chondrocytes to closely simulate the effects of continuous passive motion. The chondrocytes used in these studies were superficial layers of cartilage from 70 to 100 μm thick. These cells thus contained chondrocytes primarily from the superficial zone and from the upper middle zone of the cartilage (32–34). Additionally, we have used TNFα as an inflammatory agent because it is one of the major cytokines found in rheumatoid synovial fluids (2,6–8). Furthermore, chondrocytes are known to be sensitive to TNFα and respond to it via induction of proinflammatory molecules (5–8). Therefore, this system provided defined parameters to examine the biochemical responses of chondrocytes to CTS in the presence and absence of mediators of inflammation.
CTS suppresses TNFα-dependent induction of mRNA for multiple proteins responsible for cartilage destruction in chondrocytes, in a sustained manner. CTS abrogates NO and PGE2 production by reducing the mRNA abundance of 2 pivotal proinflammatory enzymes, iNOS and COX-2. Both of these molecules mediate cytokine-induced reduction of proteoglycan synthesis (10,35–38). Earlier studies indicated that CTS inhibits IL-1β actions over a wide range of concentrations, encompassing those frequently found in synovial fluids (7,8). Here we show that CTS also inhibits TNFα actions over a wide range of concentrations, thus emphasizing the potential of CTS as an antiinflammatory agent of a broader specificity. However, CTS actions are cytokine dependent, since CTS alone failed to induce a response. This verifies earlier reports that CTS of a low magnitude alone is not sufficient to induce synthesis of proinflammatory mediators.
Recently, a significant amount of information has been made available in the areas of mechanical behavior of the cartilage and the mechanisms of mechanotransduction in chondrocytes (32–34,39,40). There is evidence that cartilage is exposed to ~15% compressive strain under physiologic conditions (39). Furthermore, based upon their location within the cartilage, chondrocytes respond to compressive strain disparately (32,33). Specifically, chondrocytes in the superficial layers of cartilage explants, when subjected to 15% compressive loading, are submitted to 1–5% tensile strain perpendicular to the split-line pattern (33). Thus, the use of 6% CTS in our experiments is close to the magnitude of cyclic tensile strain, experienced by chondrocytes under physiologic conditions. Furthermore, at this magnitude of tensile strain, cell deformation is not affected temporally over a period of 6 days (40).
We have observed that 6% CTS inhibits TNFα-induced induction of iNOS and NO production. Interestingly, 15–19% cyclic compressive loading of chondrocytes seeded in agarose gels, which lie within physiologic range, inhibits NO production under noninflammatory conditions. However, in these studies, compressive strain inhibited constitutive NOS production (39,41). On the other hand, a magnitude of tensile strain of 12.5% or higher appears to be proinflammatory in nature and promotes iNOS and COX-2 synthesis (22,23,42). Considering that 15% compressive loading in cartilage explants exercises 1–5% tensile strain on chondrocytes (40), it is tempting to speculate that magnitudes of mechanical strain of 12.5% or higher exerted directly upon cells in these studies may be sufficiently above physiologic range to evoke an inflammatory response.
Cartilage breakdown in rheumatic diseases occurs via breakdown of extracellular matrix and the inhibition of its synthesis. During inflammation of joints, chondrocytes exhibit chronic collagenase production, which is attributed to the presence of proinflammatory cytokines such as IL-1β and TNFα (11,14,37). Therefore, for the effective control of cartilage catabolism, prolonged suppression of collagenase production is required. In this respect, CTS appears to be an effective inhibitor of collagenase synthesis because exposure of cells to CTS results in a significant and prolonged suppression of TNFα-dependent collagenase synthesis via inhibition of its mRNA expression. Nevertheless, a low magnitude of CTS alone was not sufficient to induce collagenase synthesis in these studies.
TNFα and IL-1β both down-regulate production of TIMPs and thereby augment collagen breakdown in chondrocytes (29,30,43), while applications of TIMP-1 and TIMP-2 inhibit IL-1β–induced collagen degradation in cartilage (30,43). The results described here show that, parallel with the inhibition of collagenase production, CTS abrogates the TNFα-mediated inhibition of TIMP-2 production. Moreover, in the presence of TNFα, CTS induces a 2–2.5-fold increase in TIMP-2 induction over the untreated control cells. Whether all of the TIMP-2 induced by CTS is in its active form is not yet clear. TIMP-2 has been shown to block collagenase activity effectively at less than an equimolar ratio, suggesting that the observed hyperinduction of TIMP-2 in its active form by CTS for prolonged periods may be an effective mechanism to reduce metalloproteinase-mediated extracellular matrix degradation. As noted with the other proinflammatory mediators, the increased TIMP-2 transcription by CTS takes place exclusively in the presence of an inflammatory signal such as TNFα, while exposure of chondrocytes to CTS alone is not sufficient for the induction of TIMP-2. Constitutive expression of TIMP-1 in these studies was affected by neither TNFα nor CTS.
The synthesis of the major component of the cartilaginous extracellular matrix, CS proteoglycan, is known to be dramatically reduced in inflamed joints (7–9,14, 44). On the other hand, moderate exercise leads to an increase in the proteoglycan content of inflamed articular cartilage (45–47), while immobilization of joints leads to a reversible decrease in proteoglycan contents. Our data show that biochemical signals generated by mechanical strain play a critical role in the up-regulation of proteoglycan synthesis in inflamed joints. Specifically, mechanotransduction leads to abrogation of specific TNFα-dependent signal pathways that lead to the inhibition of proteoglycan synthesis. Since NO inhibits sulfation of proteoglycans (48), it is also possible that CTS negates the effects of TNFα-dependent suppression of proteoglycan synthesis via inhibition of NO production. Thus, by counteracting TNFα-mediated inhibition of proteoglycans and iNOS synthesis, CTS may play a dynamic role in matrix deposition. These actions of CTS are similar to those observed during inflammation caused by IL-1β, where CTS has been shown to hyperinduce aggrecan synthesis in the presence of IL-1β (23).
We have also observed that CTS alone can decrease proteoglycan synthesis by 10–15%. In response to cyclic loading, intact articular cartilage has also been shown to synthesize proteoglycans of low molecular weight at a disproportionately higher rate than the proteoglycan synthesized by unstressed cartilage (49,50). Proteoglycans of low molecular weight can be dispersed in the medium in response to stress, whereas macromolecular aggregates remain attached to the tissue (48,51). These findings may explain the observed decrease in proteoglycan synthesis in chondrocytes treated with CTS alone. In this context, mechanotransduction pathways induced by dynamic compressive loading at different frequencies inhibit glycosaminoglycan (GAG) synthesis disparately. For example, dynamic compressive loading of low frequencies (0.3 Hz) inhibits GAG synthesis, whereas a frequency of 1 Hz stimulates GAG production in chondrocytes (52). Therefore, the observed reduction in GAG synthesis in our studies may occur due to the low frequency (0.05 Hz) of CTS used.
In summary, we have shown that CTS at physiologic levels is an important regulator of chondrocyte metabolic activities. CTS acts as an effective antagonist of TNFα actions on chondrocytes, and its intracellular actions are mediated through transcriptional regulation of multiple proinflammatory genes activated by TNFα. By down-regulating induction of catabolic proteins and up-regulating induction of extracellular matrix proteins, CTS acts not only as an antiinflammatory signal but also as a reparative signal in IL-1β–treated chondrocytes. Since CTS also inhibits actions of IL-1β, it appears that the actions of CTS involve disruption or regulation of critical step(s) common to the IL-1β and TNFα signal transduction cascade. These in vitro studies may thus explain the mechanisms underlying the reparative actions of moderate exercise and continuous passive motion during joint inflammation.
Likewise, the present findings are also consistent with observations that physiologic levels of exercise do not cause alterations in the structure and mechanical properties of articular cartilage (47). This is emphasized by the fact that the presence of IL-1β and/or TNFα is essential for the actions of CTS, since CTS at physiologic levels is neither proinflammatory nor induces increased matrix synthesis. Our results thus not only provide molecular evidence for the biochemical signals generated by CTS, but also provide crucial leads to further unveil the pathways regulated by mechanical strain, for a better understanding of the mechanisms of continuous passive motion–mediated reparative actions on inflamed joints.
Acknowledgments
Supported by grants from the National Center for Complementary and Alternative Medicine, NIH (grant AT-00646), and the Oral and Maxillofacial Surgery Foundation.
References
- 1.Tanaka SC, Hamanishi C, Kikuchi H, Fukuda K. Factors related to degradation of articular cartilage in osteoarthritis: a review. Semin Arthritis Rheum. 1998;27:392–9. doi: 10.1016/s0049-0172(98)80019-x. [DOI] [PubMed] [Google Scholar]
- 2.Flugge LA, Miller-Deist LA, Petillo PA. Towards a molecular understanding of arthritis. Chem Biol. 1999;6:R157–66. doi: 10.1016/S1074-5521(99)80043-X. [DOI] [PubMed] [Google Scholar]
- 3.Van den Berg WB. Lessons for joint destruction from animal models. Curr Opin Rheumatol. 1997;9:221–8. doi: 10.1097/00002281-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Bandara G, Georgescu HI, Lin CW, Evans CH. Synovial activation of chondrocytes: evidence for complex cytokine interactions. Agents Actions. 1991;34:285–8. doi: 10.1007/BF01993304. [DOI] [PubMed] [Google Scholar]
- 5.Carteron NL. Cytokines in rheumatoid arthritis: trials and tribulations. Mol Med Today. 2000;6:315–23. doi: 10.1016/s1357-4310(00)01757-3. [DOI] [PubMed] [Google Scholar]
- 6.Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40:1–11. doi: 10.3109/03008209909005273. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berg WB, Joosten LAB, Kollias G, van De Loo FAJ. Role of tumor necrosis factor α in experimental arthritis: separate activity of interleukin-1β in chronicity and cartilage destruction. Ann Rheum Dis. 1999;58(Suppl 1):I40–8. doi: 10.1136/ard.58.2008.i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, et al. Transgenic mice expressing human tumor necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–31. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maini RN, Elliott M, Brennan FM, Williams RO, Feldmann M. TNF blockade in rheumatoid arthritis: implications for therapy and pathogenesis. APMIS. 1997;105:257–63. doi: 10.1111/j.1699-0463.1997.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 10.Richardson DW, Dodge GR. Effects of interleukin-1beta and tumor necrosis factor-alpha on expression of matrix-related genes by cultured equine articular chondrocytes. Am J Vet Res. 2000;61:624–30. doi: 10.2460/ajvr.2000.61.624. [DOI] [PubMed] [Google Scholar]
- 11.Shlopov BV, Gumanovskaya ML, Hasty KA. Autocrine regulation of collagenase 3 (matrix metalloproteinase 13) during osteoarthritis. Arthritis Rheum. 2000;43:195–205. doi: 10.1002/1529-0131(200001)43:1<195::AID-ANR24>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Morisset S, Patry C, Lora M, de Brum-Fernandes AJ. Regulation of cyclooxygenase-2 expression in bovine chondrocytes in culture by interleukin-1α, tumor necrosis factor-α, glucocorticoids, and 17β-estradiol. J Rheumatol. 1998;25:1146–53. [PubMed] [Google Scholar]
- 13.Dodge GR, Diaz A, Sanz-Rodriguez C, Reginato AM, Jimenez SA. Effects of interferon-γ and tumor necrosis factor α on the expression of the genes encoding aggrecan, biglycan, and decorin core proteins in cultured human chondrocytes. Arthritis Rheum. 1998;41:274–83. doi: 10.1002/1529-0131(199802)41:2<274::AID-ART11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Kozaci LD, Buttle DJ, Hollander AP. Degradation of type II collagen, but not proteoglycan, correlates with matrix metalloproteinase activity in cartilage explant cultures. Arthritis Rheum. 1997;40:164–74. doi: 10.1002/art.1780400121. [DOI] [PubMed] [Google Scholar]
- 15.McCarty WL, Jr, Darnell MW. Rehabilitation of the temporomandibular joint through the application of motion. Cranio. 1993;11:298–307. doi: 10.1080/08869634.1993.11677982. [DOI] [PubMed] [Google Scholar]
- 16.Kim HK, Kerr RG, Cruz TF, Salter RB. Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J Rheumatol. 1995;22:1714–21. [PubMed] [Google Scholar]
- 17.Salter RB. The physiologic basis of continuous passive motion for articular cartilage healing and regeneration. Hand Clin. 1994;10:211–9. [PubMed] [Google Scholar]
- 18.Colwell CW, Jr, Morris BA. The influence of continuous passive motion on the results of total knee arthroplasty. Clin Orthop. 1992;276:225–8. [PubMed] [Google Scholar]
- 19.Koob TJ, Clark PE, Hernandez DJ, Thurmond FA, Vogel KG. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch Biochem Biophys. 1992;298:303–12. doi: 10.1016/0003-9861(92)90127-i. [DOI] [PubMed] [Google Scholar]
- 20.Williams JM, Moran M, Thonar EJ, Salter RB. Continuous passive motion stimulates repair of rabbit knee articular cartilage after matrix proteoglycan loss. Clin Orthop. 1994;304:252–62. [PubMed] [Google Scholar]
- 21.Von Schroeder HP, Coutts RD, Billings E, Jr, Mai MT, Aratow M. The changes in intramuscular pressure and femoral vein flow with continuous passive motion, pneumatic compressive stockings, and leg manipulations. Clin Orthop. 1991;266:218–26. [PubMed] [Google Scholar]
- 22.Gassner R, Buckley MJ, Georgescu H, Studer R, Stefanovich-Racic M, Piesco NP, et al. Cyclic tensile stress exerts antiinflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163:2187–92. [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of interleukin-1β actions in chondrocytes. J Immunol. 2000;165:453–60. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedewa MM, Oegema TR, Jr, Schwartz MH, MacLeod A, Lewis JL. Chondrocytes in culture produce a mechanically functional tissue. J Orthop Res. 1998;16:227–36. doi: 10.1002/jor.1100160210. [DOI] [PubMed] [Google Scholar]
- 25.Enomoto-Iwamoto M, Iwamoto M, Nakashima K, Mukudai Y, Boettiger D, Pacifici M, et al. Involvement of alpha5beta1 integrins in matrix interactions and proliferation of chondrocytes. J Bone Miner Res. 1997;12:1124–32. doi: 10.1359/jbmr.1997.12.7.1124. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Grynpas M, Kandel RA. Composition of cartilaginous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials. 1997;18:1425–31. doi: 10.1016/s0142-9612(97)00071-9. [DOI] [PubMed] [Google Scholar]
- 27.Lee AA, Delhaas T, Waldman LK, MacKenna DA, Villarreal FJ, McCulloch AD. An equibiaxial strain system for cultured cells. Am J Physiol. 1996;271:C1400–8. doi: 10.1152/ajpcell.1996.271.4.C1400. [DOI] [PubMed] [Google Scholar]
- 28.Borden P, Solymar D, Sucharczuk A, Lindman B, Cannon P, Heller RA. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J Biol Chem. 1996;271:23577–81. doi: 10.1074/jbc.271.38.23577. [DOI] [PubMed] [Google Scholar]
- 29.Cawston T, Billington C, Cleaver C, Elliott S, Hui W, Koshy P, et al. The regulation of MMPs and TIMPs in cartilage turnover. Ann N Y Acad Sci. 1999;878:120–9. doi: 10.1111/j.1749-6632.1999.tb07678.x. [DOI] [PubMed] [Google Scholar]
- 30.Shingu M, Nagai Y, Isayama T, Naono T, Nobunaga M, Nagai Y. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin Exp Immunol. 1993;94:145–9. doi: 10.1111/j.1365-2249.1993.tb05992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joosten LAB, Helsen MMA, van de Loo FAJ, van den Berg WB. Anticytokine treatment of established type II collagen–induced arthritis in DBA/1 mice: a comparative study using anti-TNFα, anti–IL-1α/β, and IL-1Ra. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 32.Lee DA, Noguchi T, Knight MM, O’Donnell L, Bentley G, Bader DL. Response of chondrocyte subpopulations cultured within unloaded and loaded agarose. J Orthop Res. 1998;16:726–33. doi: 10.1002/jor.1100160615. [DOI] [PubMed] [Google Scholar]
- 33.Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13:410–21. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- 34.Broom ND, Myers DB. A study of the structural response of wet hyaline cartilage to various loading situations. Connect Tissue Res. 1980;7:227–37. doi: 10.3109/03008208009152358. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi T, Abe E, Yamate T, Taguchi Y, Jasin HE. Nitric oxide production by superficial and deep articular chondrocytes. Arthritis Rheum. 1997;40:261–9. doi: 10.1002/art.1780400210. [DOI] [PubMed] [Google Scholar]
- 36.Fukuda K, Ohtani K, Dan H, Tanaka S. Interleukin-1 inhibits keratan sulfate production by rabbit chondrocytes: possible role of prostaglandin E2. Inflamm Res. 1995;44:178–81. doi: 10.1007/BF01782816. [DOI] [PubMed] [Google Scholar]
- 37.Taskiran D, Stefanovic-Racic M, Georgescu H, Evans C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem Biophys Res Commun. 1994;200:142–8. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 38.Lane NE, Williams RJ, III, Schurman DJ, Smith RL. Inhibition of interleukin 1 induced chondrocyte protease activity by a corticosteroid and a nonsteroidal antiinflammatory drug. J Rheumatol. 1992;19:135–9. [PubMed] [Google Scholar]
- 39.Lee DA, Noguchi T, Frean SP, Lees P, Bader DL. The influence of mechanical loading on isolated chondrocytes seeded in agarose constructs. Biorheology. 2000;37:149–61. [PubMed] [Google Scholar]
- 40.Lee DA, Bader DL. The development and characterization of an in vitro system to study strain-induced cell deformation in isolated chondrocytes. In Vitro Cell Dev Biol Anim. 1995;31:828–35. doi: 10.1007/BF02634565. [DOI] [PubMed] [Google Scholar]
- 41.Lee DA, Frean SP, Lees P, Bader DL. Dynamic mechanical compression influences nitric oxide production by articular chondrocytes seeded in agarose. Biochem Biophys Res Commun. 1998;251:580–5. doi: 10.1006/bbrc.1998.9520. [DOI] [PubMed] [Google Scholar]
- 42.Fujisawa T, Hattori T, Takahashi K, Kuboki T, Yamashita A, Takigawa M. Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J Biochem (Tokyo) 1999;125:966–75. doi: 10.1093/oxfordjournals.jbchem.a022376. [DOI] [PubMed] [Google Scholar]
- 43.Ellis AJ, Curry VA, Powell EK, Cawston TE. The prevention of collagen breakdown in bovine nasal cartilage by TIMP, TIMP-2 and a low molecular weight synthetic inhibitor. Biochem Biophys Res Commun. 1994;201:94–101. doi: 10.1006/bbrc.1994.1673. [DOI] [PubMed] [Google Scholar]
- 44.Häuselmann HJ, Flechtenmacher J, Michal L, Thonar EJ-MA, Shinmei M, Kuettner KE, et al. The superficial layer of human articular cartilage is more susceptible to interleukin-1–induced damage than the deeper layers. Arthritis Rheum. 1996;39:478–88. doi: 10.1002/art.1780390316. [DOI] [PubMed] [Google Scholar]
- 45.Burton-Wurster N, Vernier-Singer M, Farquhar T, Lust G. Effect of compressive loading and unloading on the synthesis of total protein, proteoglycan, and fibronectin by canine cartilage explants. J Orthop Res. 1993;11:717–29. doi: 10.1002/jor.1100110514. [DOI] [PubMed] [Google Scholar]
- 46.Kim YJ, Sah RLY, Grodzinsky AJ, Plaas AHK, Sandy JD. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys. 1994;311:1–12. doi: 10.1006/abbi.1994.1201. [DOI] [PubMed] [Google Scholar]
- 47.Newton PM, Mow VC, Gardner TR, Buckwalter JA, Albright JP. The effect of lifelong exercise on canine articular cartilage. Am J Sports Med. 1997;25:282–7. doi: 10.1177/036354659702500302. [DOI] [PubMed] [Google Scholar]
- 48.Hickery MS, Bayliss MT. Interleukin-1 induced nitric oxide inhibits sulphation of glycosaminoglycan chains in human articular chondrocytes. Biochim Biophys Acta. 1998;1425:282–90. doi: 10.1016/s0304-4165(98)00080-4. [DOI] [PubMed] [Google Scholar]
- 49.Campbell MA, Handley CJ, D’Souza SE. Turnover of proteoglycans in articular-cartilage cultures: characterization of proteoglycans released into the medium. Biochem J. 1989;259:21–5. doi: 10.1042/bj2590021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Kampen GP, Korver GH, van de Stadt RJ. Modulation of proteoglycan composition in cultured anatomically intact joint cartilage by cyclic loads of various magnitudes. Int J Tissue React. 1994;16:171–9. [PubMed] [Google Scholar]
- 51.Steinmeyer J, Knue S. The proteoglycan metabolism of mature bovine articular cartilage explants superimposed to continuously applied mechanical loading. Biochem Biophys Res Commun. 1997;240:216–21. doi: 10.1006/bbrc.1997.7641. [DOI] [PubMed] [Google Scholar]
- 52.Lee DA, Bader DL. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181–8. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]